Abstract
Improvised explosive devices (IEDs) are one of the main causes for casualties among civilians and military personnel in the present war against terror. Mild traumatic brain injury from IEDs induces various degrees of cognitive, emotional and behavioral disturbances but knowledge of the exact brain pathophysiology following exposure to blast is poorly understood. The study was aimed at establishing a murine model for a mild BI-TBI that isolates low-level blast pressure effects to the brain without systemic injuries. An open-field explosives detonation was used to replicate, as closely as possible, low-level blast trauma in the battlefield or at a terror-attack site. No alterations in basic neurological assessment or brain gross pathology were found acutely in the blast-exposed mice. At 7 days post blast, cognitive and behavioral tests revealed significantly decreased performance at both 4 and 7 meters distance from the blast (5.5 and 2.5 PSI, respectively). At 30 days post-blast, clear differences were found in animals at both distances in the object recognition test, and in the 7 m group in the Y maze test. Using MRI, T1 weighted images showed an increased BBB permeability one month post-blast. DTI analysis showed an increase in fractional anisotropy (FA) and a decrease in radial diffusivity. These changes correlated with sites of up-regulation of manganese superoxide dismutase 2 in neurons and CXC-motif chemokine receptor 3 around blood vessels in fiber tracts. These results may represent brain axonal and myelin abnormalities. Cellular and biochemical studies are underway in order to further correlate the blast-induced cognitive and behavioral changes and to identify possible underlying mechanisms that may help develop treatment- and neuroprotective modalities.
Introduction
Mechanisms and consequences of brain injuries have always been a matter of debate and speculation (Ratiu et al., 2004), and even more so when the injury may not easily be identified by technologies used in clinical practice such as in the case of mild traumatic brain injury (mTBI; Sterr et al., 2006). Since the actual mechanisms of injury are still unclear (Bigler 2008; Ruff, 2005), there is controversy regarding diagnostic criteria for mTBI as well as the relationship between mTBI and some manifestations of posttraumatic stress disorder (PTSD; Andreasen, 2004).. Routine and extended laboratory and clinical evaluation of mTBI patients fail to show any clear morphological brain defects, but the patients frequently suffer lasting cognitive and emotional difficulties, including various degrees of amnesia, difficulty in concentration and executive functions, depression, apathy and anxiety (Arciniegas et al., 1999; Kibby and Long, 1996; Levin et al. 1987). Although clinical signs and symptoms of mTBI usually resolve within the first year after injury, persistent ‘post-concussive syndrome’ in not uncommon (Finset et al., 1999; Margulies, 2002). For blast mTBI, signs and symptoms differ in the acute (< 72 hours), sub-acute (72 hours-30 days) and chronic (30–360 days) periods after initial exposure (Hoffer et al. 2010). Specifically, there was an elevated prevalence of vertigo, post-blast exercise induced dizziness and post-blast induced dizziness (with or without vertigo) in the subacute and chronic periods, and the new presentation of balance disorders tended to be more severe with latency from injury. To further complicate the issue, different spectra of clinical manifestations can appear with mTBI after a direct mechanical blow to the head (blunt injury induced mTBI; Ryan and Warden, 2003), or by a blast wave (blast induced mTBI; Thompson et al., 2008) or mixed injuries (Hoffer et al., 2009).
There is a need to develop reliable and “real world” animal models of blast-induced brain injury, in order to identify pathophysiological processes in blast-induced mTBI. The existing models, including shock-tubes from which widespread fiber degeneration was found that was at least partially due to cardiovascular homeostatic mechanisms (Long et al, 2009), electro-magnetic blast waves (Cheng et al., 2010) that demonstrated changes of brain tissue water content and neuron-specific enolase (NSE) expression along with vascular damage in the cortex or the use of explosives (reviewed in Cernak and Noble-Haeusslein, 2010), only partially imitate real life conditions as most of these studies tested much higher blast levels that only partially imitate real life conditions. Our previous work has utilized a mouse model of mechanical mTBI (Tashlykov et al., 2007; Tashlykov et al., 2009; Zohar et al., 2003) to study neural bases for behavioral alterations. This communication describes findings in a rodent model for low level, open field blast exposures that replicate features of our clinical experience with civilian survivors of suicide bombings (Dolberg et al., 2007; Schreiber et al., 2007).
Materials and methods
Experimental design and animal procedures
Male ICR mice weighing 25–30 g were kept five per cage under a constant 12-h light/dark cycle at room temperature. Food and water were available ad libitum. For each time point in each experimental group, at least 10 different mice were used (except the 7m group, 30d, n=8). Each mouse was used for one time point only. The ethics committee of the Sackler Faculty of Medicine approved the experimental protocol (M-07-055), in compliance with the guidelines for animal experimentation of the National Institutes of Health.
Blast trauma model
The blast-induced procedures took place in the experimental site of “Tamar Explosives” and of “Sadwin Consultancy” in central Israel. Mice (both blast and sham) were anesthetized with an i.p. mixture of ketamine (100mg/kg) and xylazine (10mg/ml), a combination that induces deep anesthesia and still enables spontaneous respiration. Anesthetized mice were placed in individual compartments on a platform and covered with a plastic net (Figure 1a), which fixed their position but did not shield them from the blast wave. Free-Field ICP® Blast Pressure Sensor “pencil gauges” (PCB Piezoelectronics, Depew, NY, USA; Model 137) at the ends of each platform (D) measured ‘side-on’ blast overpressure (in PSI) and temperature. The platforms were clamped to a stand that was weighted with sandbags (Figure 1b). The platforms were raised 1 meter above the ground (Figure 1b) and were positioned either 4 m (B) or 7 m (C) from a cast of 500g TNT (A). which was also elevated 1 m above the ground level.. . The pressure-time curves were recorded for each experiment at a sampling rate was 61.25 kHz (16 microseconds/sample) for each recording channel. The recorded blast waves showed the “direct” shock wave and the reflected wave from the ground, as expected. The mice at a distance of 7 m were exposed to 2.5 PSI peak over-pressure; at a distance of 4 m they were exposed to 5.5 PSI peak over-pressure. . . . .
Fig. 1. Blast experiment setting.
(a) The anesthetized mice were placed in a loose restraint device on a platform, which was covered with white plastic mesh. Each platform had space for 12 anesthetized mice. (b) Photograph of the explosive charge (A) and mice immediately prior to detonation. A cast of 500g TNT (A) was placed on a pedestal 1 meter above the ground. The 1 meter high platforms constraining the anesthetized mice were situated 4 meters (B) and 7 meters (C) meters from the TNT charge). Two pressure gauges were mounted at the ends of each platform (D).
Acute pathological assessment
A total of 12 mice were euthanized for standard gross and histopathological assessment at one hour and 24 hours post blast (n= 6 mice, 2 from the 2.5 PSI blast group, 2 from the 5 PSI blast group and 2 from the sham group for each time point). Paraffin-embedded tissue blocks of the kidney, liver, lung, spleen, intestine and brain were examined. Formalin-fixed hematoxylin-eosin stained 6-micron slices were examined by light microscopy by a pathologist, who was “blinded” as to animal status.
Neurological and cognitive/behavioral procedure for acute and chronic assessment
The neurological status of each of the experimental mice was assessed 1, 24 hours and 1 week post blast. The tests examined hind-leg flexion reflex, righting reflex, corneal reflex, secretory signs, strength, beam balance performance, a beam walking coordination task, exploration, and locomotor activity, as described previously (Zohar et al., 2003). Behavioral testing was performed at a subacute (7 day) and chronic (30 day) time point. Half of the mice in each exposure group (sham, 2.5 PSI and 5 PSI) were given the staircase, object recognition and Y maze tests 7 days post-blast; the other half were tested at 30 days post-blast.
A staircase was constructed as described previously (Simiand et al., 1984). A step was considered climbed only if the mouse placed all four paws on it. Rearing was recorded when the mouse rose on its hind legs either on the step or against the wall. At the end of 3 minutes, the mouse was removed and the staircase was cleaned to eliminate any residual odors.
An object recognition test was used to evaluate recognition memory (Messier 1997). This task is based on the tendency of rodents to discriminate between a familiar object and a new object. Data was analyzed and expressed as time spent exploring the novel object compared with the familiar object. The Aggelton discrimination index (ADI) was used as an outcome measure.
Spatial memory was assessed by using a Y-maze (Dellu et al., 1992), a task requiring hippocampal function and spatial memory (Conrad et al., 1996). Data were analyzed and expressed as the time spent at each arm and as a modified ADI (Aggleton et al., 1997).
In vivo MRI
MRI assessments were performed at the same times as the behavioral testing (7 or 30 days post blast). The mice were given inhalational isoflurane (1%–2%; Nicholas Piramal) anesthesia in 98% oxygen. Body temperature was maintained by circulating water at 37°C. Respiration was kept at 60–80 breath cycles/min. The imaging procedures were conducted on a 7T BioSpec 70/30 USR system (Bruker, Rheinstetten, Germany) using a volume coil for excitation and a dedicated quadrature coil for acquisition. Three MRI protocols were used: (1) Contrast enhanced T1 weighted images for assessing the blood brain barrier (BBB) were acquired before and 3, 6 and 9 min post i.p. injection of 0.5 mmol/kg Gadopentetate Dimeglumine (Gd-DTPA), 150 μl, Magnetol, Soreq, Israel) using a spin-echo pulse sequence: TR 600 ms, TE 12 ms, matrix size 256 × 128 (zero filled to 256 × 256), two averages, field of view (FOV) 2cm, 12 axial contiguous slices of 1mm thickness, spatial resolution 78μm2, acquisition time 2min33s. (2) Diffusion Tensor Imaging (DTI) with a diffusion-weighted echo planar (DWI-EPI) protocol. The parameters were TR/TE = 3000/25 ms, 4 EPI segments, Δ/δ = 10/4.5 ms, b0 of 1.7 s/mm2 and maximal b values of 1000 s/mm2 acquired at 15 non-collinear gradient directions, matrix size 128×128 and the same geometry as the T1w images. (3) Anatomical T2 weighted, with a multi echo spin echo protocol. The parameters were TR 3000ms and 16 TE incremented from 10 to 160ms, matrix dimension of 256×128 (zero filled to 256×256), two averages, scan time 9.5 min and same geometry as T1w images. On each MRI session, both treated and control mice were scanned
BBB analyses: A brain barrier permeability index (BBPi) was defined as the number of voxels with contrast enhancement larger than 0.1% multiplied by the mean of the enhancement (in %) divided by the area of the region of interest (ROI). Contrast enhancement maps were calculated as post-contrast image minus pre-contrast image divided by the pre-contrast image. ROIs were extracted by performing manual segmentation on 3 consecutive brain slices, from approximately bregma 0.26 to −1.7. Each mouse BBPi is represented as the mean value of the 3 slices. Data (pre vs. 9 min post Gd-DTPA injection, a time that shows maximal difference between groups) were normalized by multiplying with a ‘signal factor’ (mean of all the controls divided by the mean of the control on the same experimental day).
The DTI data were analyzed using in-house software to calculate fractional anisotropy (FA) and apparent diffusion (ADC) maps as well as axial (λ1) and radial (λ2 and λ3) diffusivity indexed maps (Basser et al., 1996). The calculated maps were then co-registered and normalized with one of the experimental brains using SPM8b (Wellcome Department of Imaging Neuroscience, University College London).
Histopathological Analysis of Cranial Tissues in the Acute and Subacute Time Frames
Three mice from each blast exposure (Sham, 2.5 PSI and 5.5 PSI) and were euthanized and perfused transcardially at survival times of 2 hr, 24 hr and 72 hr with phosphate-buffered saline (PBS; 0.9% NaCl in 50 mM phosphate buffer, pH 7.3), followed by phosphate-buffered formalin. The heads were removed, skinned and post-fixed in 10% neutral buffered formalin. The heads were decalcified in 10% formic acid then neutralized overnight in 5% sodium sulfite by standard methods prior to trimming and paraffin embedding. Paraffin embedded sections were cut in the horizontal plane with a rotary microtome at 8–10 μm and mounted on subbed slides.
Every 25th section was de-paraffinized and stained with hematoxylin and eosin for histopathological evaluation. Additional sets of sections were treated for 10 min with 30% H2O2 in dH2O and then rinsed thoroughly with distilled H2O. They were heated for 20 min at 90–98 °C in a low pH (0.80–3.06) sodium citrate–citric acid buffer (antigen retrieval) and rinsed thoroughly with PBS. Sections were incubated overnight with a primary polyclonal antibody raised to either (1) full length rat manganese superoxide dismutase 2 (SOD2) protein (Abcam Inc., ab13534) at 1:1000 dilution or (2) amino acids 1–95 of human CXC Chemokine Receptor 3 (CXCR3) protein (Santa Cruz Biotechnology, sc-13951, 1:1000 dilution). The SOD2 antibody has a broad spectrum of species reactivity (Drosophila and vertebrate) and its specificity has been confirmed by western blotting at 1/2000 dilution to a single band (Saha et al., 2007; Bidmon et al., 1998; Liu et al., 1993). A biotinylated secondary anti-rabbit antibody and standard ABC-peroxidase reagents (Vector Laboratories) were applied. After buffer rinses, the slides were incubated in diaminobenzidine chromogen. Normal serum was substituted for primary antibody as negative controls. Bone marrow staining was used as a positive control in each section.
Statistical analysis
Behavioral results were analyzed with Statistica 8 software (StatSoft, Tulsa, USA). Two-Way ANOVA was used to compare the blast and sham groups. P values of post hoc tests were adjusted using the LSD test.
For the MRI experiments, the permeability index of the control group and the time course of both blast distances (4 and 7 meters) were compared between and within the groups with one-way ANOVA (SPSS 11 Software, Genius Systems, Petah Tikva, Israel), followed by post-hoc Fischer LSD comparisons where appropriate. Whole brain, voxel-wise DTI analyses were performed using SPM5. Regions that displayed a statistical difference after a one-way-ANOVA (p<0.05 with false discovery rate [FDR] correction for multiple comparisons) between groups were highlighted on the FA template image. Highlighted regions of interest were evaluated by Post-hoc Fischer LSD comparison using SPSS as noted above. A one-way ANOVA test was used since there is only one pooled control group. Statistical significance was set at p<0.05 throughout and data are presented as mean ± SEM.
Results
A total of 126 male ICR mice were subjected to a blast (Fig 1; 64 mice at 7m (2.5 PSI peak overpressure) and 62 mice at 4m (5.5 PSI peak overpressure)). Less then 3% of the animals died as a consequence of the procedure. The blast animals survived and recovered from anesthesia in a similar manner as the sham group. The survival rate was not different from that of the blunt mTBI model in our lab. However, potential causes of death include anesthesia complications and climate conditions in the field, in addition to the blast effects.
General pathology in the acute time frame
No gross pathological visual damage was found in any internal organ 1-hour post blast, mice (n=6; 2 control and 2 from each blast group). Further, a thorough histopathological examination that included the brain, lungs, stomach, liver, kidney and the GI tract 24 hours post blast again revealed no visible pathological damage in any of the blast-exposed mice (n=6; 2 control and 2 from each blast group).
Neurological scores
No differences were found in neurological scores (NSS, Zohar et al., 2003) between the blast-exposed groups and sham animals at either 1 hour, 24 hours or 7 days after exposure. Hence, any neurological effects of these low level exposures are subclinical in these animals.
The staircase test
The staircase test was used to assess the effects of mild blast exposure on motor and anxiety-like behaviors. The the number of rearing events (NR) and steps ascended (NSA) were measured over a 3-min period in separate groups of animal at 7 days and 30 days after blast exposure (Fig. 2). Both blast exposures produced a similar, significant elevation in the NR outcome measure at at both assessment times. Seven days post blast, a significant elevation in NR was found in mice that were either 7 meters (2.5 PSI, n=15) or 4 meters (5 PSI, n=12) from the explosion site. The NR measure remained significantly elevated at 30 days after blast exposure: Two-way ANOVA of the NR data revealed a significant effect of blast intensity (F (2,90)=14.21; p<0.01), but not of time after exposure (F (1,90)=3.017; n.s). No intensity x time interaction was found.
Fig. 2. Staircase test- the effect of blast on the number of rearing events (NR) and steps ascended (NSA) in a 3-min period.
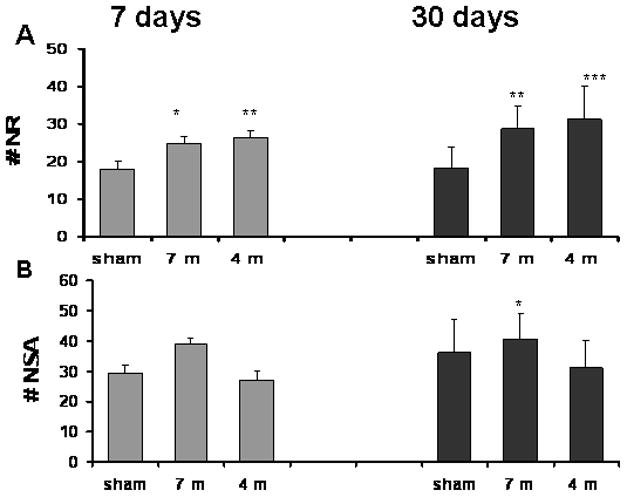
A. Rearing events (NR): Significant elevation NR was found in blasted mice both after 7 days (24.8±1.73 for 7m and 26.4±1.96 for 4m, compared with 18±1.83 in sham mice) and 30 days (28.8±6 for 7 m and 31.2±8.66 for 4 m, compared to 18.2±5.5 in sham mice). B. Steps ascended (NSA): The difference in the number of NSA reached statistical significance only in the 7 m group 7 days post blast (39±1.9 compared with 29.6±2.44 in sham mice). *p<0.05, **p<0.01 or ***p<0.001.
By contrast, only the 2.5 PSI exposure group showed a significant NSA elevation at 7 days post blast. There was no effect of blast intensity (either 2,5 or 5 PSI peak exposure meters) in number of steps ascended (NSA) at 30 days. Two-way ANOVA revealed a significant main effect of blast intensity (F (2,90)=3.090; p<0.01), but not of time (F (1,90)=3.109; n.s). No group x time interaction was found. Since the NSA was only slightly altered, we conclude that motor activity remained relatively intact after blast exposure. Because mice were anesthetized during the blast, this suggests that these behavioral changes in exploration/anxiety behavior were likely to physiological changes rather than a consequence of exposure to fear.
The novel object recognition task
The relative preference of a novel object compared with a familiar one was used to assess the effect of blast on visual memory (Fig. 3). The preference for novel objects by the mice was abolished in mice 7 and 30 days post blast. Two-way ANOVA revealed a significant effect of blast intensity: (F (2,55)=12.9; p<0.01) and time after exposure (F (2,55)=12.9; p≪0.01). No interaction effect was found. This finding suggests a persistent deficit in the blast animals’ visual memory and/or recall that was of similar magnitude at both blast intensities.
Fig. 3. The effect of blast on visual memory as assessed by the novel object recognition test.
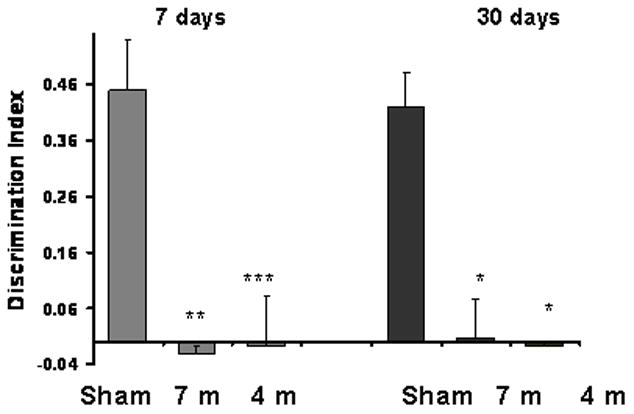
The preference for novel objects was significantly reduced in all the blast groups both at 7 days(−0.02±0.01 for 7 m group, −0.008±0.009 for 4 m group and 0.45±0.09 for sham group) and 30 days (0.007±0.07 for 7 m group, −0.008±0.01 for 4 m group and 0.42±0.06 for sham group). *p<0.05,**p<0.01 or ***p<0.001.
Y-maze test
The Y-maze test was used to quantify the preference for a new path/location based on memory (Fig. 4). The preference for the new maze arm was reduced significantly for both the 2.5 and 5 PSI blast exposures after 7 days. The same tendency was found 30 days post blast but reached statistical significance only after the 5 PSI exposure group.; Two-way ANOVA revealed a significant main effect of blast intensity [(F (2,62)=7.209; p<0.01) but not of time after exposure (F (1,62)=1.389; n.s). No interaction effect was found. The Y maze performance deficit persisted longer for the 5 PSI (4 m) exposure than the 2.5 PSI (7 m) exposure. In combination with the novel object recognition test results, the Y maze data suggest a long-term cognitive/recall deficit in the 5 PSI peak blast wave exposure group. This deficit varied with the blast intensity. The 2.5 PSI peak blast wave exposure, produced less impairment, which reached statistical significance only with the object recognition test, and a was a decrease of 31% for the Y maze, which did not reach significance.
Fig. 4. The effect of blast on spatial memory as assessed by the Y-maze test.

Preference for the new arm was significantly reduced in mice 7 days post blast in both groups (0.2±0.07 for 7 m group and 0.22±0.07 for 4 m in comparison with the sham group 0.51±0.15). Similar impaired memory was found after 30 days for the 4 m group (0.033±0.01 for the 4 m group and 0.361±0.09 for the sham group). *p<0.05,**p<0.01 or ***p<0.001.
In vivo MRI
a) Contrast enhanced T1 weighted images
The average Brain Barrier permeability index (BBpi) of each mouse was calculated for blast-exposed and control mice at 7 and 30 days after exposure. The control mice from all groups were pooled together for these analyses because one-way ANOVA yielded no significant difference between them (F (4,12)= 0.1, p= 0.98). One-way ANOVA showed a main effect of blast intensity between all experimental groups (F(4,40)=5.9, p<0.001). However, the BBPi increased significantly only in the blast group placed 7 meters (2.5 PSI peak exposure) from the explosion site 30 days post blast (Figs 5 & 6), suggesting a delayed effect.
Fig 5. BBB permeability.
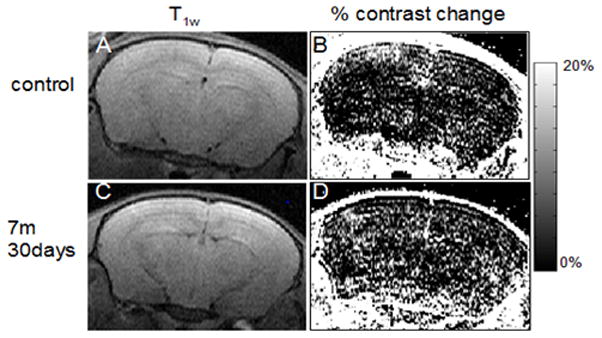
Contrast enhanced MRI and % change of contrast of a control mouse (A, B) and 7m 30 days post-blast mouse (D, E). The scale shows the % change of contrast enhancement 9 min post i.p. Gd-DTPA injection. There were more bright points in the brain region in E as compared to B (approximately Bregma −2.4 mm). (C, F) Respective T2w anatomical images of the same slices (TR/TE 3000/80).
Fig. 6. Blast effect on BBB permeability index (BBPi).
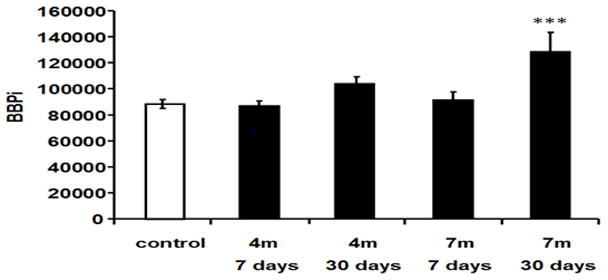
Permeability increased after blast-explosion at 30 days at 7 m compared with control group (F(4,40)=5.9, P<0.001). *** indicates significant difference between the 7m 30 days post-blast group compared to the control group, p<0.001.
b) Diffusion Tensor Imaging (DTI)
Fractional anisotropy (FA) values increased in blast mice compared with controls, particularly in the hypothalamus (Fig. 7A, C, D). Five control cohorts were pooled in one group for this analysis because the control FA values did not differ significantly in the analyzed regions (F(4,9)= 0.92, p= 0.5). The ventral hypothalamus (including median eminence and mammillary bodies) showed significant FA alteration patterns (F(4,42)=7.2, p<0.0002) that varied with blast intensity (Figure 7C). For the lower intensity exposure (7 m distance, 2.5 PSI peak), there was a significant increase in FA at 7 days after blast exposure (Fisher LSD comparison, p<0.01), which resolved by 30 days post-exposure (n.s. re: controls). For the higher intensity exposure group (7 m distance, 5 PSI peak), by contrast, the significant FA increase at 7 days persisted (or was exacerbated) at 30 days post-blast exposure (Fisher LSD comparison, p<0.01), The dorsal hypothalamus showed a similar pattern. The only significant FA increase (re:control values) in rats assessed 30 days after a 5 PSI (4 m distance) blast exposure (p<0.0001). One-way ANOVA showed a main effect of blast intensity (F(4,42)=6.08, P<0.0006). These effects suggested a temporally evolving structural alteration in these hypothalamic regions, with increased severity at the 5 PSI peak blast exposure. Diffuse cortical regions revealed a significant FA increase between control and the 4m 30 days group (p<0.0005).
Figure 7. Voxel-wise statistical analysis of the whole brain DTI.

Representative statistical maps overlaid on the fractional anisotropy (FA) (A) and radial diffusivity (λ3) (B) (approximately Bregma −1.3 mm). The color coded clusters show regions that present significant differences between control and blast-exposed mice (p<0.05, FDR corrected). (C-E) Quantitative values at the indicated regions of interest for control and blast-exposed mice groups of FA (C&D) at the hypothalamus and thalamus, respectively and λ3 (E) at the hypothalamus. Significant difference between the blast group and the control group *p<0.05, **p<0.01, ***p<0.001(one-way ANOVA). Control n=19, 4m 7 days n=12, 4m 7d n=7, 4m 30d n=8 and 7m 30d n=12.
The λ3 values also showed temporal evolving alterations with increasing blast intensity. These values differed significantly from control values for both exposure intensities and at both assessment times. The control group cohorts were pooled for these analyses because they did not differ significantly (one-way ANOVA between the λ3 values in the five control cohort groups (F(4,9)= 0.28, NS). λ3 values decreased in blast exposed mice (2.5 PSI and 5 PSI exposures at both 7 and 30 days) compared with the control group, especially in the hypothalamus (post-hoc LSD comparisons, p<0.02) (Fig 7B, E). One-way ANOVA showed a main effect of the exposure and survival groups (F(4,41)=12.591, p<0.00001). No significant differences were found in ADC, λ1 or λ2 maps between the control or blast-exposed groups at either assessment time.
Since the number of animals in each of the groups was relatively small, we performed exploratory analyses to identify trends in the data that might usefully inform future DTI studies. Setting an uncorrected threshold of p<0.01, revealed widespread, nonlocalized increases in FA and decreases in λ3 between groups, consistent with the more localized effect reported above after correcting for multiple comparisons and suggesting a potentially more global, nonspecific damage profile following blast injury (Fig. 8A-C).
Figure 8. FA statistical maps overlaid on the fractional anisotropy (FA) maps.

Apparent regions of FA abnormality are diffusely spread over most of the brain including the cortex and the thalamus (approximately Bregma 1 mm, −1.4 mm and −2.3 mm - from left to right A-C). One-way ANOVA with significance of p<0.01 uncorrected.
Histological evaluation of cranial contents
In addition to the brain and upper cervical spinal cord, the sectioned heads permitted histological evaluation of the status of blood vessels in the subarachnoid space, particularly in regions of the hypothalamus that displayed imaging alterations after blast exposure. There were no signs of subdural or subarachnoid hemorrhage from these low level blasts. For example, intact small veins and arteries are shown in the interpeducular fossa 72 hr after a 5.5 PSI (4 m distance) exposure in Figure 9. The brain parenchyma appeared normal in these regions that showed BBpi, FA and λ3 changes in MRI. However, immunohistochemistry revealed a prominent up-regulation of mitochondrial superoxide dismutase 2 expression in the mammillary bodies at 72 hr post-exposure in the group exposed to a 5.5 PSI wave at 4 m from the explosive charge (Figure 9), but the shorter survival times and lower blast exposure groups showed no difference from tissues in the control (unexposed) mice. This finding is suggestive a localized mitochondrial response to oxidative stress.
Figure 9. Histopathology and distribution of MnSOD2 in the interpeduncular region and mammillary body after blast exposure.
The left column shows low magnification photomicrographs of horizontal sections through the hypothalamus and interpeduncular nucleus (IP) of mice exposed 72h earlier to a single 5.5 PSI overpressure blast. The left upper panel is stained with hematoxylin and eosin and shows normal vasculature and brain parenchyma. The lower left panels are stained immunohistochemically for MnSOD2. Note the strong up-regulation of MnSOD2 in the mammillary body (Mm) after blast exposure, shown at higher magnification in the right panels. The calibration bar is 500 microns for the left panels and 50 microns for the right panels. Abbreviations: IP-interpeduncular nucleus, CC-crus cerebri, Mm-mammillary body, f-fornix.
Expression of the CXC motif chemokine receptor 3 (CXCR3) was also assessed as one indicator of vascular remodeling activity. Immunoreactivity for CXCR3 was upregulated widely in the regions showing in vivo imaging changes after blast exposure. The immunoreactivity appeared to be associated with blood vessels in fiber tracts within 72 hours after blast exposure at either 2.5 PSI (7 m distance, not shown) or 5.5 PSI (4 m distance, Figure 10). In horizontal sections through the ventral aspect of the hypothalamus, this increased staining was prominent in the crus cerebri lateral to the interpeduncular fossa, the fornix, and in the optic tract ventral to the lateral geniculate body. These features are suggestive of recruitment of vascular mechanisms associated with angiostasis and angiogenesis after low-level blast exposure, in the absence of brain parenchymal changes.
Figure 10.
Expression of C-X-C motif chemokine receptor 3 in fiber tracts after blast exposure. Photomicrographs show the emergence of immunoreactivity in association with blood vessels within 72h of a single low level blast exposure in the crus cerebri (upper row), fornix (middle row) and optic tract (lower row). The positive control staining of bone marrow from the same blast exposed and control sections are shown in the insert. The calibration bar represents 100 microns.
Discussion
The complex neurological and neuropsychiatric clinical picture presented by injured soldiers and civilians exposed to IEDs far exceed the physical injuries following exposure to low-level blast waves (Crabtree, 2006; Mekel et al., 2009). The spectrum of moderate to severe brain injury is easily detectable, both clinically by focal neurological signs and by neuroimaging (Mintz et al., 2002) as well as by altered (deficient functional synchronization) EEG activity (Sponheim et al., 2011). However, the affective, cognitive, and behavioral changes that frequently follow mild, or mild-to moderate brain injury are more problematic, particularly in cases with absent or transient focal neurological signs and neuroimaging studies are show no evidence of abnormalities. In these cases, the mere existence of a clinical entity has been controversial (Brenner et al., 2009; Pietrzak et al., 2009). As a consequence, symptom reports can be attributed erroneously to motives for secondary gain (Hoge et al., 2009). Various laboratory models of blast induced mild TBI have addressed this gap in knowledge, but none of them used “real-world” conditions. In order to mimic the exposure to a mild blast in as-realistically-as-possible, found that mice exposed to very low intensity explosions exhibit both behavioral and MRI results compatible with diffuse mild brain injury.
Study of the pathophysiological mechanisms that underlie blast-induced brain injury is needed both for a better understanding of the clinical entity, and for the further development of possible treatments. This can be done best in an animal model that controls for as many variables as possible as well as resembles, as much as possible, “real life” conditions. We recognize that inferences from an animal-model are limited by factors that include the structural differences between murine and human brains and biophysical factors related to head morphology (including skull thickness). However, the mechanisms of injury and responses can provide insights for rational translational research in humans.
Our mouse-model of blast-induced brain injury fulfills the requirements of replicating key features of clinical blast-related mTBI. Almost all mice exposed to blast (64 mice for a 7m distance/2.5PSI and 62 mice for a 4m distance/5.5PSI) survived and recovered from anesthesia in a manner similar to the control mice. Moreover, upon visual and light microscopic examination, no gross anatomical damage was found acutely in the blast-exposed mice. By seven days after blast exposure, there was only a marginal trend toward alteration of blood-brain barrier integrity. However, the neurological score test, no differences between the blast exposed and the sham group during the first week after blast exposure. These findings suggest that the degree of injury in this model is comparable with human ‘mild’ blast induced brain injury.
The results from mice in our open field model are consistent with findings from other species that show damage after low overpressure exposures in shock tube models of brain blast injury. Pioneering studies by Saljo and co-workers (Saljo et al., 2001, 2002a, 2002b, 2003) documented hippocampal and cerebral cortical signs after single relatively high level shock wave exposure (198–202 dB peak overpressure re: 20 μ Pa; 25–35 PSI) to rats in a blast tube. These changes included evidence of apoptotic neurons, persistent changes in phosphorylated neurofilament proteins and transcription factors and a proliferation of microglia and astrocytes for at least 3 weeks after exposure. The mouse model did not exhibit small parenchymal hemorrhages that have reported in the occipital cortex, cerebellar cortex and medulla of 30–40% pigs exposed to impulse noise in the 1.3–6 PSI range (Saljo et al., 2008). However, the behavioral findings from our mouse model are consistent with the 174 dB SPL (1.5 PSI) over-pressure threshold for abnormal Morris water maze behavior from single blast exposure in rats (Saljo et al. 2009), Despite potential differences due to species and experimental design and, the mouse, rat and pig data all indicate that low blast exposures have persistent and evolving neurobehavioral consequences.
The combination of the behavioral studies and the MRI findings suggests diffuse but subtle CNS damage. This might be expected to appear following the double impact of the blast wave: a high-pressure phase, followed closely by a low-pressure phase. (Courtney and Courtney, 2009; Elder and Cristian, 2009; Moss et al., 2009). Behavioral and cognitive testing at one week and at one-month post exposure, suggest that there are both persistent and progressive deficits after only a single, low-level blast exposure. The rearing behavior on the staircase test and the discrimination index in the novel object recognition test showed deficits at 7 days that persisted at 30 days after blast exposure, but did not vary in severity between the two low level (2.5 and 5 PSI) blast exposures. However, the discrimination index for Y-maze performance varies with blast exposure. After the lower 2.5 PSI peak exposure a deficit at 7 days resolved partially by 30 days after the blast. A single 5 PSI peak exposure, though, produced a deficit at 7 days that persisted at 30 days.
We suggest the following interpretation of these abnormal tests. A recent evaluation of the novel object recognition test (McTighe et al., 2010) has indicated that,, rather than a loss of memory for the familiar object or deficit in executive function, there is a “false memory” of the novel object as familiar after perirhinal cortex damage. Furthermore, although no motor impairments were found at the blast group (as seen in the “steps ascended” parameter of the staircase test), the equivalent of a typical clinical picture of restlessness/agitation recognized in people with blast induced mTBI, was evident in the blasted mice from the “rearing” parameter of this test. The fact that the blast-induced changes in these two different tests were not identical might be due to the fact that these are two separate systems. Together, these results suggest a clear and pronounced, combined cognitive and behavioral deficit in the blast-exposed mice that develops during a subacute to chronic post-exposure period.
In the MRI study, significant alterations were found with T1 weighted images showing an increased BBB permeability one month post-blast. BBB rupture is a common consequence of traumatic brain injury and a leading cause for secondary brain damage immediately after injury (Beaumont et al., 2000; Vajtr et al., 2009). However, we did not see BBB disruption until one month after these very low intensity blast exposures, and only in the mice exposed to the lower blast level (2.5 PSI/7 m). Because factors such as inflammatory processes can interfere with repair of leakage in some neuroinflammatory conditions (de Vries et al., 1997), an explanation may come from elucidation the long- term time course of inflammatory mechanisms after similar exposures.
Diffusion Tensor Imaging (DTI) has been suggested as a diagnostic tool for microstructural alteration in brain tissue, especially for axonal and myelin pathologies (Jiang, Q et al., 2006; Jiang, Y et al., 2010; Knake et al., 2010; Mori et al., 2006; Song et al., 2002). DTI investigates tissue microstructure of the brain and allows extraction of FA indices (fractional anisotropy), which relates to the level of white matter organization; ADC map (apparent diffusion coefficient), which is related to the isotropic mobility of water; and axial λ1 and radial λ2, λ3, are related to the diffusivity along the long and short axes of the axons, respectively.
Our data revealed a significant increase in FA values with a concomitant decrease in λ3 values in blasted mice compared with controls, particularly in the hypothalamus,. These results suggest blast-induced microstructural changes. Elevated FA values are correlated with axonal cytotoxicity and edema (Bazarian et al., 2007; Wilde et al., 2008). Reduced λ3 values are compatible with myelin abnormalities. Both FA and λ3 values exhibit a more pronounced change over time (30 days>/< 7 days, respectively). This may reflect a time dependent process. These DTI results are in agreement with some of the cognitive and behavioral results, where more deficits are found at 30 days post blast. These findings correlate with Sponheim et al., (2011) who recently published an elegant study where blast injured patients exhibited diminished EEG phase synchrony of lateral frontal sites with contralateral frontal brain regions, suggesting diminished inter-hemispheric coordination of brain activity following the blast injury. A similar trend in the DTI test was recently found in mTBI patients after blunt force (Chu et al., 2010) and blast trauma (MacDonald et al., 2011), suggesting a possible apoptosis of synapses as the underlying mechanism of damage (Coleman and Perry, 2002). However, a similar study with blast mTBI patients showing no changes in DTI parameters, possibly due to the length of time (29 months) from the injury (Levin et al., 2010). Reduced λ3 values might reflect an increased affinity of myelin to the axon (due to alteration in ganglioside composition) thus preventing its regeneration following injury. It is noteworthy that an abnormal brain condition is known as “axonal outgrowth- inhibition” suggested previously in other models of TBI (Schnaar 2010; Vyas et al., 2002).
Examination of histological sections of decalcified heads showed normal structure of the brain, spinal cord, vasculature and meninges at survival times up to 72 hours. However, immunohistochemical observations revealed changes suggestive of early inflammatory and oxidative stress responses in the hypothalamic regions that showed later changes in the imaging studies. Neuronal immunoreactivity for the mitochondrial anti-oxidant enzyme, manganese superoxide dismutase 2, was augmented markedly in the ventral aspect of the hypothalamus at 72 hours after the higher blast exposure. Increased immunoreactivity for the CXC chemokine receptor 3 (CXCR3) was also increased in association with blood vessel profiles in the fornix, optic tract and crus cerebri. The apparent upregulation of CXCR3 is of interest because this receptor has been linked to both vascular remodeling and development of autoimmune responses, such as endocrine autoimmunity and multiple sclerosis (Omari et al 2005; Rotondi et al. 2007; Lacotte et al. 2009). Because the primary ligands for CXCR3 (CXCL9, CXCL10 and CXCL11) are induced by interferon-γ, it will be important to explore the interplay between inflammatory and vascular remodeling mechanisms as factors in the chronic behavioral and cognitive consequences of mild blast TBI., The importance of such studies for longer-term neurological disorders, such as dementia and Alzheimer’s disease, has recently been emphasized (DeKosky et al, 2010).
CONCLUSION
In conclusion, our initial study shows long term cognitive and affective behavioral abnormalities after low-level blasts in a “real life” environment. There are correlative BBB changes and changes in expression of some biomarkers oxidative stress (MnSOD2) and endovascular remodeling (CXCR3) that may presage chronic neurologic complications, but no gross pathological alterations in brain or periphery. Moreover, no short-term neurological assessment changes were seen. We thus conclude that blast-induced mTBI may represent a distinct neurological disorder that requires considerable further research to define pathophysiology.
Research Highlights.
Blast-induced traumatic brain injury elicit long term behavioral abnormalities in spite of little or no gross anatomical changes in brain or periphery
Correlative MRI studies show white matter and BBB changes after blast
Overpressure/underpressure brain injury produces significant functional changes in brain without gross anatomical damage
Acknowledgments
We thank Dr. Letizia Schreiber, Dr. Galia Tsarfati and Dr. Ronit Satchi-Fainaro for their help. In addition, we would like to express thanks to Mr. Boaz Hayun, Mr. Avi Icar, Mr. Lippe Sadwin. Ms. Gloria Limetti and Ms. Cynthia Stone for their expert technical assistance and support. This study was partially supported by the Intramural Research Programs at the NIH (National Institute on Drug Abuse), by Taiwan NSC grants NSC98-2321-B-038-003-MY3 and NSC98-2314-B-038-012-MY3, and by DOD – EOARD grant FA8655-08-1-3010.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aggleton JP, Keen S, Warburton EC, Bussey TJ. Extensive cytotoxic lesions involving both the rhinal cortices and area TE impair recognition but spare spatial alternation in the rat. Brain Research Bulletin. 1997;43:279–287. doi: 10.1016/s0361-9230(97)00007-5. [DOI] [PubMed] [Google Scholar]
- Andreasen NC. Acute and delayed posttraumatic stress disorders: a history and some issues. The American Journal of Psychiatry. 2004;161:1321–1323. doi: 10.1176/appi.ajp.161.8.1321. [DOI] [PubMed] [Google Scholar]
- Arciniegas D, et al. Attention and memory dysfunction after traumatic brain injury: cholinergic mechanisms, sensory gating, and a hypothesis for further investigation. Brain Inj. 1999;13:1–13. doi: 10.1080/026990599121827. [DOI] [PubMed] [Google Scholar]
- Barnum CJ, Tansey MG. (in press) The duality of TNF signaling outcomes in the brain: Potential mechanisms? Experimental Neurology. 2011 doi: 10.1016/j.expneurol.2011.02.016. (in press) [DOI] [PubMed] [Google Scholar]
- Basser PJ, Pierpaoli C. Microstructural and physiological features of tissues elucidated by quantitative-diffusion-tensor MRI. Journal of Magnetic Resonance. 1996;111:209–219. doi: 10.1006/jmrb.1996.0086. [DOI] [PubMed] [Google Scholar]
- Bazarian JJ, Zhong J, Blyth B, et al. Diffusion tensor imaging detects clinically important axonal damage after mild traumatic brain injury: a pilot study. Journal of Neurotrauma. 2007;24:1447–1459. doi: 10.1089/neu.2007.0241. [DOI] [PubMed] [Google Scholar]
- Beaumont A, Marmarou A, Hayasaki K, et al. The permissive nature of blood brain barrier (BBB) opening in edema formation following traumatic brain injury. Acta Neurochirurgica. 2000;76:125–129. doi: 10.1007/978-3-7091-6346-7_26. [DOI] [PubMed] [Google Scholar]
- Bigler ED. Neuropsychology and clinical neuroscience of persistent post-concussive syndrome. J Int Neuropsychol Soc. 2008;14:1–22. doi: 10.1017/S135561770808017X. [DOI] [PubMed] [Google Scholar]
- Brenner LA, Vanderploeg RD, Terrio H. Assessment and diagnosis of mild traumatic brain injury, posttraumatic stress disorder, and other polytrauma conditions: burden of adversity hypothesis. Rehabilitation Psychology. 2009;54:239–246. doi: 10.1037/a0016908. [DOI] [PubMed] [Google Scholar]
- Cernak I, Noble-Haeusslein LJ. Traumatic brain injury: an overview of pathobiology with emphasis on military populations. J Cereb Blood Flow Metab. 2010;30:255–266. doi: 10.1038/jcbfm.2009.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng J, Gu J, Ma Y, et al. Development of a rat model for studying blast induced traumatic brain injury. Journal of the Neurological sciences. 2010;294:23–28. doi: 10.1016/j.jns.2010.04.010. [DOI] [PubMed] [Google Scholar]
- Chu Z, Wilde EA, Hunter JV, et al. Voxel-based analysis of diffusion tensor imaging in mild traumatic brain injury in adolescents. AJNR. 2010;31:340–346. doi: 10.3174/ajnr.A1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman MP, Perry VH. Axon pathology in neurological disease: a neglected therapeutic target. Trends in Neuroscience. 2002;25:53. doi: 10.1016/s0166-2236(02)02255-5. [DOI] [PubMed] [Google Scholar]
- Conrad CD, Galea LA, Kuroda Y, McEwen BS. Chronic stress impairs rat spatial memory on the Y maze, and this effect is blocked by tianeptine pretreatment. Behavioral Neuroscience. 1996;110:1321–1334. doi: 10.1037//0735-7044.110.6.1321. [DOI] [PubMed] [Google Scholar]
- Courtney AC, Courtney MW. A thoracic mechanism of mild traumatic brain injury due to blast pressure waves. Medical Hypotheses. 2009;72:76–83. doi: 10.1016/j.mehy.2008.08.015. [DOI] [PubMed] [Google Scholar]
- Crabtree J. Terrorist homicide bombings: a primer for preparation. J Burn Care Res. 2006;27:576–588. doi: 10.1097/01.BCR.0000235459.60000.A5. [DOI] [PubMed] [Google Scholar]
- DeKosky ST, Ikonomovic MD, Gandy S. Traumatic Brain Injury - Football, Warfare, and Long-Term Effects. N Engl Med. 2010;363:14. doi: 10.1056/NEJMp1007051. [DOI] [PubMed] [Google Scholar]
- Dellu F, Mayo W, Cherkaoui J, Le Moal M, Simon H. A two-trial memory task with automated recording: study in young and aged rats. Brain Research. 1992;588:132–139. doi: 10.1016/0006-8993(92)91352-f. [DOI] [PubMed] [Google Scholar]
- de Vries HE, Kuiper J, de Boer AG, et al. The blood-brain barrier in neuroinflammatory diseases. Pharmacological Reviews. 1997;49:143–155. [PubMed] [Google Scholar]
- Dolberg OT, Barkai G, Leor A, et al. Injured civilian survivors of suicide bomb attacks: From partial PTSD to recovery or to traumatisation. Where is the turning point? World J Biol Psychiatry. 2007:1–8. doi: 10.3109/15622970701624579. [DOI] [PubMed] [Google Scholar]
- Elder GA, Cristian A. Blast-related mild traumatic brain injury: mechanisms of injury and impact on clinical care. The Mount Sinai Journal of Medicine, New York. 2009;76:111–118. doi: 10.1002/msj.20098. [DOI] [PubMed] [Google Scholar]
- Finset A, et al. Cognitive performance in multiple trauma patients 3 years after injury. Psychosom Med. 1999;61:576–583. doi: 10.1097/00006842-199907000-00024. [DOI] [PubMed] [Google Scholar]
- Hoffer ME, Donaldson C, Gottshall KR, Balaban CD, Balough BJ. Blunt and blast trauma: different entities. International Tinnitus J. 2009;15:115–118. [PubMed] [Google Scholar]
- Hoffer ME, Balaban C, Gottshall KR, et al. Blast exposure: vestibular consequences and associated characteristics. Otol Neurotol. 2010;31:232–236. doi: 10.1097/MAO.0b013e3181c993c3. [DOI] [PubMed] [Google Scholar]
- Hoge CW, Goldberg HM, Castro CA. Care of war veterans with mild traumatic brain injury--flawed perspectives. The New England Journal of Medicine. 2009;360:1588–1591. doi: 10.1056/NEJMp0810606. [DOI] [PubMed] [Google Scholar]
- Jiang Q, Zhang ZG, Ding GL, et al. MRI detects white matter reorganization after neural progenitor cell treatment of stroke. NeuroImage. 2006;32:1080–1089. doi: 10.1016/j.neuroimage.2006.05.025. [DOI] [PubMed] [Google Scholar]
- Jiang Y, Johnson GA. Microscopic diffusion tensor imaging of the mouse brain. NeuroImage. 2010;50:465–471. doi: 10.1016/j.neuroimage.2009.12.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kibby MY, Long CJ. Minor head injury: attempts at clarifying the confusion. Brain Inj. 1996;10:159–186. doi: 10.1080/026990596124494. [DOI] [PubMed] [Google Scholar]
- Knake S, Belke M, Menzler K, et al. In vivo demonstration of microstructural brain pathology in progressive supranuclear palsy: a DTI study using TBSS. Mov Disord. 2010 March 10; doi: 10.1002/mds.23054. [DOI] [PubMed] [Google Scholar]
- Kurrelmeyer KM, Lloyd H, Michael LH, Baumgarten G, Taffet GE, Peschon JJ, Sivasubramanian N, Entman ML, Mann DL. Endogenous tumor necrosis factor protects the adult cardiac myocyte against ischemic-induced apoptosis in a murine model of acute myocardial infarction. PNAS. 2000;97(10):5456–5461. doi: 10.1073/pnas.070036297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin HS, et al. Neurobehavioral outcome following minor head injury: a three-center study. J Neurosurg. 1987;66:234–243. doi: 10.3171/jns.1987.66.2.0234. [DOI] [PubMed] [Google Scholar]
- Levin HS, Wilde E, Troyanskaya M, et al. Diffusion tensor imaging of mild to moderate blast-related traumatic brain injury and its sequelae. Journal of Neurotrauma. 2010;27:683–694. doi: 10.1089/neu.2009.1073. [DOI] [PubMed] [Google Scholar]
- Long JB, Bentley TL, Wessner KA, Cerone C, Sweeney S, Bauman RA. Blast overpressure in rats: recreating a battlefield injury in the laboratory. Journal of Neurotrauma. 2009;26(6):827–840. doi: 10.1089/neu.2008.0748. [DOI] [PubMed] [Google Scholar]
- Lacotte S, Brun S, Muller S, et al. CXCR3, inflammation and autoimmune diseases. Contemporary Challenges in Autoimmunity: Annals of the New York Academy of Sciences. 2009;1173:310–317. doi: 10.1111/j.1749-6632.2009.04813.x. [DOI] [PubMed] [Google Scholar]
- MacDonald CL, Johnson AM, Cooper D, Nelson EC, Werner NJ, Shimony JS, Snyder AZ, Raichle ME, Witherow JR, Fang R, Flaherty SF, Brody DL. Detection of blast related traumatic brain injury in U.S.military personnel. NEngl J Med. 2011;364:2091–2100. doi: 10.1056/NEJMoa1008069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margulies S. The postconcussion syndrome after mild head trauma: is brain damage over diagnosed? J Clin Neurosci. 2002;7:400–408. doi: 10.1054/jocn.1999.0681. [DOI] [PubMed] [Google Scholar]
- McTighe Stephanie M, Cowell Rosemary A, Winters Boyer D, et al. Paradoxical False Memory for Objects After Brain Damage. Science. 2010;330:1408–1410. doi: 10.1126/science.1194780. [DOI] [PubMed] [Google Scholar]
- Mekel M, Bumenfeld A, Feigenberg Z, et al. Terrorist suicide bombings: lessons learned in Metropolitan Haifa from September 2000 to January 2006. American Journal of Disaster Medicine. 2009;4:233–248. [PubMed] [Google Scholar]
- Messier C. Object recognition in mice: improvement of memory by glucose. Neurobiology of Learning and Memory. 1997;67:172–175. doi: 10.1006/nlme.1996.3755. [DOI] [PubMed] [Google Scholar]
- Mintz Y, Shapira SC, Pikarsky AJ, et al. The experience of one institution dealing with terror: the El Aqsa Intifada riots. Isr Med Assoc J. 2002;4:554–556. [PubMed] [Google Scholar]
- Mori S, Zhang J, Bulte JW. Magnetic resonance microscopy of mouse brain development. Methods in Molecular Medicine. 2006;124:129–147. doi: 10.1385/1-59745-010-3:129. [DOI] [PubMed] [Google Scholar]
- Moss WC, King MJ, Blackman EG. Skull flexure from blast waves: a mechanism for brain injury with implications for helmet design. Physical Review Letters. 2009;103:108702. doi: 10.1103/PhysRevLett.103.108702. [DOI] [PubMed] [Google Scholar]
- Omari KM, John GR, Sealfon SC, et al. CXC chemokine receptors on human oligodendrocytes: implications for multiple sclerosis. Brain. 2005;128:1003–1015. doi: 10.1093/brain/awh479. [DOI] [PubMed] [Google Scholar]
- Pietrzak RH, Johnson DC, Goldstein MB, et al. Posttraumatic stress disorder mediates the relationship between mild traumatic brain injury and health and psychosocial functioning in veterans of Operations Enduring Freedom and Iraqi Freedom. The Journal of Nervous and Mental Disease. 2009;197:748–753. doi: 10.1097/NMD.0b013e3181b97a75. [DOI] [PubMed] [Google Scholar]
- Ratiu P, Talos IF, Haker S, et al. The tale of Phineas Gage, digitally remastered. Journal of Neurotrauma. 2004;21:637–643. doi: 10.1089/089771504774129964. [DOI] [PubMed] [Google Scholar]
- Rotondi M, Chiovato L, Romagnani S, Serio M, Romagnani P. Role of chemokines in endocrine autoimmune diseases. Endocrine Reviews. 2007;28:492–520. doi: 10.1210/er.2006-0044. [DOI] [PubMed] [Google Scholar]
- Ruff R. Two decades of advances in understanding of mild traumatic brain injury. The Journal of Head Trauma Rehabilitation. 2005;20:5–18. doi: 10.1097/00001199-200501000-00003. [DOI] [PubMed] [Google Scholar]
- Ryan LM, Warden DL. Post concussion syndrome. International Review of Psychiatry, Abingdon, England. 2003;15:310–316. doi: 10.1080/09540260310001606692. [DOI] [PubMed] [Google Scholar]
- Saljo A, Bao F, Haglid KG, Hansson HA. Blast exposure causes a redistribution of phosphorylated neurofilament subunits in neurons of the adult rat brain. J Neurotrauma. 2000;17:719–726. doi: 10.1089/089771500415454. [DOI] [PubMed] [Google Scholar]
- Saljo A, Bao F, Hamberger A, Haglid KG, Hansson HA. Exposure to short-lasting impulse noise causes microglial and astroglial cell activation in the adult rat brain. Pathophysiology. 2001;8:105–111. doi: 10.1016/s0928-4680(01)00067-0. [DOI] [PubMed] [Google Scholar]
- Saljo A, Bao F, Shi J, Hamberger A, Hansson HA, Haglid KG. Expression of c-Fos and c-Myc and deposition of beta-APP in neurons in the adult rat brain as a result of exposure to short-lasting impulse noise. J Neurotrauma. 2002a;19:379–385. doi: 10.1089/089771502753594945. [DOI] [PubMed] [Google Scholar]
- Saljo A, Jingshan S, Hamberger A, Hansson HA, Haglid KG. Exposure to short-lasting impulse noise causes neuronal c-Jun expression and induction of apoptosis in the adult rat brain. J Neurotrauma. 2002b;19:985–991. doi: 10.1089/089771502320317131. [DOI] [PubMed] [Google Scholar]
- Saljo A, Huang YL, Hansson HA. Impulse noise transiently increased the permeability of nerve and glial cell membranes, an effect accentuated by a recent brain injury. J Neurotrauma. 2003;20:787–794. doi: 10.1089/089771503767870014. [DOI] [PubMed] [Google Scholar]
- Saljo A, Arrhen F, Bolouri H, Mayorga M, Hamberger A. Neuropathology and pressure in the pig brain resulting from low-impulse noise exposure. J Neurotrauma. 2008;25:1397–1406. doi: 10.1089/neu.2008.0602. [DOI] [PubMed] [Google Scholar]
- Saljo A, Svensson B, Mayorga M, Hamberger A, Bolouri H. Low-level blasts raise intracranial pressure and impair cognitive function in rats. J Neurotrauma. 2009;26:1345–1352. doi: 10.1089/neu.2008-0856. [DOI] [PubMed] [Google Scholar]
- Schnaar RL. Brain gangliosides in axon-myelin stability and axon regeneration. FEBS Letters. 2010;584:1741–1747. doi: 10.1016/j.febslet.2009.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber S, Dolberg OT, Barkai, et al. Primary intervention for memory structuring and meaning acquisition (PIMSMA): study of a mental health first-aid intervention in the ED with injured survivors of suicide bombing attacks. American Journal of Disaster Medicine. 2007;2:307–320. [PubMed] [Google Scholar]
- Simiand J, Keane PE, Morre M. The staircase test in mice: a simple and efficient procedure for primary screening of anxiolytic agents. Psychopharmacology. 1984;84:48–53. doi: 10.1007/BF00432023. [DOI] [PubMed] [Google Scholar]
- Song SK, Sun SW, Ramsbottom MJ, et al. Dysmyelination revealed through MRI as increased radial (but unchanged axial) diffusion of water. NeuroImage. 2002;17:1429–1436. doi: 10.1006/nimg.2002.1267. [DOI] [PubMed] [Google Scholar]
- Sponheim SR, McGuire KA, Kang SS, et al. Evidence of disrupted functional connectivity in the brain after combat-related blast injury. NeuroImage. 2011;54:S21–S29. doi: 10.1016/j.neuroimage.2010.09.007. [DOI] [PubMed] [Google Scholar]
- Sterr A, Herron KA, Hayward C, Montaldi D. Are mild head injuries as mild as we think? Neurobehavioral concomitants of chronic post-concussion syndrome. BMC Neurology. 2006;6:7. doi: 10.1186/1471-2377-6-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tashlykov V, Katz Y, Gazit, et al. Apoptotic changes in the cortex and hippocampus following minimal brain trauma in mice. Brain Research. 2007;1130:197–205. doi: 10.1016/j.brainres.2006.10.032. [DOI] [PubMed] [Google Scholar]
- Tashlykov V, Katz Y, Volkov A, et al. Minimal traumatic brain injury induce apoptotic cell death in mice. J Mol Neurosci. 2009;37:16–24. doi: 10.1007/s12031-008-9094-2. [DOI] [PubMed] [Google Scholar]
- Thompson JM, Scott KC, Dubinsky L. Battlefield brain: unexplained symptoms and blast-related mild traumatic brain injury. Canadian Family Physician Medecin de famille canadien. 2008;54:1549–1551. [PMC free article] [PubMed] [Google Scholar]
- Vajtr D, Benada O, Kukacka J, et al. Correlation of ultrastructural changes of endothelial cells and astrocytes occurring during blood brain barrier damage after traumatic brain injury with biochemical markers of BBB leakage and inflammatory response. Physiological Research/Academia Scientiarum Bohemoslovaca. 2009;58:263–268. doi: 10.33549/physiolres.931253. [DOI] [PubMed] [Google Scholar]
- Vyas AA, Patel HV, Fromholt SE, et al. Gangliosides are functional nerve cell ligands for myelin-associated glycoprotein (MAG), an inhibitor of nerve regeneration. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:8412–8417. doi: 10.1073/pnas.072211699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilde EA, McCauley SR, Hunter JV, et al. Diffusion tensor imaging of acute mild traumatic brain injury in adolescents. Neurology. 2008;70:948–955. doi: 10.1212/01.wnl.0000305961.68029.54. [DOI] [PubMed] [Google Scholar]
- Zohar O, Schreiber S, Getslev V, et al. Closed-head minimal traumatic brain injury produces long-term cognitive deficits in mice. Neuroscience. 2003;118:949–955. doi: 10.1016/s0306-4522(03)00048-4. [DOI] [PubMed] [Google Scholar]





