Abstract
Background and Purpose
Although epidemiological and experimental studies suggest that dietary intake of soy may be cardioprotective, use of isoflavone soy protein (ISP) supplementation as a primary preventive therapy remains unexplored. We determined whether ISP reduces subclinical atherosclerosis assessed as carotid artery intima-media thickness (CIMT) progression.
Methods
In a double-blind, placebo-controlled trial, 350 postmenopausal women 45–92 years of age without diabetes and cardiovascular disease (CVD) were randomized to 2 evenly divided daily doses of 25 g soy protein containing 91 mg aglycon isoflavone equivalents or placebo for 2.7-years.
Results
Overall, mean (95% confidence interval) CIMT progression rate was 4.77(3.39–6.16) μm/year in the ISP group and 5.68(4.30–7.06) μm/year in the placebo group. Although CIMT progression was reduced on average by 16% in the ISP group relative to the placebo group, this treatment effect was not statistically significant (p=0.36). Among the subgroup of women who were randomized within 5 years of menopause, ISP participants had on average a 68% lower CIMT progression rate than placebo participants 2.16(−1.10–5.43) vs. 6.79(3.56–10.01) μm/year, p=0.05). ISP supplementation had a null effect on women who were >5 years beyond menopause when randomized. There were no major adverse events from ISP supplementation.
Conclusion
ISP supplementation did not significantly reduce subclinical atherosclerosis progression in postmenopausal women. Subgroup analysis suggest that ISP supplementation may reduce subclinical atherosclerosis in healthy young (median age, 53 years) women at low-risk for CVD who were <5 years postmenopausal. These first trial results of their kind warrant further investigation.
Keywords: Atherosclerosis, Cardiovascular disease, Intima-media thickness, Isoflavones, Menopause, Soy, Women
More than 40 million American women are currently postmenopausal and as the United States population ages, the number of women entering menopause will steadily increase by more than 1 million American women per year (1). Fear of estrogen-based hormone therapy has resulted in an escalating use of nutraceuticals, specifically soy protein products and soy-rich diets containing isoflavones as a postmenopausal therapeutic option (2,3).
Soy isoflavones are estrogen-like compounds that are structurally similar to 17β-estradiol and possess selective estrogen receptor modulator (SERM)-like activity (4,5). Evidence from epidemiological (6–8) and non-human primate studies (9–11) indicates that isoflavone-rich soy protein (ISP) has anti-atherogenic activity, evidence supported by a large body of data that demonstrates mechanistic and biologic plausibility (12–15).
As such, ISP may provide a safe and effective strategy for extending premenopausal cardioprotection afforded by endogenous estrogen into menopause without the increased risk of breast and uterine cancer and thromboembolic events associated with traditional hormone therapy, and without the side effects limiting sex steroid hormone use (16–18). In particular, a population-based 12.5-year prospective study in 40,462 Japanese (27,435 women) has shown that a high soy and isoflavone dietary intake is associated with reduced risk of cerebral and myocardial infarctions and cardiovascular disease (CVD) mortality among postmenopausal women (8). Asian populations consume approximately 30–50 g/d of soy protein (19) and approximately 20–200 mg/d of isoflavones (20) whereas Americans ingest <5 g/d of soy protein (21) and <1 mg/d of isoflavones (22). As such, increased dietary intake of ISP in the American diet has been recommended (23).
However, whether ISP can be used for the prevention of CVD is unknown. We hypothesized that ISP supplementation designed to provide the principle isoflavones similar in composition and exposure (150 mg/d) to a soy-based Asian diet will reduce atherosclerosis in postmenopausal women.
The Women’s Isoflavone Soy Health (WISH) trial is a randomized controlled trial designed to determine the impact of ISP supplementation on health outcomes (atherosclerosis, osteoporosis, cognition and breast density) in a healthy population of postmenopausal women without pre-existing CVD. The aim of this study was to determine the effect of ISP supplementation on subclinical atherosclerosis progression.
MATERIALS AND METHODS
Study Population and Design
WISH was a randomized, double-blind, placebo-controlled trial conducted from April 12, 2004 to March 19, 2009. Participants were postmenopausal women without vaginal bleeding >1 year and serum estradiol <20 pg/ml. Exclusion criteria were clinical signs, symptoms or personal history of CVD, diabetes mellitus or fasting serum glucose >6.99 mmol/L (126 mg/dl), fasting triglycerides >5.64 mmol/L (500 mg/dL), systolic blood pressure ≥160 mmHg and/or diastolic blood pressure ≥110 mmHg, untreated thyroid disease, serum creatinine >2 mg/dL, life-threatening illness with prognosis <5 years, alcohol intake >5 drinks/day or substance abuse, taking menopausal hormone therapy and soy, nut or related food allergies. Participants were recruited from the general population from the Greater Los Angeles area predominantly through media advertisement. The University of Southern California Institutional Review Board approved the study protocol; all participants provided written informed consent.
Participants were randomly assigned in a 1:1 ratio to daily 25 g soy protein containing 91 mg aglycon equivalents of naturally occurring isoflavones and its glycosides (154 mg total isoflavone conjugates plus aglycons): genistein 52 mg aglycon equivalents (88 mg total), daidzein 36 mg aglycon equivalents (61 mg total) and glycitein 3 mg aglycon equivalents (5 mg total) or daily total milk protein matched placebo (0 isoflavones) within 2 strata of carotid artery intima-media thickness (CIMT) (<0.75 mm, ≥0.75 mm). The placebo and active treatments were taken in 2 evenly divided doses daily delivered in either beverage powder-food packs or food bars to provide variety and to maintain compliance. ISP and placebo products were prepared without charge by the Solae Company (St. Louis, MO) and were identical in taste and appearance. Within each stratum, blocked randomization was implemented with a masked block size. Participants, investigators, staff, imaging specialists and data monitors were masked to treatment assignment. The randomization procedure is more fully described in the Supplemental Material.
Clinic visits occurred every month for the first 6 months and then every other month for the remainder of the trial. At every clinic visit, data regarding dietary intake, product compliance, non-study medications and nutritional products, clinical adverse events as well as vital signs were ascertained. Every 6 months, laboratory determinations were performed (including ultrasound determinations of CIMT, lipids and isoflavones) and lifestyle and medical questionnaires administered. DXA bone scans, mammograms, pelvic examinations with Pap smears, transvaginal ultrasounds (and endometrial biopsies when indicated) and chemistry panels and complete blood counts were performed at baseline and annually. Cognitive assessments were completed at baseline and at the final follow-up visit (2.5-years).
The initial 2.5-year treatment period was increased to 3 years (an optional additional 6 month study visit) by the External Data and Safety Monitoring Board to increase the chance of detecting treatment group differences on the primary endpoint. Interim analyses of the primary trial endpoint were not performed.
The primary trial endpoint was the rate of change in the right distal common carotid artery intima-media thickness (CIMT). Sample size based on CIMT progression required 150 participants/arm (including an anticipated 10% annual dropout rate) to detect a difference in the rate of CIMT progression of 12.4 μm/year (based on unopposed estrogen randomized controlled trial data (24)) at 0.05-significance (2-sided) with 90% power. A total of 350 participants were recruited.
Assessment of Atherosclerosis Progression
High-resolution B-mode ultrasound images of the right common carotid artery were obtained with a 7.5-MHz linear array transducer attached to an ATL Apogee ultrasound system (Bothell, WA). Ultrasound imaging and measurement of far wall CIMT were completed as previously described masked to treatment group and to participant characteristics (Patents 2005, 2006, 2011) (24–29). Ultrasound imaging and CIMT measurement are more fully described in the Supplemental Material.
Laboratory Measurements
Participants fasted 8 hours before sample collections. Plasma lipids were measured using an enzymatic method under the CDC Standardization Program; LDL-C was calculated (30). Plasma isoflavone and equol levels were measured by high-pressure liquid chromatography (see Supplemental Material for further details) (31). Among ISP participants, plasma equol (a daidzein metabolite) levels at post-randomization visits were used to determine equol-producer status: equol never >20 nmol/L (non-producer), equol >20 nmol/L at some visits (intermittent producer) or equol >20 nmol/L at all visits (consistent producer). Protein and isoflavone (soy products) content was determined by analytical testing in all production lots before release to assure that the products met the target levels for macro- and micro-nutrients (31).
Statistical Analysis
Pre-randomization socio-demographic and clinical characteristics (including CIMT) and percentage product compliance were compared between treatment groups with 2-sample t-tests (or Wilcoxon rank sum) for continuous variables and chi-square tests for categorical variables. On-trial changes from baseline in plasma lipids, isoflavone levels, glucose, blood pressure and weight were compared using generalized estimating equations (dependent variables with repeated measurements) using an exchangeable correlation structure; independent variables were treatment groups and the CIMT randomization strata. Triglycerides were log transformed prior to analysis.
An intention-to-treat analysis was performed for all participants who had carotid ultrasonography at baseline and at least 1 follow-up visit. A linear mixed effects model was used to compare treatment groups on average CIMT change rates. CIMT was regressed on follow-up time (in years), with adjustment for the randomization stratification factor (baseline CIMT). The regression coefficient associated with trial follow-up time estimated the average CIMT annual rate of change. A treatment × follow-up time interaction term evaluated whether the treatment groups differed in average CIMT progression rates. Post-hoc subgroup analyses examined treatment group differences on CIMT progression rates by participant age (<55, 56–60 or >60 years), time-since-menopause (<5, 5–10 or >10 years), equol production status (non-producer, intermittent producer or consistent producer) and ethnicity.
In ancillary analyses, mixed effects models were used to evaluate the association of baseline and on-trial plasma isoflavone and equol levels (all modeled as continuous variables) with the CIMT progression rate. Interaction terms of isoflavone levels with follow-up time evaluated in the overall sample whether isoflavone levels modified CIMT progression.
Treatment group comparisons on adverse events among all randomized participants used the Fisher’s exact test. Major adverse events included deaths, cardiovascular events, cerebrovascular events, arterial revascularization procedures and cancers.
Statistical analyses used SAS 9.2 software (SAS, Inc., Cary, North Carolina); statistical testing was conducted at a 2-tailed 0.05 significance level.
This was an investigator-initiated and -conducted trial. The authors were solely responsible for the design, conduct, data collection, data management, statistical analysis and data interpretation. The funding source played no role in these functions. The funding source had no role in deciding whether or where the study would be submitted for publication.
RESULTS
Baseline Characteristics
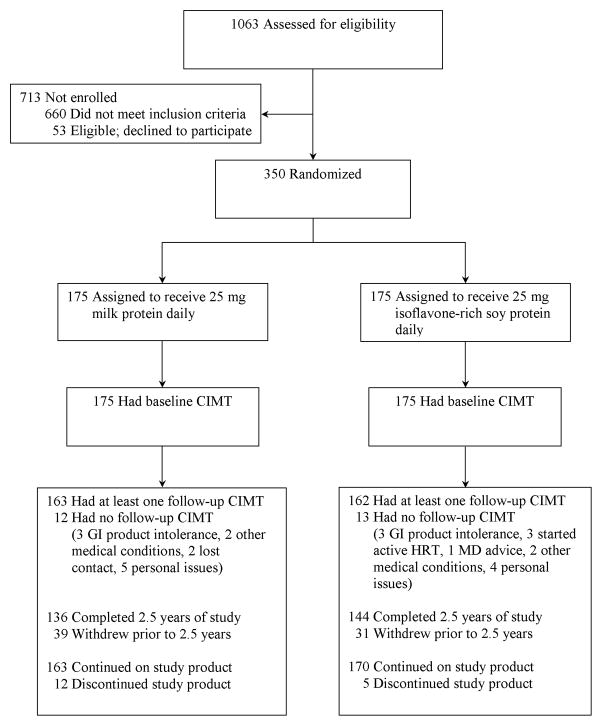
Of the 1063 individuals screened (Figure 1), 350 were randomized (175 placebo, 175 ISP). Of the randomized participants, 280(136 placebo, 144 ISP) completed the initially planned 2.5-year trial period; of these, 165(81 placebo, 84 ISP) participated in the trial extension. 325 participants (163 placebo, 162 ISP) contributed to the primary end point analysis.
Figure 1.
Women’s Isoflavone Soy Health (WISH) trial flow for carotid artery intima-media thickness outcome.
Treatment groups did not significantly differ at baseline for demographic, clinical and atherosclerosis characteristics (Table 1). The average age was 60.9 years and 36% were from an ethnic minority.
Table 1.
Baseline Characteristics*
| Variable | Placebo n = 163 |
ISP n = 162 |
No CIMT Follow-up n = 25 |
|---|---|---|---|
| CIMT – mm | 0.811(0.098) | 0.810 (0.105) | 0.813 (0.082) |
| Age – yrs | 60.9 (6.9) | 60.8 (7.2) | 62.3 (7.8) |
| Ethnicity | |||
| White (non-Hispanic) | 110 (67%) | 97 (60%) | 16 (64%) |
| Black (non-Hispanic) | 10 (6%) | 12 (7%) | 3 (12%) |
| Hispanic | 21 (13%) | 31 (19%) | 4 (16%) |
| Asian | 22 (14%) | 21 (13%) | 2 (8%) |
| Unknown | - | 1 (<1%) | - |
| Education | |||
| ≤ High school | 5 (3%) | 13 (8%) | 1 (4%) |
| > High school | 158 (97%) | 149 (92%) | 24 (96%) |
| Smoking history | |||
| Current | 5 (3%) | 2 (1%) | 1 (4%) |
| Former | 59 (36%) | 68 (42%) | 8 (32%) |
| Never smoked | 99 (61%) | 92 (57%) | 16 (64%) |
| Body mass index – kg/m2 | 25.8 (23.2, 30.0) | 26.0 (22.8, 29.6) | 25.0 (23.0, 27.6) |
| Past use of hormone therapy | |||
| Yes | 111 (68%) | 118 (73%) | 16 (64%) |
| No | 52 (32%) | 44 (27%) | 9 (36%) |
| Age at menopause – yrs† | 50.0 (48.0, 52.0) | 50.0 (47.0, 52.0) | 48.5 (46.0, 50.0) |
| Time-since-menopause – yrs | |||
| <5 | 35 (22%) | 33 (20%) | 5 (20%) |
| 5–10 | 43 (26%) | 46 (28%) | 6 (24%) |
| >10 | 65 (40%) | 69 (43%) | 11 (44%) |
| Unknown | 20 (12%) | 14 (9%) | 3 (12%) |
| Type of menopause | |||
| Natural | 144 (88%) | 150 (93%) | 20 (80%) |
| Surgical | 18 (11%) | 12 (7%) | 5 (20%) |
| Unknown | 1 (<1%) | - | - |
Mean (SD) or median (25th, 75th percentile) for continuous variables and n (%).
Age at menopause could not be determined in 37 subjects.
ISP = isoflavone soy protein treatment group.
CIMT = carotid artery intima-media thickness.
CIMT Progression Rates
The 325 participants with CIMT data had a median(range) of 2.8(0.5–3.6) years of follow-up in the placebo group and 2.7(0.5–3.6) years of follow-up in the ISP group (p=0.83). Participants contributed an average of 5.8(range, 2–8) CIMT measures in the placebo group and 6.1(range, 2–8) measures in the ISP group (p=0.07). Although the progression rate of CIMT was reduced on average by 16% in the ISP group relative to the placebo group (Figure 2), this treatment effect was not statistically significant overall (p=0.36) (Table 2). Treatment groups did not significantly differ on CIMT progression among post-hoc analysis of age, ethnic and equol-production subgroups (Tables 2 and 3). Among women who had experienced menopause within the past 5 years (median age = 53 years, range = 45–62 years), ISP participants had on average a 68% lower CIMT progression rate than placebo participants (p=0.05) (Table 2). Asian participants receiving ISP had on average a 64% lower CIMT progression rate than those Asian participants receiving placebo (p=0.08). In mixed effects models in the entire sample of 325 participants, baseline and on-trial plasma isoflavone or equol levels were not significantly associated with CIMT progression rate.
Figure 2.
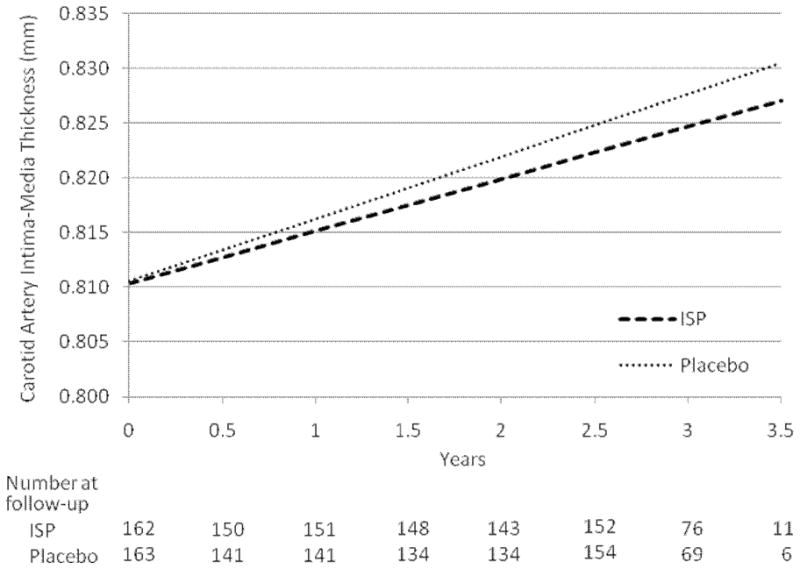
Carotid artery intima-media thickness progression rates estimated by linear mixed models (by treatment group) with numbers of subjects evaluated at each follow-up ultrasound scanning time; p=0.35 for difference between treatment groups. ISP = isoflavone soy protein treatment group.
Table 2.
Carotid Artery Intima-Media Thickness Progression Rates*
| Subject group | N† | Placebo | ISP | Differences in rates | p-value‡ |
|---|---|---|---|---|---|
| All Subjects | 325 (163/162) | 5.68 (4.30–7.06) | 4.77 (3.39–6.16) | −0.91(−2.86–1.05) | 0.36 |
| Age at randomization – yrs | |||||
| ≤55 | 82 (44/38) | 5.41 (2.70–8.11) | 2.92 (0.05–5.79) | −2.49 (−6.44–1.45) | 0.21 |
| 56–60 | 81 (40/41) | 5.76 (3.21–8.32) | 4.19 (1.64–6.74) | −1.57 (−5.18–2.04) | 0.39 |
| >60 | 162 (79/83) | 5.78 (3.74–7.82) | 5.89 (3.90–7.88) | 0.11 (−2.74–2.96) | 0.94 |
| Time-since-menopause – yrs | |||||
| <5 | 68 (35/33) | 6.79 (3.56–10.00) | 2.16 (−1.10–5.43) | −4.62 (−9.21– −0.04) | 0.05 |
| 5–10 | 89 (43/46) | 6.46 (4.10–8.83) | 5.36 (3.08–7.64) | −1.10 (−4.39–2.19) | 0.51 |
| >10 | 134 (65/69) | 5.24 (2.98–7.50) | 4.78 (2.58–6.98) | −0.46 (−3.62–2.70) | 0.77 |
| Ethnicity§ | |||||
| White (non-Hispanic) | 207 (110/97) | 5.89 (4.16–7.62) | 5.19 (3.35–7.03) | −0.70 (−3.22–1.83) | 0.59 |
| Black (non-Hispanic) | 22 (10/12) | 6.69 (1.25–12.13) | 6.27 (1.31–11.22) | −0.43 (−7.79–6.93) | 0.91 |
| Hispanic | 52 (21/31) | 3.42 (−0.29–7.12) | 4.63 (1.55–7.70) | 1.21 (−3.60–6.02) | 0.62 |
| Asian | 43 (22/21) | 6.37 (2.95–9.79) | 2.07 (−1.40–5.55) | −4.30 (−9.18–0.58) | 0.08 |
Mean (95% confidence interval) change rate in μm/year.
Total sample size (placebo treatment group sample size/isoflavone soy protein treatment group sample size).
P-value for treatment group differences in carotid artery intima-media thickness rate analyzed by linear mixed effects models adjusting for carotid artery intima-media thickness randomization strata.
Ethnicity unknown for 1 participant and excluded from the model.
ISP = isoflavone soy protein treatment group.
Table 3.
Carotid Artery Intima-Media Thickness Progression Rate by Isoflavone Soy Protein Treatment Group Equol Producer Status versus Placebo Treatment
| Study group | N* | CIMT† | p-value‡ | p-value§ |
|---|---|---|---|---|
| Placebo-treatment | 163 | 5.68 (4.29–7.07) | Reference | 0.84 |
| Isoflavone soy protein-treatment | ||||
| Non-equol producer | 76 | 5.09 (3.07–7.12) | 0.64 | |
| Intermittent equol producer | 35 | 4.52 (1.55–7.50) | 0.49 | |
| Consistent equol producer | 39 | 4.56 (1.74–7.39) | 0.49 | |
Sample size; isoflavone soy protein treatment group sample size does not total 162 subjects since 12 participants did not have sufficient follow-up equol measures to be classified.
Mean (95% confidence interval) carotid artery intima-media thickness change rate in μm/year.
p-value for difference from placebo.
Overall p-value across isoflavone soy protein treatment equol producer categories compared to placebo treatment adjusted for CIMT randomization strata.
Compliance
Median (inter-quartile range) compliance assessed over the 3-year treatment period by package and bar count was 86.5% (47.5%–96.0%) among the placebo-treated participants and 91.0% (75.0%–98.0%) among the ISP-treated participants (p=0.008). For subjects who were lost to follow-up, compliance was censored at last contact. For the participants who discontinued study product, zero adherence was assigned from the time they discontinued the study product until the last follow-up visit. Compliance was confirmed by plasma and urine isoflavone measurements (Table 4) (31). No participant reported on-trial use of soy/isoflavone supplements.
Table 4.
Absolute Change in Blood Pressure, Weight and Plasma Lipids, Glucose and Isoflavone Levels
| Placebo | ISP | p-value* | |
|---|---|---|---|
| Total Cholesterol – mg/dl | −0.1 (−4.6-4.3) | −1.4 (−5.4-2.5) | 0.55 |
| HDL-Cholesterol – mg/dl | 1.3 (0.2-2.5) | 2.7 (1.4-3.9) | 0.03 |
| LDL-Cholesterol – mg/dl | −2.3 (−6.7-2.0) | −4.0 (−7.8- −0.2) | 0.41 |
| Total Triglycerides – mg/dl | 8.0 (−3.5-19.4) | 1.6 (−6.3-9.5) | 0.09 |
| Plasma genistein – nmol/L | −7.5 (−76.7-61.7) | 467.1 (366.4-567.8) | <.0001 |
| Plasma daidzein – nmol/L | 11.8 (−36.3-59.8) | 337.9 (273.5-402.3) | <.0001 |
| Plasma glycitein – nmol/L | 2.9 (−0.04-5.9) | 10.3 (7.3-13.3) | 0.0002 |
| Glucose – mg/dl | −2.7 (−4.4- −0.9) | −0.8 (−2.7-1.0) | 0.12 |
| Systolic Blood Pressure – mmHg | −1.7 (−3.7-0.3) | −2.3 (−4.3- −0.3) | 0.51 |
| Diastolic Blood Pressure – mmHg | −2.0 (−3.5- −0.6) | −2.5 (−4.0- −1.0) | 0.53 |
| Weight – lbs | 0.3 (−0.9-1.6) | 0.4 (−0.8-1.5) | 0.93 |
Mean (95% confidence interval) change from baseline, adjusted for randomization stratum.
Treatment groups compared using generalized estimating equations with identity link function and exchangeable correlation structure.
- Total triglycerides: Placebo = 2.5 (−14.5, 21.0); ISP = 1.0 (−19.5, 19.0).
- Plasma genistein: Placebo = 0.6 (−6.1, 21.2); ISP = 273.9 (70.3, 679.3).
- Plasma daidzein: Placebo = 2.1 (−6.4, 28.3); ISP = 202.9 (49.9, 484.9).
- Plasma glycitein: Placebo = 0.0 (−0.6, 2.1); ISP = 4.2 (0, 14.3).
ISP = isoflavone soy protein treatment group.
Metabolic and Blood Pressure Variables
Treatment groups did not differ on average baseline levels of fasting plasma lipids, isoflavone levels or glucose, blood pressure or body weight (Supplemental Material Table 1). Over the trial, average changes in fasting glucose, blood pressure and weight did not significantly differ between treatment groups (Table 4). Compared to placebo, ISP participants on average increased HDL-C levels (p=0.03).
Clinical Events
There were no deaths and 1 CVD event (stroke in an ISP participant) during the trial. Five cancers were reported in placebo participants (colon, breast, uterus and 2 squamous cell of the skin), none were reported in ISP participants, p=0.06. Overall categorical differences (p<0.05) in adverse events occurred for the respiratory and urinary systems. The adverse events in the ISP vs. placebo participants that accounted for the categorical differences were cold/influenza symptoms (32.6% vs. 16.6%), pharyngitis/pharyngodynia (6.3% vs. 2.3%) and bronchitis/respiratory infection (9.1% vs. 4.0%) for the respiratory system and urinary tract infection (12.0% vs. 2.9%) for the urinary system. There were no other differences by treatment group across any other category including the gastrointestinal and gynecological systems although breast tenderness (5.7% vs. 1.1%) and vaginal bleeding/spotting (10.3% vs. 2.9%) were more evident in the ISP vs. placebo participants; endometrial hyperplasia (0.003% vs. 0%) and endometrial biopsies (2.9% vs. 3.4%) were not different between treatment groups.
DISCUSSION
In the overall cohort of healthy postmenopausal women, ISP supplementation did not significantly reduce the progression of subclinical atherosclerosis relative to placebo. In subgroup analysis, ISP supplementation significantly reduced progression of subclinical atherosclerosis in women who were within 5 years of menopause with a null effect on the progression of subclinical atherosclerosis in the women who were >5 years beyond menopause when randomized.
The age range for this trial was developed to address the effect of ISP supplementation over the postmenopausal age range for potential generalization to all postmenopausal women. Although the trial was not specifically designed to test the effects of ISP supplementation in younger women, the trial results indicate that a beneficial treatment effect may be limited to women who initiate ISP supplementation when close to menopause (within <5 years). This suggests a potential benefit over a narrower age range. The approximate 46% reduction in progression of CIMT in the ISP versus placebo group in the women <55 years of age is consistent with this possibility. It is possible that the broad age range of our population may have diluted a possible treatment effect of ISP supplementation in younger postmenopausal women.
Findings from WISH are consistent with data that support the timing hypothesis that posits that CVD is reduced in young postmenopausal women who initiate hormone therapy in close proximity to menopause versus a null CVD effect in women who initiate therapy when distant from menopause (24,32,33). Accumulating data from human and animal studies indicate that postmenopausal hormone therapy has little effect in reversing atherosclerosis once it is established, whereas it significantly reduces the extent of atherosclerosis if initiated at an early stage (24,32). Animal studies have demonstrated the same phenomenon whereby soy isoflavone administration does not reverse established atherosclerosis (34) but prevents atherosclerosis development (35,36). Recently, the timing hypothesis has been shown to be operative with the SERM raloxifene whereby women <60 years of age who received SERM therapy had a statistically significant reduction in CVD whereas women older than 60 years of age had no CVD benefit relative to placebo (37). Our subgroup analysis suggests that ISP may be the third class of estrogen receptor-binding molecules (4,5) to behave in accordance with the timing hypothesis (33).
Equol, a product of intestinal bacterial metabolism of daidzein is superior to all other isoflavones in its antioxidant activity (38,39). However, equol is not produced by all individuals and it has been suggested that the maximal clinical responses to ISP are observed in equol-producers versus equol non-producers (38). Since the clinical effectiveness of ISP may be a function of the ability to biotransform daidzein to the more potent estrogenic isoflavone equol, we examined the effect of ISP on atherosclerosis progression in equol producers versus equol non-producers. We found no difference in atherosclerosis progression comparing placebo to treatment groups defined by equol production.
Although shown to require the estrogen receptor (ER) to be anti-atherogenic (40–43), soy isoflavones may also have non-ER-mediated anti-atherogenic effects through traditional CVD risk factors including alteration of lipids and blood pressure (12,13,44). The ISP effects on these 2 major CVD risk factors are inconsistent across the literature (45). We found no effect of ISP on blood pressure but did detect a 2-fold greater increase in HDL-C in the ISP-treated group relative to the placebo-treated group. We previously reported that 17β-estradiol therapy significantly reduced CIMT progression with approximately 30% of the variability of this effect due to lipid alteration including the HDL-C raising effect of estrogen therapy (24).
Epidemiological, cross-cultural and migrant studies indicate that the Western diet plays an important role in the incidence of coronary heart disease as well as breast, ovary, colon and prostate cancer (7,45). Among Pacific Rim (Asian) countries where the intake of ISP is very high, the incidence of these chronic diseases is much lower than in Western countries (6–8). In the context of non-human primate studies in which dietary intake was completely replaced with ISP and epidemiological studies of Asian populations, the effects of ISP may not have been fully expressed in WISH where ISP was used as a supplement to rather than a replacement of a Western diet. Longer or sole exposure to ISP as in Asian populations may be required for the full expression of the effects of ISP on atherosclerosis progression. Although we did not find a difference in treatment effect across ethnic groups, the treatment group difference in CIMT progression was of borderline significance in the small subgroup of Asian women. Additionally, within the ISP-treatment group, the rate of CIMT progression in Asians was less than half the CIMT rate as the other ethnic groups. Taken together, the cumulated data suggest that a potential gene-ISP interaction may exist for the expression of a beneficial effect of ISP.
WISH was the largest and longest randomized controlled trial of ISP supplementation examining CVD in general and progression of atherosclerosis specifically; isoflavone dosage was high, tolerable and without serious adverse events. As such, participant withdrawal was low and compliance with study products was excellent (about 90%) with confirmed high plasma concentrations (31). However, sample size and duration of therapy may not have been sufficient to detect a smaller change in atherosclerosis progression than that originally hypothesized and used to guide our sample size estimates. As the first large-scale randomized controlled trial examining the effects of ISP on atherosclerosis progression, certain limitations result from the disadvantage of not having antecedent trials upon which to guide design. Practicality mandated use of only 1 readily available form of high-dose ISP such that the results may not be necessarily generalizable to isolated individual isoflavones, other classes of phytoestrogens more abundant in non-soy food products (e.g., flavones, coumestans and lignans) or to the more recently developed equol products. Duration of therapy may be a limitation since the cardioprotective effect of ISP observed in epidemiological studies is in populations of individuals who are exposed over a life-time to high levels of soy beginning in-utero and to a large dietary ingestion of soy from birth. Another limitation of the trial results from the disadvantage of experience in administration of ISP in different populations of women since our results suggest that certain populations of women such as Asians and women in close proximity to menopause (younger women in a narrower age range) may have a more effective response to ISP supplementation. While these latter findings are consistent with those predicted from epidemiological studies our findings are limited by the number of subgroup analyses performed.
SUMMARY
In conclusion, ISP supplementation over a 2.7-year period did not significantly reduce the progression of subclinical atherosclerosis in postmenopausal women. Subgroup analyses suggest a possible benefit of ISP supplementation among women who were randomized within 5 years of menopause; this hypothesis will require further study. ISP supplementation appears to be safe and long-term high compliance feasible. Mechanistically, the effect of ISP is consistent with postmenopausal hormone therapy and partly accounted for by a rise in HDL-C. These results warrant further investigation.
Supplementary Material
Acknowledgments
The authors thank the WISH participants for their invaluable time and dedication to this study.
SOURCES OF FUNDING
This study was supported by NIH grant U01AT-001653 from the National Center for Complementary and Alternative Medicine, the Office of Dietary Supplements and the Office of Research on Women’s Health and grant P30 CA-71789. Solae LLC (St. Louis, MO) provided the study products gratis.
APPENDIX
WISH Research Group Members
Study Chairman: Howard N. Hodis MD*; Clinical Center Staff: Martha Charlson RD, Irma Flores MA, Martha Huerta, Thelma LaBree MA, Sonia Lavender MA, Violetta McElreath RN, Janie Teran, Liny Zurbrugg RN, Philip Zurbrugg; Ultrasound Image Acquisition and Processing Laboratory: Robert H. Selzer MS* (Director), Mei Feng MD, Yanjie Li MD, Lora Whitfield-Maxwell RN, Ming Yan MD, PhD; Data Coordinating Center: Wendy J. Mack PhD* (Director), Stanley P. Azen PhD*, Farzana Choudhury MS, Carlos Carballo, Chun Ju-Chien, Laurie Dustin MS, Adrian Herbert, Michael Hutchinson, Naoko Kono MPH, George Martinez, Nitya Mathew, Olga Morales, Connie Wu MS, Mingzhu Xiang, MS; Core Lipid/Lipoprotein Laboratory: Juliana Hwang-Levine PharmD* (Director), Gail Izumi CLS, Arletta Ramirez CLS, Luci Rodriguez; Gynecology: Donna Shoupe MD*; Neurocognition: Victor W. Henderson MD*, Carol A. McCleary, PhD, Jan A. St. John MPH; Bone Density and Metabolic Laboratory: Robert Rude MD* (Director), Livia Y. Wei; Mammography Breast Density Laboratory: Anna H. Wu PhD* (Director), Chiu-chen Tseng MS, Giske Ursin PhD; Isoflavone Laboratory: Adrian A. Franke PhD* (Director), Sandra M. Hebshi, Ian Pagano; Data Safety Monitoring Board: Meir Stampfer MD (Chairman), Ronald M. Krauss MD, J. Christopher Gallagher MD; Josh Berman MD, PHD, Catherine Stoney PhD and Shan S. Wong PhD (NCCAM ex-officios); Lisa Begg, DrPH, RN and Rebecca B. Costello PhD (ODS ex-officios).
*Primary trial investigators
Footnotes
Clinical Trial Registration Information: http://www.ClinicalTrials.gov, NCT00118846.
DISCLOSURES
None
References
- 1.Spencer Gregory., editor. U.S. Bureau of the Census Population Reports. Series P-25, No. 1018, Projections of the Population of the United States, by Age, Sex, and Race: 1988 to 2080. U.S. Government Printing Office; Washington, D.C.: 1989. [Google Scholar]
- 2.Eisenberg DM, Kessler RC, Foster C, Norlock FE, Calkins DR, Delbanco TL. Unconventional medicine in the United States: prevalence, costs and patterns of use. N Engl J Med. 1993;328:246–252. doi: 10.1056/NEJM199301283280406. [DOI] [PubMed] [Google Scholar]
- 3.Stadberg E, Mattsson LA, Milsom I. The prevalence and severity of climacteric symptoms and the use of different treatment regimens in a Swedish population. Acta Obstet Gynecol Scand. 1997;76:442–448. doi: 10.3109/00016349709047826. [DOI] [PubMed] [Google Scholar]
- 4.Kuiper GGJM, Carlson B, Grandien K, Enmark E, Haggblad J, Nilsson S, et al. Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors α and β. Endocrinology. 1997;138:863–870. doi: 10.1210/endo.138.3.4979. [DOI] [PubMed] [Google Scholar]
- 5.Kuiper GGJM, Lemmen JG, Carlsson B, Corton JC, Safe SH, van der Saag PT, et al. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor β. Endocrinology. 1998;139:4252–4263. doi: 10.1210/endo.139.10.6216. [DOI] [PubMed] [Google Scholar]
- 6.Zhang X, Shu XO, Gao YT, Yang G, Li Q, Li H, et al. Soy food consumption is associated with lower risk of coronary heart disease in Chinese women. J Nutr. 2003;133:2874–2878. doi: 10.1093/jn/133.9.2874. [DOI] [PubMed] [Google Scholar]
- 7.Menotti A, Kromhout D, Blackburn H, Fidanza F, Buzina R, Nissinen A. Food intake patterns and 25-year mortality from coronary heart disease: cross-cultural correlations in the Seven Countries Study. Eur J Epidemiol. 1999;15:507–515. doi: 10.1023/a:1007529206050. [DOI] [PubMed] [Google Scholar]
- 8.Kokubo Y, Iso H, Ishihara J, Okada K, Inoue M, Tsugane S for the JPHC Study Group. Association of dietary intake of soy, beans, and isoflavones with risk of cerebral and myocardial infarctions in Japanese populations: the Japan Public Health Center-Based (JPHC) study cohort I. Circulation. 2007;116:2553–2562. doi: 10.1161/CIRCULATIONAHA.106.683755. [DOI] [PubMed] [Google Scholar]
- 9.Anthony MS, Clarkson TB, Bullock BC, Wagner JD. Soy protein versus soy phytoestrogens in the prevention of diet-induced coronary artery atherosclerosis of male cynomolgus monkeys. Arterioscler Thromb Vasc Biol. 1997;17:2524–2531. doi: 10.1161/01.atv.17.11.2524. [DOI] [PubMed] [Google Scholar]
- 10.Wagner JD, Cefalu WT, Anthony MS, Litwak KN, Zhang L, Clarkson TB. Dietary soy protein and estrogen replacement therapy improve cardiovascular risk factors and decrease aortic cholesteryl ester content in ovariectomized cynomolgus monkeys. Metabolism. 1997;46:698–705. doi: 10.1016/s0026-0495(97)90016-0. [DOI] [PubMed] [Google Scholar]
- 11.Clarkson TB, Anthony MS, Morgan TM. Inhibition of postmenopausal atherosclerosis progression: a comparison of the effects of conjugated equine estrogens and soy phytoestrogens. J Clin Endocrinol Metab. 2001;86:41–47. doi: 10.1210/jcem.86.1.7151. [DOI] [PubMed] [Google Scholar]
- 12.Nagarajan S. Mechanisms of anti-atherosclerotic functions of soy-based diets. J Nutr Biochem. 2010;21:255–260. doi: 10.1016/j.jnutbio.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 13.Omoni AO, Aluko RE. Soybean food and their benefits: potential mechanisms of action. Nutr Rev. 2005;63:272–283. doi: 10.1111/j.1753-4887.2005.tb00141.x. [DOI] [PubMed] [Google Scholar]
- 14.Hwang J, Sevanian A, Hodis HN, Ursini F. Synergistic inhibition of LDL oxidation by phytoestrogens and ascorbic acid. Free Rad Biol Med. 2000;29:79–89. doi: 10.1016/s0891-5849(00)00322-1. [DOI] [PubMed] [Google Scholar]
- 15.Hwang J, Hodis HN, Sevanian A. Soy and alfalfa phytoestrogen extracts become potent low-density lipoprotein antioxidants in the presence of acerola cherry extract. J Agric Food Chem. 2001;49:308–314. doi: 10.1021/jf0007028. [DOI] [PubMed] [Google Scholar]
- 16.Rios DRA, Rodrigues ET, Cardoso APZ, Montes MBA, Franceschini SA, Toloi MRT. Effects of isoflavones on the coagulation and fibrinolytic system of postmenopausal women. Nutrition. 2008;24:120–126. doi: 10.1016/j.nut.2007.10.009. [DOI] [PubMed] [Google Scholar]
- 17.Teede HJ, Dalais FS, Kotsopoulos D, McGrath BP, Malan E, Gan TE, et al. Dietary soy containing phytoestrogens does not activate the hemostatic system in postmenopausal women. J Clin Endocrinol Metab. 2005;90:1936–1941. doi: 10.1210/jc.2004-1428. [DOI] [PubMed] [Google Scholar]
- 18.Cassidy A, Albertazzi P, Nielsen IL, Hall W, Williamson G, Tetens I, et al. Critical review of health effects of soyabean phyto-oestrogens in post-menopausal women. Proced Nutr Soc. 2006;65:76–92. doi: 10.1079/pns2005476. [DOI] [PubMed] [Google Scholar]
- 19.Adlercreutz H, Honjo H, Higashi A, Fotsis T, Hamalainen E, Hasegawa T, et al. Urinary excretion of lignans and isoflavonoid phytoestrogens in Japanese men and women consuming a traditional Japanese diet. Am J Clin Nutr. 1991;54:1093–1100. doi: 10.1093/ajcn/54.6.1093. [DOI] [PubMed] [Google Scholar]
- 20.Murkies AL, Wilcox G, Davis SR. Phytoestrogens. J Clin Endocrinol Metab. 1998;83:297–303. doi: 10.1210/jcem.83.2.4577. [DOI] [PubMed] [Google Scholar]
- 21.Fournier DB, Erdman JW, Gordon GB. Soy, its components, and cancer prevention: a review of the in-vitro, animal, and human data. Cancer Epidemiol Biomarkers Prev. 1998;7:1055–1065. [PubMed] [Google Scholar]
- 22.de Kleijn MJJ, van der Schouw YT, Wilson PWF, Adlercreutz H, Mazur W, Grobbee DE, et al. Intake of dietary phytoestrogens is low in postmenopausal women in the United States: the Framingham Study. J Nutr. 2001;131:1826–1832. doi: 10.1093/jn/131.6.1826. [DOI] [PubMed] [Google Scholar]
- 23.Sacks FM, Lichtenstein A, Van Horn L, Harris W, Kris-Etherton P, Winston M. Soy protein, isoflavones, and cardiovascular health: an American Heart Association Science Advisory for Professionals From the Nutrition Committee. Circulation. 2006;113:1034–1044. doi: 10.1161/CIRCULATIONAHA.106.171052. [DOI] [PubMed] [Google Scholar]
- 24.Hodis HN, Mack WJ, Lobo RA, Shoupe D, Sevanian A, Mahrer PR, et al. Estrogen in the prevention of atherosclerosis: a randomized, double-blind, placebo-controlled trial. Ann Intern Med. 2001;135:939–953. doi: 10.7326/0003-4819-135-11-200112040-00005. [DOI] [PubMed] [Google Scholar]
- 25.Hodis HN, Mack WJ, LaBree L, Mahrer PR, Sevanian A, Liu CR, et al. Alpha tocopherol supplementation in healthy individuals reduces low-density lipoprotein oxidation but not atherosclerosis: the Vitamin E Atherosclerosis Prevention Study (VEAPS) Circulation. 2002;106:1453–1459. doi: 10.1161/01.cir.0000029092.99946.08. [DOI] [PubMed] [Google Scholar]
- 26.Selzer RH, Hodis HN, Kwong-Fu H, Mack WJ, Lee PL, Liu CR, et al. Evaluation of computerized edge tracking for quantifying intima-media thickness of the common carotid artery from B-mode ultrasound images. Atherosclerosis. 1994;111:1–11. doi: 10.1016/0021-9150(94)90186-4. [DOI] [PubMed] [Google Scholar]
- 27.Selzer RH, Mack WJ, Lee PL, Kwong-Fu H, Hodis HN. Improved common carotid elasticity and intima-media thickness measurement from computer analysis of sequential ultrasound frames. Atherosclerosis. 2001;154:185–193. doi: 10.1016/s0021-9150(00)00461-5. [DOI] [PubMed] [Google Scholar]
- 28.Mack WJ, LaBree L, Liu CL, Liu CH, Selzer RH, Hodis HN. Correlations between measures of atherosclerosis change using carotid ultrasonography and coronary angiography. Atherosclerosis. 2000;150:371–379. doi: 10.1016/s0021-9150(99)00383-4. [DOI] [PubMed] [Google Scholar]
- 29.Hodis HN, Mack WJ, LaBree L, Selzer RH, Liu CL, Liu CH, et al. The role of carotid arterial intima-media thickness in predicting clinical coronary events. Ann Intern Med. 1998;128:262–269. doi: 10.7326/0003-4819-128-4-199802150-00002. [DOI] [PubMed] [Google Scholar]
- 30.Lipid Research Clinics Program. DHEW Publication No. N.I.H. Bethesda, MD: National Institutes of Health; 1974. The Manual of Laboratory Operations: Lipid and Lipoprotein Analysis; pp. 75–628. [Google Scholar]
- 31.Franke AA, Hebshi SM, Pagano I, Kono N, Mack WJ, Hodis HN. Urine accurately reflects circulating isoflavonoids and ascertains compliance during soy intervention. Cancer Epidemiol Biomarkers and Prev. 2010;19:1775–1783. doi: 10.1158/1055-9965.EPI-10-0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hodis HN, Mack WJ, Lobo RA, Shoupe D, Mahrer PR, Faxon DP, et al. Hormone therapy and progression of coronary artery atherosclerosis in postmenopausal women. N Engl J Med. 2003;349:535–545. doi: 10.1056/NEJMoa030830. [DOI] [PubMed] [Google Scholar]
- 33.Hodis HN, Mack WJ. A window of opportunity: the reduction of coronary heart disease and total mortality with menopausal therapies is age and time dependent. Brain Research. 2011;1379:244–252. doi: 10.1016/j.brainres.2010.10.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Averill MM, Bennett BJ, Rattazzi M, Rodmyre RM, Kirk EA, Schwartz SM, et al. Neither antioxidants nor genistein inhibit the progression of established atherosclerotic lesions in older apoE deficient mice. Atherosclerosis. 2009;203:82–88. doi: 10.1016/j.atherosclerosis.2008.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ni W, Tsuda Y, Sakono M, Imaizumi K. Dietary soy protein isolate, compared with casein, reduces atherosclerotic lesion area in apolipoprotein E-deficient mice. J Nutr. 1998;128:1884–1889. doi: 10.1093/jn/128.11.1884. [DOI] [PubMed] [Google Scholar]
- 36.Adams MR, Golden DL, Anthony MS, Register TC, Williams JK. The inhibitory effect of soy protein isolate on atherosclerosis in mice does not require the presence of LDL receptors or alteration of plasma lipoproteins. J Nutr. 2002;132:43–49. doi: 10.1093/jn/132.1.43. [DOI] [PubMed] [Google Scholar]
- 37.Collins P, Mosca L, Geiger MJ, Grady D, Kornitzer M, Amewou-Atisso MG, et al. Effects of the selective estrogen receptor modulator raloxifene on coronary outcomes in the raloxifene use for the heart trial: results of subgroup analyses by age and other factors. Circulation. 2009;119:922–930. doi: 10.1161/CIRCULATIONAHA.108.817577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Setchell KDR, Brown NM, Lydeking-Olsen E. The clinical importance of the metabolite equol: a clue to the effectiveness of soy and its isoflavones. J Nutr. 2002;132:3577–3584. doi: 10.1093/jn/132.12.3577. [DOI] [PubMed] [Google Scholar]
- 39.Hwang J, Wang J, Morazzoni P, Hodis HN, Sevanian A. The phytoestrogen equol increases nitric oxide availability by inhibiting superoxide production: an antioxidant mechanism for cell-mediated LDL modification. Free Rad Biol Med. 2003;34:1271–1282. doi: 10.1016/s0891-5849(03)00104-7. [DOI] [PubMed] [Google Scholar]
- 40.Adams MR, Golden DL, Register TC, Anthony MS, Hodgin JB, Maeda N, et al. The atheroprotective effect of dietary soy isoflavones in apolipoprotein E−/− mice requires the presence of estrogen receptor-α. Arterioscler Thromb Vasc Biol. 2002;22:1859–1864. doi: 10.1161/01.atv.0000042202.42136.d0. [DOI] [PubMed] [Google Scholar]
- 41.Morito K, Hirose T, Kinjo J, Hirakawa T, Okawa M, Nohara T, et al. Interaction of phytoestrogens with estrogen receptors α and β. Biol Pharm Bull. 2001;24:351–356. doi: 10.1248/bpb.24.351. [DOI] [PubMed] [Google Scholar]
- 42.Makela S, Savolainen H, Aavik E, Myllarniemi M, Strauss L, Taskinen E, et al. Differentiation between vasculoprotective and uterotrophic effects of ligands with different binding affinities to estrogen receptors α and β. Proc Natl Acad Sci USA. 1999;96:7077–7082. doi: 10.1073/pnas.96.12.7077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Register TC, Adams MR. Coronary artery and cultured aortic smooth muscle cells express mRNA for both the classical estrogen receptor and the newly described estrogen receptor beta. J Steroid Biochem Molec Biol. 1998;64:187–191. doi: 10.1016/s0960-0760(97)00155-6. [DOI] [PubMed] [Google Scholar]
- 44.Taku K, Lin N, Cai D, Hu J, Zhao X, Zhang Y, et al. Effects of soy isoflavone extract supplements on blood pressure in adult humans: systematic review and meta-analysis of randomized placebo-controlled trials. J Hypertension. 2010;28:1971–1982. doi: 10.1097/HJH.0b013e32833c6edb. [DOI] [PubMed] [Google Scholar]
- 45.Adlercreutz H, Mazur W. Phyto-oestrogens and western diseases. Annals of Medicine. 1997;29:95–120. doi: 10.3109/07853899709113696. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.