Abstract
BACKGROUND
In Kenya and much of sub-Saharan Africa, nearly half of all couples affected by HIV are discordant. Antiretroviral therapy (ART) slows disease progression in HIV-1-infected individuals, and reduces transmission to uninfected partners. We examined time to ART initiation and factors associated with delayed initiation in HIV-1-discordant couples in Nairobi.
METHODS
HIV-1-discordant couples were enrolled and followed quarterly for up to 2 years. Clinical staff administered questionnaires and conducted viral loads and CD4 counts. Participants with a CD4 count meeting ART criteria were referred to a nearby PEPFAR-funded treatment center. Barriers to ART initiation among participants with a CD4 count eligible for ART were assessed by Cox regression.
RESULTS
Of 439 HIV-1-infected participants (63.6% females and 36.4% males) 146 met CD4 count criteria for ART during follow-up. Median time from meeting CD4 criteria until ART initiation was 8.9 months, with 42.0% of eligible participants on ART by 6 months and 63.4% on ART by 1 year. The CD4 count at the time of eligibility was inversely associated with time to ART initiation (HR=0.49, p< 0.001). Compared to homeowners, those paying higher rents started ART 48% more slowly (p=0.062) and those paying lower rents started 71% more slowly (p=0.002).
CONCLUSIONS
Despite access to regular health care, referrals to treatment centers, and free access to ART, over a third of participants with an eligible CD4 count had not started ART within 1 year. Factors of lower socioeconomic status may slow ART initiation and targeted approaches are needed to avoid delays in treatment initiation.
Keywords: HIV, discordant couples, serodiscordant, antiretroviral, ART, HAART
Introduction
The benefits of antiretroviral therapy (ART) for improving survival and reducing transmission are well established,1-3 yet only an estimated 44% of people in sub-Saharan Africa needing ART are receiving it.4 Much attention has been placed on system-level barriers to ART initiation, with notable improvements in the availability of affordable treatment. Less attention has been devoted to individual-level barriers that may interfere with timely ART initiation despite the availability of drugs.
Prompt initiation of ART is of particular importance in HIV-discordant couples, where effective treatment not only benefits the infected partner, but also significantly reduces the risk of transmission to their uninfected partner.3, 5-7 While risk reduction in discordant couples must be a multifaceted approach, the dominant role of viral load in determining transmission risk argues strongly for a major focus on strategies to reduce viral load in the infected partner.8 Effective viral suppression through ART therefore has great potential as a prevention strategy, particularly when targeted at discordant couples.
Studies from South Africa showed that by 1 year after being diagnosed with HIV-1 and receiving a CD4 count eligible to start ART, only 39% had started treatment,9, 10 and one important obstacle to maximizing ART coverage is poor retention in pre-ART care programs.11 New initiatives to promote provider-initiated testing are increasing the number of people who are aware of their HIV status, but patients tested in this way tend to have a lower rate of linkage to ongoing HIV care and ART initiation compared to those who initiate testing on their own.12 These findings highlight the great need to improve monitoring and ongoing care of patients through pre-ART programs, and to expedite ART initiation rates once eligibility criteria are met.
In this study, we followed a cohort of HIV-1-infected participants in HIV-1-discordant relationships to determine the ART initiation rate. Both members of these couples were aware of their partner’s HIV-1 status, and thus these couples represent a group of particular interest as a target for strategies to expedite ART initiation. We focused on factors that may serve as barriers to ART initiation in an attempt to better understand the decision process surrounding ART initiation and to identify potential intervention targets to improve initiation rates.
Methods
Study participants
HIV-1-discordant couples were recruited from voluntary counseling and testing (VCT) centers in Nairobi, Kenya from September 2007 to December 2009. Participants consented to 2 years of follow-up with quarterly study visits as part of a study of HIV-1-specific cellular immune responses and HIV-1 transmission. Eligible couples reported sex ≥3 times in the 3 months prior to screening and planned to remain together for the duration of the study. Women could not be pregnant at enrollment and HIV-1-infected participants could not have a history of clinical AIDS (WHO stage IV) and were not currently on ART. At enrollment and follow-up visits, clinical staff administered a questionnaire including sociodemographic, sexual behavior, and medical history characteristics. Questionnaires were presented in English or Kiswahili depending on participant preference, and were administered individually to ensure confidentiality. Low education was defined as less than a primary education (<8 years). Rent was categorized as lower rent (less than the median) or higher rent (greater than or equal to the median) and income was calculated as the combined income for both partners in the couple.
Distance between the participant’s residence and the treatment center to which participants were referred for ART assessment was estimated using locater information provided by each participant. Based on this information, a study team member mapped the residences using a web-based mapping application. The resulting coordinates were used to estimate driving distances using a geospatial algorithm to generate driving routes (Google, Inc., Mountain View, CA).
Laboratory methods
CD4 cell counts were measured on blood samples collected at enrollment and every 6 months during follow-up using a FACSCalibur flow cytometer (BD Bioscience, Franklin Lakes, NJ), and HIV-1 RNA levels were measured in plasma from blood samples collected at enrollment using the Gen-Probe HIV-1 viral load assay (Gen-Probe Incorporated, San Diego, CA, USA) as previously described.13 Urine pregnancy tests (Quick Vue One Step hCG Urine Pregnancy kit, Quidel Corp., San Diego) were done on all women at enrollment and at each quarterly follow-up visit.
ART eligibility
Eligibility to start ART was based on CD4 count, with the eligibility threshold set by Kenyan national guidelines. These guidelines changed during the follow-up period, resulting in a change in the CD4 level at which participants were referred for ART evaluation. Between September 1, 2007 and October 1 2009, ART eligibility was based on a CD4 count <200 cells/μL and between October 2, 2009 and November 30, 2010, ART eligibility was based on a CD4 count <250 cells/μL. Participants with an eligible CD4 count were referred to a Comprehensive Care Center (CCC) funded by the U.S President’s Emergency Plan for AIDS Relief (PEPFAR) and located approximately 500 meters from the study clinic. Evaluation for ART initiation at the CCC involved a CD4 count conducted independently of the CD4 count provided by the study.
ART initiation
At each quarterly follow-up visit, participants were asked if they had started ART since their last study visit. Additionally, at the end of the study, participants were asked if they had started ART during the study period, and if so, when they had started therapy. The date of ART initiation was based on the self-reported date of starting ART, or the midpoint between the last visit not on ART and the first visit on ART if an exact date was not available. The definition of starting ART did not include short-course use of antiretroviral drugs for prevention of mother-to-child transmission of HIV during pregnancy or breastfeeding.
Statistical methods
The Kaplan-Meier product-limit estimate was used to determine the cumulative incidence of starting ART. Cox proportional hazards regression was used to assess time to ART initiation and to investigate barriers to ART initiation among participants with an eligible CD4 count. In these models, a hazard ratio (HR) >1 indicates faster ART initiation while a HR <1 indicates slower initiation. Participants were administratively censored at the end of their scheduled follow-up period. Participants that were lost to follow-up or died prior to ART initiation were censored after the last attended study visit.
In a secondary analysis, we also determined if time-varying ART use was associated with a women becoming pregnant. A Cox proportional hazards model was used, which allowed ARV status to change over time from “pre-ART” to “on-ART” following ART initiation. In the resulting model, the hazard ratio for ART indicates the likelihood of a woman becoming pregnant once on ART relative to the likelihood of becoming pregnant while not on ART at a give time since enrollment. In a related longitudinal analysis, generalized estimating equation (GEE) regression with a log link was used to compare the likelihood of reporting current hormonal contraceptive use between women on ART and those not yet on ART.
Results
Participant Characteristics
We followed 439 HIV-1-infected participants from HIV-1-discordant couples, of which 279 (63.6%) were female. At enrollment, the median CD4 count was 405 cells/μL (interquartile range 280 to 586), with 51 (11.7%) below 200 cells/μL, 115 (26.4%) in the range of 200 to 350 cells/μL, 112 (25.7%) in the range of 350 to 500 cells/μL, and 158 (36.2%) ≥500 cells/μL. There were some relevant differences between the male and female HIV-1-infected participants (Table 1). Females tended to be younger (median age 28 years versus 36 years) and reported a shorter relationship duration with their uninfected partner (median duration 4.8 years versus 6.0 years) compared to males. Female CD4 counts tended to be higher (median CD4 count 450 versus 356) and viral loads tended to be lower among females (median log10 viral load 4.6 versus 4.8 copies/mL).
Table 1.
Baseline characteristics of HIV-1-infected partners in HIV-1-discordant couples enrolled in the prospective cohort study, by gender.
| Characteristic | Overall N=439 | Male N=160 | Female N=279 |
|---|---|---|---|
|
n (%)*
|
|||
| Desire additional children | 216 (49.4) | 84 (52.8) | 132 (47.5) |
| Number of living children | |||
| 0 | 46 (10.5) | 13 (8.1) | 33 (11.8) |
| 1-2 | 245 (55.8) | 76 (47.5) | 169 (60.6) |
| ≥3 | 148 (33.7) | 71 (44.4) | 77 (27.6) |
| Any unprotected sex† | 77 (17.6) | 24 (15.1) | 53 (19.1) |
| Hormonal contraceptive use | – | – | 53 (19.0) |
| Low Education (< primary school) | 88 (20.0) | 23 (14.4) | 65 (23.3) |
| Own Home | 36 (8.5) | 15 (9.6) | 21 (7.8) |
|
median (interquartile range)
|
|||
| CD4 count | 405 (280, 586) | 356 (253, 513) | 450 (298, 632) |
| Log10 viral load | 4.7 (3.9, 5.3) | 4.8 (4.0, 5.4) | 4.6 (3.7, 5.2) |
| Age | 31 (26, 37) | 36 (32, 42.5) | 28 (24, 34) |
| Lifetime partners | 4 (3, 6) | 5 (4, 8) | 3 (2, 4) |
| Relationship duration (years) | 5.2 (2.3, 10.6) | 6.0 (2.4, 10.9) | 4.8 (2.3, 9.6) |
| Sex acts with study partner† | 5 (3, 8.5) | 5 (2.25, 8) | 5.5 (3, 9) |
| Couple’s combined income‡ | 8,000 (4,000, 15,000) | 9000 (3750, 16000) | 8000 (4000, 14000) |
| Rent‡ | 2,000 (1,200, 3,000) | 2,000 (1,500, 3,500) | 1800 (1,200, 2,700) |
Numbers may not add to total because of missing data
In the month before enrollment
In Kenyan shillings ($1 equals approximately 67 Kenyan shillings)
ART Initiation Rates in the Study Population
We assessed ART initiation among the entire cohort, whether the participant had an eligible CD4 count or not. Of the 439 HIV-1-infected participants followed, 130 (30%) reported starting ART during the study period, 278 (63%) were administratively censored at the end of follow-up, 7 (2%) died prior to ART initiation, and 24 (5%) were lost to follow-up. The cumulative incidence of ART initiation was 18.7% by 1 year and 31.9% by 2 years. Lower baseline CD4 count (HR=1.92 per 100 cells/μL decline, 95% CI 1.69 to 2.18) and higher baseline viral load (HR=1.63 per log10 copies/mL, 95% CI 1.35 to 1.97) were associated with significantly faster ART initiation. After adjusting for baseline CD4 count, faster ART initiation was observed among participants with higher viral loads (HR=1.24 per log10 copies/mL, 95% CI 1.03 to 1.49), those that reported owning their own homes compared to non-homeowners (HR=2.09, 95% CI 1.25 to 3.49), and those reporting longer relationship durations (HR=1.11 per 5 years, 95% CI 1.00 to 1.25). Additionally, women on hormonal contraceptives at enrollment initiated ART more slowly than women not on hormonal contraceptives (HR=0.50, 95% CI 0.25 to 0.99).
Time to an Eligible CD4 Count
At 1 year of follow-up, 28.5% of participants had a CD4 count consistent with eligibility to start ART (<200 cells/μL prior to 1 Oct 2009 and <250 cells/μL after 1 Oct 2009). The strongest predictor of time to an eligible CD4 count was lower baseline CD4 count (HR=2.84 per 100 cells/μL decline, 95% CI 2.43 to 3.31). After adjusting for baseline CD4 count, those with higher viral loads had shorter time to an eligible CD4 count (HR=1.21 per log10 copies, 95% CI 1.02 to 1.43). There were no significant associations between time to an eligible CD4 count and age (HR=1.01, 95% CI 0.99 to 1.03, p=0.560), female gender (HR=0.87, 95% CI 0.63 to 1.19, p=0.378), relationship duration (HR=1.01, 95% CI 0.98 to 1.03, p=0.658), or homeownership (HR=1.30, 95% CI 0.77 to 2.20, p=0.319).
Barriers to ART Initiation Among Those with an Eligible CD4 Count
After a CD4 count that met guidelines to begin ART, HIV-1-infected participants were followed for ART initiation. A total of 146 participants had an eligible CD4 count, of which 81 (55.5%) were female. Of those with a qualifying CD4 count, 94 (64%) started ART during follow-up, 47 (32%) were administratively censored at the end of follow-up, 3 (2%) died prior to ART initiation, and 2 (1%) were lost to follow-up. The median time from meeting CD4 criteria until ART initiation was 8.9 months (95% CI 6.0 to 10.0). By 6 months from the eligible CD4 count, 42.0% of participants had started ART and by 1 year 63.4% were on ART. The magnitude of the eligible CD4 count was strongly inversely related to the time until ART initiation following an eligible CD4 count, with 56.3% on ART by 6 months among those with an eligible CD4 count <100 compared to 42.6% among those with a CD4 count of 100 to 200 (HR=0.40, 95% CI 0.22 to 0.70), and 32.2% among those with a CD4 count of 200 to 250 (HR=0.38, 95% CI 0.19 to 0.76) (Figure 1).
Figure 1.
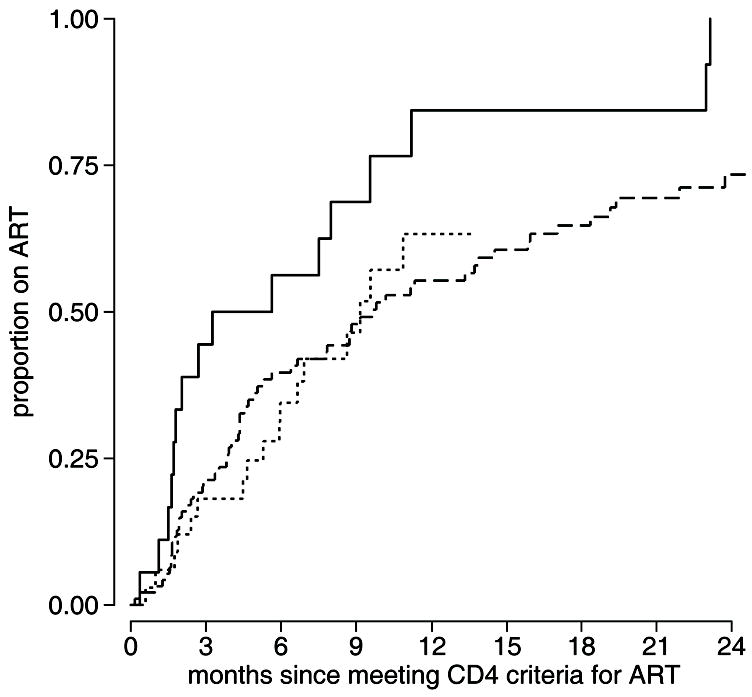
Participants meeting CD4 count criteria to start ART were followed for ART initiation and plotted separately based on the CD4 count at the time of meeting CD4 count criteria for ART. Estimated cumulative incidence is shown for [▬] those with a CD4 count <100, [
 ] those with a CD4 count 100–200, and [
] those with a CD4 count 100–200, and [
 ] those with a CD4 count 200–250.
] those with a CD4 count 200–250.
Factors related to socioeconomic status were investigated as potential barriers to ART initiation. After adjusting for eligible CD4 count, homeownership (HR=1.94, 95% CI 1.07 to 3.53) was significantly associated with faster ART initiation. When further stratified by rent, home owners started ART at the fastest rate, those paying higher rents started ART at an intermediate rate, and those paying lower rents started at the slowest rate (Figure 2). A multivariate model was fitted that included socioeconomic factors (Supplemental Digital Content 1), further supporting the relationship between the rate of ART initiation and home ownership and the amount of rent paid, with a stepwise decline in ART initiation from homeowners to those paying higher rents (HR=0.52, 95% CI 0.26 to 1.03, p=0.062) to those paying lower rents (HR=0.29, 95% CI 0.13 to 0.64). Additionally, there was a trend toward significance indicating that, after adjusting for the other factors in the model, those living greater than the median distance from the treatment center started ART at a slower rate than those at less than the median distance (HR=0.69, 95% CI 0.43 to 1.12, p=0.134).
Figure 2.
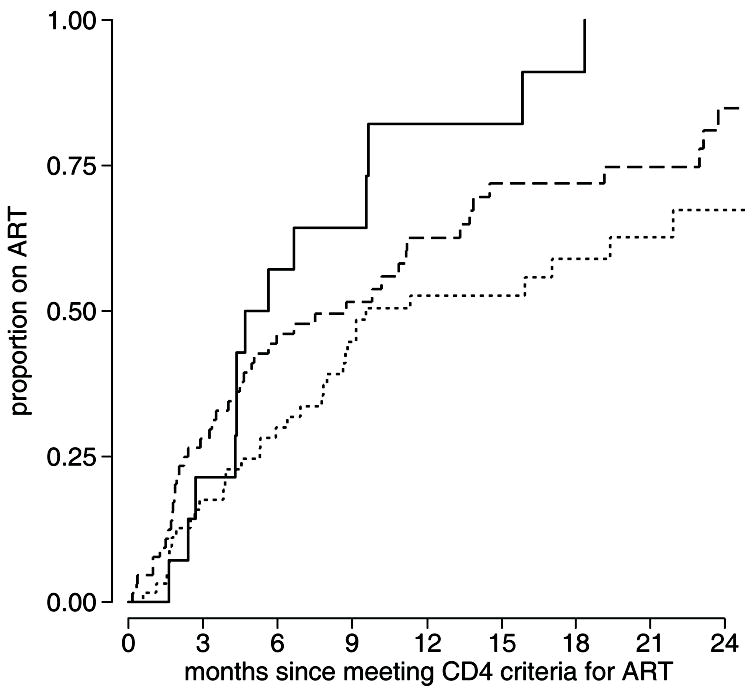
Cumulative incidence of ART initiation following an eligible CD4 count is shown for [▬] participants that report owning their own home, [
 ] those who do not own their own home and pay greater than the median rent, and [
] those who do not own their own home and pay greater than the median rent, and [
 ] those who do not own their own home and pay less than the median rent.
] those who do not own their own home and pay less than the median rent.
While there was no significant difference in the initiation rate between males and females after adjusting for eligible CD4 count (HR=0.88, 95% CI 0.58 to 1.33, p=0.534), there was a trend among females indicating that women who were using hormonal contraception at baseline were less likely to start ART (HR=0.42, 95% CI 0.17 to 1.07, p=0.068) (Table 2). In a multivariate model including baseline hormonal contraceptive use, reported desire for additional children, and eligible CD4 count, women on hormonal contraceptives initiated ART significantly more slowly (HR=0.39, 95% CI 0.15 to 1.00), but there was no significant association between reporting a desire for additional children and time to ART initiation (HR=0.79, 95% CI 0.44 to 1.39, p=0.409) (Figure 3a).
Table 2.
Correlates of antiretroviral therapy (ART) initiation following a CD4 count that met criteria for ART.
| Characteristic | Crude | Adjusted* | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p-value | HR | 95% CI | p-value | |
| 1st eligible CD4 count¶ | 0.49 | (0.33–0.73) | <0.001 | NA | ||
| Log10 viral load | 1.18 | (0.97–1.44) | 0.104 | 1.13 | (0.93–1.37) | 0.223 |
| Age | 1.01 | (0.99–1.04) | 0.289 | 1.01 | (0.99–1.04) | 0.349 |
| Female gender | 0.78 | (0.52–1.17) | 0.237 | 0.88 | (0.58–1.33) | 0.534 |
| Desire additional children§ | 0.97 | (0.55–1.69) | 0.906 | 0.94 | (0.54–1.64) | 0.825 |
| Relationship duration (per 5 years) | 1.15 | (1.01–1.30) | 0.029 | 1.12 | (0.98–1.28) | 0.089 |
| Number of living children | ||||||
| 0 | 1 | reference | 1 | reference | ||
| 1-2 | 1.83 | (0.83–4.05) | 0.136 | 1.51 | (0.67–3.38) | 0.316 |
| ≥3 | 1.95 | (0.87–4.36) | 0.104 | 1.58 | (0.70–3.57) | 0.275 |
| Lifetime partners | 1.00 | (0.98–1.02) | 0.842 | 1.00 | (0.97–1.02) | 0.703 |
| History of STI | 0.94 | (0.62–1.42) | 0.768 | 0.90 | (0.60–1.36) | 0.627 |
| Total sex acts with study partner† | 0.94 | (0.90–0.98) | 0.004 | 0.95 | (0.91–0.99) | 0.015 |
| Any unprotected sex† | 1.27 | (0.81–2.01) | 0.294 | 1.29 | (0.82–2.03) | 0.269 |
| Hormonal contraceptive use§ | 0.42 | (0.17–1.06) | 0.067 | 0.42 | (0.17–1.07) | 0.068 |
| Couple’s combined income‡ | 0.94 | (0.84–1.06) | 0.306 | 0.95 | (0.84–1.06) | 0.356 |
| Low Education (< primary school) | 1.07 | (0.69–1.65) | 0.756 | 1.19 | (0.77–1.85) | 0.439 |
| Own Home | 1.95 | (1.07–3.54) | 0.029 | 1.94 | (1.07–3.53) | 0.030 |
Adjusted for first eligible CD4 count
Per 100 cells/μL
In the month before enrollment
Female participants only, at enrollment
Per 10,000Ksh
Figure 3.
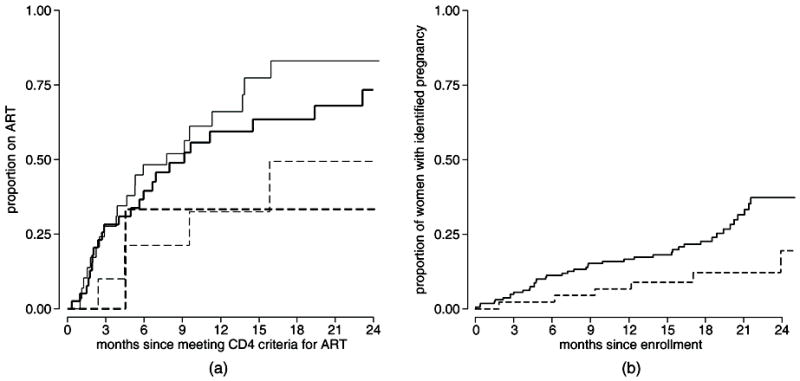
a: Cumulative incidence of ART initiation following an eligible CD4 count is shown for [—] women who did not state a desire for additional children and were not on hormonal contraceptives at enrollment, [▬] women who stated a desire for additional children and were not on hormonal contraceptives at enrollment, [
 ] women who did not state a desire for additional children and were on hormonal contraceptives at enrollment, and [
] women who did not state a desire for additional children and were on hormonal contraceptives at enrollment, and [
 ] women who stated a desire for additional children and were on hormonal contraceptives at enrollment.
] women who stated a desire for additional children and were on hormonal contraceptives at enrollment.
b: Cumulative incidence of pregnancy among women comparing [▬] the period before ART initiation to [
 ] the period after ART initiation.
] the period after ART initiation.
Pregnancy Rates Before and After ART Initiation
We previously reported an overall pregnancy rate of 10.0 per 100 women-years, with a rate of 10.4 per 100 woman-years among HIV-1-infected women.14 To investigate the relationship between ART and the decision to become pregnant, we assessed the pregnancy rate before and after ART initiation (Figure 3b). Based on a Cox proportional hazards model for the time from enrollment until first detected pregnancy, we included ART initiation as a time-varying covariate. We observed a pregnancy rate of 10.7 per 100 woman-years prior to ART initiation compared to a rate of 4.5 per 100 woman-years after initiation, with a nearly statistically significant association of lower pregnancy rates after ART initiation (HR=0.43, 95% CI 0.18 to 1.00, p=0.051). When adjusted for age and baseline CD4 count the hazard ratio was not significant, but the magnitude of the estimate did not change substantially (HR=0.50, 95% CI 0.20 to 1.24, p=0.133), indicating minimal confounding by age or CD4 count. There was further evidence that women chose to avoid becoming pregnant following ART initiation. Among women who started ART during follow-up, women were 81% more likely to report being on hormonal contraception after starting ART compared to women not yet on ART (RR=1.81, 95% CI 1.02 to 3.19). The association was even stronger after adjusting for age and baseline self reported desire for additional children (RR=1.98, 95% CI 1.04 to 3.77).
Discussion
We found delays in ART initiation among a cohort of discordant couples, despite regular CD4 testing, referrals to local treatment programs, counseling on the importance of ART, and free access to drugs. At 1 year after an eligible CD4 count, 36.6% of participants were still not on ART. We found that, not surprisingly, participants with lower CD4 counts initiated ART more quickly. These findings may predict future challenges to be faced as guidelines recommend starting ART at higher CD4 counts. Based on the results of this study, patients with higher CD4 counts may need additional counseling to emphasize the benefits of starting ART earlier.
Prompt initiation of ART is particularly important in discordant couples, where therapy benefits both the infected partner by delaying disease progression and the uninfected partner by reducing the risk of transmission. A couple-centered approach, in which the uninfected partner is involved in the decision to start ART and provides support once treatment is started, may be a productive strategy to accelerate ART initiation and improve treatment outcomes.
The strongest predictor of starting ART in this study was the CD4 count at the time of ART eligibility, with 56.3% of participants with an eligible CD4 count <100 on ART by 6 months after eligibility, compared to 42.6% of those with a CD4 count of 100-200 and 32.2% of those with a CD4 count of 200-250. A number of factors may be involved in patients with lower CD4 counts starting ART earlier. Those with lower CD4 counts are likely to be sicker, and therefore both the patient and the ART counselor are more likely to push for rapid treatment initiation. Similarly, patients with higher CD4 counts may believe that because they still feel well, they can delay starting ART. Patients may be concerned about side effects and other real or perceived implications of starting therapy, and may therefore believe that it is best to delay therapy for as long as possible. Understanding these concerns will enable treatment counselors to provide better information to patients to maximize the benefits of ART while minimizing any potential negative effects. In locations with strict CD4 count thresholds for starting ART, small variations in measured CD4 count may result in an eligible CD4 count on one day but not on the next. Thus, patients with CD4 counts near the eligibility threshold are more likely to have an eligible CD4 count at a clinic visit but not at a subsequent visit when they are evaluated to start ART. In response to this natural variability, treatment centers may consider using CD4 counts from referring sources to judge eligibility rather than relying only on the CD4 count measured at the time of the evaluation visit. Additionally, the trend of raising the threshold for starting ART to higher CD4 counts should result in greater margins of error when determining treatment eligibility. The consequence of misclassifying a person with a CD4 count below but near a cutoff of 200 cells/μL as ineligible to start ART is likely to have greater consequences than misclassifying a person below but near a cutoff of 350 cells/μL. The likelihood of significant disease progression during the time until next CD4 count testing is far less in the latter scenario.
Unlike previous studies, we did not find evidence that men started ART more slowly than women.9, 10, 15 Across many settings, men are more resistant to engaging with medical care and preventive services and tend to present for care later in the course of disease. The similarity in ART initiation rates between men and women in this study may be due to the nature of the study population. The discordant couples recruited in this study demonstrated a requisite willingness to engage with the health system by agreeing to participate in the study, and therefore may have selected for men that are more willing to seek care. We found that among the women, those on hormonal contraceptives may be less likely to start ART, and that after starting ART, women are less likely to become pregnant. We hypothesize that a number of factors may be involved in delaying ART in women. Concerns about the safety of ART during future pregnancies and potential interactions between contraceptive use and antiretroviral drugs, including the concern that ART could reduce the effectiveness or hormonal contraception, may discourage some women from starting ART.16 These barriers are of particular concern because they may represent important gaps in knowledge and potential targets for educational interventions to make women aware that being on ART can be safe during pregnancy and that the risk of both horizontal and vertical transmission is significantly reduced by ART.17 Additional behavioral research is needed to better understand the decision-making process among women surrounding reproductive desires and ART initiation.
Our findings that women are less likely to become pregnant after starting ART compared to those not yet on ART and that women on ART are more likely to report hormonal contraceptive use contradicts previous findings of increased fertility desire and higher pregnancy rates among women on ART.18-21 This difference may be explained in part by differences in the study populations and the duration of follow-up. A previous study of women recruited from an enhanced prevention of mother-to-child transmission program (PMTCT) at 11 sites across sub-Saharan Africa reported pregnancy rates following an “index” pregnancy of 6.5 per 100 woman-years during the “pre-ART” period and 9.0 per 100 woman-years during the “on-ART” period compared to 10.7 and 4.5 per 100 woman-years respectively in our study population.21 The lower “pre-ART” pregnancy rate in the previous study may be due to the short time since last pregnancy. Additionally, women in our study were followed for less time after starting ART. It is possible that pregnancy rates are reduced for some period after starting ART, but that pregnancy rates increase after women have a chance to adjust to being on ART. There is clearly complexity in the relationship between reproductive desires, ART initiation, and pregnancy incidence. Management of women living with HIV should include access to family planning resources and a full discussion of the risks, benefits, and implications of being pregnant before, during, and after starting ART.
The treatment center to which study participants with an eligible CD4 count were referred is a PEPFAR-funded site that provide ART at no cost to the patient. Nevertheless, we found evidence of socioeconomic barriers to ART initiation. Even though there is no direct cost associated with ART, indirect costs such as transportation may pose a sufficient barrier to ART initiation despite eligibility and referral to a treatment center.22 This is supported by the findings in this study that, compared to homeowners, those paying higher rents started ART at a 48% slower rate and those paying lower rents started at a 71% slower rate. The additional finding of a trend indicating that those living further from the treatment center to which participants were referred started ART 31% more slowly than those living closer to the treatment center gives some support to the hypothesis that transportation barriers may interfere with prompt ART initiation. The potential indirect costs of ART, including transportation and lost time at work, should be considered when addressing barriers to ART initiation.
This study benefited from a relatively large sample size and extended period of follow-up; however, a number of limitations exist that may affect the generalizability of the findings. The study population was a research cohort of discordant couples in which the infected partner had disclosed their HIV status to their uninfected partner. Infected partners in this population may have more support than those that have not disclosed their status, leading to faster ART initiation, particularly among men that may be more resistant to seeking care. We relied on self-report to determine if and when participants started ART. In general, this is likely to underestimate the true initiation rate. Eligibility for ART was based on a CD4 count performed by the study. Because the treatment center to which study participant were referred used an independent CD4 count to assess eligibility, participants with a CD4 count near the eligibility threshold may not have had an eligible CD4 count when tested by the treatment center. Finally, this study cohort may not be directly comparable to the general population of HIV-1-infected persons in discordant relationships. The study participants agreed to 2 years of follow-up and received regular counseling on reducing transmission risk and the importance of starting ART. However, it is likely that this cohort would have a higher uptake of ART, and thus the delays in starting ART observed here may underestimate the true problem in the general population.
From these findings we conclude that even in locations where ART is accessible and available at low or no cost, there are delays in ART initiation after a patient meets the eligibility criteria. It is likely that a number of factors influence a person’s decision to start ART, including direct and indirect costs of therapy, concerns about the sustainability of therapy, side-effects and drug interactions, and implications for family planning. The relative importance of these factors will vary between individuals, and will depend to a large degree on the counseling and support that patients receive and the information they have available while making decisions about treatment. Additional behavioral research is needed to understand how these and others factors interact in the decision-making process surrounding ART initiation. A number of these factors represent potential gaps in knowledge and opportunities for interventions to address concerns and accelerate ART in patients. Accelerated treatment initiation will benefit both the health of the infected individual as well as their sexual partners through the decreased risk of sexual transmission.
Supplementary Material
Acknowledgments
We thank the couples who participated in the study and the clinical, laboratory, and data management personnel that made this research possible. Funding for this study was provided by the NIH/NIAID research grant R01 AI068431. Funding was also provided by the University of Washington (UW) STD/AIDS training grant, grant T32 AI07140 and by the UW Center for AIDS research (CFAR).
Sources of Support Fogarty grant (D43 TW000007)
Footnotes
These data were reported, in part, at the XVIII International AIDS conference, Vienna, Austria, July 2010 (TUAB0206)
Legend of Supplemental Digital Content Supplemental Digital Table 1: Multivariate model of socioeconomic factors associated with delays in ART initiation.
References
- 1.Palella FJ, Jr, Delaney KM, Moorman AC, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N Engl J Med. 1998;338:853–860. doi: 10.1056/NEJM199803263381301. [DOI] [PubMed] [Google Scholar]
- 2.Vittinghoff E, Scheer S, O’Malley P, Colfax G, Holmberg SD, Buchbinder SP. Combination antiretroviral therapy and recent declines in AIDS incidence and mortality. J Infect Dis. 1999;179:717–720. doi: 10.1086/314623. [DOI] [PubMed] [Google Scholar]
- 3.Attia S, Egger M, Muller M, Zwahlen M, Low N. Sexual transmission of HIV according to viral load and antiretroviral therapy: systematic review and meta-analysis. AIDS. 2009;23:1397–1404. doi: 10.1097/QAD.0b013e32832b7dca. [DOI] [PubMed] [Google Scholar]
- 4.Joint United Nations Programme on HIV/AIDS. AIDS epidemic update. 2009 [PubMed] [Google Scholar]
- 5.Donnell D, Baeten JM, Kiarie J, et al. Heterosexual HIV-1 transmission after initiation of antiretroviral therapy: a prospective cohort analysis. Lancet. 2010;375:2092–2098. doi: 10.1016/S0140-6736(10)60705-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lingappa JR, Hughes JP, Wang RS, et al. Estimating the Impact of Plasma HIV-1 RNA Reductions on Heterosexual HIV-1 Transmission Risk. PLoS One. 2010;5:e12598. doi: 10.1371/journal.pone.0012598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reynolds SJ, Makumbi F, Nakigozi G, et al. HIV-1 transmission among HIV-1 discordant couples before and after the introduction of antiretroviral therapy. AIDS. 2011;25:473–477. doi: 10.1097/QAD.0b013e3283437c2b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Quinn TC, Wawer MJ, Sewankambo N, et al. Viral load and heterosexual transmission of human immunodeficiency virus type 1. Rakai Project Study Group. N Engl J Med. 2000;342:921–929. doi: 10.1056/NEJM200003303421303. [DOI] [PubMed] [Google Scholar]
- 9.Bassett IV, Regan S, Chetty S, et al. Who starts antiretroviral therapy in Durban, South Africa?… not everyone who should. AIDS. 2010;24(Suppl 1):S37–44. doi: 10.1097/01.aids.0000366081.91192.1c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bassett IV, Wang B, Chetty S, et al. Loss to care and death before antiretroviral therapy in Durban, South Africa. J Acquir Immune Defic Syndr. 2009;51:135–139. doi: 10.1097/qai.0b013e3181a44ef2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Larson BA, Brennan A, McNamara L, et al. Early loss to follow up after enrolment in pre-ART care at a large public clinic in Johannesburg, South Africa. Trop Med Int Health. 2010;15(Suppl 1):43–47. doi: 10.1111/j.1365-3156.2010.02511.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dahab M, Charalambous S, Karstaedt AS, et al. Contrasting predictors of poor antiretroviral therapy outcomes in two South African HIV programmes: a cohort study. BMC Public Health. 2010;10:430. doi: 10.1186/1471-2458-10-430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Emery S, Bodrug S, Richardson BA, et al. Evaluation of performance of the Gen-Probe human immunodeficiency virus type 1 viral load assay using primary subtype A, C, and D isolates from Kenya. J Clin Microbiol. 2000;38:2688–2695. doi: 10.1128/jcm.38.7.2688-2695.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guthrie BL, Choi RY, Bosire R, et al. Predicting pregnancy in HIV-1-discordant couples. AIDS Behav. 2010;14:1066–1071. doi: 10.1007/s10461-010-9716-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Keiser O, Anastos K, Schechter M, et al. Antiretroviral therapy in resource-limited settings 1996 to 2006: patient characteristics, treatment regimens and monitoring in sub-Saharan Africa, Asia and Latin America. Trop Med Int Health. 2008;13:870–879. doi: 10.1111/j.1365-3156.2008.02078.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Laher F, Todd CS, Stibich MA, et al. A qualitative assessment of decisions affecting contraceptive utilization and fertility intentions among HIV-positive women in Soweto, South Africa. AIDS Behav. 2009;13(Suppl 1):47–54. doi: 10.1007/s10461-009-9544-z. [DOI] [PubMed] [Google Scholar]
- 17.Sturt AS, Dokubo EK, Sint TT. Antiretroviral therapy (ART) for treating HIV infection in ART-eligible pregnant women. Cochrane Database Syst Rev. 2010;3:CD008440. doi: 10.1002/14651858.CD008440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Taulo F, Berry M, Tsui A, et al. Fertility intentions of HIV-1 infected and uninfected women in Malawi: a longitudinal study. AIDS Behav. 2009;13(Suppl 1):20–27. doi: 10.1007/s10461-009-9547-9. [DOI] [PubMed] [Google Scholar]
- 19.Homsy J, Bunnell R, Moore D, et al. Reproductive intentions and outcomes among women on antiretroviral therapy in rural Uganda: a prospective cohort study. PLoS ONE. 2009;4:e4149. doi: 10.1371/journal.pone.0004149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Myer L, Morroni C, Rebe K. Prevalence and determinants of fertility intentions of HIV-infected women and men receiving antiretroviral therapy in South Africa. AIDS Patient Care STDS. 2007;21:278–285. doi: 10.1089/apc.2006.0108. [DOI] [PubMed] [Google Scholar]
- 21.Myer L, Carter RJ, Katyal M, Toro P, El-Sadr WM, Abrams EJ. Impact of antiretroviral therapy on incidence of pregnancy among HIV-infected women in Sub-Saharan Africa: a cohort study. PLoS Med. 2010;7:e1000229. doi: 10.1371/journal.pmed.1000229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Duff P, Kipp W, Wild TC, Rubaale T, Okech-Ojony J. Barriers to accessing highly active antiretroviral therapy by HIV-positive women attending an antenatal clinic in a regional hospital in western Uganda. J Int AIDS Soc. 2010;13:37. doi: 10.1186/1758-2652-13-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


