Abstract
Human palatal clefting is debilitating and difficult to rectify surgically. Animal models enhance our understanding of palatogenesis and are essential in strategies designed to ameliorate palatal malformations in humans. Recent studies have shown that the zebrafish palate, or anterior neurocranium, is under similar genetic control to the amniote palatal skeleton. We extensively analyzed palatogenesis in zebrafish to determine the similarity of gene expression and function across vertebrates. By 36 hpf palatogenic cranial neural crest cells reside in homologous regions of the developing face compared to amniote species. Transcription factors and signaling molecules regulating mouse palatogenesis are expressed in similar domains during palatogenesis in zebrafish. Functional investigation of a subset of these genes, fgf10a, tgfb2, pax9 and smad5 revealed their necessity in zebrafish palatogenesis. Collectively, these results suggest that the gene regulatory networks regulating palatogenesis may be conserved across vertebrate species, demonstrating the utility of zebrafish as a model for palatogenesis.
Keywords: Cranial Neural Crest Cells, Palatal Skeleton, Zebrafish, Anterior Neurocranium, Gene Regulatory Network, Fate Mapping
Introduction
In humans disruption of palatal development can result in the congenital disorder cleft palate. Cleft palate has a prevalence of approximately 1 in 700 live births and leads to problems with feeding, speaking, hearing and social integration (Dixon et al., 2011). Our understanding of human palatogenesis and cleft palate relies on studies of palate development across diverse vertebrate species.
The morphology of the palatal skeleton varies greatly in extinct and extant vertebrate species (Ferguson, 1988; Kimmel et al., 2009). Morphogenesis of the palate has been studied extensively in alligators, chicken, mice, and human. Recently, the zebrafish anterior neurocranium, the functional equivalent of the mammalian palate (discussed in more detail below), has been used as a model for palatogenesis. Although there are inter-species differences in palatogenesis, the earliest events are highly similar. Segmented streams of cranial neural crest cells (CNCC) migrate from the dorsal neural tube to populate the pharyngeal arches in all vertebrate species. In this manuscript we focus on mouse and zebrafish for simplicity.
In both mouse and zebrafish the first stream of CNCC splits and a portion migrate over the eye to reside just below the eye and are referred to as frontonasal CNCC (Osumi-Yamashita et al., 1994; Wada et al., 2005; Eberhart et al., 2006; Kimmel and Eberhart, 2008) (Fig. 1A,B). The remainder of the first stream of migrating CNCC move into the first pharyngeal arch and populate two domains (Osumi-Yamashita et al., 1994; Trainor et al., 2003; Wada et al., 2005; Eberhart et al., 2006). The maxillary domain occupies the space ventral to the eye and dorsal to the oral ectoderm while the mandibular domain occupies the space ventral to the oral ectoderm to the limit of the first arch (Fig. 1A,B). Within the pharyngeal arches of both species CNCC condense on the oral ectoderm where reciprocal signals between the neural crest and the ectoderm drive fusion events necessary for palatogenesis.
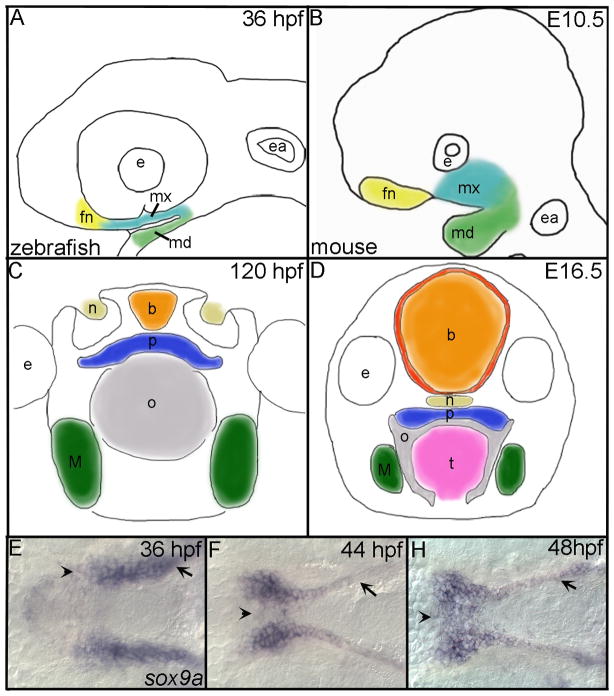
Fig. 1. Comparison of zebrafish and mouse palatogenesis with a detailed look at zebrafish palate formation.
(A,B) Outlines of embryos (lateral view), after CNCC condensation in the frontonasal (yellow), maxillary (turquoise) and mandibular (green) domains of (A) zebrafish and (B) mouse. (C,D) Drawings of cross sections through heads of (C) zebrafish and (D) mouse after formation of the palate. Colors correspond to similar structures across both species. (E,F,H) In situ hybridization of sox9a expression marking the palatal CNCC in zebrafish, ventral view with anterior to the left is shown and mandibular crest have been removed to allow full view of the developing palate. (E) At 36 hpf, sox9a expression marks the CNCC that line the maxillary (arrow) and the frontonasal (arrowhead) domains prior to their migration to the midline. (F) Migration of frontonasal cells to the midline to begin the formation of the ethmoid plate (arrowhead) at 44 hpf and maxillary cells (arrow) begin rearrangements to form the trabeculae rods of the palate. (H) By 48 hpf, most, if not all, frontonasal CNCC have migrated to the midline (arrowhead) and trabeculae rods are well formed in the palate (arrow). fn, frontonasal CNCC; mx, maxillary CNCC; md, mandibular CNCC; e, eye; ea, ear; b, brain; n, nasal opening; p, palate; o, oral opening; t, tongue; M, Meckel’s.
The mammalian palatal skeleton forms by the fusion of a series of CNCC-derived bones in the primary and secondary palate. In mice, the palatal shelves initiate from the maxillary processes on embryonic day (E) 11 and grow vertically, lateral to the tongue between E12 and E13. At E14.5, the palatal shelves rapidly re-orientate to a horizontal position above the tongue and contact one another at the midline. The medial edge epithelia of the apposed palatal shelves adhere to form a midline epithelial seam, which subsequently degenerates to allow mesenchymal continuity across the palate by E15. The palatal skeleton is formed by fusion of the secondary palate with the primary palate and the nasal septum, creating a separation between the oral and nasal cavities (Dixon et al., 2011) (Fig. 1D).
In fish, a series of bones forms in the roof of the mouth (Kesteven, 1922; Shah et al., 1995; Cubbage and Mabee, 1996), separating the oral cavity from the brain (Fig. 1C). In fish species where palatogenesis has been studied, the fusion events appear to happen directly, without a midline epithelial seam, although the presence of rudimentary palatal shelves has been suggested in salmon (Shah et al., 1990). In the early larval zebrafish a simple palatal skeleton which consists of the cartilaginous paired trabeculae and ethmoid plate as well as the dermal parasphenoid bone, forms by 4 dpf, the later two being midline structure (Fig. 2) (Schilling and Kimmel, 1997). These elements are either maintained or contribute to the juvenile and adult palatal skeleton (Cubbage and Mabee, 1996; Kimmel et al., 1998). The oral cavity of zebrafish contains teeth that are situated in the back of the oral cavity on the fifth ceratobrachial (Huysseune et al., 1998). Recently, investigation of the oral cavity in zebrafish has revealed a muscular, pseudo-stratified epithelia covered tongue initially present by 1 dpf and maintained into adulthood (Abbate et al., 2006; Hu et al., 2010). The nasal canals, used in olfactory reception, are functionally similar to other vertebrates and are situated dorsal to the palate and anterior to the eyes (Weth et al., 1996), but do not appear to connect to the pharyngeal cavity as they do in amniotes. Some authors have argued for homology of individual elements in the palatal skeletons of fish and amniotes (Kesteven, 1922; Shah et al., 1990; Shah et al., 1995). While we make no such claims for homology of palatal skeletal elements, there is growing evidence that the amniote and fish palatal skeletons are under similar genetic control, warranting a deeper exploration of the palatogenic gene regulatory network in zebrafish.
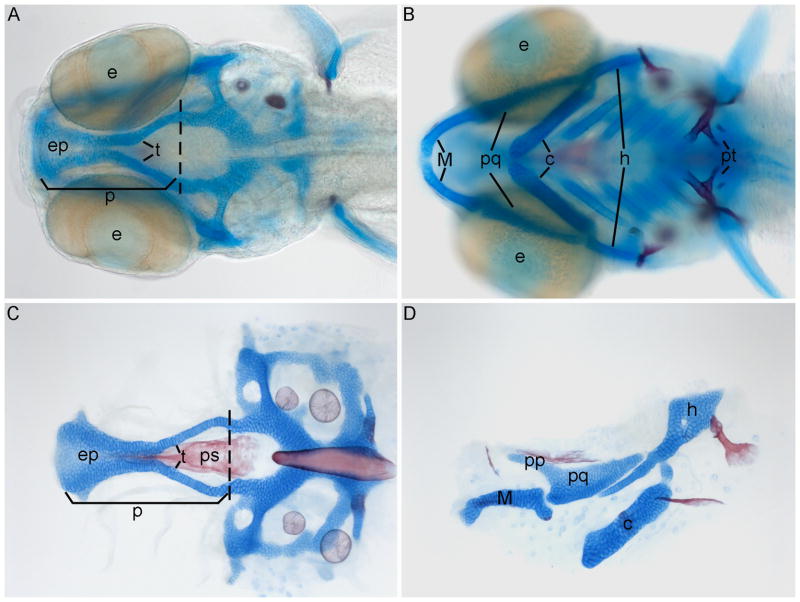
Fig. 2. Wild-type craniofacial cartilage and bone.
(A–D) Alcian blue and Alizarin red-stained cartilages and bones, respectively, of 5 dpf zebrafish, anterior is to the left. (A) Whole-mount dorsal view showing the neurocranium. (B) Ventral view showing the pharyngeal elements. (C) Dorsal flat mount view of the neurocranium. (D) Lateral view of first and second arch pharyngeal elements, dorsal is up. (A,C) Palate is anterior to the dashed line and comprised of the ethmoid plate and trabeculae. e, eye; ep, ethmoid plate; t, trabeculae; p, palate; M, Meckel’s cartilage; pq, palatoquadrate; c, ceratohyl; h, hyosymplectic; pt, pharyngeal teeth; pp, pterogoid process (upperjaw); ps, paresphenoid.
A number of recent genetic studies have illustrated the usefulness of zebrafish in the study of palate development. For example, disruption of SHH or SATB2 causes defects in the palatal skeleton in human, mouse and zebrafish (Belloni et al., 1996; Chiang et al., 1996; Roessler et al., 1996; FitzPatrick et al., 2003; Wada et al., 2005; Britanova et al., 2006; Eberhart et al., 2006; Sheehan- Rooney, unpublished). Ghassibe-Sabbagh and colleagues recently revealed the importance of FAF1, for proper palate formation in humans and its importance was verified in zebrafish by morpholino analysis, highlighting the utility of quick verification of human studies in zebrafish (2011). Importantly, work in zebrafish has recently been shown to be predictive for genes contributing to cleft palate in humans. Mutation of Pdgfra leads to cleft palate in both zebrafish and mouse (Xu et al., 2005; Eberhart et al., 2008). Research by Eberhart and colleagues (2008) showed that the microRNA mir140 negatively regulates pdgfra, thereby providing the first evidence of a specific microRNA involved in palatogenesis. Subsequently, sequence variants in this same microRNA were found to be associated with cleft palate in humans (Li et al., 2010), demonstrating the importance of zebrafish as a model of palatogenesis.
In amniotes, such as mouse, a highly regulated gene network drives palatogenesis (Cobourne and Sharpe, 2003; Hilliard et al., 2005). Mouse mutants have revealed many genes involved in palatogenesis such as the transcription factors Msx1, Pax9, Lhx8, Tbx22 and Osr2 and signaling molecules including Tgfb3, Bmp4, Shh, Fgf10 and Bmp2 (Satokata and Maas, 1994; Kaartinen et al., 1995; Proetzel et al., 1995; Peters et al., 1998; Zhao et al., 1999; Zhang et al., 2002b; Lan et al., 2004; Rice et al., 2004; Liu et al., 2005; Pauws et al., 2009). Notably, rescue of the cleft palate in Msx1 knockout mice via Bmp4 expression revealed a gene regulatory network involving several factors. In the anterior palate, interplay between Bmp4 and Msx1 in the mesenchyme maintains Shh in the ectoderm. In turn, Shh then induces Bmp2 in the mesenchyme to promote proliferation and palatal shelf growth (Zhang et al., 2002b). Subsequent research demonstrated that Fgf10 regulates Shh, adding to this network of genes (Rice et al., 2004). Despite these observations, a comprehensive understanding of the gene regulatory networks regulating palatogenesis has not been achieved.
Zebrafish has proven to be an extremely useful model system in which to achieve a deeper understanding of craniofacial development, particularly, with regards to anterior/posterior and dorsal/ventral patterning (David et al., 2002; Hunter and Prince, 2002; Crump et al., 2004a; Crump et al., 2004b; Miller et al., 2004; Crump et al., 2006; Walker et al., 2006; Miller et al., 2007; Walker et al., 2007; Talbot et al., 2010). In the current study, we sought to determine the usefulness of zebrafish for studies of palatal development by examining homologs of members of the amniote palatogenic gene regulatory network in zebrafish. Our results show that the vast majority of the signaling molecules and transcription factors known to be expressed in the developing palate of mouse are similarly expressed in zebrafish. The genes osr1 and osr2 show partitioning of gene expression in the maxillary domain. Both the maxillary and mandibular domains express the zebrafish homolog of Msx1, msxe (Postlethwait, 2006). Other transcription factors including the paralogs lhx6 and lhx8 as well as tbx22 are expressed in a similar pattern to mouse with expression in the maxillary and mandibular domains. We also show that many of the signaling molecules, such as Bmps and Tgfbs that have been shown to be important in mouse palatogenesis have similar expression patterns in zebrafish.
Not only are there similarities in gene expression but these same genes drive development of the zebrafish palate. Knock-down of the signaling molecules fgf10a and tgfb2 causes disruptions to the palate. Pharyngeal pouch endoderm and CNCC of the maxillary and mandibular domains express pax9 in zebrafish. Morpholino (MO) knock-down of pax9 in zebrafish causes highly penetrant defects in the palate, hyoid arch and teeth. Furthermore, our analysis of our smad5 mutant zebrafish clearly shows that CNCC require the reception of Bmp signaling for proper palatogenesis. Taken together, our results suggest conservation of gene expression and function in the palatogenic gene regulatory network across vertebrates.
Results
The zebrafish larval skeleton develops rapidly with trabecular cartilages appearing as early as 45 hpf (Schilling and Kimmel, 1997). Soon thereafter, the ethmoid plate appears followed by the jaw, jaw support cartilages and parasphenoid bone. The neurocranium and pharyngeal skeleton form the basic larval head skeleton by 4 dpf (Cubbage and Mabee, 1996; Schilling and Kimmel, 1997) Fig. 2A,B). The larval palate consists of paired trabeculae, the ethmoid plate and parasphenoid while the posterior neurocranium consists of the otic vesicles and the parachordal cartilages lateral to the notochord (Fig. 2A,C). The jaw can best be visualized in a flat mount view (Fig. 2D). Meckel’s cartilage forms the lower jaw and the pterygoid process of the palatoquadrate forms the upper jaw, a joint with the palate and pterygoid process forms by 90 hpf (Cubbage and Mabee, 1996; Schilling and Kimmel, 1997). Fate mapping has shown that CNCC populate the first arch region below the eye, and condense on the roof of the oral ectoderm (Eberhart et al., 2006). These regions of CNCC, homologous to the frontonasal and maxillary prominences in amniotes (Kimmel and Eberhart, 2008), give rise to the palate and the upper jaw. CNCC ventral to the oral ectoderm within the first arch populate the mandibular domain which gives rise to the lower jaw.
Frontonasal and Maxillary domain fate mapping
CNCC populations relating to frontonasal and maxillary crest have been observed in zebrafish (Wada et al., 2005; Eberhart et al., 2006), however, their relative locations within the arches following initial crest condensation are unknown. To track these CNCC and relate them to the expression analyses that follow, we photo-converted subpopulations of CNCC in sox10:KikGR embryos at 30, 36, and 48 hpf and followed their lineage to 60 hpf when the palatal skeleton is readily identifiable. Progeny from photo-converted CNCC residing adjacent to the olfactory epithelium and anterior to the choroid fissure of the eye eventually come to populate the midline of the ethmoid plate consistent with the distribution of frontonasal crest in amniotes (Fig. 3A,B). In zebrafish, maxillary CNCC migrate below the eye to populate the lateral ethmoid plate and the trabeculae (Wada et al., 2005; Eberhart et al., 2006). In mouse, maxillary CNCC have been further subdivided on the basis of gene expression into anterior and posterior maxillary CNCC (Hilliard et al., 2005) although the final location of these cells is unknown. We find that in zebrafish labeled CNCC that have migrated below the eye just posterior to the choroid fissure populate the lateral ethmoid plate and portions of the trabeculae at 60 hpf (Fig. 3C,D). CNCC labeled immediately posterior to this domain populate only the trabeculae at 60 hpf (Fig. 3E,F) demonstrating that the anterior-posterior location in the maxillary domain correlates with the eventual localization in the zebrafish palatal skeleton.
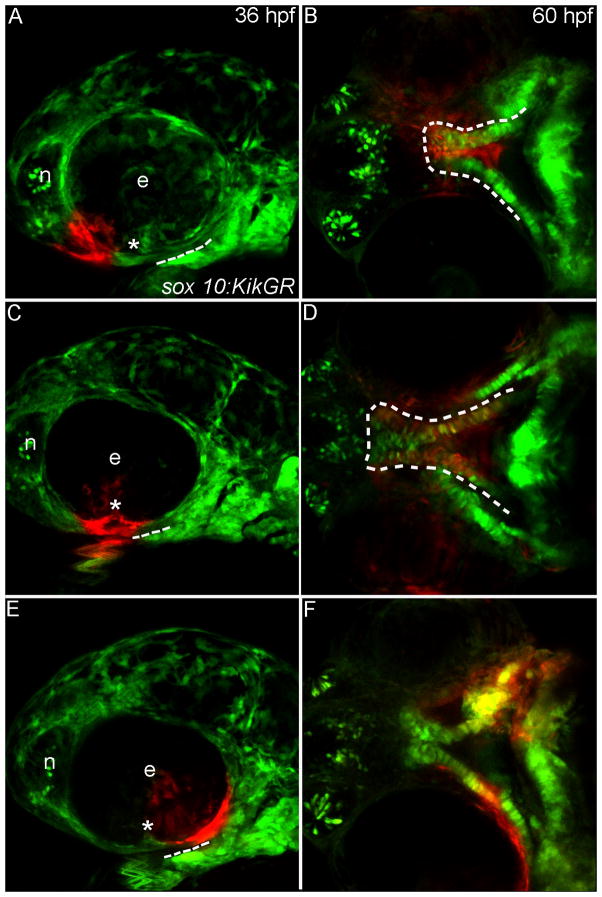
Fig. 3. Progenitor domains for the zebrafish palate.
(A–F) Sox10:KikGR photoconverted CNCC in red. (A,C,E) Lateral images of the zebrafish head at 36 hpf dashed lines indicate the oral ectoderm, asterisk marks location of the choroid fissure. (B,D,F) Ventral view of the developing palatal skeleton at 60 hpf, the developing palate is outlined by the dashed white lines. In some cases other CNCC-derived tissues were labeled as the UV penetrated through the embryo, but here we focus on the palatal skeleton. (A) CNCCs adjacent to the eye and nasal epithelium (termed frontonasal CNCC) were labeled. (B) Labeled cells occupy the midline of the ethmoid plate. (C) CNCCs adjacent to and anterior to the choroid fissure (anterior maxillary CNCC) were labeled. (D) Photoactivated CNCC populate the lateral ethmoid and anterior portion of the trabeculae. (E) CNCCs labeled posterior to the choroid fissure (posterior maxillary CNCC) (F) will contribute to the posterior portion of the trabeculae. e, eye; n, nasal opening.
Transcription factor expression analysis
To encompass the period from CNCC condensation through the morphogenesis that prefigures the palatal skeleton, we performed expression analyses from 30 hpf to 72 hpf. Within this developmental window, we focused on the 36-48 hpf interval as a particularly relevant time period to analyze gene expression during zebrafish development. Two lines of evidence were used in selecting this time period. First, static images of sox9a-labeled CNCC and live imaging of fli1:EGFP transgenics between 36 hpf and by 48 hpf indicated that the CNCC were rearranging to prefigure the palate (Fig. 1 and data not shown). Second our fate mapping indicated that CNCC marked at 36 hpf migrated to the palate by 48 hpf (Fig. 3 and data not shown).
We initially analyzed whole-mount expression patterns to detect global distribution of these genes and then collected 2 μm confocal optical sections of a large subset of these whole-mount stained embryos to verify the tissue distribution of transcripts. Because the expression patterns of the transcription factors were less ambiguous and more static than the signaling molecules we have presented only the 40 hpf time point in optical section. For the signaling molecules we included both the 40 and 44 hpf time points in optical sections. In parallel we used antibodies against EGFP to label CNCC in our fli1:EGFP transgenic embryos. At the 40 hpf time point, we found that there was significant quenching of the anti-EGFP signal therefore in some cases the EGFP signal only highlights the edges of the CNCC field. To aid in orientation for the expression data to follow we have included a schematic of the regions we have imaged in this manuscript (top panels in figures 4, 5,6 and 7).
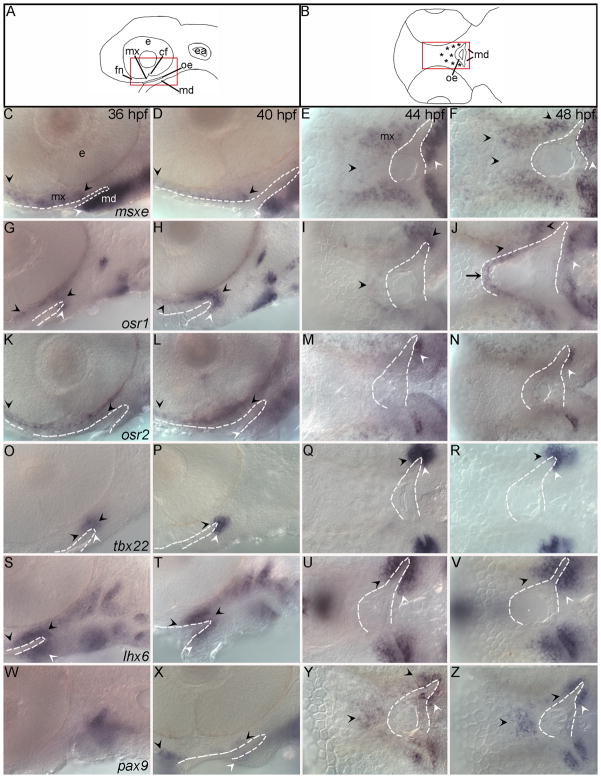
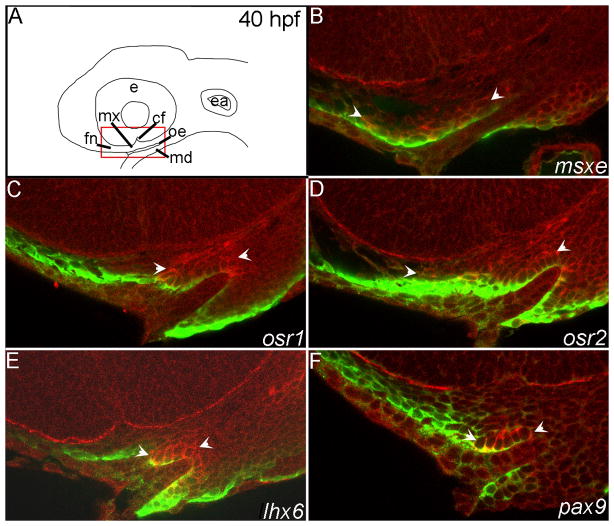
Fig. 4. Transcription factors important in amniote palatogenesis are expressed during development of the zebrafish palate.
(A,B) Schematics representing lateral view of 36, 40 hpf and 44, 48 hpf RNA in situ hybridizations respectively, asterisks mark palate-forming CNCC. (C,D,G,H,K,L,O,P,S,T,W,X) Lateral views at 36 and 40 hpf and (E,F,I,J,M,N,Q,R,U,V,Y,Z) ventral views at 44 and 48 hpf are shown. Arrowheads indicate areas of CNCC expression domains, black for palatal precursor CNCC and white for mandibular CNCC, while arrows mark expression in oral ectoderm. In all images the oral ectoderm is outlined by white dashes to aid in its visualization. (C–F) msxe is expressed in frontonasal, maxillary and mandibular cranial neural crest cells (CNCC) from 36 hpf to 48 hpf. (G–J) osr1 expression is restricted to maxillary and a small portion of mandibular CNCC from 36 to 40 hpf and then also includes dorsal oral ectodermal cells at 44 to 48 hpf. (K–N) osr2 is expressed in the frontonasal and maxillary CNCC at 36 and 40 hpf. At 44 and 48 hpf osr2 expression becomes restricted to a subpopulation of the mandibular CNCC expression. (O–R) The expression domain of tbx22 in CNCC increases with time and is limited to the posterior maxillary and mandibular domains, wrapping around the oral ectoderm. (S–V) CNCC express lhx6, wrapping around the oral ectoderm. This expression includes both the maxillary and mandibular domains and extends further than tbx22 into more anterior crest cells within each domain. (W–Z) pax9a is not expressed at 36hpf in the first arch, but is expressed in the frontonasal and posterior maxillary CNCC from 40 to 48 hpf with a small area of expression in the oral ectoderm. e,eye; mx, maxillary domain; md, mandibular domain; fn, frontonasal CNCC; cf, choroid fissure; oe, oral ectoderm; ea, ear.
Fig. 5. Fluorescent in situ hybridization optical sections verify the expression patterns of select transcription factors at 40 hpf.
(A) Schematic diagrams of a lateral view of the zebrafish head at 40 hpf, red box indicates the magnified view of the in situ sagittal sections. (B–F) mRNA localization detected by fluorescence of the NBT/BCIP precipitate (red) and anti-EGFP immunostaining labels CNCC in the fli1:EGFP transgenic background (green). Arrowheads indicate areas of CNCC expression domains. (B) msxe is expressed in frontonasal and maxillary CNCC. (C) osr1 is expressed in maxillary CNCC. (D) osr2 is expressed in the frontonasal and maxillary CNCC. (E) Maxillary CNCC express lhx6. (F) The posterior maxillary CNCC express pax9. e,eye; mx, maxillary domain; md, mandibular domain; fn, frontonasal CNCC; cf, choroid fissure; oe, oral ectoderm; ea, ear.
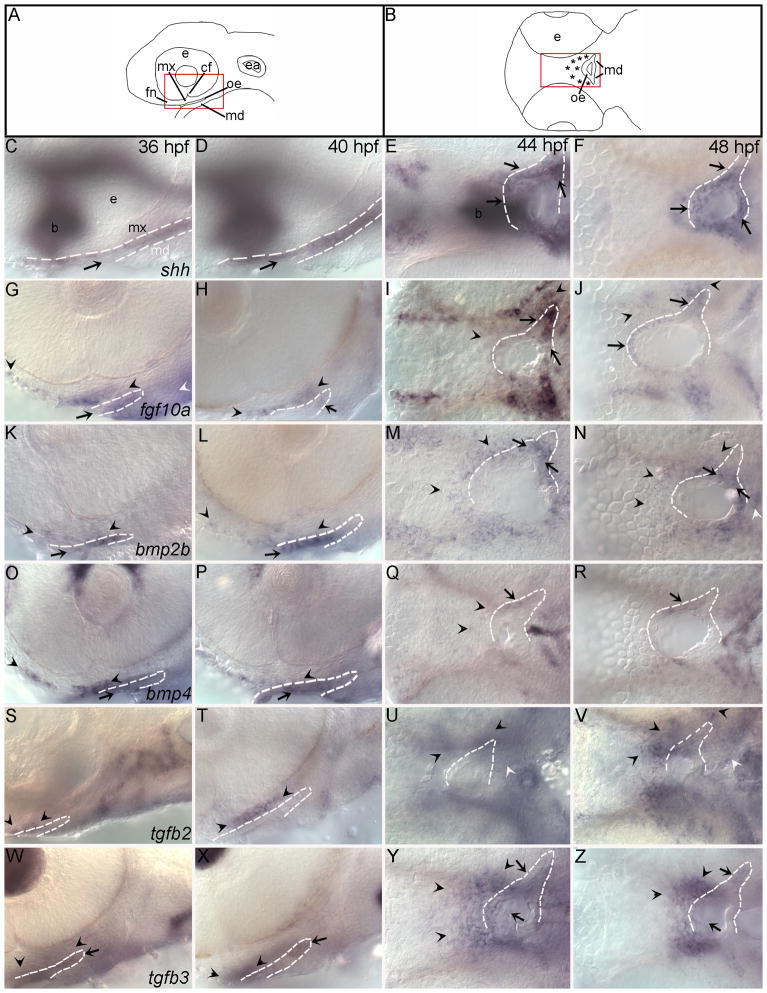
Fig. 6. The expression of signaling molecules involved in amniote palatogenesis is conserved in zebrafish.
(A,B) Schematic diagrams representing lateral view of 36, 40 hpf and 44, 48 hpf RNA in situ hybridizations respectively. Red boxes outline location of images, asterisks mark palate-forming CNCC.(C,D,G,H,K,L,O,P,S,T,W,X) Lateral views at 36 and 40 hpf (E,F,I,J,M,N,Q,R,U,V,Y,Z) ventral views at 44 and 48 hpf. Arrowheads indicate areas of CNCC expression black for maxillary and white for mandibular, while arrows indicate expression in oral ectoderm. In all images the oral ectoderm is outlined by white dashes to aid in its visualization, ventral views, only one side of the embryo is outlined. (C–F) The oral ectoderm expresses shh at all time points examined, out of focus expression is shh expression in the brain (b). (G–J) Both anterior oral ectoderm and frontonasal and maxillary CNCC express fgf10a from 36 to 40 hpf. fgf10a CNCC expression and oral ectodermal expression remains at 44 hpf. (K–N) A subpopulation of anterior CNCCs and the oral ectoderm express bmp2b at 36 and 40 hpf. By 44 hpf this expression expands to include more posterior CNCCs. Likewise, over time an anterior oral ectoderm expression domain of bmp2b expands to include more posterior oral ectoderm. (O–R) At 36 and 40 hpf bmp4 expression is in the oral ectoderm (arrow) and the frontonasal and maxillary CNCC, although there appears to be a gap between the maxillary and frontonasal expression domains. The oral ectodermal expression of bmp4 does not expand and CNCCs lose expression by 48 hpf. (S–V) tgfb2 is initially weakly expressed in an anterior domain of maxillary CNCC at 36 and by 40 hpf the expression is stronger. (W–Z) The oral ectoderm expresses tgfb3 at all time points. At 36 and 40 hpf, a small population of maxillary CNCC express tgfb3. By 44 hpf this CNCC expression has greatly expanded to include most, if not all, palatal CNCCs. e,eye; mx maxillary domain; md mandibular domain; fn, frontonasal CNCC; cf, choroid fissure; oe, oral ectoderm; ea, ear.
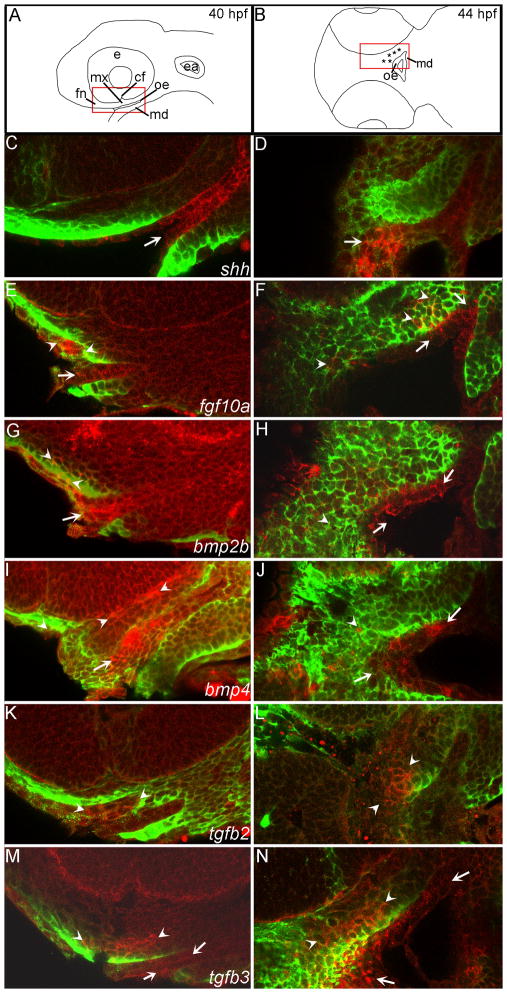
Fig. 7. Fluorescent in situ hybridization optical sections verify the expression patterns of signaling molecules at 40 and 44 hpf.
(A) Schematic lateral view of the zebrafish head at 40 hpf, red box indicates the magnified view of the in situ sagittal optical sections. (B) Schematic diagramof the zebrafish head in ventral view, red box indicates area of in situ horizontal optical sections. (C–N) mRNA localization is detected by fluorescence of NBT/BCIP precipitate in red and anti-EGFP immunostaining labels CNCC in the fli1:EGFP transgenic background in green. Arrowheads indicate areas of CNCC expression domains, arrows indicate expression in the oral ectoderm. (C–D) shh is expressed in the oral ectoderm at both time points. (E) Both anterior oral ectoderm and frontonasal and maxillary CNCC express fgf10a at 40 hpf. (F) fgf10a CNCC expression and oral ectodermal expression remains at 44 hpf. (G) Anterior CNCCs and oral ectoderm express bmp2b at 40 hpf. (H) By 44 hpf bmp2b is expressed more broadly in the oral ectoderm and less in the CNCC. (I) bmp4 expression is in the oral ectoderm and the CNCC. (J) At 44 hpf only a few CNCC express bmp4 while much of the oral ectoderm maintains bmp4 expression. (K–L) Anterior CNCC express tgfb2 at 40 and 44 hpf. (M–N) At both 40 and 44 hpf tgfb3 is expressed in anterior CNCC and the weak expression in the oral ectoderm.
In both humans and mouse, mutation of the homeobox-containing transcription factor MSX1 causes cleft palate (Satokata and Maas, 1994; van den Boogaard et al., 2000). In zebrafish msxe is the homolog of Msx1 (Postlethwait, 2006). At 30 hpf, only the ventral regions of the pharyngeal arches express msxe (Fig. S1A for schematic view and S2A), however, by 36 hpf frontonasal and anterior maxillary CNCC also express this gene (Fig. 4C). As palatogenesis proceeds, these CNCC continue to express msxe (Figs. 4D–F, 5B, see S4 for images of the separate channels in Fig 5) until at least 60 hpf, but by 72 hpf msxe expression is absent in the palate (Fig. S2C,D).
The related zinc-finger transcription factors Osr1 and Osr2 have both been implicated in palate development in mouse (Lan et al., 2004; Wang et al., 2005), although their role in human development is unclear. Immediately following CNCC condensation at 30 hpf, zebrafish osr1 is only expressed in the pharyngeal endoderm (Fig. S2E). By 36 hpf and continuing to 40 hpf, a highly restricted group of CNCC that wrap around the oral ectoderm from the posterior maxillary domain to the posterior mandibular domain express osr1 (Figs. 4G,H, 5C and S4C,D) and at 48 hpf onward the transcript additionally becomes expressed in the anterior oral ectoderm (Figs. 4J and S2F–H). From 36 to 40 hpf CNCC immediately anterior to those expressing osr1 express osr2 (compare Figs 4G,H with K,L, 5C,D, S4C,D,E,F). At 44 hpf, these CNCC down-regulate osr2 expression and the mandibular CNCC upregulate this gene (Fig. 4M,N).
Mutation of the T-box transcription factorTBX22 causes cleft palate in both human and mouse (Braybrook et al., 2002; Pauws et al., 2009). In zebrafish, tbx22 expression appears to be largely unchanged throughout the period of our analyses (Fig. 4O–R) (Jezewski et al., 2009). Similar to osr1, the posterior maxillary and mandibular domains express tbx22. Expression of tbx22 initiates at 30 hpf (Fig. S2M) and is maintained in this population of CNCC at least until 72 hpf (Figs. 4O–R, S2M–P), consistent with the persistent expression of Tbx22 in maxillary and mandibular domains throughout palatogenesis in mouse (Braybrook et al., 2002; Bush et al., 2002).
The related LIM homeodomain transcription factors Lhx6 and Lhx8 are known to be involved in palatogenesis in mouse (Zhang et al., 2002a). We found that there is considerable overlap in the expression pattern of zebrafish lhx6 and lhx8. As in mouse, both genes are expressed in maxillary and mandibular CNCC. The anterior extent of expression of both genes within the maxillary domain stops at the choroid fissure (Figs. 4S–V, 5E and S3A–D). This pattern of expression initiates by at least 30 hpf and persists with strong expression until 55 hpf (Fig. S2V,Z).
In human and mouse, palatal morphogenesis and tooth development require the paired box transcription factor PAX9 (Peters et al., 1998). Prior to 40 hpf, pharyngeal endoderm and potentially some second pharyngeal arch CNCC, express pax9 in zebrafish (Figs. 4W, S2Y). First arch CNCC do not express pax9 until 40 hpf (Figs. 4X, 5F and S4I,J). The most robust expression at 40 hpf is in the frontonasal CNCC with weaker expression in the anterior and posterior maxillary CNCC as well as some mandibular CNCC. We were unable to obtain a single section including all these domains of expression together and so chose to show the section with maxillary and mandibular CNCC labeling. By 44 and 48 hpf, as CNCC rearrange to form the palate, they continue to express pax9 (Figs 4Y,Z and S2Z–BB).
Signaling molecule expression
A complex of signaling molecules that regulate human and mouse palate development, including Shh, Bmps, Fgfs and Tgfbs, has been described in recent years. We therefore examined the expression patterns of these genes to determine their potential involvement in zebrafish palatogenesis.
It is known that, as in mouse, the oral ectoderm expresses shh in zebrafish (Miller et al., 2000). Previous analyses were limited to expression domains at 48 hpf and 54 hpf. Here we present a more detailed investigation of its expression pattern during palatogenesis and show that the oral ectoderm strongly expresses shh throughout palatogenesis (Figs. 6C–F, 7C,D; see S6 and S7 for images of the separate channels).
Fgf signaling has been shown to maintain Shh expression during mouse palatogenesis (Rice et al., 2004). In zebrafish, anterior CNCCs which are adjacent to shh-expressing oral ectoderm express fgf10a from 36 to 40 hpf (Figs. 6G,H, 7E,F, S6C,D arrowheads). At 36 and 40hpf, a posterior region of the oral ectoderm also expresses fgf10a (Fig. 6G,H 7E,F, S6C,D arrows). From 44 to 72 hpf, this expression pattern changes to one in which posterior CNCC as well as anterior and posterior oral ectoderm expresses fgf10a (Figs. 6I,J, 7F, S5F–H, S7C,D).
In addition to FGF signaling, oral ectodermal expression of Shh is critical to induce Bmp2 expression in mouse (Zhang et al., 2002b). Therefore, we next examined bmp2 expression in zebrafish. Both the anterior crest and the oral ectoderm express bmp2b from 36 to 48 hpf (Figs. 6K–N, 7G,H, S6E,F, S7E,F). Similar to bmp2b, bmp4 is expressed in anterior CNCC and the oral ectoderm from 36 to 40 hpf (Figs. 6O,P, 7G, S6E,F). At 44 and 48 hpf, a small population of posterior CNCC also express bmp4 (Figs. 6Q, S7G,H, arrowheads) and from 44 to 72 hpf, the oral ectoderm weakly expresses bmp4 (Figs. 6Q,R, 7I,J, S5B–D, S7G–H).
Tgfb family members, notably Tgfb2 and Tgfb3, exhibit dynamic expression patterns and are essential for appropriate palate formation (Fitzpatrick et al., 1990; Kaartinen et al., 1995; Proetzel et al., 1995). Prior to fusion, Tgfb2 and Tgfb3 transcripts are expressed in opposing tissues: CNCC expresses Tgfb2, the midline epithelial seam expressing Tgfb3 (Sanford et al., 1997). After fusion of the palatal shelves, both transcripts are up-regulated in the mesenchyme and it has been suggested that these genes play a later role in chondrogensis and osteogenesis (Fitzpatrick et al., 1990). Similar expression patterns are found in zebrafish, with tgfb2 found exclusively in the CNCC throughout palatogenesis (Figs. 6S–V, 7K,l, S6I,J, S7I,J). At 36 and 40 hpf, the oral ectoderm strongly expresses tgfb3 and a small patch of anterior CNCC expresses tgfb3 more weakly (Figs. 6W,X, 7M, S6K,L). From 44 to 72 hpf, a small region of the oral ectoderm continues to express tgfb3 and most if not all palatal CNCCs express tgfb3, consistent with an early and a late role for tgfb3 in zebrafish palatogenesis (Figs. 6Y,Z, 7N, S5J–L, S7K,L).
Morpholino knockdown analysis
Given the similarity of gene expression between mouse and zebrafish, we chose several genes with which to perform functional analyses in zebrafish. We used morpholino injection to examine the effects of loss-of-gene function in two signaling molecules, fgf10a and tgfb2, and one transcription factor, pax9 (Fig. 8), with complementary analysis of mutant zebrafish to investigate the involvement of bmp signaling in palatogenesis (Fig. 9).
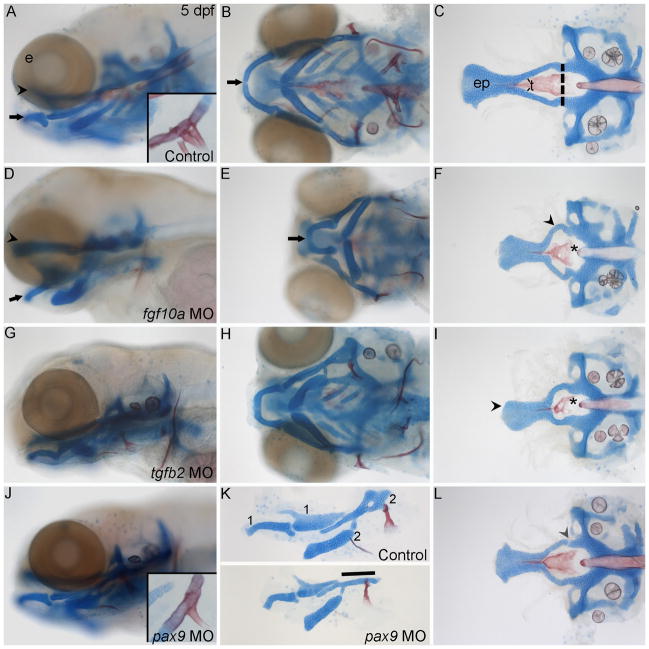
Fig. 8. Loss of signaling molecules and transcription factors important in amniote palatogenesis causes craniofacial defects in zebrafish.
(A–L) Alcian blue and Alizarin red stained the cartilages and bones, respectively, of 5 dpf zebrafish. (A) Whole-mount lateral and (B) ventral views of an uninjected control craniofacial skeleton (arrowhead, palate; black arrow, Meckel’s cartilage) (C) Flat mounted neurocranium. The anterior neurocranium or zebrafish palate is anterior to the dashed lines. (A) Inset is a magnified view showing the three pharyngeal teeth present at this stage. (D–F) fgf10a morpholino-injected fish have severely misshaped jaws and palate. (D,E) Lateral and ventral views highlight the shortened ethmoid plate and jaw. (F) Flatmount view of the neurocranium reveals the shortened ethmoid plate and misshaped trabeculae (arrowhead) and parasphenoid bone (asterisk). (G–I) tgfb2 knockdown also causes shortening and misshaping of the jaw, ethmoid plate, trabeculae and parasphenoid. (I) Misshaped shortened palate marked by arrowhead, misshaped parsasphenoid marked by asterisk. (J–L) pax9a morpholino injection causes defects of the jaw, hyomandibular cartilage, palatal skeleton and teeth. (J) Inset, shows the reduction in the number of teeth. (K) Lateral view of flat mounted first and second arch-derived pharyngeal skeletal elements, control, (top half of panel) and pax9a morpholino-injected embryos (bottom half of panel). The second arch hyomandibular cartilage is reduced to a rod-like structure in this example (line). (L) The palatal skeleton fails to fuse to the posterior neurocranium, arrowhead. Anterior is to the left in all panels. e, eye; ep, ethmoid plate; t, trabeculae.
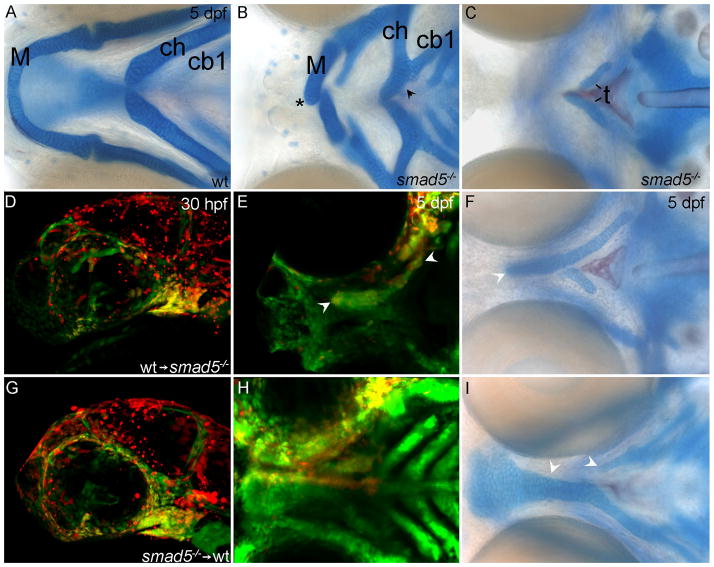
Fig. 9. Neural crest cells require the reception of Bmp signaling for palatogenesis in zebrafish.
(A,B,C,F,I) Whole-mount Alcian Blue/Alizarin Red-stained 5dpf zebrafish. (D,E,G,H) Genetic mosaics in which Alexa-568 dextran labels donor cells (red). (A,B) Ventral views (C,F,I) dorsal views (D,G) lateral views (E,H) ventral views focused on the palatal skeleton. (A) Wild-type and (B, C) smad5 mutant zebrafish larvae. (B) In the pharyngeal skeleton, Meckel’s cartilages are reduced and fail to meet at the midline symphysis, asterisk. Additionally, the ceratohyal cartilage is fused to the first ceratobranchial cartilage black arrowhead. (C) Only small remnants of the trabeculae remain in the palatal skeleton of smad5 mutants. (D–F) Transplantation of wild-type neural crest cells into smad5 mutants partially rescues the palatal skeleton. (D) At 30 hpf, wild-type neural crest cells (yellow) contribute readily to the first pharyngeal arch of smad5 mutant embryos. (E,F) By 5 dpf these wild-type cells have populated the palatal skeleton (white arrowheads in E) and substantially rescued the trabeculae and partially rescued the ethmoid plate (white arrowhead in F). (G–I) In reciprocal transplants, (G) smad5 mutant neural crest cells readily condense on the oral ectoderm in the maxillary and frontonasal domains. (H) At 5 dpf, many smad5 mutant cells remain in the region of the developing palate; however few of these cells contribute to the palatal skeleton and (I) the trabeculae and ethmoid plate are both disrupted on the side of the transplant (white arrowheads). M, Meckel’s cartilage; ch, ceratohyal; cb1, first ceratobranchial; t, trabeculae.
Compared to control fish, knockdown of fgf10a produced a shortened and squared palatal skeleton (Fig. 8C,F). Trabeculae and the parasphenoid in the morpholino-injected fish were also shortened and misshaped (arrowhead, arrow respectively). Likewise, Meckel’s cartilage and the palatoquadrate are also misshaped (Fig. 8D,E). Because we observed a complete absence of the pectoral fin (Nechiporuk and Raible, 2008), we are confident that we had a near complete loss of Fgf10a function. Therefore, the lack of a complete cleft palatal skeleton could either be due to possible roles of fgf10b, which was not examined in this study, or differences in palatogenesis between zebrafish and mouse.
Tgfb signaling is critical for palatogenesis in mouse and loss of either Tgfb2 or Tgfb2 causes cleft palate. Morpholino knockdown of tgfb3 in zebrafish has been shown to cause craniofacial defects, including disruption of the palate (Cheah et al., 2010). In our hands, injection of a different tgfb3 morpholino, even at very low doses, caused severe edema that precluded analysis of the palatal skeleton (data not shown). However, morpholino knockdown of tgfb2 induced craniofacial defects. At high morpholino doses, knock down of tgfb2 caused severe edema similar to that seen with tgfb3 morpholino injection (data not shown). At lower concentrations, we obtained partial loss of the wild-type transcript (Fig. S8) and palatal defects were observed (Fig. 8G–I) without coincident edema. This phenotype is not rescued by co-injection of tp53 morpholino demonstrating that the phenotype is not due to non-specific toxicity (data not shown). The craniofacial phenotype consists of a highly reduced and misshaped palate and a reduced Meckel’s cartilage (Fig. 8G,H). This morpholino-induced phenotype is consistent with the expression of tgfb2 being restricted to maxillary crest and a few mandibular crest cells early in palatal development. Consistent with our results, Tgfb2-null mice also have highly penetrant retrognathia in addition to partially penetrant cleft palate (Sanford et al., 1997).
Pax9 knockout mice have cleft palate, missing teeth buds and defects in which the hyoid bone is rod-like in appearance (Peters et al., 1998). Because the first and second arches, as well as the pharyngeal endoderm of zebrafish, express pax9, we examined the function of this gene in zebrafish craniofacial development using splice-blocking morpholinos. Splicing of the transcript was completely disrupted following injection of the pax9 morpholino (Fig. S9). Compared to uninjected controls, morpholino-injected fish exhibited several cartilage and bone defects (Fig. 8J–L). A highly penetrant hyomandibular cartilage defect was apparent. Three categories of phenotypes were seen (n=54): 72% of cartilages had a rod-like shape (Fig. 8K), 17% were missing the dorsal portion of the hyomandibular resulting in a forked appearance (data not shown) and, in 5.5% of injected embryos, the commissure in the hyomandibular cartilage was enlarged (data not shown) and 5.5% appeared wild-type. In zebrafish, unlike mouse, the teeth appear as dermal outgrowths from the seventh arch. Despite this difference, loss of pax9 in zebrafish results in the loss of teeth (Fig. 8A,J). The entire neurocranium also appears shorter with disorganized cells in the lateral ethmoid plate and trabeculae. Furthermore, the trabeculae often fail to fuse to the posterior neurocranium resulting in a gap between the anterior and posterior neurocranium (Fig. 8L). The parasphenoid, a dermal bone that arises from first arch CNCC, is also misshaped (Fig. 8L). Taken together, these results show that proper development of the zebrafish palate, hyomandibular and teeth all require pax9 function.
Cranial neural crest require the reception of Bmp signaling for proper palatogenesis in zebrafish
Bmp signaling plays crucial roles in palatogenesis in mouse (Liu et al., 2005). Our expression analyses suggested that Bmp signaling may also be necessary during zebrafish palatal development. To test the role of Bmp signaling during zebrafish palate development, we analyzed smad5 mutant fish in which signaling downstream of the Bmp receptor is disrupted. In smad5 mutant embryos the palatal skeleton is almost entirely lost, with only small remnants of the trabeculae remaining (Fig. 9C). The bilateral Meckel’s cartilages are greatly shortened and fail to meet at the midline symphysis, instead projecting past one another (Fig. 9B, asterisk). There are also fusions of normally separate cartilage elements near the midline of the second and third, as well as occasionally the fourth, pharyngeal arch (Fig. 9B, arrowhead). Thus, as in other species, Bmp signaling is necessary for craniofacial development, including palatogenesis, in zebrafish.
During mouse palatogenesis, CNCC-derived mesenchyme requires the reception of Bmp signaling. We generated genetic mosaics to determine if CNCC must receive Bmp signaling for palate development in zebrafish. Wild-type CNCC transplanted into smad5 mutant embryos readily contributed to the trabeculae and the ethmoid plate, resulting in a partial rescue of the mutant phenotype (Fig. 9D–F). Reciprocal transplants, from smad5 mutants into wild-type embryos, show that smad5 mutant crest readily condense in the pharyngeal arch, but tend not to contribute to the palatal skeleton, resulting in a partial loss of the trabeculae and ethmoid plate (Fig. 9G–I). Collectively, these results demonstrate clearly that CNCC must receive Bmp signals for proper palatogenesis. It is likely that Bmp signaling mediates differentiation and/or survival/proliferation of palatal precursors following condensation upon the oral ectoderm.
Discussion
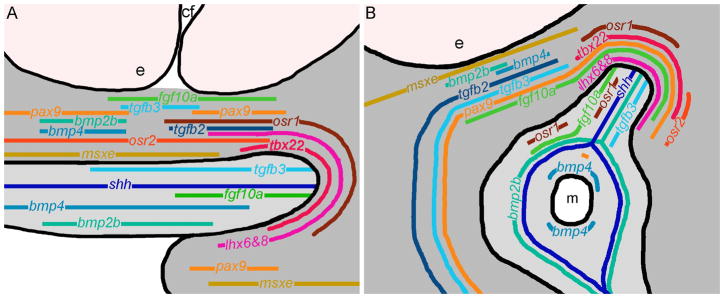
Here, we have examined the process of palatogenesis in zebrafish. As in amniotes, portions of the first stream of CNCC come to occupy frontonasal and maxillary domains in zebrafish (Osumi-Yamashita et al., 1994; Wada et al., 2005; Eberhart et al., 2006). We show first that the cells in these regions subsequently give rise to specific subsets of the palate at 60 hpf and that particularly between 36 hpf and 48 hpf dynamic cell rearrangements are occurring, driving the morphogenesis of the zebrafish palate. Second, using the well-annotated zebrafish genome, we identified homologues of many genes that have been implicated in mouse palatogenesis. We show by extensive expression analyses from 30 hpf to 72 hpf that a group of 13 signaling molecules and transcription factors that are involved in amniote palatogenesis are similarly expressed in zebrafish. We summarize these gene expression patterns at 40 and 44 hpf (Fig. 9). These time points are representative of early and late gene expression patterns, respectively, covering the time points we examined. Additionally, our data in combination with that of others (Cubbage and Mabee, 1996; Schilling and Kimmel, 1997; Wada et al., 2005; Eberhart et al., 2006) shows that a substantial proportion of cell rearrangements during palatogenesis is completed around these time points. Third, we showed that at least a subset of these genes also functions during zebrafish palatogenesis. Collectively, our results support a model where the gene regulatory networks responsible for palatogenesis in amniotes are largely intact in zebrafish.
Zebrafish palate progenitor domains and general gene expression patterns
The migratory pathways of CNCCs evolved early in the vertebrate lineage (Kuratani, 2005) and our results, along with others, strongly suggest that the progenitor domains for regions of the palatal skeleton are also conserved in vertebrate species (Wada et al., 2005; Eberhart et al., 2006). Our fate map analyses show that frontonasal CNCC adjacent to the nasal epithelium will populate the midline of the ethmoid plate. We also show that the anterior and posterior maxillary domains will populate the lateral ethmoid plate and trabeculae, respectively. In mouse, authors have suggested that there are anterior to posterior differences in palate development, for example the review by Hillard et al. (2005). However, fate maps that would provide insight into the lineage of these anterior and posterior populations, and thus the functional relevance of these differences, are absent.
Anterior to posterior differences in murine palate development are strongly implicated by differential gene expression (Hilliard et al., 2005). We find remarkable similarities in the expression of the zebrafish homologues of these differentially expressed genes. The posterior maxillary and mandibular prominence express Osr1, Lhx6, Lhx8 and Tbx22 in mouse and, likewise, we find a clear segregation of zebrafish osr1, lhx6, lhx8 and tbx22 transcripts to the posterior maxillary and mandibular domain. At early outgrowth stages, Msx1, Bmp4, Bmp2, Fgf10 and Shox2 are all expressed in the anterior palate in mouse (Hilliard et al., 2005). Likewise, the early zebrafish anterior maxillary domain expressed msxe, bmp4, bmp2b and fgf10a. Of these anteriorly expressed genes fgf10a extends the most posteriorly, it is unclear from the literature how this would compare to mouse expression. We did not detect shox2 expression in the developing palate (data not shown). Thus, the regional differences of the palatogenic CNCCs are largely shared between mouse and zebrafish.
Other complementary patterns of gene expression are also shared between mouse and zebrafish. In mouse, Osr2 and Osr1 have largely non-overlapping expression domains (Lan et al., 2004). Similarly, zebrafish frontonasal and maxillary CNCC express osr2 and this expression domain appears to be largely non-overlapping with osr1. Once CNCC have populated the midline at 48 hpf, they down-regulate osr2 expression; limiting osr2 expression to a few mandibular CNCC. Interestingly, in mouse Osr2 expression is downregulated in the palatal mesenchyme following palatal fusion. Despite these similarities in expression, Osr2 has not been described in the mouse oral ectoderm while 3 to 4 cells of the zebrafish oral ectoderm clearly express osr2 (Lan et al., 2004; Wang et al., 2005). Due to the limited number of these osr2- expressing ectoderm cells, this potential difference is difficult to interpret. Collectively, our expression analyses will provide a foundation for future studies determining the gene regulatory networks during palatogenesis using the zebrafish as a model system.
Because of the conservation in progenitor domains, similarities in gene expression profiles hint at a conservation of function. To date, there are relatively few studies of palatogenesis in zebrafish, although using zebrafish to understand the function of genes involved in human palatogenesis is becoming more popular (Ghassibe-Sabbagh et al., 2011). Previous work in mouse and zebrafish has shown that signaling pathways including Shh, Pdgf and Wnt9, and transcription factors including Satb2 and Irf6 have similar expression and function in both species (Belloni et al., 1996; Soriano, 1997; Wada et al., 2005; Xu et al., 2005; Dobreva et al., 2006; Eberhart et al., 2006; Juriloff et al., 2006; Lan et al., 2006; Eberhart et al., 2008; Richardson et al., 2009; Sabel et al., 2009; Curtin et al., 2010; Sheehan-Rooney et al., 2010; Sheehan-Rooney, unpublished). Our current work adds pax9, tgfb2, fgf10 and the Bmp pathway to this growing list of genes with important functions during palatogenesis in both amniotes and zebrafish.
Functional importance of pax9 during zebrafish craniofacial development
We chose to examine pax9 because, in mouse, Pax9 is thought to control the shape of the palate and the hyoid bone, a second pharyngeal arch derivative, and is also necessary for tooth development (Peters et al., 1998). In 40 hpf zebrafish patches of CNCC in the frontonasal, anterior maxillary, posterior maxillary and mandibular domains express pax9. As palatal morphogenesis continues pax9 expression expands to all CNCC in the frontonasal and maxillary domains. In pax9 morpholino-injected zebrafish embryos, the shape of the palatal skeleton and the hyosymplectic, a second arch derivative, was altered. Additionally, there was a loss of teeth in these embryos (Fig. 7J–L). The similarity of Pax9 expression and function between mouse and zebrafish suggests a model where the role of Pax9 in palatogenesis is conserved across these divergent species. Future experiments will test this model.
Tgfb function in mouse and zebrafish
Tgfb signaling is crucial for palatogenesis in mouse (Kaartinen et al., 1995; Proetzel et al., 1995) and we have found striking similarities in Tgfb expression and function between zebrafish and mouse. In mouse, CNCC of the developing palate express Tgfb2 and the medial edge epithelium alone expresses Tgfb3 until after fusion of the palatal shelves when palatal mesenchymal expression of this gene is up-regulated. This later expression of Tgfb3 in the mesenchyme is thought to play a role in skeletogenesis (Fitzpatrick et al., 1990). This pattern of expression is largely recapitulated in zebrafish. Zebrafish tgfb2 transcript is expressed solely by CNCC throughout palatogenesis. A small population of CNCC as well as the oral ectoderm expresses tgfb3 at 40 hpf and expression of the transcript in CNCC expands at 44 hpf, again suggesting dual roles for tgfb3 in the formation of the palate. The fact that the zebrafish oral ectoderm expresses tgfb3 is intriguing since palatal shelves have not been described in this species. Future analyses examining the regulation of Tgfb3 in mouse and zebrafish could be very insightful to our understanding of the evolution of the secondary palate.
Tgfb2 and Tgfb3 play important roles in fusion of the palatal shelves in mice, with loss of gene function resulting in cleft palate with low penetrance in Tgfb2 mutants and almost complete penetrance in Tgfb3 mutants. Others have implicated tgfb3 signaling in zebrafish craniofacial development (Cheah et al., 2010). However, we were unable to test the role of tgfb3 in zebrafish palatogenesis because the morpholino-injected zebrafish embryos died from what appeared to be cardiac defects. Our results do, however, indicate that tgfb2 plays a very similar role in development of the palate, jaw and heart in both mouse and zebrafish. Embryos injected with tgfb2 morpholino display retrognathia, and a reduced palatal skeleton. We were only able to achieve partial knock-down of the wild-type transcript because higher doses yielded embryos with cardiac edema. Morpholino-injected embryos had smaller misshaped palates, not cleft palate. While this could reflect an incomplete loss of Tgfb2, we also believe it is likely that many gene mutations that would cause cleft palate in amniotes would cause smaller palatal skeletons in fish. In amniotes, mutations that cause a loss of proliferation or an increase in CNCC apoptosis can cause the palatal shelves to be too small to contact across the midline. In zebrafish, the palate is formed by direct growth of skeletal elements across the midline. Therefore, the size of the CNCC condensation may have less influence on the skeletal elements joining across the midline in zebrafish. This may cause mutations that result in cleft palate in amniotes to result in smaller, misshaped, palatal skeletons in fish.
Potential presence of an amniote palatogenic gene regulatory network in zebrafish
In mouse, palatogenesis requires a well-established gene network that includes Bmps, Msx1 and Shh. This pathway may function in zebrafish given that our fate mapping data in combination with our expression analyses shows that these molecules are expressed similarly in mouse and zebrafish. Consistent with this prediction, our smad5 mutant analyses show that, as in amniotes, palatogenesis requires the reception of Bmp signaling in the palatal mesenchyme. While functional analysis of msxe has not been performed, shh signaling is involved in the development of the zebrafish palate (Wada et al., 2005; Eberhart et al., 2006). Collectively, these data support a bmp, msx, shh pathway being involved in zebrafish palatogenesis.
In mouse Fgf10 signaling feeds into this Bmp, Msx, Shh pathway because Fgf10 mutants down-regulate Shh (Rice et al., 2004). Throughout palatogenesis in zebrafish, CNCC that subsequently populate the trabeculae and lateral ethmoid plate express fgf10a and the oral ectoderm expresses shh. We show that the disruption of fgf10a causes shape changes to the palate. Furthermore, over-expression of Fgf10 causes deformation and lateral overgrowth of cultured rat Meckel’s cartilage (Terao et al., 2010), demonstrating the importance of Fgf10 in shaping Meckel’s cartilage. This function of fgf10a appears conserved because our fgf10a morpholino-injected zebrafish have the reciprocal defect: a lateral hypoplasia of Meckel’s cartilage. Regulation of shh by Fgf10a also seems possible in zebrafish given their spatial relationships, although since shh transcript is more widely distributed then fgf10a appears to be, additional inputs seem likely. Therefore, there is a role for Fgf10 in palatogenesis and lower jaw development in zebrafish and mouse although the exact mechanism is unclear in both species. Further work will be needed to elucidate these signaling interactions.
Studies have shown that zebrafish is an important model system in other aspects of craniofacial development, such as dorsal-ventral patterning. Our data here suggests that by utilizing the strengths of zebrafish as a model organism, including forward genetic screens and live imaging of transgenics for fate mapping and elucidating cell behaviors, future work in the study of palatogenesis will be enhanced.
Experimental Procedures
Fish maintenance and fish lines
Zebrafish were raised and staged as described previously (Westerfield, 1993; Kimmel et al., 1995) under IACUC approved protocols (AUP 08080601). AB wild-type and tg(fli1a:EGFP)y1 transgenic embryos, termed fli1:GFP here after (Lawson and Weinstein, 2002) zebrafish were used for morpholino injections and in situ hybridization. The stable Tg(sox10:KikGR)el2 (sox10:KikGR) transgenic line was a generous gift from J.G. Crump. This line consists of the KikGR cDNA (MBL International) expressed under the −4.9 kb sox10 promoter (Carney et al., 2006) and will be described in more detail elsewhere. The b1100 allele was generated in a forward genetic screen carried out at the University of Oregon. Complementation analysis verified that it is an allele of smad5. Full analysis of the b1100 allele will be described elsewhere. Transplantations were performed according to established protocols (Eberhart et al., 2008).
Tissue labeling and imaging
Staining of cartilage with Alcian Blue and bone with Alizarin Red was done according to the protocol outlined in Walker and Kimmel (2007). Dissected facial cartilages and bones were flat mounted according to Kimmel et al. (1998). GFP antibody labeling was performed as described in Maves et al. (2002). The anti- GFP antibody (Santa Cruz Biotechnology) was used at a 1:200 dilution. Alexa Fluor 488 secondary antibody (Invitrogen) was used at a 1:500 dilution. RNA in situ hybridization was performed as reported in Miller et al. (2000). Supplemental Tables S1, S2 provide details of all probes used. shh is duplicated in zebrafish (Krauss et al., 1993), here we report the expression of shha, which we refer to as shh for clarity. Wild-type AB fish were treated with 0.0015% PTU (1-phenyl 2- thiourea) to inhibit the production of melanin (Westerfield, 1993). Bright field images of flat mounts and DIC images of in situ hybridizations were collected on a Zeiss Axioimager. Confocal fluorescent 2um optical sections of NBT/BCIP precipitate (Trinh le et al., 2007) and anti-EGFP stained embryos were taken using a Leica TCS SP5 II.
Morpholino analysis
The fgf10a and tp53 morpholinos have been described (Robu et al., 2007; Nechiporuk and Raible, 2008), respectively. Disruption of both tgfb2 and pax9 was achieved using splice-blocking morpholinos. The tgfb2 gene was targeted at the second exon and second intron boundary: 5′ GGATTTACCTGGTAAAGCTCGATGC 3′. The pax9 morpholino was designed to disrupt splicing of the first intron and second exon boundary: 5′ CCAAAGGCTGGCTCTAGTTATGCAG 3′. One-cell embryos were pressure injected with approximately 3 or 5 nl of a 3 mg/ml solution of the tgfb2 morpholino and a 3nl bolus of a 5 mg/ml solution of the pax9 morpholino, according to the procedure outlined by Maves et al. (2002). Primarily we examined embryos injected with the lower dose of tgfb2 morpholino because they were non-edematous. pax9 morpholino-injected embryos were sorted according to their severity. All embryos were either flat mounted or whole mounted and imaged on a Zeiss Axioimager. In addition, pax9-deficient embryos were flat mounted and imaged to obtain teeth counts. To check for the effectiveness of the tgfb2 and pax9 morpholinos, total RNA was extracted from control and morpholino-injected 48 hpf fish using Trizol reagent (Invitrogen). Synthesis of cDNA was performed using first strand synthesis kit from Invitrogen. Splicing of tgfb2 and pax9 was assessed using forward primer (5′-CCTCCTTCTGCTTTTGGATTTAGC-3′) and reverse (5′-TTGGGGGTCTTGCCGATGTAGTAG-3′) and forward primer (5′-GCTAAACTGGACTCGGAACAGGTC-3′) reverse (5′-TTTGCTTCAGGTTCTAAGTGAGGC-3′), respectively. Products were then resolved on 2% and 3% agarose gels, respectively.
Fate mapping
Fate map analysis was performed using the sox10:KikGR Kaede transgenic line. Labeling of cells was performed at 30, 36 and 48 hpf as described in Eberhart et al. (2006) using a Zeiss 710 confocal microscope equipped with a UV lamp. Confocal images were collected immediately after photoconversion and at 60 hpf.
Supplementary Material
(A,B) Red box represents the magnified view of the in situ images in supplementary Figures 2,4. (A) Lateral view of embryo representing the 30, 36 and 40 hpf time points. (B) Ventral view of embryo representing the 44,48,55,60 and 72 hpf time points. Asterisks mark palate forming CNCC, eye; cf, choroid fissure; ea, ear; mx maxillary domain; fn, frontonasal crest; oe, oral ectoderm; md, mandibular domain.
(A,E,I,M,Q,U,Y) Lateral views at 30, (B,C,D,F,G,H,J,K,L,N,O,P,R,S,T,V,W,X,Z,AA,BB) ventral views at 55, 60 and 72 hpf. (A) msxe is expressed in mandibular cranial neural crest cells at 30 hpf and (B) at 55 hpf it is expressed in palatal precursors and mandibular crest; (C,D) thereafter expression is lost. (E) osr1 is weakly expressed in second arch CNCC at 30 hpf. (F–H) Dorsal oral ectodermal cells express osr1 from 55 to 72 hpf. (I–L) CNCC weakly express osr2 at 30 hpf and (J–K) from 55 hpf onward osr2 expression becomes restricted to a small group of mandibular CNCC. (M–P) The expression domain of tbx22 in CNCC increases in size over time but remains limited to the posterior maxillary and mandibular domains, wrapping around the oral ectoderm. (Q–T) CNCC express lhx6 and (U–X) lhx8 in a similar pattern, wrapping around the oral ectoderm. This expression includes both the maxillary and mandibular domains with decreasing amounts of signal over time. (Y) At 30hpf, pax9 is not expressed in the first arch, (Z–BB) but it is expressed in palatal precursor CNCC from 55 to 72 hpf. (BB) At 72 hpf, a small area of the dorsal oral ectoderm expresses pax9.
(A,B) Lateral views at 36 and 40 hpf, (C,D) ventral views at 44 and 48 hpf. CNCC express lhx8, wrapping around the oral ectoderm including expression in both the maxillary and mandibular domains.
(A–J) Lateral views of the zebrafish head in sagittal sections at 40 hpf. (A–I) Anti-EGFP immunostaining labels CNCC in the fli1:EGFP transgenic background (green). (B–J) mRNA localization detected by fluorescence of the NBT/BCIP precipitate (red). Arrowheads indicate areas of CNCC expression domains. (A,B) msxe is expressed in frontonasal and maxillary CNCC. (C,D) osr1 is expressed in maxillary CNCC. (E,F) osr2 is expressed in the frontonasal and maxillary CNCC. (G,H) Maxillary CNCC express lhx6. (I,J) The posterior maxillary CNCC express pax9.
(A,E,I) Lateral views at 30 hpf (B,C,D,F,G,H,J,K,L) and ventral views at 55, 60 and 72 hpf. (A) At 30 hpf, a small group of maxillary CNCC and mandibular neural crest cells express bmp4. (B–D) From 55 hpf onward scattered CNCC weakly express bmp4. (E) At 30hpf, fgf10a is expressed in what is likely to be surface ectoderm. (F–H) At later time points, CNCC and dorsal oral ectoderm continue to express fgf10a transcript. (I) The lens and a small population of maxillary CNCC express tgfb3 at 30 hpf. (J–L) From 55 to 72 hpf, CNCC strongly express tgfb3.
(A–J) Lateral views of the zebrafish head in sagittal sections at 40 hpf. (A–K) Anti-EGFP immunostaining labels CNCC in the fli1:EGFP transgenic background (green). (B–L) mRNA localization detected by fluorescence of the NBT/BCIP precipitate (red). Arrowheads indicate areas of CNCC expression domains, arrows indicate areas of oral ectoderm expression. (A,B) shh is expressed in the oral ectoderm at both time points. (C,D) Both anterior oral ectoderm and frontonasal and maxillary CNCC express fgf10a at 40 hpf. (E,F) Anterior CNCCs and oral ectoderm express bmp2b at 40 hpf. (G,H) bmp4 expression is in the oral ectoderm and the CNCC. (I,J) Anterior CNCC express tgfb2. (K,L) tgfb3 is expressed in anterior CNCC and the oral ectoderm.
Ventral views of the zebrafish head in horizontal optical sections. (A–K) Anti-EGFP immunostaining labels CNCC in the fli1:EGFP transgenic background in green. (B–L) mRNA localization is detected by fluorescence of NBT/BCIP precipitate in red. Arrowheads indicate areas of CNCC expression domains, arrows mark expression in the oral ectoderm. (A,B) shh is expressed in the oral ectoderm. (C,D) fgf10a is expressed in both the CNCC and the oral ectoderm. (E,F) bmp2b is expressed more broadly in the oral ectoderm and less in the CNCC. (G,H) CNCC and oral ectoderm express bmp4. (I,J) Anterior CNCC express tgfb2. (K,L) tgfb3 is expressed in the CNCC and lightly in the oral ectoderm.
RT-PCR was performed on RNA isolated from 48 hpf uninjected controls and embryos that were injected with 9ng or 15ng of the tgfb2 morpholino at the one cell stage. Primers designed to amplify a portion of zebrafish cyp26b1 were used as a control. Control primers yielded products that were of equal intensity across the conditions. tgfb2 primers revealed a decrease in the amount of wild-type transcript across the conditions.
RT-PCR was performed on RNA that was isolated from 48 hpf uninjected control embryos and embryos that were injected with 15ng of the pax9 morpholino at the one cell stage. Primers designed to amplify a portion of zebrafish cyp26b1 were used as a control. Control primers yielded products that were of equal intensity across the conditions. Single asterisk indicates the wildtype product, double asterisks indicate the improperly spliced variant.
Fig. 10. Model of zebrafish gene expression at 40 and 44 hpf, reveals a conserved palatogenic gene program.
(A and B) CNCC in dark grey, oral ectoderm in light grey and eye in light pink. The relative distribution of transcription factors is shown in shades of yellow, orange and red while signaling molecules appear in shades of green and blue. (A) Lateral view at 40 hpf. (B) Ventral view at 44 hpf. m, mouth opening; cf, choroid fissure; e, eye.
Acknowledgments
Grant Sponsor: NIH/NIDCR
Grant Numbers; DE018088-JKE, HD22486-CBK.
We would like to thank the members of the Eberhart lab for discussions and suggestions in the preparation of this manuscript. We especially want to thank Briana Schroeder for her excellent fish husbandry. For help with section imaging thanks to the Patterson Microscopy Center at the University of Texas at Austin. We are grateful to J.G. Crump for the Tg(sox10:KikGR)el2 line. Thanks also to Charles Kimmel and the University of Oregon fish researchers and staff for their contributions to the forward genetic screens. Research support was provided by NIH/NIDCR; DE018088-JKE, HD22486-CBK.
References
- Abbate F, Germana GP, De Carlos F, Montalbano G, Laura R, Levanti MB, Germana A. The Oral Cavity of the Adult Zebrafish (Danio rerio) Anatomy Histology Embryology. 2006:299–304. doi: 10.1111/j.1439-0264.2006.00682.x. [DOI] [PubMed] [Google Scholar]
- Belloni E, Muenke M, Roessler E, Traverso G, Siegel-Bartelt J, Frumkin A, Mitchell HF, Donis-Keller H, Helms C, Hing AV, Heng HH, Koop B, Martindale D, Rommens JM, Tsui LC, Scherer SW. Identification of Sonic hedgehog as a candidate gene responsible for holoprosencephaly. Nat Genet. 1996;14:353–356. doi: 10.1038/ng1196-353. [DOI] [PubMed] [Google Scholar]
- Braybrook C, Lisgo S, Doudney K, Henderson D, Marcano AC, Strachan T, Patton MA, Villard L, Moore GE, Stanier P, Lindsay S. Craniofacial expression of human and murine TBX22 correlates with the cleft palate and ankyloglossia phenotype observed in CPX patients. Hum Mol Genet. 2002;11:2793–2804. doi: 10.1093/hmg/11.22.2793. [DOI] [PubMed] [Google Scholar]
- Britanova O, Depew MJ, Schwark M, Thomas BL, Miletich I, Sharpe P, Tarabykin V. Satb2 haploinsufficiency phenocopies 2q32-q33 deletions, whereas loss suggests a fundamental role in the coordination of jaw development. Am J Hum Genet. 2006;79:668–678. doi: 10.1086/508214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush JO, Lan Y, Maltby KM, Jiang R. Isolation and developmental expression analysis of Tbx22, the mouse homolog of the human X-linked cleft palate gene. Dev Dyn. 2002;225:322–326. doi: 10.1002/dvdy.10154. [DOI] [PubMed] [Google Scholar]
- Carney TJ, Dutton KA, Greenhill E, Delfino-Machin M, Dufourcq P, Blader P, Kelsh RN. A direct role for Sox10 in specification of neural crest-derived sensory neurons. Development. 2006;133:4619–4630. doi: 10.1242/dev.02668. [DOI] [PubMed] [Google Scholar]
- Cheah FS, Winkler C, Jabs EW, Chong SS. Tgfbeta3 regulation of chondrogenesis and osteogenesis in zebrafish is mediated through formation and survival of a subpopulation of the cranial neural crest. Mech Dev. 2010;127:329–344. doi: 10.1016/j.mod.2010.04.003. [DOI] [PubMed] [Google Scholar]
- Chiang C, Litingtung Y, Lee E, Young KE, Corden JL, Westphal H, Beachy PA. Cyclopia and defective axial patterning in mice lacking Sonic hedgehog gene function. Nature. 1996;383:407–413. doi: 10.1038/383407a0. [DOI] [PubMed] [Google Scholar]
- Cobourne MT, Sharpe PT. Tooth and jaw: molecular mechanisms of patterning in the first branchial arch. Arch Oral Biol. 2003;48:1–14. doi: 10.1016/s0003-9969(02)00208-x. [DOI] [PubMed] [Google Scholar]
- Crump JG, Maves L, Lawson ND, Weinstein BM, Kimmel CB. An essential role for Fgfs in endodermal pouch formation influences later craniofacial skeletal patterning. Development. 2004a;131:5703–5716. doi: 10.1242/dev.01444. [DOI] [PubMed] [Google Scholar]
- Crump JG, Swartz ME, Eberhart JK, Kimmel CB. Moz-dependent Hox expression controls segment-specific fate maps of skeletal precursors in the face. Development. 2006;133:2661–2669. doi: 10.1242/dev.02435. [DOI] [PubMed] [Google Scholar]
- Crump JG, Swartz ME, Kimmel CB. An integrin-dependent role of pouch endoderm in hyoid cartilage development. PLoS Biol. 2004b;2:E244. doi: 10.1371/journal.pbio.0020244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cubbage CC, Mabee PM. Development of the cranium and paired fins in the zebrafish Danio rerio (Ostariophysi, Cyprinidae. Journal of Morphology. 1996;229:121–160. doi: 10.1002/(SICI)1097-4687(199608)229:2<121::AID-JMOR1>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Curtin E, Hickey G, Kamel G, Davidson AJ, Liao EC. Zebrafish wnt9a is expressed in pharyngeal ectoderm and is required for palate and lower jaw development. Mech Dev. 2010 doi: 10.1016/j.mod.2010.11.003. [DOI] [PubMed] [Google Scholar]
- David NB, Saint-Etienne L, Tsang M, Schilling TF, Rosa FM. Requirement for endoderm and FGF3 in ventral head skeleton formation. Development. 2002;129:4457–4468. doi: 10.1242/dev.129.19.4457. [DOI] [PubMed] [Google Scholar]
- Dixon MJ, Marazita ML, Beaty TH, Murray JC. Cleft lip and palate: understanding genetic and environmental influences. Nat Rev Genet. 2011;12:167–178. doi: 10.1038/nrg2933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobreva G, Chahrour M, Dautzenberg M, Chirivella L, Kanzler B, Farinas I, Karsenty G, Grosschedl R. SATB2 is a multifunctional determinant of craniofacial patterning and osteoblast differentiation. Cell. 2006;125:971–986. doi: 10.1016/j.cell.2006.05.012. [DOI] [PubMed] [Google Scholar]
- Eberhart JK, He X, Swartz ME, Yan YL, Song H, Boling TC, Kunerth AK, Walker MB, Kimmel CB, Postlethwait JH. MicroRNA Mirn140 modulates Pdgf signaling during palatogenesis. Nat Genet. 2008;40:290–298. doi: 10.1038/ng.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberhart JK, Swartz ME, Crump JG, Kimmel CB. Early Hedgehog signaling from neural to oral epithelium organizes anterior craniofacial development. Development. 2006;133:1069–1077. doi: 10.1242/dev.02281. [DOI] [PubMed] [Google Scholar]
- Ekker M, Akimenko MA, Allende ML, Smith R, Drouin G, Langille RM, Weinberg ES, Westerfield M. Relationships among msx gene structure and function in zebrafish and other vertebrates. Mol Biol Evol. 1997;14:1008–1022. doi: 10.1093/oxfordjournals.molbev.a025707. [DOI] [PubMed] [Google Scholar]
- Ferguson MW. Palate development. Development. 1988;103(Suppl):41–60. doi: 10.1242/dev.103.Supplement.41. [DOI] [PubMed] [Google Scholar]
- FitzPatrick DR, Carr IM, McLaren L, Leek JP, Wightman P, Williamson K, Gautier P, McGill N, Hayward C, Firth H, Markham AF, Fantes JA, Bonthron DT. Identification of SATB2 as the cleft palate gene on 2q32-q33. Hum Mol Genet. 2003;12:2491–2501. doi: 10.1093/hmg/ddg248. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick DR, Denhez F, Kondaiah P, Akhurst RJ. Differential expression of TGF beta isoforms in murine palatogenesis. Development. 1990;109:585–595. doi: 10.1242/dev.109.3.585. [DOI] [PubMed] [Google Scholar]
- Ghassibe-Sabbagh M, Desmyter L, Langenberg T, Claes F, Boute O, Bayet B, Pellerin P, Hermans K, Backx L, Mansilla MA, Imoehl S, Nowak S, Ludwig KU, Baluardo C, Ferrian M, Mossey PA, Noethen M, Dewerchin M, Francois G, Revencu N, Vanwijck R, Hecht J, Mangold E, Murray J, Rubini M, Vermeesch JR, Poirel HA, Carmeliet P, Vikkula M. FAF1, a gene that is disrupted in cleft palate and has conserved function in zebrafish. Am J Hum Genet. 2011;88:150–161. doi: 10.1016/j.ajhg.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilliard SA, Yu L, Gu S, Zhang Z, Chen YP. Regional regulation of palatal growth and patterning along the anterior-posterior axis in mice. J Anat. 2005;207:655–667. doi: 10.1111/j.1469-7580.2005.00474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu H, Zhang C, Baawo K, Qin R, Cole C, Lee J, Chen XY. Zebrafish K5 promoter driven GFP expression as a transgenic system for oral research. Oral Oncology. 2010;46:31–37. doi: 10.1016/j.oraloncology.2009.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter MP, Prince VE. Zebrafish hox paralogue group 2 genes function redundantly as selector genes to pattern the second pharyngeal arch. Dev Biol. 2002;247:367–389. doi: 10.1006/dbio.2002.0701. [DOI] [PubMed] [Google Scholar]
- Huysseune A, Van der heyden C, Sire JY. Early development of the zebrafish (Danio rerio) pharyngeal dentition (Teleostei, Cyprinidae) Anat Embryol (Berl) 1998;198:289–305. doi: 10.1007/s004290050185. [DOI] [PubMed] [Google Scholar]
- Jackman WR, Draper BW, Stock DW. Fgf signaling is required for zebrafish tooth development. Dev Biol. 2004;274:139–157. doi: 10.1016/j.ydbio.2004.07.003. [DOI] [PubMed] [Google Scholar]
- Jezewski PA, Fang PK, Payne-Ferreira TL, Yelick PC. Alternative splicing, phylogenetic analysis, and craniofacial expression of zebrafish tbx22. Dev Dyn. 2009;238:1605–1612. doi: 10.1002/dvdy.21962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juriloff DM, Harris MJ, McMahon AP, Carroll TJ, Lidral AC. Wnt9b is the mutated gene involved in multifactorial nonsyndromic cleft lip with or without cleft palate in A/WySn mice, as confirmed by a genetic complementation test. Birth Defects Res A Clin Mol Teratol. 2006;76:574–579. doi: 10.1002/bdra.20302. [DOI] [PubMed] [Google Scholar]
- Kaartinen V, Voncken JW, Shuler C, Warburton D, Bu D, Heisterkamp N, Groffen J. Abnormal lung development and cleft palate in mice lacking TGF-beta 3 indicates defects of epithelial-mesenchymal interaction. Nat Genet. 1995;11:415–421. doi: 10.1038/ng1295-415. [DOI] [PubMed] [Google Scholar]
- Kesteven HL. A New Interpretation of the Bones in the Palate and upper Jaw of Fishes: Part I. Journal of Anatomy. 1922;56:307–324. [PMC free article] [PubMed] [Google Scholar]
- Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, Schilling TF. Stages of embryonic development of the zebrafish. Dev Dyn. 1995;203:253–310. doi: 10.1002/aja.1002030302. [DOI] [PubMed] [Google Scholar]
- Kimmel CB, Eberhart JK. The midline, oral ectoderm, and the arch-0 problem. Integr Comp Biol. 2008;48:668–680. doi: 10.1093/icb/icn048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimmel CB, Miller CT, Kruze G, Ullmann B, BreMiller RA, Larison KD, Snyder HC. The shaping of pharyngeal cartilages during early development of the zebrafish. Dev Biol. 1998;203:245–263. doi: 10.1006/dbio.1998.9016. [DOI] [PubMed] [Google Scholar]
- Kimmel CB, Sidlauskas B, Clack JA. Linked morphological changes during palate evolution in early tetrapods. J Anat. 2009;215:91–109. doi: 10.1111/j.1469-7580.2009.01108.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krauss S, Concordet JP, Ingham PW. A functionally conserved homolog of the Drosophila segment polarity gene hh is expressed in tissues with polarizing activity in zebrafish embryos. Cell. 1993;75:1431–1444. doi: 10.1016/0092-8674(93)90628-4. [DOI] [PubMed] [Google Scholar]
- Kuratani S. Cephalic neural crest cells and the evolution of craniofacial structures in vertebrates: morphological and embryological significance of the premandibular-mandibular boundary. Zoology (Jena) 2005;108:13–25. doi: 10.1016/j.zool.2004.12.001. [DOI] [PubMed] [Google Scholar]
- Lan Y, Ovitt CE, Cho ES, Maltby KM, Wang Q, Jiang R. Odd-skipped related 2 (Osr2) encodes a key intrinsic regulator of secondary palate growth and morphogenesis. Development. 2004;131:3207–3216. doi: 10.1242/dev.01175. [DOI] [PubMed] [Google Scholar]
- Lan Y, Ryan RC, Zhang Z, Bullard SA, Bush JO, Maltby KM, Lidral AC, Jiang R. Expression of Wnt9b and activation of canonical Wnt signaling during midfacial morphogenesis in mice. Dev Dyn. 2006;235:1448–1454. doi: 10.1002/dvdy.20723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson ND, Weinstein BM. In vivo imaging of embryonic vascular development using transgenic zebrafish. Dev Biol. 2002;248:307–318. doi: 10.1006/dbio.2002.0711. [DOI] [PubMed] [Google Scholar]
- Li L, Meng T, Jia Z, Zhu G, Shi B. Single nucleotide polymorphism associated with nonsyndromic cleft palate influences the processing of miR-140. Am J Med Genet A. 2010;152A:856–862. doi: 10.1002/ajmg.a.33236. [DOI] [PubMed] [Google Scholar]
- Liu W, Sun X, Braut A, Mishina Y, Behringer RR, Mina M, Martin JF. Distinct functions for Bmp signaling in lip and palate fusion in mice. Development. 2005;132:1453–1461. doi: 10.1242/dev.01676. [DOI] [PubMed] [Google Scholar]
- Maves L, Jackman W, Kimmel CB. FGF3 and FGF8 mediate a rhombomere 4 signaling activity in the zebrafish hindbrain. Development. 2002;129:3825–3837. doi: 10.1242/dev.129.16.3825. [DOI] [PubMed] [Google Scholar]
- Miller CT, Maves L, Kimmel CB. moz regulates Hox expression and pharyngeal segmental identity in zebrafish. Development. 2004;131:2443–2461. doi: 10.1242/dev.01134. [DOI] [PubMed] [Google Scholar]
- Miller CT, Schilling TF, Lee K, Parker J, Kimmel CB. sucker encodes a zebrafish Endothelin-1 required for ventral pharyngeal arch development. Development. 2000;127:3815–3828. doi: 10.1242/dev.127.17.3815. [DOI] [PubMed] [Google Scholar]
- Miller CT, Swartz ME, Khuu PA, Walker MB, Eberhart JK, Kimmel CB. mef2ca is required in cranial neural crest to effect Endothelin1 signaling in zebrafish. Dev Biol. 2007;308:144–157. doi: 10.1016/j.ydbio.2007.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nechiporuk A, Raible DW. FGF-dependent mechanosensory organ patterning in zebrafish. Science. 2008;320:1774–1777. doi: 10.1126/science.1156547. [DOI] [PubMed] [Google Scholar]
- Nikaido M, Tada M, Saji T, Ueno N. Conservation of BMP signaling in zebrafish mesoderm patterning. Mech Dev. 1997;61:75–88. doi: 10.1016/s0925-4773(96)00625-9. [DOI] [PubMed] [Google Scholar]
- Osumi-Yamashita N, Ninomiya Y, Doi H, Eto K. The contribution of both forebrain and midbrain crest cells to the mesenchyme in the frontonasal mass of mouse embryos. Dev Biol. 1994;164:409–419. doi: 10.1006/dbio.1994.1211. [DOI] [PubMed] [Google Scholar]
- Pauws E, Hoshino A, Bentley L, Prajapati S, Keller C, Hammond P, Martinez-Barbera JP, Moore GE, Stanier P. Tbx22null mice have a submucous cleft palate due to reduced palatal bone formation and also display ankyloglossia and choanal atresia phenotypes. Hum Mol Genet. 2009;18:4171–4179. doi: 10.1093/hmg/ddp368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters H, Neubuser A, Kratochwil K, Balling R. Pax9-deficient mice lack pharyngeal pouch derivatives and teeth and exhibit craniofacial and limb abnormalities. Genes Dev. 1998;12:2735–2747. doi: 10.1101/gad.12.17.2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postlethwait JH. The zebrafish genome: a review and msx gene case study. Genome Dyn. 2006;2:183–197. doi: 10.1159/000095104. [DOI] [PubMed] [Google Scholar]
- Proetzel G, Pawlowski SA, Wiles MV, Yin M, Boivin GP, Howles PN, Ding J, Ferguson MW, Doetschman T. Transforming growth factor-beta 3 is required for secondary palate fusion. Nat Genet. 1995;11:409–414. doi: 10.1038/ng1295-409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice R, Spencer-Dene B, Connor EC, Gritli-Linde A, McMahon AP, Dickson C, Thesleff I, Rice DP. Disruption of Fgf10/Fgfr2b-coordinated epithelial-mesenchymal interactions causes cleft palate. J Clin Invest. 2004;113:1692–1700. doi: 10.1172/JCI20384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson RJ, Dixon J, Jiang R, Dixon MJ. Integration of IRF6 and Jagged2 signalling is essential for controlling palatal adhesion and fusion competence. Hum Mol Genet. 2009;18:2632–2642. doi: 10.1093/hmg/ddp201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robu ME, Larson JD, Nasevicius A, Beiraghi S, Brenner C, Farber SA, Ekker SC. p53 activation by knockdown technologies. PLoS Genet. 2007;3:e78. doi: 10.1371/journal.pgen.0030078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roessler E, Belloni E, Gaudenz K, Jay P, Berta P, Scherer SW, Tsui LC, Muenke M. Mutations in the human Sonic Hedgehog gene cause holoprosencephaly. Nat Genet. 1996;14:357–360. doi: 10.1038/ng1196-357. [DOI] [PubMed] [Google Scholar]
- Sabel JL, d’Alencon C, O’Brien EK, Van Otterloo E, Lutz K, Cuykendall TN, Schutte BC, Houston DW, Cornell RA. Maternal Interferon Regulatory Factor 6 is required for the differentiation of primary superficial epithelia in Danio and Xenopus embryos. Dev Biol. 2009;325:249–262. doi: 10.1016/j.ydbio.2008.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanford LP, Ormsby I, Gittenberger-de Groot AC, Sariola H, Friedman R, Boivin GP, Cardell EL, Doetschman T. TGFbeta2 knockout mice have multiple developmental defects that are non-overlapping with other TGFbeta knockout phenotypes. Development. 1997;124:2659–2670. doi: 10.1242/dev.124.13.2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satokata I, Maas R. Msx1 deficient mice exhibit cleft palate and abnormalities of craniofacial and tooth development. Nat Genet. 1994;6:348–356. doi: 10.1038/ng0494-348. [DOI] [PubMed] [Google Scholar]
- Schilling TF, Kimmel CB. Musculoskeletal patterning in the pharyngeal segments of the zebrafish embryo. Development. 1997;124:2945–2960. doi: 10.1242/dev.124.15.2945. [DOI] [PubMed] [Google Scholar]
- Shah RM, Donaldson EM, Scudder GG. Toward the origin of the secondary palate. A possible homologue in the embryo of fish, Onchorhynchus kisutch, with description of changes in the basement membrane area. Am J Anat. 1990;189:329–338. doi: 10.1002/aja.1001890405. [DOI] [PubMed] [Google Scholar]
- Shah RM, Young AV, Feeley JE, Donaldson EM. Growth and Differentiation of the Secondary Palate in a Teleostean Fish, Oncorhyrtchus kisutch. The Journal of Experimental Zoology. 1995;271:220–227. [Google Scholar]
- Sheehan-Rooney K. unpublished. [Google Scholar]
- Sheehan-Rooney K, Palinkasova B, Eberhart JK, Dixon MJ. A cross-species analysis of Satb2 expression suggests deep conservation across vertebrate lineages. Dev Dyn. 2010;239:3481–3491. doi: 10.1002/dvdy.22483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soriano P. The PDGF alpha receptor is required for neural crest cell development and for normal patterning of the somites. Development. 1997;124:2691–2700. doi: 10.1242/dev.124.14.2691. [DOI] [PubMed] [Google Scholar]
- Talbot JC, Johnson SL, Kimmel CB. hand2 and Dlx genes specify dorsal, intermediate and ventral domains within zebrafish pharyngeal arches. Development. 2010;137:2507–2517. doi: 10.1242/dev.049700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tena JJ, Neto A, de la Calle-Mustienes E, Bras-Pereira C, Casares F, Gomez- Skarmeta JL. Odd-skipped genes encode repressors that control kidney development. Dev Biol. 2007;301:518–531. doi: 10.1016/j.ydbio.2006.08.063. [DOI] [PubMed] [Google Scholar]
- Terao F, Takahashi I, Mitani H, Haruyama N, Sasano Y, Suzuki O, Takano- Yamamoto T. Fibroblast growth factor 10 regulates Meckel’s cartilage formation during early mandibular morphogenesis in rats. Dev Biol. 2010;350:337–347. doi: 10.1016/j.ydbio.2010.11.029. [DOI] [PubMed] [Google Scholar]
- Tittle RK, Sze R, Ng A, Nuckels RJ, Swartz ME, Anderson RM, Bosch J, Stainier DY, Eberhart JK, Gross JM. Uhrf1 and Dnmt1 are required for development and maintenance of the zebrafish lens. Dev Biol. 2010 doi: 10.1016/j.ydbio.2010.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trainor PA, Melton KR, Manzanares M. Origins and plasticity of neural crest cells and their roles in jaw and craniofacial evolution. Int J Dev Biol. 2003;47:541–553. [PubMed] [Google Scholar]
- Trinh le A, McCutchen MD, Bonner-Fraser M, Fraser SE, Bumm LA, McCauley DW. Fluorescent in situ hybridization employing the conventional NBT/BCIP chromogenic stain. Biotechniques. 2007;42:756–759. doi: 10.2144/000112476. [DOI] [PubMed] [Google Scholar]
- van den Boogaard MJ, Dorland M, Beemer FA, van Amstel HK. MSX1 mutation is associated with orofacial clefting and tooth agenesis in humans. Nat Genet. 2000;24:342–343. doi: 10.1038/74155. [DOI] [PubMed] [Google Scholar]
- Wada N, Javidan Y, Nelson S, Carney TJ, Kelsh RN, Schilling TF. Hedgehog signaling is required for cranial neural crest morphogenesis and chondrogenesis at the midline in the zebrafish skull. Development. 2005;132:3977–3988. doi: 10.1242/dev.01943. [DOI] [PubMed] [Google Scholar]
- Walker MB, Kimmel CB. A two-color acid-free cartilage and bone stain for zebrafish larvae. Biotech Histochem. 2007;82:23–28. doi: 10.1080/10520290701333558. [DOI] [PubMed] [Google Scholar]
- Walker MB, Miller CT, Coffin Talbot J, Stock DW, Kimmel CB. Zebrafish furin mutants reveal intricacies in regulating Endothelin1 signaling in craniofacial patterning. Dev Biol. 2006;295:194–205. doi: 10.1016/j.ydbio.2006.03.028. [DOI] [PubMed] [Google Scholar]
- Walker MB, Miller CT, Swartz ME, Eberhart JK, Kimmel CB. phospholipase C, beta 3 is required for Endothelin1 regulation of pharyngeal arch patterning in zebrafish. Dev Biol. 2007;304:194–207. doi: 10.1016/j.ydbio.2006.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Lan Y, Cho ES, Maltby KM, Jiang R. Odd-skipped related 1 (Odd 1) is an essential regulator of heart and urogenital development. Dev Biol. 2005;288:582–594. doi: 10.1016/j.ydbio.2005.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerfield M. The Zebrafish Book: A Guide for the Laboratory Use of Zebrafish (Brachydanio rerio) Eugene: University of Oregon Press; 1993. [Google Scholar]
- Weth F, Nadler W, Korsching S. Nested expression domains for odorant receptors in zebrafish olfactory epithelium. Proc Natl Acad Sci U S A. 1996;93:13321–13326. doi: 10.1073/pnas.93.23.13321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Bringas P, Jr, Soriano P, Chai Y. PDGFR-alpha signaling is critical for tooth cusp and palate morphogenesis. Dev Dyn. 2005;232:75–84. doi: 10.1002/dvdy.20197. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Mori T, Takaki H, Takeuch M, Iseki K, Hagino S, Murakawa M, Yokoya S, Wanaka A. Comparison of the expression patterns of two LIM-homeodomain genes, Lhx6 and L3/Lhx8, in the developing palate. Orthod Craniofac Res. 2002a;5:65–70. doi: 10.1034/j.1600-0544.2002.02198.x. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Song Y, Zhao X, Zhang X, Fermin C, Chen Y. Rescue of cleft palate in Msx1-deficient mice by transgenic Bmp4 reveals a network of BMP and Shh signaling in the regulation of mammalian palatogenesis. Development. 2002b;129:4135–4146. doi: 10.1242/dev.129.17.4135. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Guo YJ, Tomac AC, Taylor NR, Grinberg A, Lee EJ, Huang S, Westphal H. Isolated cleft palate in mice with a targeted mutation of the LIM homeobox gene lhx8. Proc Natl Acad Sci U S A. 1999;96:15002–15006. doi: 10.1073/pnas.96.26.15002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A,B) Red box represents the magnified view of the in situ images in supplementary Figures 2,4. (A) Lateral view of embryo representing the 30, 36 and 40 hpf time points. (B) Ventral view of embryo representing the 44,48,55,60 and 72 hpf time points. Asterisks mark palate forming CNCC, eye; cf, choroid fissure; ea, ear; mx maxillary domain; fn, frontonasal crest; oe, oral ectoderm; md, mandibular domain.
(A,E,I,M,Q,U,Y) Lateral views at 30, (B,C,D,F,G,H,J,K,L,N,O,P,R,S,T,V,W,X,Z,AA,BB) ventral views at 55, 60 and 72 hpf. (A) msxe is expressed in mandibular cranial neural crest cells at 30 hpf and (B) at 55 hpf it is expressed in palatal precursors and mandibular crest; (C,D) thereafter expression is lost. (E) osr1 is weakly expressed in second arch CNCC at 30 hpf. (F–H) Dorsal oral ectodermal cells express osr1 from 55 to 72 hpf. (I–L) CNCC weakly express osr2 at 30 hpf and (J–K) from 55 hpf onward osr2 expression becomes restricted to a small group of mandibular CNCC. (M–P) The expression domain of tbx22 in CNCC increases in size over time but remains limited to the posterior maxillary and mandibular domains, wrapping around the oral ectoderm. (Q–T) CNCC express lhx6 and (U–X) lhx8 in a similar pattern, wrapping around the oral ectoderm. This expression includes both the maxillary and mandibular domains with decreasing amounts of signal over time. (Y) At 30hpf, pax9 is not expressed in the first arch, (Z–BB) but it is expressed in palatal precursor CNCC from 55 to 72 hpf. (BB) At 72 hpf, a small area of the dorsal oral ectoderm expresses pax9.
(A,B) Lateral views at 36 and 40 hpf, (C,D) ventral views at 44 and 48 hpf. CNCC express lhx8, wrapping around the oral ectoderm including expression in both the maxillary and mandibular domains.
(A–J) Lateral views of the zebrafish head in sagittal sections at 40 hpf. (A–I) Anti-EGFP immunostaining labels CNCC in the fli1:EGFP transgenic background (green). (B–J) mRNA localization detected by fluorescence of the NBT/BCIP precipitate (red). Arrowheads indicate areas of CNCC expression domains. (A,B) msxe is expressed in frontonasal and maxillary CNCC. (C,D) osr1 is expressed in maxillary CNCC. (E,F) osr2 is expressed in the frontonasal and maxillary CNCC. (G,H) Maxillary CNCC express lhx6. (I,J) The posterior maxillary CNCC express pax9.
(A,E,I) Lateral views at 30 hpf (B,C,D,F,G,H,J,K,L) and ventral views at 55, 60 and 72 hpf. (A) At 30 hpf, a small group of maxillary CNCC and mandibular neural crest cells express bmp4. (B–D) From 55 hpf onward scattered CNCC weakly express bmp4. (E) At 30hpf, fgf10a is expressed in what is likely to be surface ectoderm. (F–H) At later time points, CNCC and dorsal oral ectoderm continue to express fgf10a transcript. (I) The lens and a small population of maxillary CNCC express tgfb3 at 30 hpf. (J–L) From 55 to 72 hpf, CNCC strongly express tgfb3.
(A–J) Lateral views of the zebrafish head in sagittal sections at 40 hpf. (A–K) Anti-EGFP immunostaining labels CNCC in the fli1:EGFP transgenic background (green). (B–L) mRNA localization detected by fluorescence of the NBT/BCIP precipitate (red). Arrowheads indicate areas of CNCC expression domains, arrows indicate areas of oral ectoderm expression. (A,B) shh is expressed in the oral ectoderm at both time points. (C,D) Both anterior oral ectoderm and frontonasal and maxillary CNCC express fgf10a at 40 hpf. (E,F) Anterior CNCCs and oral ectoderm express bmp2b at 40 hpf. (G,H) bmp4 expression is in the oral ectoderm and the CNCC. (I,J) Anterior CNCC express tgfb2. (K,L) tgfb3 is expressed in anterior CNCC and the oral ectoderm.
Ventral views of the zebrafish head in horizontal optical sections. (A–K) Anti-EGFP immunostaining labels CNCC in the fli1:EGFP transgenic background in green. (B–L) mRNA localization is detected by fluorescence of NBT/BCIP precipitate in red. Arrowheads indicate areas of CNCC expression domains, arrows mark expression in the oral ectoderm. (A,B) shh is expressed in the oral ectoderm. (C,D) fgf10a is expressed in both the CNCC and the oral ectoderm. (E,F) bmp2b is expressed more broadly in the oral ectoderm and less in the CNCC. (G,H) CNCC and oral ectoderm express bmp4. (I,J) Anterior CNCC express tgfb2. (K,L) tgfb3 is expressed in the CNCC and lightly in the oral ectoderm.
RT-PCR was performed on RNA isolated from 48 hpf uninjected controls and embryos that were injected with 9ng or 15ng of the tgfb2 morpholino at the one cell stage. Primers designed to amplify a portion of zebrafish cyp26b1 were used as a control. Control primers yielded products that were of equal intensity across the conditions. tgfb2 primers revealed a decrease in the amount of wild-type transcript across the conditions.
RT-PCR was performed on RNA that was isolated from 48 hpf uninjected control embryos and embryos that were injected with 15ng of the pax9 morpholino at the one cell stage. Primers designed to amplify a portion of zebrafish cyp26b1 were used as a control. Control primers yielded products that were of equal intensity across the conditions. Single asterisk indicates the wildtype product, double asterisks indicate the improperly spliced variant.