Abstract
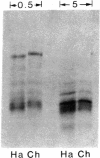
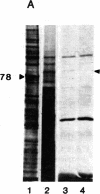
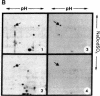
A temperature-sensitive mutant K12 derived from a Chinese hamster fibroblast has been shown to overproduce three specific proteins of Mr 94,000, 78,000, and 58,000 when incubated at the nonpermissive temperature (40.5 degrees C). We previously identified these proteins as glucose-regulated proteins similar to those observed in chicken embryo fibroblasts when the cells are starved of glucose. In this report, we show that the Mr 78,000 proteins isolated from the hamster K12 cell line and from chicken embryo fibroblasts have identical electrophoretic mobilities in two-dimensional isoelectric focusing gels and nearly identical peptide maps. However, these proteins are different from heat-shock proteins previously described for animal cells. We have constructed a library of cDNA clones by using the RNA extracted from the hamster K12 cells incubated at 40.5 degrees C. Clones that hybridize preferentially with cDNA made from RNA at 40.5 degrees C were selected. By using the hybrid-selection technique, followed by in vitro translation, a cDNA clone containing a 2550-nucleotide insert coding for the hamster Mr 78,000 protein has been identified.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bramwell M. E., Harris H. An abnormal membrane glycoprotein associated with malignancy in a wide range of different tumours. Proc R Soc Lond B Biol Sci. 1978 Apr 13;201(1142):87–106. doi: 10.1098/rspb.1978.0034. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Harpold M. M., Evans R. M., Salditt-Georgieff M., Darnell J. E. Production of mRNA in Chinese hamster cells: relationship of the rate of synthesis to the cytoplasmic concentration of nine specific mRNA sequences. Cell. 1979 Aug;17(4):1025–1035. doi: 10.1016/0092-8674(79)90341-6. [DOI] [PubMed] [Google Scholar]
- Hightower L. E. Cultured animal cells exposed to amino acid analogues or puromycin rapidly synthesize several polypeptides. J Cell Physiol. 1980 Mar;102(3):407–427. doi: 10.1002/jcp.1041020315. [DOI] [PubMed] [Google Scholar]
- Isaka T., Yoshida M., Owada M., Toyoshima K. Alterations in membrane polypeptides of chick embryo fibroblasts induced by transformation with avian sarcoma viruses. Virology. 1975 May;65(1):226–237. doi: 10.1016/0042-6822(75)90023-9. [DOI] [PubMed] [Google Scholar]
- Jacobs S., Hazum E., Cuatrecasas P. The subunit structure of rat liver insulin receptor. Antibodies directed against the insulin-binding subunit. J Biol Chem. 1980 Jul 25;255(14):6937–6940. [PubMed] [Google Scholar]
- Jacobs S., Hazum E., Shechter Y., Cuatrecasas P. Insulin receptor: covalent labeling and identification of subunits. Proc Natl Acad Sci U S A. 1979 Oct;76(10):4918–4921. doi: 10.1073/pnas.76.10.4918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley P. M., Schlesinger M. J. The effect of amino acid analogues and heat shock on gene expression in chicken embryo fibroblasts. Cell. 1978 Dec;15(4):1277–1286. doi: 10.1016/0092-8674(78)90053-3. [DOI] [PubMed] [Google Scholar]
- Lai E. C., Woo S. L., Bordelon-Riser M. E., Fraser T. H., O'Malley B. W. Ovalbumin is synthesized in mouse cells transformed with the natural chicken ovalbumin gene. Proc Natl Acad Sci U S A. 1980 Jan;77(1):244–248. doi: 10.1073/pnas.77.1.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasky L. A., Lev Z., Xin J. H., Britten R. J., Davidson E. H. Messenger RNA prevalence in sea urchin embryos measured with cloned cDNAs. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5317–5321. doi: 10.1073/pnas.77.9.5317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee A. S. The accumulation of three specific proteins related to glucose-regulated proteins in a temperature-sensitive hamster mutant cell line K12. J Cell Physiol. 1981 Jan;106(1):119–125. doi: 10.1002/jcp.1041060113. [DOI] [PubMed] [Google Scholar]
- McCormick P. J., Keys B. J., Pucci C., Millis A. J. Human fibroblast-conditioned medium contains a 100K dalton glucose-regulated cell surface protein. Cell. 1979 Sep;18(1):173–182. doi: 10.1016/0092-8674(79)90366-0. [DOI] [PubMed] [Google Scholar]
- Melero J. A., Smith A. E. Possible transcriptional control of three polypeptides which accumulate in a temperature-sensitive mammalian cell line. Nature. 1978 Apr 20;272(5655):725–727. doi: 10.1038/272725a0. [DOI] [PubMed] [Google Scholar]
- Pouysségur J., Shiu R. P., Pastan I. Induction of two transformation-sensitive membrane polypeptides in normal fibroblasts by a block in glycoprotein synthesis or glucose deprivation. Cell. 1977 Aug;11(4):941–947. doi: 10.1016/0092-8674(77)90305-1. [DOI] [PubMed] [Google Scholar]
- Pouysségur J., Yamada K. M. Isolation and immunological characterization of a glucose-regulated fibroblast cell surface glycoprotein and its nonglycosylated precursor. Cell. 1978 Jan;13(1):139–140. doi: 10.1016/0092-8674(78)90145-9. [DOI] [PubMed] [Google Scholar]
- Ricciardi R. P., Miller J. S., Roberts B. E. Purification and mapping of specific mRNAs by hybridization-selection and cell-free translation. Proc Natl Acad Sci U S A. 1979 Oct;76(10):4927–4931. doi: 10.1073/pnas.76.10.4927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roscoe D. H., Read M., Robinson H. Isolation of temperature sensitive mammalian cells by selective detachment. J Cell Physiol. 1973 Dec;82(3):325–331. doi: 10.1002/jcp.1040820302. [DOI] [PubMed] [Google Scholar]
- Shields D., Blobel G. Efficient cleavage and segregation of nascent presecretory proteins in a reticulocyte lysate supplemented with microsomal membranes. J Biol Chem. 1978 Jun 10;253(11):3753–3756. [PubMed] [Google Scholar]
- Shiu R. P., Pouyssegur J., Pastan I. Glucose depletion accounts for the induction of two transformation-sensitive membrane proteinsin Rous sarcoma virus-transformed chick embryo fibroblasts. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3840–3844. doi: 10.1073/pnas.74.9.3840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sogin D. C., Hinkle P. C. Immunological identification of the human erythrocyte glucose transporter. Proc Natl Acad Sci U S A. 1980 Oct;77(10):5725–5729. doi: 10.1073/pnas.77.10.5725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Stone K. R., Smith R. E., Joklik W. K. Changes in membrane polypeptides that occur when chick embryo fibroblasts and NRK cells are transformed with avian sarcoma viruses. Virology. 1974 Mar;58(1):86–100. doi: 10.1016/0042-6822(74)90143-3. [DOI] [PubMed] [Google Scholar]
- Storti R. V., Scott M. P., Rich A., Pardue M. L. Translational control of protein synthesis in response to heat shock in D. melanogaster cells. Cell. 1980 Dec;22(3):825–834. doi: 10.1016/0092-8674(80)90559-0. [DOI] [PubMed] [Google Scholar]
- Sutcliffe J. G. pBR322 restriction map derived from the DNA sequence: accurate DNA size markers up to 4361 nucleotide pairs long. Nucleic Acids Res. 1978 Aug;5(8):2721–2728. doi: 10.1093/nar/5.8.2721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zain S., Sambrook J., Roberts R. J., Keller W., Fried M., Dunn A. R. Nucleotide sequence analysis of the leader segments in a cloned copy of adenovirus 2 fiber mRNA. Cell. 1979 Apr;16(4):851–861. doi: 10.1016/0092-8674(79)90100-4. [DOI] [PubMed] [Google Scholar]