Abstract
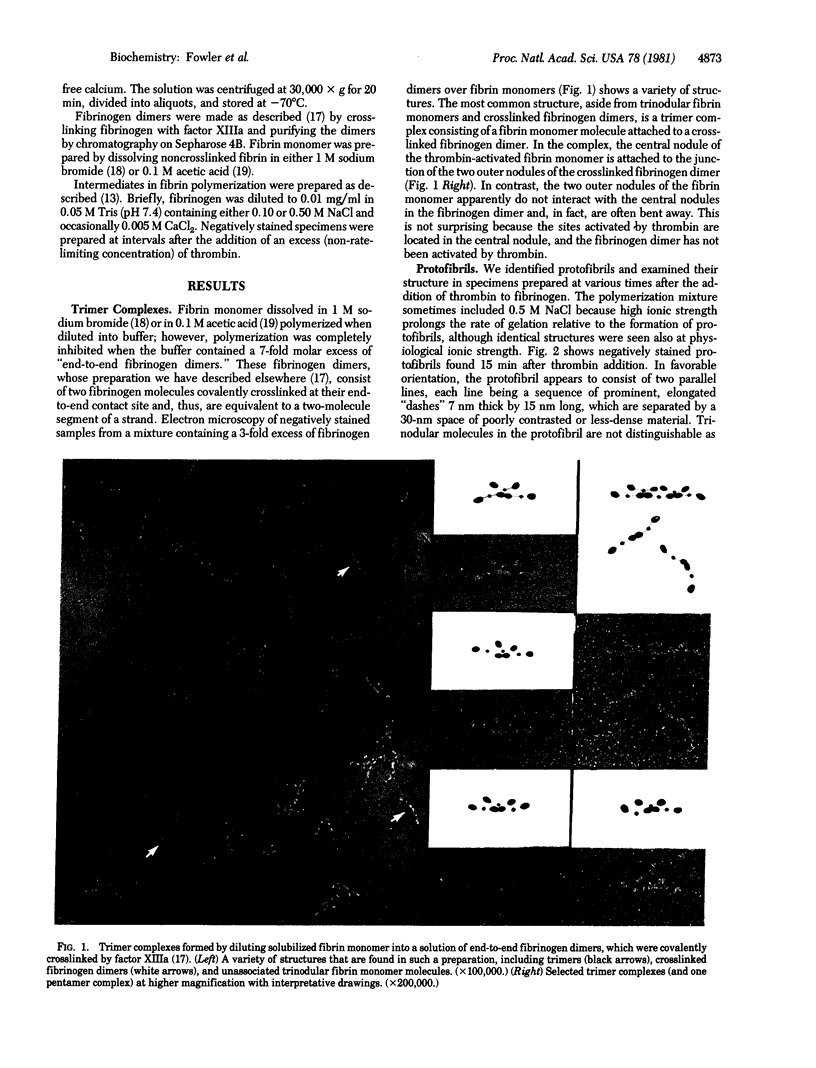
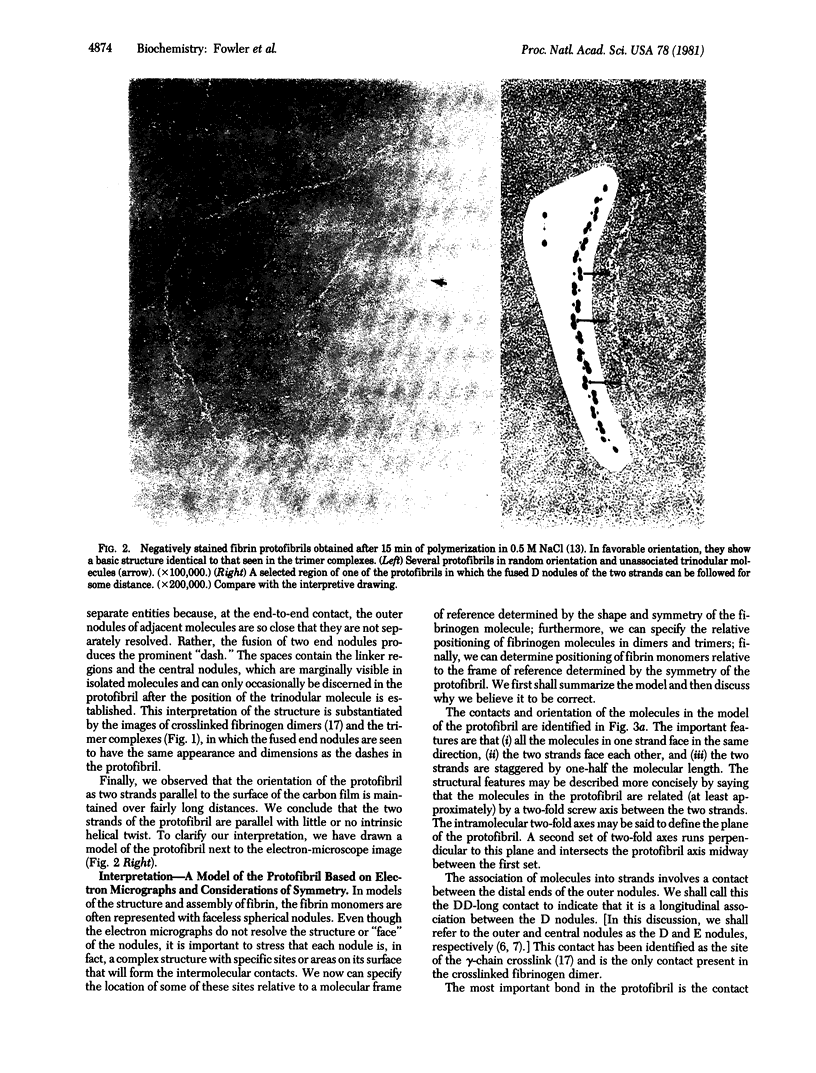
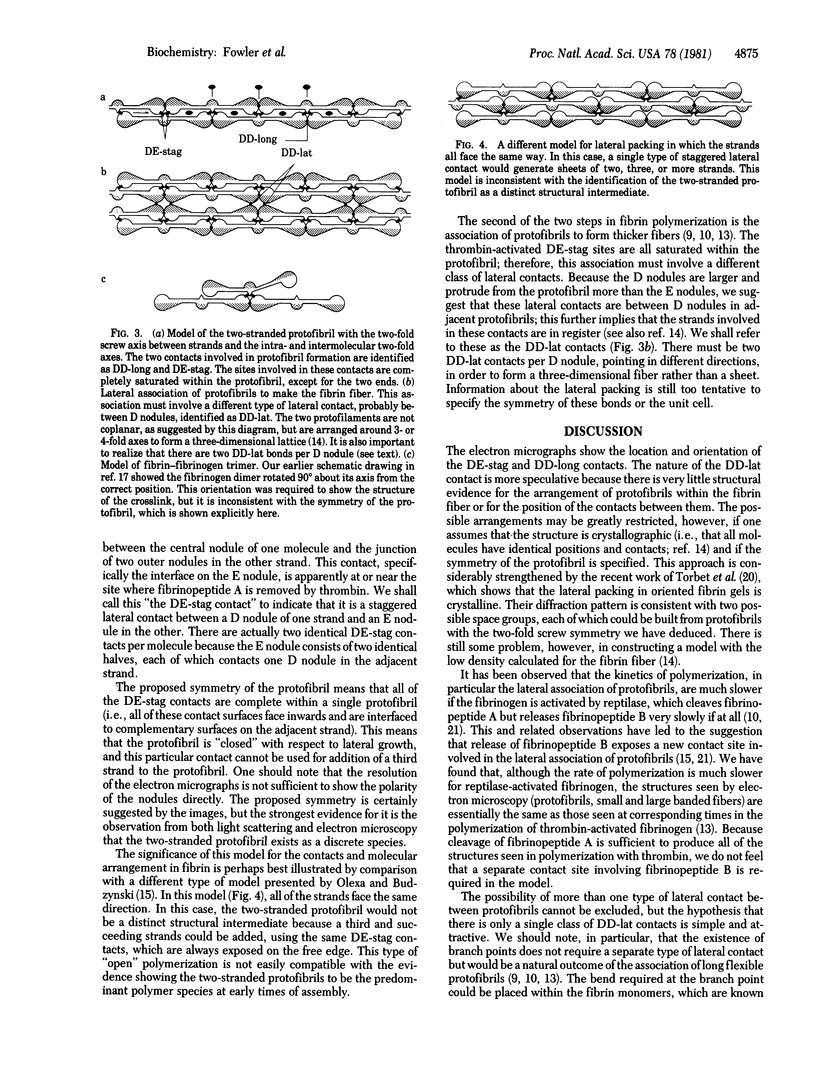
We identified the two-stranded fibrin protofibril and studied its structure in electron micrographs of negatively stained specimens. Based on these images and on considerations of symmetry, we constructed a model of the protofibril in which the two strands of trinodular fibrin molecules are related by a two-fold screw axis between the strands and two-fold axes perpendicular to them. The two strands are held together by staggered lateral contacts between the central nodules of one strand and outer nodules of the other. The molecules within a strand are joined by longitudinal contacts between outer nodules. This interpretation of the structure of protofibrils is supported by images of trimer complexes whose preparation and structure are described here, in which the central nodule of a fibrin monomer is attached to the crosslinked outer nodules of two other molecules. We conclude that the association of protofibrils to form thicker fibers must involve a second type of lateral contact, probably between outer nodules of adjacent, in-register strands. In total, we identify three intermolecular contacts involved in the polymerization of fibrin.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Belitser V. A., Varetskaja T. V., Malneva G. V. Fibrinogen-fibrin interaction. Biochim Biophys Acta. 1968 Feb 19;154(2):367–375. doi: 10.1016/0005-2795(68)90051-2. [DOI] [PubMed] [Google Scholar]
- Blombäck B., Hessel B., Hogg D., Therkildsen L. A two-step fibrinogen--fibrin transition in blood coagulation. Nature. 1978 Oct 12;275(5680):501–505. doi: 10.1038/275501a0. [DOI] [PubMed] [Google Scholar]
- Carr M. E., Jr, Hermans J. Size and density of fibrin fibers from turbidity. Macromolecules. 1978 Jan-Feb;11(1):46–50. doi: 10.1021/ma60061a009. [DOI] [PubMed] [Google Scholar]
- Doolittle R. F. Structure and function of fibrinogen. Horiz Biochem Biophys. 1977;3:164–191. [PubMed] [Google Scholar]
- Erickson H. P., Pantaloni D. The role of subunit entropy in cooperative assembly. Nucleation of microtubules and other two-dimensional polymers. Biophys J. 1981 May;34(2):293–309. doi: 10.1016/S0006-3495(81)84850-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferry J. D. The Mechanism of Polymerization of Fibrinogen. Proc Natl Acad Sci U S A. 1952 Jul;38(7):566–569. doi: 10.1073/pnas.38.7.566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler W. E., Erickson H. P., Hantgan R. R., McDonagh J., Hermans J. Cross-linked fibrinogen dimers demonstrate a feature of the molecular packing in fibrin fibers. Science. 1981 Jan 16;211(4479):287–289. doi: 10.1126/science.6108612. [DOI] [PubMed] [Google Scholar]
- Fowler W. E., Erickson H. P. Trinodular structure of fibrinogen. Confirmation by both shadowing and negative stain electron microscopy. J Mol Biol. 1979 Oct 25;134(2):241–249. doi: 10.1016/0022-2836(79)90034-2. [DOI] [PubMed] [Google Scholar]
- Fowler W. E., Fretto L. J., Erickson H. P., McKee P. A. Electron microsocpy of plasmic fragments of human fibrinogen as related to trinodular structure of the intact molecule. J Clin Invest. 1980 Jul;66(1):50–56. doi: 10.1172/JCI109834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HALL C. E., SLAYTER H. S. The fibrinogen molecule: its size, shape, and mode of polymerization. J Biophys Biochem Cytol. 1959 Jan 25;5(1):11–16. doi: 10.1083/jcb.5.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hantgan R. R., Hermans J. Assembly of fibrin. A light scattering study. J Biol Chem. 1979 Nov 25;254(22):11272–11281. [PubMed] [Google Scholar]
- Hermans J. Models of fibrin. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1189–1193. doi: 10.1073/pnas.76.3.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krakow W., Endres G. F., Siegel B. M., Scheraga H. A. An electron microscopic investigation of the polymerization of bovine fibrin monomer. J Mol Biol. 1972 Oct 28;71(1):95–103. doi: 10.1016/0022-2836(72)90403-2. [DOI] [PubMed] [Google Scholar]
- Olexa S. A., Budzynski A. Z. Evidence for four different polymerization sites involved in human fibrin formation. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1374–1378. doi: 10.1073/pnas.77.3.1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STRYER L., COHEN C., LANGRIDGE R. Axial period of fibrinogen and fibrin. Nature. 1963 Feb 23;197:793–794. doi: 10.1038/197793a0. [DOI] [PubMed] [Google Scholar]
- Slayter H. S. High-resolution metal replication of macromolecules. Ultramicroscopy. 1976 Sep-Oct;1(4):341–357. doi: 10.1016/0304-3991(76)90050-4. [DOI] [PubMed] [Google Scholar]
- Smith G. F. Fibrinogen-fibrin conversion. The mechanism of fibrin-polymer formation in solution. Biochem J. 1980 Jan 1;185(1):1–11. doi: 10.1042/bj1850001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telford J. N., Nagy J. A., Hatcher P. A., Scheraga H. A. Location of peptide fragments in the fibrinogen molecule by immunoelectron microscopy. Proc Natl Acad Sci U S A. 1980 May;77(5):2372–2376. doi: 10.1073/pnas.77.5.2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tooney N. M., Cohen C. Crystalline states of a modified fibrinogen. J Mol Biol. 1977 Feb 25;110(2):363–385. doi: 10.1016/s0022-2836(77)80077-6. [DOI] [PubMed] [Google Scholar]
- Torbet J., Freyssinet J. M., Hudry-Clergeon G. Oriented fibrin gels formed by polymerization in strong magnetic fields. Nature. 1981 Jan 1;289(5793):91–93. doi: 10.1038/289091a0. [DOI] [PubMed] [Google Scholar]
- Weisel J. W., Phillips G. N., Jr, Cohen C. A model from electron microscopy for the molecular structure of fibrinogen and fibrin. Nature. 1981 Jan 22;289(5795):263–267. doi: 10.1038/289263a0. [DOI] [PubMed] [Google Scholar]




