Abstract
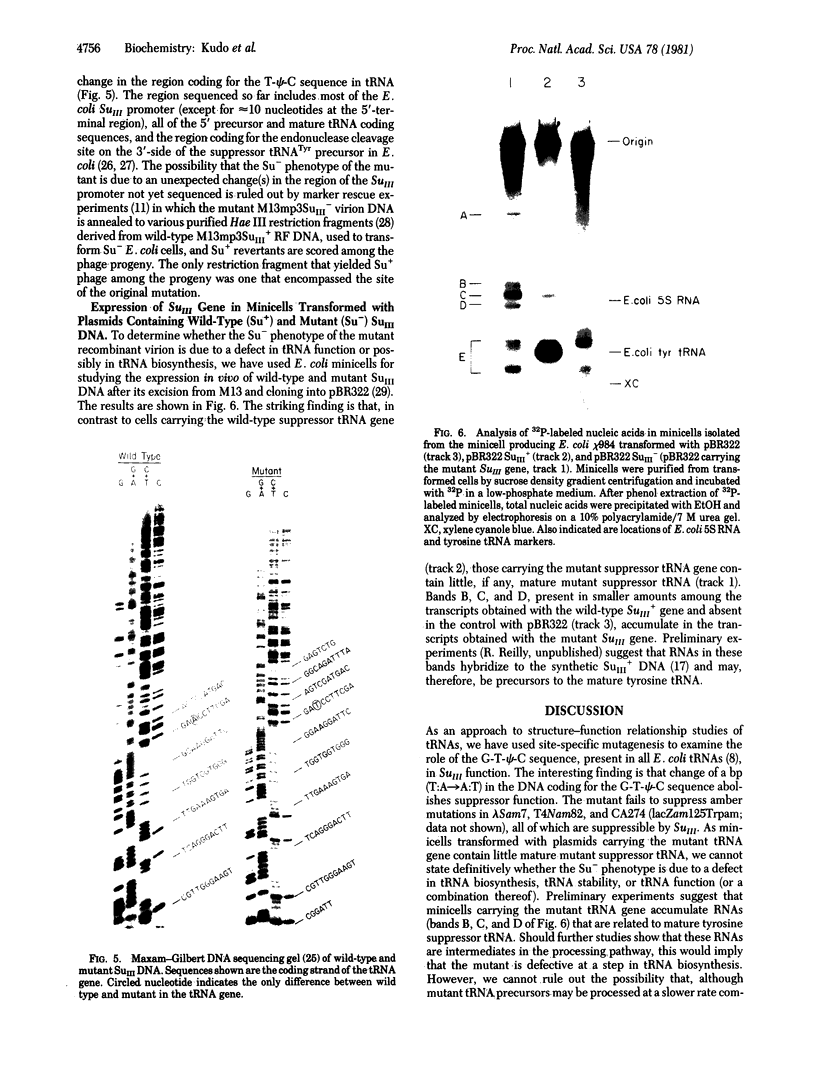
We have cloned the Escherichia coli tyrosine-inserting amber suppressor tRNA gene into the recombinant single-strand phage M12mp3. By using the M13mp3SuIII+ recombinant phage DNA as template and an oligonucleotide bearing a mismatch as primer, we have synthesized in vitro an M13mp3SuIII heteroduplex DNA that has a single mismatch at a predetermined site in the tRNA gene. Transformation of E. coli with the heteroduplex DNA yielded M13 recombinant phages carrying a mutant suppressor tRNA gene in which the sequence G-T-T-C, corresponding to the universal G-T-pseudouracil-C sequence in E. coli tRNAs, is changed to G-A-T-C. The mutant DNA has been characterized by restriction mapping and by sequence analysis. In contrast to results with the wild-type suppressor tRNA gene, cells transformed with recombinant plasmids carrying the mutant tRNA gene are phenotypically Su-. Thus, the single nucleotide change introduced has inactivated the function of the tRNA gene. By using E. coli minicells for studying the expression in vivo of cloned tRNA genes, we have found that cells transformed with recombinant plasmids carrying the mutant tRNA gene contain very little, if any, mature mutant suppressor tRNA. In contrast, the predominant low molecular weight RNA in cells transformed with recombinant plasmids carrying the wild-type suppressor tRNA gene is the mature tyrosine suppressor tRNA. Thus, while our results imply an important role for the G-T-pseudouracil-C sequence common to all E. coli tRNAs, whether this sequence is essential for tRNA biosynthesis, tRNA stability in vivo, or tRNA function remains to be determined. The procedures used to generate the mutant should be of general application toward site-specific mutagenesis on cloned DNAs, including regions that possess high degrees of secondary structure. In addition, the frequency of mutants among the progeny is high enough to enable one to identify and isolate site-specific mutants on any cloned DNA without requiring phenotypic selection.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abelson J. RNA processing and the intervening sequence problem. Annu Rev Biochem. 1979;48:1035–1069. doi: 10.1146/annurev.bi.48.070179.005131. [DOI] [PubMed] [Google Scholar]
- Altman S. Isolation of tyrosine tRNA precursor molecules. Nat New Biol. 1971 Jan 6;229(1):19–21. doi: 10.1038/newbio229019a0. [DOI] [PubMed] [Google Scholar]
- Baumstark B. R., Roberts R. J., RajBhandary U. L. Use of short synthetic DNA duplexes as substrates for the restriction endonucleases Hpa II and Mno I. J Biol Chem. 1979 Sep 25;254(18):8943–8950. [PubMed] [Google Scholar]
- Bhanot O. S., Khan S. A., Chambers R. W. A new system for studying molecular mechanisms of mutation by carcinogens. J Biol Chem. 1979 Dec 25;254(24):12684–12693. [PubMed] [Google Scholar]
- Celis J. E. Collection of mutant tRNA sequences. Nucleic Acids Res. 1980 Jan 11;8(1):r23–r29. doi: 10.1093/nar/8.1.197-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cudny H., Deutscher M. P. Apparent involvement of ribonuclease D in the 3' processing of tRNA precursors. Proc Natl Acad Sci U S A. 1980 Feb;77(2):837–841. doi: 10.1073/pnas.77.2.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgell M. H., Hutchison C. A., 3rd, Sclair M. Specific endonuclease R fragments of bacteriophage phiX174 deoxyribonucleic acid. J Virol. 1972 Apr;9(4):574–582. doi: 10.1128/jvi.9.4.574-582.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan J., Landy A. Structural analysis of the tRNA1Tyr gene of Escherichia coli. A 178 base pair sequence that is repeated 3.14 times. J Biol Chem. 1978 May 25;253(10):3607–3622. [PubMed] [Google Scholar]
- Goodman H. M., Abelson J., Landy A., Brenner S., Smith J. D. Amber suppression: a nucleotide change in the anticodon of a tyrosine transfer RNA. Nature. 1968 Mar 16;217(5133):1019–1024. doi: 10.1038/2171019a0. [DOI] [PubMed] [Google Scholar]
- Goulian M., Kornberg A., Sinsheimer R. L. Enzymatic synthesis of DNA, XXIV. Synthesis of infectious phage phi-X174 DNA. Proc Natl Acad Sci U S A. 1967 Dec;58(6):2321–2328. doi: 10.1073/pnas.58.6.2321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gronenborn B., Messing J. Methylation of single-stranded DNA in vitro introduces new restriction endonuclease cleavage sites. Nature. 1978 Mar 23;272(5651):375–377. doi: 10.1038/272375a0. [DOI] [PubMed] [Google Scholar]
- Heckman J. E., Sarnoff J., Alzner-DeWeerd B., Yin S., RajBhandary U. L. Novel features in the genetic code and codon reading patterns in Neurospora crassa mitochondria based on sequences of six mitochondrial tRNAs. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3159–3163. doi: 10.1073/pnas.77.6.3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchison C. A., 3rd, Phillips S., Edgell M. H., Gillam S., Jahnke P., Smith M. Mutagenesis at a specific position in a DNA sequence. J Biol Chem. 1978 Sep 25;253(18):6551–6560. [PubMed] [Google Scholar]
- Khorana H. G. Total synthesis of a gene. Science. 1979 Feb 16;203(4381):614–625. doi: 10.1126/science.366749. [DOI] [PubMed] [Google Scholar]
- Klenow H., Henningsen I. Selective elimination of the exonuclease activity of the deoxyribonucleic acid polymerase from Escherichia coli B by limited proteolysis. Proc Natl Acad Sci U S A. 1970 Jan;65(1):168–175. doi: 10.1073/pnas.65.1.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurjan J., Hall B. D., Gillam S., Smith M. Mutations at the yeast SUP4 tRNATyr locus: DNA sequence changes in mutants lacking suppressor activity. Cell. 1980 Jul;20(3):701–709. doi: 10.1016/0092-8674(80)90316-5. [DOI] [PubMed] [Google Scholar]
- Küpper H., Sekiya T., Rosenberg M., Egan J., Landy A. A rho-dependent termination site in the gene coding for tyrosine tRNA su3 of Escherichia coli. Nature. 1978 Mar 30;272(5652):423–428. doi: 10.1038/272423a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low K. B. Escherichia coli K-12 F-prime factors, old and new. Bacteriol Rev. 1972 Dec;36(4):587–607. doi: 10.1128/br.36.4.587-607.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller W., Weber H., Meyer F., Weissmann C. Site-directed mutagenesis in DNA: generation of point mutations in cloned beta globin complementary dna at the positions corresponding to amino acids 121 to 123. J Mol Biol. 1978 Sep 15;124(2):343–358. doi: 10.1016/0022-2836(78)90303-0. [DOI] [PubMed] [Google Scholar]
- RICHARDSON C. C., LEHMAN I. R., KORNBERG A. A DEOXYRIBONUCLEIC ACID PHOSPHATASE-EXONUCLEASE FROM ESCHERICHIA COLI. II. CHARACTERIZATION OF THE EXONUCLEASE ACTIVITY. J Biol Chem. 1964 Jan;239:251–258. [PubMed] [Google Scholar]
- Razin A., Hirose T., Itakura K., Riggs A. D. Efficient correction of a mutation by use of chemically synthesized DNA. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4268–4270. doi: 10.1073/pnas.75.9.4268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich A., RajBhandary U. L. Transfer RNA: molecular structure, sequence, and properties. Annu Rev Biochem. 1976;45:805–860. doi: 10.1146/annurev.bi.45.070176.004105. [DOI] [PubMed] [Google Scholar]
- Richter D., Erdmann V. A., Sprinzl M. A new transfer RNA fragment reaction: Tp psi pCpGp bound to a ribosome-messenger RNA complex induces the synthesis of guanosine tetra- and pentaphosphates. Proc Natl Acad Sci U S A. 1974 Aug;71(8):3226–3229. doi: 10.1073/pnas.71.8.3226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter D., Erdmann V. A., Sprinzl M. Specific recognition of GTpsiC loop (loop IV) of tRNA by 50S ribosomal subunits from E. coli. Nat New Biol. 1973 Dec 5;246(153):132–135. doi: 10.1038/newbio246132a0. [DOI] [PubMed] [Google Scholar]
- Roberts R. J. Structures of two glycyl-tRNAs from Staphylococcus epidermidis. Nat New Biol. 1972 May 10;237(71):44–45. doi: 10.1038/newbio237044a0. [DOI] [PubMed] [Google Scholar]
- Roozen K. J., Fenwick R. G., Jr, Curtiss R., 3rd Synthesis of ribonucleic acid and protein in plasmid-containing minicells of Escherichia coli K-12. J Bacteriol. 1971 Jul;107(1):21–33. doi: 10.1128/jb.107.1.21-33.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell R. L., Abelson J. N., Landy A., Gefter M. L., Brenner S., Smith J. D. Duplicate genes for tyrosine transfer RNA in Escherichia coli. J Mol Biol. 1970 Jan 14;47(1):1–13. doi: 10.1016/0022-2836(70)90397-9. [DOI] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R., Barrell B. G., Smith A. J., Roe B. A. Cloning in single-stranded bacteriophage as an aid to rapid DNA sequencing. J Mol Biol. 1980 Oct 25;143(2):161–178. doi: 10.1016/0022-2836(80)90196-5. [DOI] [PubMed] [Google Scholar]
- Sekiya T., Contreras R., Takeya T., Khorana H. G. Total synthesis of a tyrosine suppressor transfer RNA gene. XVII. Transcription, in vitro, of the synthetic gene and processing of the primary transcript to transfer RNA. J Biol Chem. 1979 Jul 10;254(13):5802–5816. [PubMed] [Google Scholar]
- Sekiya T., Gait M. J., Noris K., Ramamoorthy B., Khorana H. G. The nucleotide sequence in the promoter region of the gene for an Escherichia coli tyrosine transfer ribonucleic acid. J Biol Chem. 1976 Aug 10;251(15):4481–4489. [PubMed] [Google Scholar]
- Setlow P., Brutlag D., Kornberg A. Deoxyribonucleic acid polymerase: two distinct enzymes in one polypeptide. I. A proteolytic fragment containing the polymerase and 3' leads to 5' exonuclease functions. J Biol Chem. 1972 Jan 10;247(1):224–231. [PubMed] [Google Scholar]
- Shortle D., Nathans D. Local mutagenesis: a method for generating viral mutants with base substitutions in preselected regions of the viral genome. Proc Natl Acad Sci U S A. 1978 May;75(5):2170–2174. doi: 10.1073/pnas.75.5.2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simsek M., Ziegenmeyer J., Heckman J., Rajbhandary U. L. Absence of the sequence G-T-psi-C-G(A)- in several eukaryotic cytoplasmic initiator transfer RNAs. Proc Natl Acad Sci U S A. 1973 Apr;70(4):1041–1045. doi: 10.1073/pnas.70.4.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J. D. Mutants which allows accumulation of tRNATyr precursor molecules. Brookhaven Symp Biol. 1975 Jul;(26):1–11. [PubMed] [Google Scholar]
- Sprague K. U., Hagenbüchle O., Zuniga M. C. The nucleotide sequence of two silk gland alanine tRNAs: implications for fibroin synthesis and for initiator tRNA structure. Cell. 1977 Jul;11(3):561–570. doi: 10.1016/0092-8674(77)90074-5. [DOI] [PubMed] [Google Scholar]
- Sprinzl M., Grueter F., Spelzhaus A., Gauss D. H. Compilation of tRNA sequences. Nucleic Acids Res. 1980 Jan 11;8(1):r1–r22. [PMC free article] [PubMed] [Google Scholar]