Abstract
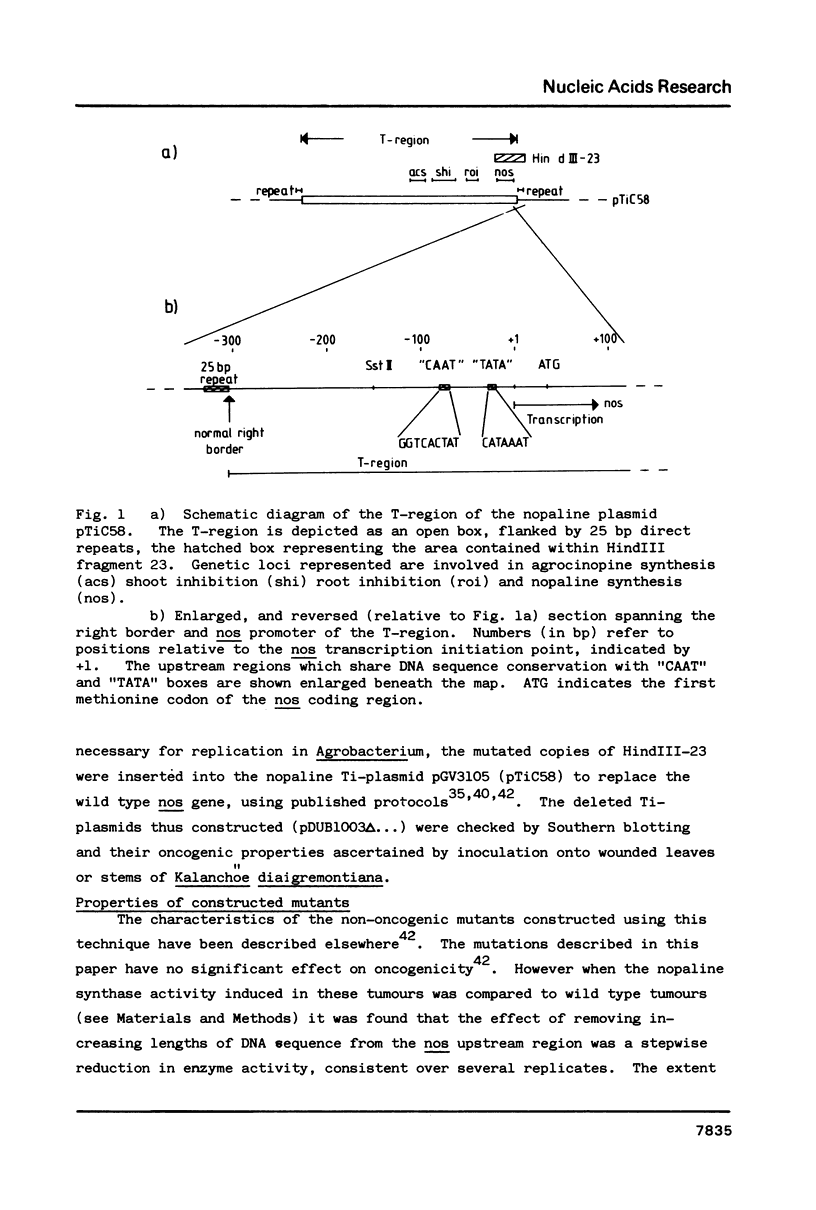
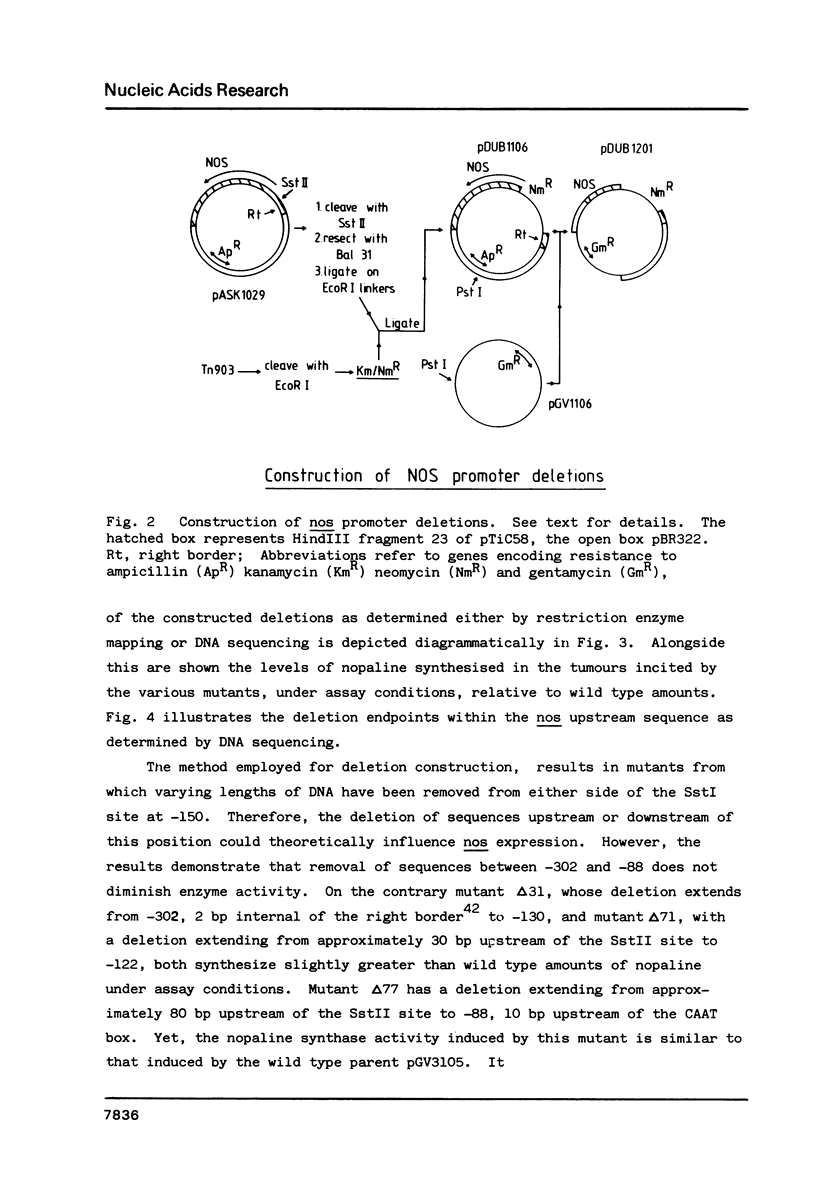
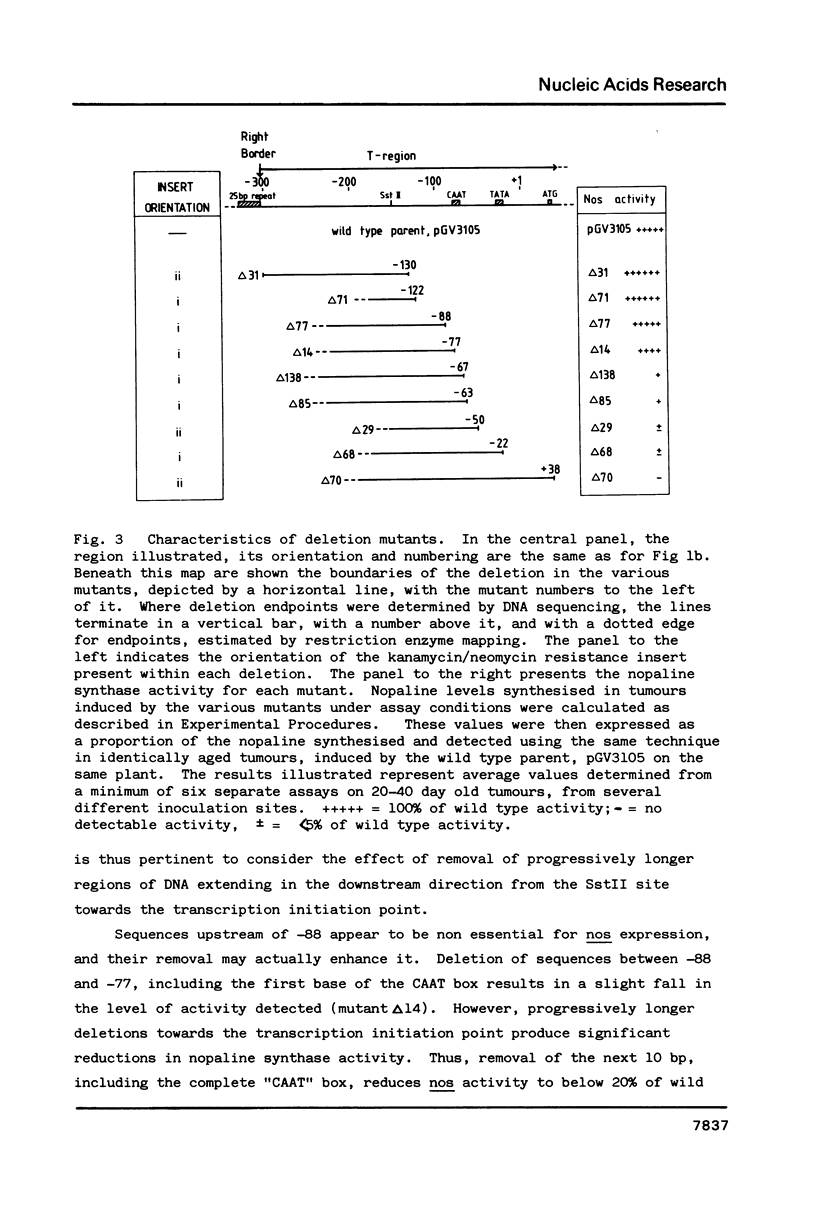
This paper describes the first functional map of a promoter expressed from the plant chromosome. We have constructed a series of overlapping deletion mutants within the region upstream of the Ti-plasmid encoded nopaline synthase (nos) gene. By monitoring nos expression in tumour tissue we have inferred a functional map of the nos promoter. The maximum length of sequence upstream of the transcription initiation point required to express wild type levels of nopaline synthase is 88 bp. Within this region, the "CAAT" box is essential for maximal activity; deletion of this sequence reduced apparent nos expression by over 80%. Presence of an intact or partial "TATA" box in the absence of the "CAAT" box supports a barely detectable level of nopaline synthase. Removal of all sequences upstream of the nos coding sequence results in no detectable activity.
Full text
PDF












Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benoist C., Chambon P. Deletions covering the putative promoter region of early mRNAs of simian virus 40 do not abolish T-antigen expression. Proc Natl Acad Sci U S A. 1980 Jul;77(7):3865–3869. doi: 10.1073/pnas.77.7.3865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry-Lowe S. L., Mc Knight T. D., Shah D. M., Meagher R. B. The nucleotide sequence, expression, and evolution of one member of a multigene family encoding the small subunit of ribulose-1,5-bisphosphate carboxylase in soybean. J Mol Appl Genet. 1982;1(6):483–498. [PubMed] [Google Scholar]
- Bevan M. W., Chilton M. D. T-DNA of the Agrobacterium Ti and Ri plasmids. Annu Rev Genet. 1982;16:357–384. doi: 10.1146/annurev.ge.16.120182.002041. [DOI] [PubMed] [Google Scholar]
- Bevan M., Barnes W. M., Chilton M. D. Structure and transcription of the nopaline synthase gene region of T-DNA. Nucleic Acids Res. 1983 Jan 25;11(2):369–385. doi: 10.1093/nar/11.2.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caplan A., Herrera-Estrella L., Inzé D., Van Haute E., Van Montagu M., Schell J., Zambryski P. Introduction of genetic material into plant cells. Science. 1983 Nov 18;222(4625):815–821. doi: 10.1126/science.222.4625.815. [DOI] [PubMed] [Google Scholar]
- Chilton M. D., Saiki R. K., Yadav N., Gordon M. P., Quetier F. T-DNA from Agrobacterium Ti plasmid is in the nuclear DNA fraction of crown gall tumor cells. Proc Natl Acad Sci U S A. 1980 Jul;77(7):4060–4064. doi: 10.1073/pnas.77.7.4060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corden J., Wasylyk B., Buchwalder A., Sassone-Corsi P., Kedinger C., Chambon P. Promoter sequences of eukaryotic protein-coding genes. Science. 1980 Sep 19;209(4463):1406–1414. doi: 10.1126/science.6251548. [DOI] [PubMed] [Google Scholar]
- De Greve H., Dhaese P., Seurinck J., Lemmers M., Van Montagu M., Schell J. Nucleotide sequence and transcript map of the Agrobacterium tumefaciens Ti plasmid-encoded octopine synthase gene. J Mol Appl Genet. 1982;1(6):499–511. [PubMed] [Google Scholar]
- Depicker A., Stachel S., Dhaese P., Zambryski P., Goodman H. M. Nopaline synthase: transcript mapping and DNA sequence. J Mol Appl Genet. 1982;1(6):561–573. [PubMed] [Google Scholar]
- Dhaese P., De Greve H., Decraemer H., Schell J., Van Montagu M. Rapid mapping of transposon insertion and deletion mutations in the large Ti-plasmids of Agrobacterium tumefaciens. Nucleic Acids Res. 1979 Dec 11;7(7):1837–1849. doi: 10.1093/nar/7.7.1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dierks P., van Ooyen A., Mantei N., Weissmann C. DNA sequences preceding the rabbit beta-globin gene are required for formation in mouse L cells of beta-globin RNA with the correct 5' terminus. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1411–1415. doi: 10.1073/pnas.78.3.1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efstratiadis A., Posakony J. W., Maniatis T., Lawn R. M., O'Connell C., Spritz R. A., DeRiel J. K., Forget B. G., Weissman S. M., Slightom J. L. The structure and evolution of the human beta-globin gene family. Cell. 1980 Oct;21(3):653–668. doi: 10.1016/0092-8674(80)90429-8. [DOI] [PubMed] [Google Scholar]
- Fraley R. T., Rogers S. G., Horsch R. B., Sanders P. R., Flick J. S., Adams S. P., Bittner M. L., Brand L. A., Fink C. L., Fry J. S. Expression of bacterial genes in plant cells. Proc Natl Acad Sci U S A. 1983 Aug;80(15):4803–4807. doi: 10.1073/pnas.80.15.4803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garfinkel D. J., Simpson R. B., Ream L. W., White F. F., Gordon M. P., Nester E. W. Genetic analysis of crown gall: fine structure map of the T-DNA by site-directed mutagenesis. Cell. 1981 Nov;27(1 Pt 2):143–153. doi: 10.1016/0092-8674(81)90368-8. [DOI] [PubMed] [Google Scholar]
- Gelvin S. B., Karcher S. J., DiRita V. J. Methylation of the T-DNA in Agrobacterium tumefaciens and in several crown gall tumors. Nucleic Acids Res. 1983 Jan 11;11(1):159–174. doi: 10.1093/nar/11.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosschedl R., Birnstiel M. L. Identification of regulatory sequences in the prelude sequences of an H2A histone gene by the study of specific deletion mutants in vivo. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1432–1436. doi: 10.1073/pnas.77.3.1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosschedl R., Birnstiel M. L. Spacer DNA sequences upstream of the T-A-T-A-A-A-T-A sequence are essential for promotion of H2A histone gene transcription in vivo. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7102–7106. doi: 10.1073/pnas.77.12.7102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosveld G. C., Rosenthal A., Flavell R. A. Sequence requirements for the transcription of the rabbit beta-globin gene in vivo: the -80 region. Nucleic Acids Res. 1982 Aug 25;10(16):4951–4971. doi: 10.1093/nar/10.16.4951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosveld G. C., Shewmaker C. K., Jat P., Flavell R. A. Localization of DNA sequences necessary for transcription of the rabbit beta-globin gene in vitro. Cell. 1981 Jul;25(1):215–226. doi: 10.1016/0092-8674(81)90246-4. [DOI] [PubMed] [Google Scholar]
- Grosveld G. C., de Boer E., Shewmaker C. K., Flavell R. A. DNA sequences necessary for transcription of the rabbit beta-globin gene in vivo. Nature. 1982 Jan 14;295(5845):120–126. doi: 10.1038/295120a0. [DOI] [PubMed] [Google Scholar]
- Hen R., Sassone-Corsi P., Corden J., Gaub M. P., Chambon P. Sequences upstream from the T-A-T-A box are required in vivo and in vitro for efficient transcription from the adenovirus serotype 2 major late promoter. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7132–7136. doi: 10.1073/pnas.79.23.7132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henikoff S., Tatchell K., Hall B. D., Nasmyth K. A. Isolation of a gene from Drosophila by complementation in yeast. Nature. 1981 Jan 1;289(5793):33–37. doi: 10.1038/289033a0. [DOI] [PubMed] [Google Scholar]
- Hentschel C. C., Birnstiel M. L. The organization and expression of histone gene families. Cell. 1981 Aug;25(2):301–313. doi: 10.1016/0092-8674(81)90048-9. [DOI] [PubMed] [Google Scholar]
- Hepburn A. G., Clarke L. E., Pearson L., White J. The role of cytosine methylation in the control of nopaline synthase gene expression in a plant tumor. J Mol Appl Genet. 1983;2(3):315–329. [PubMed] [Google Scholar]
- Hitzeman R. A., Hagie F. E., Levine H. L., Goeddel D. V., Ammerer G., Hall B. D. Expression of a human gene for interferon in yeast. Nature. 1981 Oct 29;293(5835):717–722. doi: 10.1038/293717a0. [DOI] [PubMed] [Google Scholar]
- Hu S. L., Manley J. L. DNA sequence required for initiation of transcription in vitro from the major late promoter of adenovirus 2. Proc Natl Acad Sci U S A. 1981 Feb;78(2):820–824. doi: 10.1073/pnas.78.2.820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphries R. K., Ley T., Turner P., Moulton A. D., Nienhuis A. W. Differences in human alpha-, beta- and delta-globin gene expression in monkey kidney cells. Cell. 1982 Aug;30(1):173–183. doi: 10.1016/0092-8674(82)90023-x. [DOI] [PubMed] [Google Scholar]
- Joos H., Inzé D., Caplan A., Sormann M., Van Montagu M., Schell J. Genetic analysis of T-DNA transcripts in nopaline crown galls. Cell. 1983 Apr;32(4):1057–1067. doi: 10.1016/0092-8674(83)90290-8. [DOI] [PubMed] [Google Scholar]
- Klee H., Montoya A., Horodyski F., Lichtenstein C., Garfinkel D., Fuller S., Flores C., Peschon J., Nester E., Gordon M. Nucleotide sequence of the tms genes of the pTiA6NC octopine Ti plasmid: two gene products involved in plant tumorigenesis. Proc Natl Acad Sci U S A. 1984 Mar;81(6):1728–1732. doi: 10.1073/pnas.81.6.1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koncz C., De Greve H., André D., Deboeck F., Van Montagu M., Schell J. The opine synthase genes carried by Ti plasmids contain all signals necessary for expression in plants. EMBO J. 1983;2(9):1597–1603. doi: 10.1002/j.1460-2075.1983.tb01630.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langridge P., Feix G. A zein gene of maize is transcribed from two widely separated promoter regions. Cell. 1983 Oct;34(3):1015–1022. doi: 10.1016/0092-8674(83)90559-7. [DOI] [PubMed] [Google Scholar]
- Leemans J., Deblaere R., Willmitzer L., De Greve H., Hernalsteens J. P., Van Montagu M., Schell J. Genetic Identification of functions of TL-DNA transcripts in octopine crown galls. EMBO J. 1982;1(1):147–152. doi: 10.1002/j.1460-2075.1982.tb01138.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leemans J., Langenakens J., De Greve H., Deblaere R., Van Montagu M., Schell J. Broad-host-range cloning vectors derived from the W-plasmid Sa. Gene. 1982 Oct;19(3):361–364. doi: 10.1016/0378-1119(82)90027-0. [DOI] [PubMed] [Google Scholar]
- Leemans J., Shaw C., Deblaere R., De Greve H., Hernalsteens J. P., Maes M., Van Montagu M., Schell J. Site-specific mutagenesis of Agrobacterium Ti plasmids and transfer of genes to plant cells. J Mol Appl Genet. 1981;1(2):149–164. [PubMed] [Google Scholar]
- Lichtenstein C., Klee H., Montoya A., Garfinkel D., Fuller S., Flores C., Nester E., Gordon M. Nucleotide sequence and transcript mapping of the tmr gene of the pTiA6NC octopine Ti-plasmid: a bacterial gene involved in plant tumorigenesis. J Mol Appl Genet. 1984;2(4):354–362. [PubMed] [Google Scholar]
- Lycett G. W., Croy R. R., Shirsat A. H., Boulter D. The complete nucleotide sequence of a legumin gene from pea (Pisum sativum L.). Nucleic Acids Res. 1984 Jun 11;12(11):4493–4506. doi: 10.1093/nar/12.11.4493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matzke A. J., Chilton M. D. Site-specific insertion of genes into T-DNA of the Agrobacterium tumor-inducing plasmid: an approach to genetic engineering of higher plant cells. J Mol Appl Genet. 1981;1(1):39–49. [PubMed] [Google Scholar]
- McKnight S. L., Gavis E. R., Kingsbury R., Axel R. Analysis of transcriptional regulatory signals of the HSV thymidine kinase gene: identification of an upstream control region. Cell. 1981 Aug;25(2):385–398. doi: 10.1016/0092-8674(81)90057-x. [DOI] [PubMed] [Google Scholar]
- Messing J., Vieira J. A new pair of M13 vectors for selecting either DNA strand of double-digest restriction fragments. Gene. 1982 Oct;19(3):269–276. doi: 10.1016/0378-1119(82)90016-6. [DOI] [PubMed] [Google Scholar]
- Otten L. A., Schilperoort R. A. A rapid micro scale method for the detection of lysopine and nopaline dehydrogenase activities. Biochim Biophys Acta. 1978 Dec 8;527(2):497–500. doi: 10.1016/0005-2744(78)90363-7. [DOI] [PubMed] [Google Scholar]
- Otten L., De Greve H., Hernalsteens J. P., Van Montagu M., Schieder O., Straub J., Schell J. Mendelian transmission of genes introduced into plants by the Ti plasmids of Agrobacterium tumefaciens. Mol Gen Genet. 1981;183(2):209–213. doi: 10.1007/BF00270619. [DOI] [PubMed] [Google Scholar]
- Pedersen K., Devereux J., Wilson D. R., Sheldon E., Larkins B. A. Cloning and sequence analysis reveal structural variation among related zein genes in maize. Cell. 1982 Jul;29(3):1015–1026. doi: 10.1016/0092-8674(82)90465-2. [DOI] [PubMed] [Google Scholar]
- Pelham H. R. A regulatory upstream promoter element in the Drosophila hsp 70 heat-shock gene. Cell. 1982 Sep;30(2):517–528. doi: 10.1016/0092-8674(82)90249-5. [DOI] [PubMed] [Google Scholar]
- Pintor-Toro J. A., Langridge P., Feix G. Isolation and characterization of maize genes coding for zein proteins of the 21000 dalton size class. Nucleic Acids Res. 1982 Jul 10;10(13):3845–3860. doi: 10.1093/nar/10.13.3845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saule S., Coll J., Righi M., Lagrou C., Raes M. B., Stehelin D. Two different types of transcription for the myelocytomatosis viruses MH2 and CMII. EMBO J. 1983;2(6):805–809. doi: 10.1002/j.1460-2075.1983.tb01506.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah D. M., Hightower R. C., Meagher R. B. Complete nucleotide sequence of a soybean actin gene. Proc Natl Acad Sci U S A. 1982 Feb;79(4):1022–1026. doi: 10.1073/pnas.79.4.1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw C. H., Leemans J., Shaw C. H., van Montagu M., Schell J. A general method for the transfer of cloned genes to plant cells. Gene. 1983 Sep;23(3):315–330. doi: 10.1016/0378-1119(83)90021-5. [DOI] [PubMed] [Google Scholar]
- Slightom J. L., Sun S. M., Hall T. C. Complete nucleotide sequence of a French bean storage protein gene: Phaseolin. Proc Natl Acad Sci U S A. 1983 Apr;80(7):1897–1901. doi: 10.1073/pnas.80.7.1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomashow M. F., Nutter R., Postle K., Chilton M. D., Blattner F. R., Powell A., Gordon M. P., Nester E. W. Recombination between higher plant DNA and the Ti plasmid of Agrobacterium tumefaciens. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6448–6452. doi: 10.1073/pnas.77.11.6448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokunaga K., Hirose S., Suzuki Y. In monkey COS cells only the TATA box and the cap site region are required for faithful and efficient initiation of the fibroin gene transcription. Nucleic Acids Res. 1984 Feb 10;12(3):1543–1558. doi: 10.1093/nar/12.3.1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai S. Y., Tsai M. J., O'Malley B. W. Specific 5' flanking sequences are required for faithful initiation of in vitro transcription of the ovalbumin gene. Proc Natl Acad Sci U S A. 1981 Feb;78(2):879–883. doi: 10.1073/pnas.78.2.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiborg O., Hyldig-Nielsen J. J., Jensen E. O., Paludan K., Marcker K. A. The nucleotide sequences of two leghemoglobin genes from soybean. Nucleic Acids Res. 1982 Jun 11;10(11):3487–3494. doi: 10.1093/nar/10.11.3487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willmitzer L., Dhaese P., Schreier P. H., Schmalenbach W., Van Montagu M., Schell J. Size, location and polarity of T-DNA-encoded transcripts in nopaline crown gall tumors; common transcripts in octopine and nopaline tumors. Cell. 1983 Apr;32(4):1045–1056. doi: 10.1016/0092-8674(83)90289-1. [DOI] [PubMed] [Google Scholar]
- Willmitzer L., Otten L., Simons G., Schmalenbach W., Schröder J., Schröder G., Van Montagu M., de Vos G., Schell J. Nuclear and polysomal transcripts of T-DNA in octopine crown gall suspension and callus cultures. Mol Gen Genet. 1981;182(2):255–262. doi: 10.1007/BF00269667. [DOI] [PubMed] [Google Scholar]
- Willmitzer L., Schmalenbach W., Schell J. Transcription of T-DNA in octopine and nopaline crown gall tumours is inhibited by low concentrations of alpha-amanitin. Nucleic Acids Res. 1981 Oct 10;9(19):4801–4812. doi: 10.1093/nar/9.19.4801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willmitzer L., Simons G., Schell J. The TL-DNA in octopine crown-gall tumours codes for seven well-defined polyadenylated transcripts. EMBO J. 1982;1(1):139–146. doi: 10.1002/j.1460-2075.1982.tb01137.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


