Abstract
The study of molecular clock mechanisms in psychiatric disorders is gaining significant interest due to data suggesting that a misalignment between the endogenous circadian system and the sleep-wake cycle might contribute to the clinical status of patients suffering from a variety of psychiatric disorders. Sleep disturbances in major depressive disorder (MDD) are characterized by increased sleep latency, poorer sleep efficiency, reduced latency to the first rapid eye movement (REM) sleep episode, and early-morning awakening, but there is little data to indicate a role of circadian clock genes in MDD. There is also relatively little information regarding the role of clock genes in anxiety. In contrast, a significant amount of evidence gathered in bipolar disorder (BPD) patients suggests a circadian rhythm disorder, namely an advanced circadian rhythm and state-dependent alterations of REM sleep latency. Most research on the role of clock genes in BPD has focused on polymorphisms of CLOCK, but the lithium target GSK3 may also play a significant role. A circadian phase shift is also theorized to contribute to the pathophysiology of winter seasonal affective disorder (SAD). Certain allelic combinations of NPAS2, PER3, and BMAL1 appear to contribute to the risk of SAD. In chronic shizophrenia, disturbances of sleep including insomnia and reduced sleep efficiency have been observed. Genetic studies have found associations with CLOCK, PER1, PER3, and TIMELESS. Sleep and circadian changes associated with dementia due to Alzheimer's disease suggest a functional change in the circadian master clock, which is supported by postmortem studies of clock gene expression in the brain.
Keywords: clock gene, mental disorder, mental health, major depressive disorder, bipolar affective disorder, seasonal affective disorder, schizophrenia, Alzheimer's disease, sleep-wake cycle, rest-activity cycle, single-nucleotide polymorphism
Abstract
El estudio de los mecanismos del reloj molecular en los trastornos psiquátricos tiene un interés creciente ya que la evidencia sugiere que un desajuste entre el sistema circadiano endógeno y el ciclo sueño-vigilia podría contribuir al estado clínico de pacientes que sufren diversos trastornos psiquiátricos. Las alteraciones del sueño en el trastorno depresivo mayor (TDM) están caracterizadas por un aumento en la latencia de sueño, una pobre eficiencia de sueño, una reducción de la latencia para el primer episodio de sueño de movimientos oculares rápidos (MOR) y un despertar matinal precoz, pero existe escasa evidencia para explicar el papel de los genes del reloj circadiano en el TDM. También es escasa la información sobre los genes del reloj en la ansiedad. En oposición, se ha acumulado une importante cantidad de evidencia en pacientes con trastorno del ritmo circadiano, especialmente un avance de éste y alteraciones de la latencia del sueño MOR estado dependientes. Gran parte de la investigación acerca del papel de los genes del reloj en el TAB se ha centrado en el polimorfismo de CLOCK ; pero GSK., blanco del litio, también puede tener un papel significativo. Además se postula que un cambio de fase circadiana puede contribuir a la fisiopatología del trastorno afectivo estacional invernal (TAE). Al parecer ciertas combinaciones de alelos de NPAS2, PER3, y BMAL1 contribuyen al riesgo de un TAE. En la esquizofrenia crónica se ha observado insomnio y reducción de la eficiencia del sueño. Los estudios genéticos han encontrado asociaciones con CLOCK, PER1, PER3, y TIMELESS. Los cambios circadianos y del sueño asociados con la demencia de la Enfermedad de Alzheimer sugieren un cambio funcional en el reloj maestro circadiano de acuerdo con estudios postmortem de la expresión génica del reloj en el cerebro.
Abstract
L'intérêt grandissant de l'étude des mécanismes moléculaires de l'horloge dans les troubles psychiatriques s'explique par les données qui indiquent une mauvaise synchronisation entre le système circadien endogène et le cycle veille-sommeil lors de ces troubles et par l'hypothèse que ceci jouerait un rôle dans l'état clinique des patients. L'allongement du temps d'endormissement, un sommeil moins réparateur, une diminution de la latence du premier épisode de sommeil paradoxal et un réveil matinal précoce sont caractéristiques des perturbations du sommeil dans les troubles dépressifs majeurs (TDM). Le rôle des gènes de l'horloge circadienne reste cependant mal défini dans les TDM et dans l'anxiété. En revanche, un trouble du rythme circadien chez les patients atteints de troubles bipolaires (TB) semble exister, du type d'avance du rythme circadien et d'altérations de la latence du sommeil paradoxal en relation avec l'état pathologique. La recherche sur le rôle des gènes de l'horloge dans les TB a surtout porté sur un polymorphisme du gène CLOCK, mais le GSK3, cible du lithium, pourrait aussi jouer un rôle significatif. La possibilité d'un décalage de phase des rythmes circadiens pourrait également intervenir dans la physiopathologie du trouble affectif saisonnier hivernal (TAS). Certaines combinaisons d'allèles des gènes NPAS2, PER3, et BMAL1 semblent contribuer au risque de TAS. Dans la schizophrénie, des troubles du sommeil comprenant insomnie et diminution de l'efficacité du sommeil sont présents. Des études génétiques ont trouvé des associations avec les gènes CLOCK, PER1, PER3, et TIMELESS. Des études postmortem de l'expression des gènes de l'horloge dans le cerveau soutiennent l'hypothèse d'anomalies fonctionnelles de l'horloge circadienne lors des modifications circadiennes et du sommeil associées à la maladie d'Alzheimer.
The role of the endogenous circadian System is to coordinate the body's fonctions with each other and with the external environment.1 This includes the integration of sensory information and environmental time eues, and of the organismes physiological and psychological states. When the harmony of this integrative fonction is perturbed, as could be the case with several mental disorders, disturbances of mood, a disrupted sleepwake cycle, and changes in levels and/or timing of hormones can occur. Whether these changes precede, follow, or are epiphenonrenal to the mental disorders is often difficult to determine with certainty, although several lines of evidence support a role of the endogenous circadian system in the pathophysiology of these disorders. The purpose of this review is to describe sleep and circadian rhythm disturbances in mental disorders and to summarize current research on the role of clock genes in several psychiatric disorders.
Molecular mechanism of circadian rhythmicity
Circadian rhythmiclty is a consequence of intracellular molecular mechanisms involving so-called clock genes. The products of some of these clock genes regulate their own expression, and the outcome of this feedback loop is an oscillation in the levels of messenger ribonucleic acids (mRNAs) and proteins. These mRNA and protein rhythms are observed in the suprachiasmatlc nucleus (SCN) of the hypothalamus, the master clock, as well as in other brain regions and peripheral tissues. Within the clock, other factors control the phosphorylation, stability, and localization of clock proteins, thereby regulating the oscillation, particularly the period.
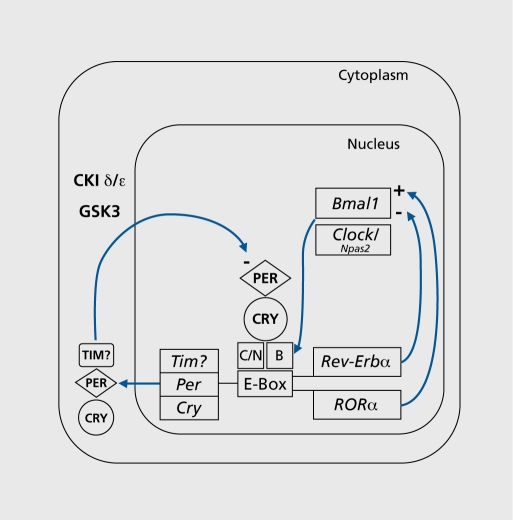
In mammals, Clock and Bmall encode transcription factors CLOCK and BMAL1 (brain and muscle ARNT-like protein 1; also known as ARNTL or MOP3),2-4 which form heterodlmers that activate the transcription of three Period genes (PER1, 2 and 3) and two Cryptochrome genes (CRY1 and 2),5-7 Rorαm and Rev-Erbα 8-10 (Figure 1). PER and CRY proteins form complexes11 that are translocated back into the nucleus and inhibit their own expression.5,7,12-15 RORα and REV-ERBα act on Bmall to activate and repress transcription respectively8,9 NPAS2 is an alternate dimerization partner for BMAL1 that may also regulate circadian rhythmiclty in the forebraln, but it has not been consistently found in the SCN.16,17 Clock proteins are phosphorylated by casein kinase I epsilon (CKIε) and delta (CKIδ), and possibly also by the Drosophila shaggy homologue glycogen synthase kinase 3 (GSK3).18 They are targeted for degradation by components of ubiqultin llgase complexes like FBXL3 and β-TRCPl,19,20 which together regulate the period of circadian oscillation by controlling the rate of accumulation, association and translocation of PER and CRY.11,15,21-23
Figure 1. Simplified schematic diagram of the molecular mechanisms of the circadian clock in mammals. See the main text for details. Positive and negative feedbacks are indicated by arrows with a + and a - sign, respectively. Genes and messenger ribonucleic acid (mRNA) are indicated by italics, proteins are in bold caps. C = CLOCK protein; N = NPAS2 protein; B = BMAL1 protein.
These genes, protein products, and enzymes work together to control clock functioning, and abnormalities such as clock gene mutations can have profound consequences for the synchronization of emotional, physiological, and behavioral processes with each other and the environment. Examples of the sometimes dramatic effects of clock gene polymorphisms in nonpsychiatric disorders are described, followed by a description of recent research on clock genes in mental disorders.
Role of circadian clock genes in disorders of the sleep-wake cycle
The evidence linking mutations of circadian clock genes and nonpsychlatric sleep/circadlan related disorders is compelling. In fact, one of the first demonstrations of a disorder directly related to the human molecular clock was a polymorphism of human PER224 identified in a family diagnosed with familial advance sleep phase disorder (FASPD).25 The resulting amino acid change in the PER2 protein affects its phosphorylation by CKIδ/ε, and its stability and intracellular localization, hence the short period and advanced sleep phase of the patients.26,27 Interestingly, in another FASPD family, a mutation was found in the gene encoding CKIδ.28
A number of studies have focused on a polymorphism In the human PER3 gene. In one study, this polymorphism was found to be associated with delayed sleep phase dis? order (DSPD).29,30 PER3 variants have also been associated with morning-evening preference, in this study, possibly through an effect on sleep structure, but not circadian timing. Viola and colleagues31 found that individuals homozygous for the PER35 allele showed marked differences in sleep compared with those homozygous for PER3,4 including greater sleep propensity, increased slow-wave sleep (SWS) and greater susceptibility to the effects of sleep deprivation. However, the circadian rhythms of melatonin, Cortisol, and activity were similar in both groups.31 This suggests that different clock genes may affect chronotype, either via direct effects on the clock, or through other mechanisms such as sleep homeostasis.
Polymorphism of the human CLOCK gene has also been associated with evening preference and delayed timing of the sleep-wake cycle32,33 (but there are also conflicting results34). Subjects carrying one or two copies of the CLOCK 31 11C allele showed increased eveningness and reduced mornlngness, while 3111T/T subjects showed higher morningness scores.32,33 Although the 3111C/C genotype is also associated with delayed sleep timing and greater daytime sleepiness in a Japanese population,33 thus far there is insufficient evidence to draw the same conclusions in Caucasians.32 There is currently no evidence to support an association between the 3111C/C genotype and DSPD.34,35
There is evidence to suggest that evening chronotype could increase the risk of psychiatric disorders.36 Both bipolar disorder (BPD) and schlzophrenla/schizoaffective patients show greater eveningness scores than controls. In BPD this observation appears to be correlated with age (ie, younger BPD patients were more extreme “evening types”), while schizophrenla/schizoaffectlve subjects tended to show greater eveningness at all ages. Being classified as an “evening type” could account for some of the sleep disturbances reported by BPD patients36 and could increase the severity of BPD as evidenced by an earlier age at onset of treatment, greater likelihood of self-reported rapid mood swings, and rapidcycling mood changes.36
Some work suggests there may be a relationship between DSPD and personality disorder.37 In one study, 16% of institutionalized mentally 111 adolescents were also diagnosed with DSPS,38 as compared with 7.3% of adolescents in Western countries.37 In these adolescents, the diagnoses tended to include an affective component, such as bipolar affective, schizoaffective or borderline personality disorder. Again, this suggests a relationship between circadian and psychiatric disorders, although whether one precedes the other or they co-occur is difficult to determine.
Sleep, circadian clock genes, and mental disorders
Major Depressive Disorder
Sleep disturbances, particularly insomnia, are an important symptom of Major Depressive Disorder (MDD).39-42 Between 80% and 90% of depressed patients report insomnia, and insomnia is also a risk factor for developing depression.39-41 Sleep disturbances are associated with impaired quality of life43 and a greater risk of relapse.44,45
Reduced rapid eye movement (REM) sleep latency (RL), an earlier distribution of REM sleep during the night, and early-morning awakening suggest a possible phase advance of the endogenous circadian system.46,47
This hypothesis is further supported by the therapeutic success of sleep phase advance therapy In depressed patients.48 Higher core body temperature (CBT),49 higher Cortisol 50- and lower melatonin secretion54,55 have been observed in depressed patients, supporting an involvement of the circadian system, although contradictory results exist,54,56-58 (see refs 46 and 47 for reviews).
To date there has been no evidence of clock gene mutations associated with MDD. The T3111C polymorphism of CLOCK was investigated, based on its association with eveningness, but no differences were found in allelic frequencies between a group of 143 people with a history of major depression and 195 controls.59 However, there has been some recent evidence suggesting that electroconvulsive therapy and antidepressant medications targeting the dopaminergic and serotonergic neurotransmitter systems, including, monoamine oxidase inhibitors (MAOIs), fluoxetine, imlpr amine, clozapine, risperidone, and haloperldol may have a common mode of action either via the direct inhibition or increased phosphorylation of the GSK3 enzyme.60 GSK3 Is involved in many cellular functions; therefore the therapeutic action may be via a number of possible routes, including regulation of monaminerglc signaling, neuroprotection, neuroplasticity, modulation of estrogen and glucocorticoid activity, regulation of brain metabolism, or regulation of the circadian system.60 This is described in more detail in the section on BPD below.
Anxiety disorders
Individuals suffering from an anxiety disorder frequently experience sleep disturbances such as insomnia. Sleep disorders are common in both generalized anxiety disorder (GAD) and post-traumatic stress disorder (PTSD), and PTSD patients frequently experience nightmares, and reduced REM sleep.61 There is relatively little evidence suggesting specific circadian disturbances or a role for clock genes in anxiety disorders. This is perhaps not surprising, given the heterogeneity of anxiety disorders and their comorbidity with other disorders such as depression.61 In contrast to studies in humans, there are a few interesting results from research on animals. One study showed a reduction of Perl mRNA levels in mouse cerebellum by antianxiety medications,62 suggesting that altering circadian clock gene levels could theoretically contribute to the therapeutic action of these drugs. Also of interest is the observed reduction in anxiety observed in mice with a mutation of the Clock gene. For example, Clock mutant animals are much more likely to spend time in open spaces, which normal mice avoid.63 However, as these mice also showed behaviors associated with mania, it is unclear how to best classify this phenotype.
Bipolar disorder
Sleep disturbances have been observed in BPD and often precede relapses into depression or mania.46,64-67 Insomnia or hypersomnia, early-morning awakenings, reduced sleep efficiency, and reduced RL are the most consistently reported changes. Irregularities in the sleep-wake cycle and daily activities can be important contributing factors in mood disruption.68 The relationship between the sleep-wake cycle and changes in mood appears to be important in patients with frequent and rapid changes in mood state, so-called “rapid cyclers,” with the switch from mania/hypomania to depression/euthymia occurring during or after sleep, while positive changes in mood from depression to hypomania/mania are more likely to occur after a period of wakefulness.69,70 Circadian disturbances have been reported in BPD that suggest a phase advance of the master clock, including a phase advance of the diurnal rhythm of plasma Cortisol,71 although negative results have been reported.72
Much of the work attempting to link BPD to clock genes has focused on the 3111T/C polymorphism of the human CLOCK gene.73-76 The C/C allele of CLOCK has been associated with greater severity of insomnia during antidepressant treatment76 and a higher recurrence rate of bipolar episodes,74 and reduced need for sleep.75 Support for a role of Clock mutation in BPD has recently come from the animal literature, where behavioral studies using CLOCK mutant mice suggest a phenotype similar to mania, with an increase in the reward value of appetitive stimuli and reduced depressive and anxiety-like behaviors.63
An analysis of 46 single nucleotide polymorphisms (SNP) in eight clock genes (BMAL1, CLOCK, PER 1, 2, 3 CRY 1,2, TIMELESS) using family-based samples with BPD or schizophrenia has been reported.77 A Mendelian transmission distortion analysis revealed association of BMAL1 (ARNTL) and TIMELESS with BPD. However, these were modest associations found using a very liberal analysis. Interestingly, an independent study using haplotype analysis seems to confirm the association with BMAL1 (ARNTL) and also finds one with PER3 (TIMELESS was not studied).78 Studies examining other genes have found negative results: screening for human PER2 mutations at the CKIδ/ε binding site showed no difference in frequency between BPD patients and controls,79 nor is there any evidence for linkage or association of CRY1 80
Although it has been known for many years that lithium is effective as a mood stabilizer, its pharmacological mode of action has remained uncertain.60 However, recent evidence suggests that the therapeutic action of lithium may be related to direct effects on the circadian clock. For example, lithium has been shown to lengthen the period of circadian rhythms in rodents,81 and can lengthen the period of neuronal firing of cultured SCN neurons in a dose-dependent manner.82 A delay of the circadian rhythm of temperature and of REM sleep has also been shown in a BPD patient.83 This suggests that the therapeutic action of lithium could be due, in part, to correcting a phase advance of the circadian system related to the illness.
One proposed molecular mechanism is via the inhibition of GSK3.18,84 Although this enzyme has a number of functions that could potentially mediate the therapeutic effects of lithium,85 one likely possibility is via its function as a central regulator of the circadian clock.60 Numerous lines of evidence support this idea; both lithium and GSK3 knockdown produce a lengthening of mPer2 period in mouse fibroblasts,86 and GSK3 phosphorylates PER2 and REV-ERBα and regulates their localization and stability, respectively.18'84 Even more interesting are findings that inhibition of GSK3 may be common to other mood-stabilizing agents such as valproate, and may even be a target of antidepressant therapies, including drugs which target the serotonergic and dopaminergic systems as well as electroconvusive therapy.60 There is also evidence for effects of allelic frequency of the GSK3/β-50 T/C SNP. Bipolar patients with the T/T allele of GSK3β show an earlier age on onset of bipolar disorder and enjoy less improvement from lithium therapy than patients with the T/C or C/C alleles.87,88 Together these results are persuasive, making GSK3 a promising target for the future development of pharmotherapeutlc agents.
Seasonal affective disorder
Seasonal affective disorder (SAD) patients frequently have sleep complaints, particularly hypersomnia, with longer polysomnographlcally-recorded non-REM (NREM) sleep and greater slow-wave activity per minute of NREM sleep.89 A chronoblological hypothesis of SAD has been suggested for a while.90,91 The phase shift hypothesis postulates that SAD patients become depressed in winter because there is a season-specific shift in their endogenous circadian system with respect to their sleep-wake cycle.90 Bright light exposure and/or exogenous melatonin have been used successfully to correct this phase shift.92
Recent studies suggest that polymorphisms of PERIOD2, NPAS2, and BMAL1 (ARNTL) could be associated with an increased risk for SAD. These three clock genes were analyzed for SNPs in a sample of 189 SAD patients and an equal number of matched controls. Specifically, polymorphisms of BMALl, PER2, and NPAS2 are associated with SAD, but together, certain allelic combinations of SNPs of these three genes have an additive effect, increasing the risk of developing SAD by 4.43 over other genotypes, and 10.67 over the most protective genotype.93 An association of the same leucine allele for NPAS2 471 had been reported previously.94 In addition, this study supported a relationship between the PER3 647 Val/Gly genotype and mornlngness /eveningness, particularly in the SAD group.94 This reinforces the suggestion of an association between certain clock gene polymorphisms and chronotype. Also, it suggests that certain abnormalities in the circadian molecular clock increase the susceptibility to SAD.
Schizophrenia
Sleep abnormalities have been consistently found in schizophrenia, although the results have not been consistent across studies.95 These include insomnia, reduced total sleep time (TST), increased sleep latency, poor sleep consolidation and sleep efficiency, and low levels of SWS, with insomnia frequently signaling relapse.96 Actigraphlc recordings of schizophrenic patients have revealed disturbed rest-activity cycles, showing either phase delays, longer periods of activity, or clrcabidlan rest-activity patterns.97-99 The study of schizophrenic patients by a forced desynchrony experiment revealed an abnormal circadian propensity for sleep suggesting a disturbed circadian regulation of sleep.100 Another study reported desynchronizatlon of CBT, pulse and blood pressure rhythms.101 The analysis of melatonin secretion demonstrated blunted circadian variation.102-104 Others have reported phase advances of prolactin, melatonin and tryptophan.105
Evidence linking circadian clock gene polymorphisms or deregulation with schizophrenia is limited. In one study, SNP analysis of the CLOCK gene demonstrated that the T3111C polymorphism showed a transmission bias in a sample of 145 Japanese schizophrenic subjects relative to healthy controls.106 The authors suggested that this polymorphism, associated with aberrant dopaminergic transmission to the SCN may underlie the pathophysiology of schizophrenia. Since dopaminergic signaling through D2 receptors appears to be associated with increased CLOCK:BMALl activity,107 this provides an interesting link between the dopaminergic hypothesis of schizophrenia and circadian abnormalities in these patients. Post-mortem studies have shown decreased expression of the PERI mRNA in the temporal lobe of schizophrenic subjects compared with age-matched normal controls.108 Associations of PER3 and TIMELESS have also been found with schizophrenla/schizoaffectlve disorder, as well as with bipolar disorder.77 The association with PER3 is interesting, given the evidence of a relationship between PER3 with DSPD and evening chronotype. However, the function of TIMELESS in mammals is not yet clear,109 making it difficult to interpret this finding. Finally, the CRY1 gene was hypothesized to be a candidate gene for schizophrenia based on its location near a linkage hotspot for schizophrenia on chromosome 12q24.110 The fact that CRY1 is expressed in dopaminergic cells in the retina and that its expression influences the effects of psychoactive drugs lends further supports to this hypothesis.
Dementia
Dementia associated with Alzheimer's disease (AD) has frequently been associated with psychological disturbances that tend to worsen with the progression of the disease.111-113 Disturbances of sleep and the rest-activity cycle are common, including “sundownlng,” consisting of increased wandering, aggression, vocalization, and agitation during the evening, as well as polysomnographic sleep measures including increased wake after sleep onset, reduced nocturnal TST, sleep efficiency, and REM sleep, and increased RL, and electroencephalogram (EEG) slowing. In addition, these changes in sleep variables may have diagnostic value as there is some evidence suggesting that sleep disturbances in AD patients correlate with lower cognitive scores.114-116 In addition, changes in circadian rhythms of a number of physiological variables have been noted in AD patients including reduced amplitude and increased fragmentation of the circadian rhythm of activity, reduced amplitude and phase delay of the CBT rythm, and reduced amplitude of the rhythms of melatonin and its metabolite 6-sulfatoxymelatonin.117-120 Although AD patients were not significantly different from healthy age-matched controls on all variables, the delay of CBT phase is of particular note because of a tendency toward phase advance of CBT in normal aging.121 Anatomical studies suggest that the changes in the circadian organization of the hormonal and sleep-activity cycles observed in AD sufferers are due to fundamental changes in the master clock itself.122
Molecular changes in clock gene expression have been identified in the pineal gland, the brain region that produces melatonin in response to timing information from the SCN master clock. Post-mortem pineal tissue from non-demented subjects shows rhythmic circadian fluctuations of BMAL1, CRY1, PER1, melatonin, melatonin synthesis, and β1-adrenerglc receptor mRNA, the receptor responsible for the circadian control of melatonin levels in the pineal. In contrast, AD patients did not show any evidence of day-night differences in clock gene expression, pineal melatonin, melatonin synthesis activity, or β1-adrenergic receptor mRNA levels, suggesting malfunction in the circadian signal from the SCN.123,124 Based on this evidence, it is possible that a weakening of the signal from the SCN may also be responsible for changes observed in CBT and the sleep-wake cycle of AD patients.
Conclusion
Evidence is mounting for a relationship between BPD and clock genes, particularly with a polymorphism of the gene CLOCK. Also of considerable interest is the relationship between mood-stabilizing and antidepressant treatments and GSK3. Although research linking clock genes and other mental disorders is still in the early stages, the findings to date suggest that this approach may be fruitful, especially in SAD and schizophrenia. Certainly the potential utility of a genetic screening tool for the differential diagnosis of mental disorders can not be underestimated. Clock genes provide a good target for this type of approach. In addition, clock genes could open up a new frontier for genetic therapies, as well as guide the development of new pharmaceuticals. Well-controlled studies in psychiatric populations must be pursued in order to increase our knowledge of sleep and circadian rhythm disturbances in mental disorders and on the genetic basis of these disturbances.
Selected abbreviations and acronyms
- AD
Alzheimer's disease
- ASPD
advanced sleep phase disorder
- BPD
bipolar disorder
- CBT
core body temperature
- DSPD
delayed sleep phase disorder
- FASPD
familial advanced sleep phase disorder
- GSK
glycogen synthase kinase
- MDD
Major Depressive Disorder
- mRNA
messenger ribonucleic acid
- REM
rapid eye movement
- SAD
seasonal affective disorder
- SCN
suprachiasmatic nucleus
- SNP
single nucleotide polymorphism
Supported by the National Alliance for Research on Schizophrenia and Depression, the Canadian Psychiatric Research Foundation, the Levinschi Foundation, the Canadian Institutes of Health Research, the “Institut de recherche Robert-Sauvé en santé et en sécurité du travail,” and the “Fonds de la Recherche en Santé du Québec.” Special thanks to Dr Valérie Mongraïn, Arï Shechter, and Dr Marïje aan het Rot for their contributions to this manuscript.
Contributor Information
Elaine Waddington Lamont, Centre for Study and Treatment of Circadian Rhythms, Douglas Mental Health University Institute, Montreal, QC,; Laboratory of Molecular Chronobiology, Douglas Mental Health University Institute, Montreal, QC, Canada; Department of Psychiatry, McGill University, Montreal, QC, Canada..
Daniel Legault-Coutu, Lady Davis Institute for Medical Research, McGill University, Montreal, QC, Canada.
Nicolas Cermakian, Laboratory of Molecular Chronobiology, Douglas Mental Health University Institute, Montreal, QC, Canada; Department of Psychiatry, McGill University, Montreal, QC, Canada.
Diane B. Boivin, Centre for Study and Treatment of Circadian Rhythms, Douglas Mental Health University Institute, Montreal, QC, Canada; Department of Psychiatry, McGill University, Montreal, QC, Canada.
References
- 1.Dunlap JC., Loros JJ., DeCoursey PJ. Chronobiology: Biological Timekeeping. Sunderland, Mass: Sinauer Associates; 2004;xix:406. [Google Scholar]
- 2.Hogenesch JB., Chan WK., Jackiw VH., Brown RC., et al. Characterization of a subset of the basic-helix-loop-helix-PAS superfamily that interacts with components of the dioxin signaling pathway. J Biol Chem. 1997;272:8581–8593. doi: 10.1074/jbc.272.13.8581. [DOI] [PubMed] [Google Scholar]
- 3.Hogenesch JB., Gu YZ., Jain S., Bradfield CA. The basic-helix-Ioop-helixPAS orphan MOP3 forms transcriptionally active complexes with circadian and hypoxia factors. Proc Natl Acad Sci U S A. 1998;95:5474–5479. doi: 10.1073/pnas.95.10.5474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.King DP., Zhao Y., Sangoram AMl., et al Positional cloning of the mouse circadian clock gene. Cell. 1997;89:641–653. doi: 10.1016/s0092-8674(00)80245-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Albrecht U., Sun ZS., Eichele G., Lee CC. A differential response of two putative mammalian circadian regulators, mperl and mper2, to light. Cell. 1997;91:1055–1064. doi: 10.1016/s0092-8674(00)80495-x. [DOI] [PubMed] [Google Scholar]
- 6.Albrecht U., Zheng B., Larkin D., Sun ZS., Lee CC. MPerl and mper2 are essential for normal resetting of the circadian clock. J Biol Rhythms. 2001;16:100–104. doi: 10.1177/074873001129001791. [DOI] [PubMed] [Google Scholar]
- 7.Kume K., Zylka MJ., Sriram S., et al. mCRY1 and mCRY2 are essential components of the negative limb of the circadian clock feedback loop. Cell. 1999;98:193–205. doi: 10.1016/s0092-8674(00)81014-4. [DOI] [PubMed] [Google Scholar]
- 8.Preitner N., Damiola F., Lopez-Molina L., et al. The orphan nuclear receptor REV-ERBalpha controls circadian transcription within the positive limb of the mammalian circadian oscillator. Cell. 2002;110:251–260. doi: 10.1016/s0092-8674(02)00825-5. [DOI] [PubMed] [Google Scholar]
- 9.Sato TK., Panda S., Miraglia LJ., Reyes TM., et al. A functional genomics strategy reveals Rora as a component of the mammalian circadian clock. Neuron. 2004;43:527–537. doi: 10.1016/j.neuron.2004.07.018. [DOI] [PubMed] [Google Scholar]
- 10.Ueda HR., Chen W., Adachî A., et al. A transcription factor response element for gene expression during circadian night. Nature. 2002;418:534–539. doi: 10.1038/nature00906. [DOI] [PubMed] [Google Scholar]
- 11.Eïde EJ., Vielhaber EL., Hinz WA., Virshup DM. The circadian regulatory proteins BMAL1 and cryptochromes are substrates of casein kinase lepsilon. J Biol Chem. 2002;277:17248–17254. doi: 10.1074/jbc.M111466200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Akashi M., Takumi T. The orphan nuclear receptor RORalpha regulates circadian transcription of the mammalian core-clock Bmall. Nat Struct Mol Biol. 2005;12:441–448. doi: 10.1038/nsmb925. [DOI] [PubMed] [Google Scholar]
- 13.Brown SA., Ripperger J., Kadener S., et al. PER10D1 -associated proteins modulate the negative limb of the mammalian circadian oscillator. Science. 2005;308:693–696. doi: 10.1126/science.1107373. [DOI] [PubMed] [Google Scholar]
- 14.Guillaumond F., Dardente H., Gïguère V., Cermakian N. Differential control of Bmall circadian transcription by REV-ERB and ROR nuclear receptors. J Biol Rhythms. 2005;20:391–403. doi: 10.1177/0748730405277232. [DOI] [PubMed] [Google Scholar]
- 15.Shearman LP., Sriram S., Weaver DR., et al. Interacting molecular loops in the mammalian circadian clock. Science. 2000;288:1013. doi: 10.1126/science.288.5468.1013. [DOI] [PubMed] [Google Scholar]
- 16.DeBruyne JP., Weaver DR., Reppert SM. CLOCK and NPAS2 have overlapping roles in the suprachiasmatic circadian clock. NatNeurosci. 2007;10:543–545. doi: 10.1038/nn1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reick M., Garcia JA., Dudley C., McKnïght SL. NPAS2: an analog of clock operative in the mammalian forebrain. Science. 2001;293:506–509. doi: 10.1126/science.1060699. [DOI] [PubMed] [Google Scholar]
- 18.litaka C., Miyazaki K., Akaike T., Ishida N. A role for glycogen synthase kinase3beta in the mammalian circadian clock. J Biol Chem. 2005;280:29397–29402. doi: 10.1074/jbc.M503526200. [DOI] [PubMed] [Google Scholar]
- 19.Godïnho SI., Maywood ES., Shaw L., et al. The after-hours mutant reveals a role for Fbxl3 in determining mammalian circadian period. Science. 2007;316:897–900. doi: 10.1126/science.1141138. [DOI] [PubMed] [Google Scholar]
- 20.Siepka SM., Yoo SH., Park J., et al. Circadian mutant Overtime reveals Fbox protein FBXL3 regulation of cryptochrome and period gene expression. Cell. 2007;129:1011–1023. doi: 10.1016/j.cell.2007.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eide EJ., Woolf MF., Kang H., et al. Control of mammalian circadian rhythm by CKlepsilon-regulated proteasome-mediated PER2 degradation. Mol Cell Biol. 2005;25:2795–2807. doi: 10.1128/MCB.25.7.2795-2807.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee C., Weaver DR., Reppert SM. Direct association between mouse PERIOD and CKIepsilon is critical for a functioning circadian clock. Mol Cell Biol. 2004;24:584–594. doi: 10.1128/MCB.24.2.584-594.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lowrey PL., Shimomura K., Antoch MP., et al. Positional syntenic cloning and functional characterization of the mammalian circadian mutation tau. Science. 2000;288:483–492. doi: 10.1126/science.288.5465.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Toh KL., Jones CR., He X., et al. An hPer2 phosphorylation site mutation in familial advanced sleep phase syndrome. Science. 2001;291:1040–1043. doi: 10.1126/science.1057499. [DOI] [PubMed] [Google Scholar]
- 25.Jones CR., Campbell SS., Zone SE., et al. Familial advanced sleep-phase syndrome: A short-period circadian rhythm variant in humans. Nat Med. 1999;5:1062–1065. doi: 10.1038/12502. [DOI] [PubMed] [Google Scholar]
- 26.Vanselow K., Vanselow JT., Westermark PO., et al. Differential effects of PER2 phosphorylation: molecular basis for the human familial advanced sleep phase syndrome (FASPS). Genes Dev. 2006;20:2660–2672. doi: 10.1101/gad.397006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xu Y., Toh KL., Jones CR., Shin JY., Fu YH., Ptacek LJ. Modeling of a human circadian mutation yields insights into clock regulation by PER2. Cell. 2007;128:59–70. doi: 10.1016/j.cell.2006.11.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xu Y., Padiath QS., Shapiro RE., et al. Functional consequences of a CKIdelta mutation causing familial advanced sleep phase syndrome. Nature. 2005;434:640–644. doi: 10.1038/nature03453. [DOI] [PubMed] [Google Scholar]
- 29.Archer SN., Robilliard DL., Skene DJ., et al. A length polymorphism in the circadian clock gene Per3 is linked to delayed sleep phase syndrome and extreme diurnal preference. Sleep. 2003;26:413–415. doi: 10.1093/sleep/26.4.413. [DOI] [PubMed] [Google Scholar]
- 30.Ebisawa T., Uchiyama M., Kajïmura N., et al. Association of structural polymorphisms in the human period3 gene with delayed sleep phase syndrome. EMBO Rep. 2001;2:342–346. doi: 10.1093/embo-reports/kve070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Viola AU., Archer SN., James LM., et al. PER3 polymorphism predicts sleep structure and waking performance. Curr Biol. 2007;17:613–618. doi: 10.1016/j.cub.2007.01.073. [DOI] [PubMed] [Google Scholar]
- 32.Katzenberg D., Young T., Finn L., et al. A CLOCK polymorphism associated with human diurnal preference. Sleep. 1998;21:569–576. doi: 10.1093/sleep/21.6.569. [DOI] [PubMed] [Google Scholar]
- 33.Mishima K., Tozawa T., Satoh K., Saitoh H., Mïshïma Y. The 3111T/C polymorphism of hClock is associated with evening preference and delayed sleep timing in a Japanese population sample. Am J Med Genet B Neuropsychiatr Genet. 2005;133:101–104. doi: 10.1002/ajmg.b.30110. [DOI] [PubMed] [Google Scholar]
- 34.Robilliard DL., Archer SN., Arendt J., et al. The 3111 Clock gene polymorphism is not associated with sleep and circadian rhythmicity in phenotypically characterized human subjects. J Sleep Res. 2002;11:305–312. doi: 10.1046/j.1365-2869.2002.00320.x. [DOI] [PubMed] [Google Scholar]
- 35.Iwase T., Kajïmura N., Uchiyama M., et al. Mutation screening of the human Clock gene in circadian rhythm sleep disorders. Psychiatry Res. 2002;109:121–128. doi: 10.1016/s0165-1781(02)00006-9. [DOI] [PubMed] [Google Scholar]
- 36.Mansour HA., Wood J., Chowdari KV., et al. Circadian phase variation in bipolar I disorder. Chronobiol Int. 2005;22:571–584. doi: 10.1081/CBI-200062413. [DOI] [PubMed] [Google Scholar]
- 37.Dagan Y., Borodkin K. Behavioral and psychiatric consequences of sleepwake schedule disorders. Dialogues Clin Neurosci. 2005;7:357–365. doi: 10.31887/DCNS.2005.7.4/ydagan. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dagan Y., Stein D., Steinbock M., Yovel I., Hallis D. Frequency of delayed sleep phase syndrome among hospitalized adolescent psychiatric patients. J Psychosom Res. 1998;45:15–20. doi: 10.1016/s0022-3999(97)00299-7. [DOI] [PubMed] [Google Scholar]
- 39.Armitage R. Sleep and circadian rhythms in mood disorders. Acta Psychiatr Scand Suppi. 2007:104–115. doi: 10.1111/j.1600-0447.2007.00968.x. [DOI] [PubMed] [Google Scholar]
- 40.Armitage R., Husain M., Hoffmann R., Rush AJ. The effects of vagus nerve stimulation on sleep EEG in depression: a preliminary report. J Psychosom Res. 2003;54:475–482. doi: 10.1016/s0022-3999(02)00476-2. [DOI] [PubMed] [Google Scholar]
- 41.Ford DE., Cooper-Patrick L. Sleep disturbances and mood disorders: an epidemiologic perspective. Depress Anxiety. 2001;14:3–6. doi: 10.1002/da.1041. [DOI] [PubMed] [Google Scholar]
- 42.Thase ME. Depression and sleep: pathophysiology and treatment. Dialogues Clin Neurosci. 2006;8:217–226. doi: 10.31887/DCNS.2006.8.2/mthase. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Motivala SJ., Levin MJ., Oxman MN., Irwin MR. Impairments in health functioning and sleep quality in older adults with a history of depression. J Am GeriatrSoc. 2006;54:1184–1191. doi: 10.1111/j.1532-5415.2006.00819.x. [DOI] [PubMed] [Google Scholar]
- 44.Kupfer DJ. Depression and associated sleep disturbances: patient benefits with agomelatine. Eur Neuropsychopharmacol. 2006;16 (suppl 5):S639–S643. [Google Scholar]
- 45.Buysse DJ., Frank E., Lowe KK., Cherry CR., Kupfer DJ. Electroencephalographs sleep correlates of episode and vulnerability to recurrence in depression. Biol Psychiatry. 1997;41:406–418. doi: 10.1016/S0006-3223(96)00041-8. [DOI] [PubMed] [Google Scholar]
- 46.Boivin DB. Influence of sleep-wake and circadian rhythm disturbances in psychiatric disorders. J Psychiatry Neurosci. 2000;25:446–458. [PMC free article] [PubMed] [Google Scholar]
- 47.Wirz-Justice A. Biological rhythm disturbances in mood disorders. Int Clin Psychopharmacol. 2006;21 Suppl 1:S11–S115. doi: 10.1097/01.yic.0000195660.37267.cf. [DOI] [PubMed] [Google Scholar]
- 48.Berger M., van Calker D., Riemann D. Sleep and manipulations of the sleep-wake rhythm in depression. Acta Psychiatr Scand Suppl. 2003:83–91. doi: 10.1034/j.1600-0447.108.s418.17.x. [DOI] [PubMed] [Google Scholar]
- 49.Persaud R. Nocturnal sweating and temperature in depression. Acta Psychiatr Scand. 2000;101:251. [PubMed] [Google Scholar]
- 50.Antonïjevïc IA., Murck H., Frïeboes RM., Uhr M., Steiger A. On the role of menopause for sleep-endocrine alterations associated with major depression. Psychoneuroendocrinology. 2003;28:401–418. doi: 10.1016/s0306-4530(02)00031-8. [DOI] [PubMed] [Google Scholar]
- 51.Gold PW., Drevets WC., Charney DS. New insights into the role of Cortisol and the glucocorticoid receptor in severe depression. Biol Psychiatry. 2002;52:381–385. doi: 10.1016/s0006-3223(02)01480-4. [DOI] [PubMed] [Google Scholar]
- 52.Keller J., Flores B., Gomez RG., Solvason HB., Kenna H., Williams GH., Schatzberg AF. Cortisol circadian rhythm alterations in psychotic major depression. Biol Psychiatry. 2006;60:275–281. doi: 10.1016/j.biopsych.2005.10.014. [DOI] [PubMed] [Google Scholar]
- 53.Swaab DF., Bao AM., Lucassen PJ. The stress system in the human brain in depression and neurodegeneration. Ageing Res Rev. 2005;4:141–194. doi: 10.1016/j.arr.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 54.Pacchierottï C., lapichino S., Bossini L., Pieraccini F., Castrogiovanni P. Melatonin in psychiatric disorders: a review on the melatonin involvement in psychiatry. Front Neuroendocrinol. 2001;22:18–32. doi: 10.1006/frne.2000.0202. [DOI] [PubMed] [Google Scholar]
- 55.Paparrigopoulos T. Melatonin response to atenolol administration in depression: indication of beta-adrenoceptor dysfunction in a subtype of depression. Acta Psychiatr Scand. 2002;106:440–445. doi: 10.1034/j.1600-0447.2002.02342.x. [DOI] [PubMed] [Google Scholar]
- 56.Carvalho LA., Gorenstein C., Moreno RA., Markus RP. Melatonin levels in drug-free patients with major depression from the southern hemisphere. Psychoneuroendocrinology. 2006;31:761–768. doi: 10.1016/j.psyneuen.2006.02.010. [DOI] [PubMed] [Google Scholar]
- 57.Rabe-Jablonska J., Szymanska A. Diurnal profile of melatonin secretion in the acute phase of major depression and in remission. Med Sci Monit. 2001;7:946–952. [PubMed] [Google Scholar]
- 58.Voderholzer U., Laakmann G., Becker U., et al. Circadian profiles of melatonin in melancholic depressed patients and healthy subjects in relation to Cortisol secretion and sleep. Psychiatry Res. 1997;71:151–161. doi: 10.1016/s0165-1781(97)00048-6. [DOI] [PubMed] [Google Scholar]
- 59.Desan PH., Oren DA., Malison R., et al. Genetic polymorphism at the CLOCK gene locus and major depression. Am J Med Genet. 2000;96:418–421. doi: 10.1002/1096-8628(20000612)96:3<418::aid-ajmg34>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 60.Gould TD., Manji HK. Glycogen synthase kïnase-3: a putative molecular target for lithium mimetic drugs. Neuropsychopharmacology. 2005;30:1223–1237. doi: 10.1038/sj.npp.1300731. [DOI] [PubMed] [Google Scholar]
- 61.Mellman TA. Sleep and anxiety disorders Abstract. Psychiatr Clin North Am. 2006;29:1047–1058. doi: 10.1016/j.psc.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 62.Akiyama M., Kïrihara T., Takahashi S., et al. Modulation of mPer1 gene expression by anxiolytic drugs in mouse cerebellum. Br J Pharmacol. 1999;128:1616–1622. doi: 10.1038/sj.bjp.0702957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Roybal K., Theobold D., Graham A., et al. Mania-like behavior induced by disruption of CLOCK. Proc Natl Acad Sci U S A. 2007;104:6406–6411. doi: 10.1073/pnas.0609625104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Duncan WC., Jr, Pettigrew KD., Gillin JC. REM architecture changes in bipolar and unipolar depression. Am J Psychiatry. 1979;136:1424–1427. doi: 10.1176/ajp.136.11.1424. [DOI] [PubMed] [Google Scholar]
- 65.Hudson JI., Lipinski JF., Keck PE., et al. Polysomnographic characteristics of young manic patients. Comparison with unipolar depressed patients and normal control subjects. Arch Gen Psychiatry. 1992;49:378–383. doi: 10.1001/archpsyc.1992.01820050042006. [DOI] [PubMed] [Google Scholar]
- 66.Reynolds CF., 3rd, Kupfer DJ. Sleep research in affective illness: state of the art circa 1987. Sleep. 1987;10:199–215. doi: 10.1093/sleep/10.3.199. [DOI] [PubMed] [Google Scholar]
- 67.Sierra P., Lïvïanos L., Arques S., Castello J., Rojo L. Prodromal symptoms to relapse in bipolar disorder. Aust N Z J Psychiatry. 2007;41:385–391. doi: 10.1080/00048670701266854. [DOI] [PubMed] [Google Scholar]
- 68.Grandin LD., Alloy LB., Abramson LY. The social zeitgeber theory, circadian rhythms, and mood disorders: review and evaluation. Clin Psychol Rev. 2006;26:679–694. doi: 10.1016/j.cpr.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 69.Feldman-Naim S., Turner EH., Leibenluft E. Diurnal variation in the direction of mood switches in patients with rapid-cycling bipolar disorder. J Clin Psychiatry. 1997;58:79–84. doi: 10.4088/jcp.v58n0205. [DOI] [PubMed] [Google Scholar]
- 70.Voderholzer U., Weske G., Ecker S., Rïemann D., Gann H., Berger M. Neurobiological findings before and during successful lithium therapy of a patient with 48-hour rapid-cycling bipolar disorder. Neuropsychobiology. 2002;45(suppl 1):13–19. doi: 10.1159/000049256. [DOI] [PubMed] [Google Scholar]
- 71.Linkowski P., Kerkhofs M., Van Onderbergen A., et al. The 24-hour profiles of Cortisol, prolactin, and growth hormone secretion in mania. Arch Gen Psychiatry. 1994;51:616–624. doi: 10.1001/archpsyc.1994.03950080028004. [DOI] [PubMed] [Google Scholar]
- 72.Cervantes P., Gelber S., Kin FN., Nair VN., Schwartz G. Circadian secretion of Cortisol in bipolar disorder. J Psychiatry Neurosci. 2001;26:411–416. [PMC free article] [PubMed] [Google Scholar]
- 73.Benedetti F., Dallaspezïa S., Fulgosi MC. Actimetric evidence that CLOCK 31 1 1 T/C SNP influences sleep and activity patterns in patients affected by bipolar depression. Am J Med Genet B Neuropsychiatr Genet. 2007;1446:631–635. doi: 10.1002/ajmg.b.30475. [DOI] [PubMed] [Google Scholar]
- 74.Benedetti F., Serretti A., Colombo C., et al. Influence of CLOCK gene polymorphism on circadian mood fluctuation and illness recurrence in bipolar depression. Am J Med Genet B Neuropsychiatr Genet. 2003;123:23–26. doi: 10.1002/ajmg.b.20038. [DOI] [PubMed] [Google Scholar]
- 75.Serretti A., Benedetti F., Mandellï L., et al. Genetic dissection of psychopathological symptoms: insomnia in mood disorders and CLOCK gene polymorphism. Am J Med Genet B Neuropsychiatr Genet. 2003;121:35–38. doi: 10.1002/ajmg.b.20053. [DOI] [PubMed] [Google Scholar]
- 76.Serretti A., Cusin C., Benedetti F., et al. Insomnia improvement during antidepressant treatment and CLOCK gene polymorphism. Am J Med Genet B Neuropsychiatr Genet. 2005;137:36–39. doi: 10.1002/ajmg.b.30130. [DOI] [PubMed] [Google Scholar]
- 77.Mansour HA., Wood J., Logue T., et al. Association study of eight circadian genes with bipolar I disorder, schizoaffective disorder and schizophrenia. Genes Brain Behav. 2006;5:150–157. doi: 10.1111/j.1601-183X.2005.00147.x. [DOI] [PubMed] [Google Scholar]
- 78.Nievergelt CM., Kripke DF., Barrett TB., et al. Suggestive evidence for association of the circadian genes PERIODS and ARNTL with bipolar disorder. Am J Med Genet B Neuropsychiatr Genet. 2006;141:234–241. doi: 10.1002/ajmg.b.30252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Shiino Y., Nakajima S., Ozeki Y., Isono T., Yamada N. Mutation screening of the human period 2 gene in bipolar disorder. Neurosci Lett. 2003;338:82–84. doi: 10.1016/s0304-3940(02)01290-9. [DOI] [PubMed] [Google Scholar]
- 80.Nievergelt CM., Kripke DF., Remïck RA., et al. Examination of the clock gene Cryptochrome 1 in bipolar disorder: mutational analysis and absence of evidence for linkage or association. Psychiatr Genet. 2005;15:45–52. doi: 10.1097/00041444-200503000-00008. [DOI] [PubMed] [Google Scholar]
- 81.LeSauter J., Silver R. Lithium lengthens the period of circadian rhythms in lesioned hamsters bearing SCN grafts. Biol Psychiatry. 1993;34:75–83. doi: 10.1016/0006-3223(93)90259-g. [DOI] [PubMed] [Google Scholar]
- 82.Abe M., Herzog ED., Block GD. Lithium lengthens the circadian period of individual suprachiasmatic nucleus neurons. Neuroreport. 2000;11:3261–3264. doi: 10.1097/00001756-200009280-00042. [DOI] [PubMed] [Google Scholar]
- 83.Campbell SS., Gillin JC., Kripke DF., Janowsky DS., Risch SC. Lithium delays circadian phase of temperature and REM sleep in a bipolar depressive: a case report. Psychiatry Res. 1989;27:23–29. doi: 10.1016/0165-1781(89)90005-x. [DOI] [PubMed] [Google Scholar]
- 84.Yin L., Wang J., Klein PS., Lazar MA. Nuclear receptor Rev-erbalpha is a critical lithium-sensitive component of the circadian clock. Science. 2006;311:1002–1005. doi: 10.1126/science.1121613. [DOI] [PubMed] [Google Scholar]
- 85.Quiroz JA., Gould TD., Manji HK. Molecular effects of lithium. Mol Interv. 2004;4:259–272. doi: 10.1124/mi.4.5.6. [DOI] [PubMed] [Google Scholar]
- 86.Kaladchïbachï SA., Doble B., Anthopoulos N., Woodgett JR., Manoukian AS. Glycogen synthase kinase 3, circadian rhythms, and bipolar disorder: a molecular link in the therapeutic action of lithium. J Circadian Rhythms. 2007;5:3. doi: 10.1186/1740-3391-5-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Benedetti F., Bernasconi A., Lorenzï C., et al. A single nucleotide polymorphism in glycogen synthase kinase 3-beta promoter gene influences onset of illness in patients affected by bipolar disorder. Neurosci Lett. 2004;355:37–40. doi: 10.1016/j.neulet.2003.10.021. [DOI] [PubMed] [Google Scholar]
- 88.Benedetti F., Serretti A., Pontiggia A., et al. Long-term response to lithium salts in bipolar illness is influenced by the glycogen synthase kinase 3-beta -50 T/C SNP. Neurosci Lett. 2005;376:51–55. doi: 10.1016/j.neulet.2004.11.022. [DOI] [PubMed] [Google Scholar]
- 89.Schwartz PJ., Rosenthal NE., Kajimura N., et al. Ultradian oscillations in cranial thermoregulation and electroencephalographic slow-wave activity during sleep are abnormal in humans with annual winter depression. Brain Res. 2000;866:152–167. doi: 10.1016/s0006-8993(00)02271-x. [DOI] [PubMed] [Google Scholar]
- 90.Lewy AJ., Sack RL., Singer CM., White DM., Hoban TM. Winter depression and the phase-shift hypothesis for bright light's therapeutic effects: history, theory, and experimental evidence. J Biol Rhythms. 1988;3:121–134. doi: 10.1177/074873048800300203. [DOI] [PubMed] [Google Scholar]
- 91.Magnusson A., Boivin D. Seasonal affective disorder: an overview. Chronobiol Int. 2003;20:189–207. doi: 10.1081/cbi-120019310. [DOI] [PubMed] [Google Scholar]
- 92.Lewy AJ., Lefler BJ., Emens JS., Bauer VK. The circadian basis of winter depression. Proc Natl Acad Sci U S A. 2006;103:7414–7419. doi: 10.1073/pnas.0602425103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Partonen T., Treutlein J., Alpman A., et al. Three circadian clock genes Per2, Arntl, and Npas2 contribute to winter depression. Ann Med. 2007;39:229–238. doi: 10.1080/07853890701278795. [DOI] [PubMed] [Google Scholar]
- 94.Johansson C., Willeit M., Smedh C., et al. Circadian clock-related polymorphisms in seasonal affective disorder and their relevance to diurnal preference. Neuropsychopharmacology. 2003;28:734–739. doi: 10.1038/sj.npp.1300121. [DOI] [PubMed] [Google Scholar]
- 95.Chouinard S., Poulin J., Stip E., Godbout R. Sleep in untreated patients with schizophrenia: a meta-analysis. Schizophr Bull. 2004;30:957–967. doi: 10.1093/oxfordjournals.schbul.a007145. [DOI] [PubMed] [Google Scholar]
- 96.Benson KL., Zarcone VP., Jr Schizophrenia. In: Kryger MH, Roth T, Dement WC, eds. Principles and Practice of Sleep Medicine. Philadelphia, Pa: Elsevier/Saunders; 2005 [Google Scholar]
- 97.Wulff K., Joyce E., Middleton B., Dijk DJ., Foster RG. The suitability of actigraphy, diary data, and urinary melatonin profiles for quantitative assessment of sleep disturbances in schizophrenia: a case report. Chronobiol Int. 2006;23:485–495. doi: 10.1080/07420520500545987. [DOI] [PubMed] [Google Scholar]
- 98.Wirz-Justice A., Cajochen C., Nussbaum P. A schizophrenic patient with an arrhythmic circadian rest-activity cycle. Psychiatry Res. 1997;73:83–90. doi: 10.1016/s0165-1781(97)00117-0. [DOI] [PubMed] [Google Scholar]
- 99.Wirz-Justice A., Haug HJ., Cajochen C. Disturbed circadian rest-activity cycles in schizophrenia patients: an effect of drugs?. Schizophr Bull. 2001;27:497–502. doi: 10.1093/oxfordjournals.schbul.a006890. [DOI] [PubMed] [Google Scholar]
- 100.Boivin DB., Morisset NJ., Lai S. Abnormal circadian rhythms of sleep propensity in chronic schizophrenia. Sleep. 2000;23:A363. [Google Scholar]
- 101.Madjirova NP., Petrova NS., Delchev NK. Daily rhythmiclty of temperature, pulse and blood pressure in schizophrenic patients. Schizophr Res. 1995;14:183. doi: 10.1016/0920-9964(95)90708-i. [DOI] [PubMed] [Google Scholar]
- 102.Bersani G., Mameli M., Garavini A., Pancheri P., Nordio M. Reduction of night/day difference in melatonin blood levels as a possible disease-related index in schizophrenia. Neuro Endocrinol Lett. 2003;24:181–184. [PubMed] [Google Scholar]
- 103.Monteleone P., Maj M., Fusco M., Kemali D., Reiter RJ. Depressed nocturnal plasma melatonin levels in drug-free paranoid schizophrenics. Schizophr Res. 1992;7:77–84. doi: 10.1016/0920-9964(92)90077-i. [DOI] [PubMed] [Google Scholar]
- 104.Shamir E., Laudon M., Barak Y., et al. Melatonin improves sleep quality of patients with chronic schizophrenia. J Clin Psychiatry. 2000;61:373–377. doi: 10.4088/jcp.v61n0509. [DOI] [PubMed] [Google Scholar]
- 105.Rao ML., Gross G., Strebel B., et al. Circadian rhythm of tryptophan, serotonin, melatonin, and pituitary hormones in schizophrenia. Biol Psychiatry. 1994;35:151–163. doi: 10.1016/0006-3223(94)91147-9. [DOI] [PubMed] [Google Scholar]
- 106.Takao T., Tachîkawa H., Kawanïshï Y., Mïzukamî K., Asada T. CLOCK gene T3111C polymorphism is associated with Japanese schizophrenics: a preliminary study. Eur Neuropsychopharmacol. 2007;17:273–276. doi: 10.1016/j.euroneuro.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 107.Yujnovsky I., Hirayama J., Doi M., Borrelli E., Sassone-Corsi P. Signaling mediated by the dopamine D2 receptor potentiates circadian regulation by CLOCK: BMALL. Proc Natl Acad Sci U S A. 2006;103:6386–6389. doi: 10.1073/pnas.0510691103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Aston C., Jiang L., Sokolov BP. Microarray analysis of postmortem temporal cortex from patients with schizophrenia. J Neurosci Res. 2004;77:858–866. doi: 10.1002/jnr.20208. [DOI] [PubMed] [Google Scholar]
- 109.Barnes JW., Tischkau SA., Barnes JA., et al. Requirement of mammalian Timeless for circadian rhythmiclty. Science. 2003;302:439–442. doi: 10.1126/science.1086593. [DOI] [PubMed] [Google Scholar]
- 110.Peng ZW., Chen XG., Wei Z. Cryptochromel maybe a candidate gene of schizophrenia. Med Hypotheses. 2007;69:849–851. doi: 10.1016/j.mehy.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 111.Levey A., Lah J., Goldstein F., Steenland K., Blïwïse D. Mild cognitive impairment: an opportunity to identify patients at high risk for progression to Alzheimer's disease. Clin Ther. 2006;28:991–1001. doi: 10.1016/j.clinthera.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 112.Reiman EM. Linking brain imaging and genomics in the study of Alzheimer's disease and aging. Ann N Y Acad Sci. 2007;1097:94–113. doi: 10.1196/annals.1379.011. [DOI] [PubMed] [Google Scholar]
- 113.Small BJ., Gagnon E., Robinson B. Early identification of cognitive deficits: preclinical Alzheimer's disease and mild cognitive impairment. Geriatrics. 2007;62:19–23. [PubMed] [Google Scholar]
- 114.Blïwïse DL. Sleep disorders in Alzheimer's disease and other dementias. Clin Cornerstone. 2004;6 (suppl 1A):S16–S28. doi: 10.1016/s1098-3597(04)90014-2. [DOI] [PubMed] [Google Scholar]
- 115.Bonanni E., Maestri M., Tognoni G., et al. Daytime sleepiness in mild and moderate Alzheimer's disease and its relationship with cognitive impairment. J Sleep Res. 2005;14:311–317. doi: 10.1111/j.1365-2869.2005.00462.x. [DOI] [PubMed] [Google Scholar]
- 116.Montplaisir J., Petit D., Gauthier S., Gaudreau H., Decary A. Sleep disturbances and eeg slowing in alzheimer's disease. Sleep Res Online. 1998;1:147–151. [PubMed] [Google Scholar]
- 117.Harper DG., Volicer L., Stopa EG., McKee AC., Nitta M., Satlin A. Disturbance of endogenous circadian rhythm in aging and Alzheimer disease. Am J Geriatr Psychiatry. 2005;13:359–368. doi: 10.1176/appi.ajgp.13.5.359. [DOI] [PubMed] [Google Scholar]
- 118.Luboshitzky R., Shen-Orr Z., Tzischichinsky O., Maldonado M., Herer P., Lavie P. Actigraphic sleep-wake patterns and urinary 6-sulfatoxymelatonin excretion in patients with Alzheimer's disease. Chronobiol Int. 2001;18:513–524. doi: 10.1081/cbi-100103973. [DOI] [PubMed] [Google Scholar]
- 119.Mïshïma K., Tozawa T., Satoh K., Matsumoto Y., Hishikawa Y., Okawa M. Melatonin secretion rhythm disorders in patients with senile dementia of Alzheimer's type with disturbed sleep-waking. Biol Psychiatry. 1999;45:417–421. doi: 10.1016/s0006-3223(97)00510-6. [DOI] [PubMed] [Google Scholar]
- 120.Satlin A., Volicer L., Stopa EG., Harper D. Circadian locomotor activity and core-body temperature rhythms in Alzheimer's disease. Neurobiol Aging. 1995;16:765–771. doi: 10.1016/0197-4580(95)00059-n. [DOI] [PubMed] [Google Scholar]
- 121.CzeislerCA, Dumont M., Duffy JF., et al. Association of sleep-wake habits in older people with changes in output of circadian pacemaker. Lancet. 1992;340:933–936. doi: 10.1016/0140-6736(92)92817-y. [DOI] [PubMed] [Google Scholar]
- 122.Swaab DF., Fliers E., Partiman TS. The suprachiasmatic nucleus of the human brain in relation to sex, age and senile dementia. Brain Res. 1985;342:37–44. doi: 10.1016/0006-8993(85)91350-2. [DOI] [PubMed] [Google Scholar]
- 123.Wu YH., Feenstra MG., Zhou JN., et al. Molecular changes underlying reduced pineal melatonin levels in Alzheimer disease: alterations in preclinical and clinical stages. J Clin Endocrinol Metab. 2003;88:5898–5906. doi: 10.1210/jc.2003-030833. [DOI] [PubMed] [Google Scholar]
- 124.Wu YH., Fischer DF., Kalsbeek A., et al. Pineal clock gene oscillation is disturbed in Alzheimer's disease, due to functional disconnection from the “master clock.”. Faseb J. 2006;20:1874–1876. doi: 10.1096/fj.05-4446fje. [DOI] [PubMed] [Google Scholar]