Abstract
Objectives
(1) To summarize the protocol used for grading features of postradiation abnormalities from fundus photographs and fluorescein angiograms of patients enrolled in the Collaborative Ocular Melanoma Study (COMS); (2) to document the prevalence of features of interest in the posterior pole of these eyes during 8 years of follow-up; and (3) to investigate baseline patient, tumor, and treatment characteristics associated with posterior pole features.
Design
Observational case series within a randomized, multicenter clinical trial.
Participants
We evaluated 650 patients who were assigned to and received iodine-125 brachytherapy in the COMS for medium-sized tumors.
Methods
Color fundus photographs and fluorescein angiograms were taken at baseline and 2, 5, and 8 years; 30 features were graded according to a standard protocol.
Main Outcome Measures
Prevalence at selected time intervals of fundus photographic features associated with retinopathy and optic neuropathy.
Results
The percentage of patients with 21 feature of interest was 49.2% at baseline, 84.4% at 2 years, 91.2% at 5 years, and 90.7% at 8 years. The most frequent findings across all follow-up examinations were macular microaneurysms (75.6% of examinations), macular angiographic leakage (75.1%), and optic disc hyperfluorescence (62.8%). The median number of features present increased significantly with each follow-up to a maximum of 7 features at 8 years. The prevalence of neovascularization of the disc at 5 years was 5.2%. The prevalence of optic neuropathy at 5 years was 27.4%. Prognostic factors for more prevalent and severe posterior pole abnormalities were diabetes, tumor location close to both optic nerve and foveal avascular zone, and greater dose of radiation to the foveola and optic nerve head.
Conclusions
The amount and severity of retinopathy and optic neuropathy after iodine-125 brachytherapy increased through 8 years of follow-up. Assessment of photographs and angiograms taken in accord with a standard protocol provided reliable estimates of rates of development of features of retinopathy and optic neuropathy in eyes treated using the COMS brachytherapy protocol. Our findings support earlier reports that tumor factors in addition to radiation treatment may contribute to posterior pole abnormalities.
INTRODUCTION
Choroidal melanoma is the most common primary intraocular tumor in adults.1–3 Enucleation had been the definitive treatment for over a century, but eye and vision preserving alternatives using radiation became increasingly popular during the 20th century.2,4 –7 Although radiation treatment was effective at tumor destruction, it was not without serious complications. Macular angiographic leakage, retinal and vitreous hemorrhages, loss of foveal vasculature, hard exudates, and optic neuropathy were noted in a significant number of these initial patients who developed radiation retinopathy and vision loss.8,9
Various sources of radiation, including cobalt 60, ruthenium, helium ion, proton beam, iridium 192, gold 198, palladium 103, and iodine 125 (I125), have been used at different institutions.7,10–27 Brachytherapy with I125 was selected for the Collaborative Ocular Melanoma Study (COMS) because of its availability, good tissue penetration, and the ability to standardize dosimetry, but more importantly, because of the ability to shield emitted radiation effectively with a thin gold plaque to protect normal ocular structures.23,24,28 It was hoped that the incidence of radiation retinopathy and optic neuropathy could be lessened with the relatively low-energy I125 when compared with other radiation sources.28
Despite these protective measures aimed at sight preservation, radiation-induced damage to the eye is a well-known iatrogenic consequence of brachytherapy.1,7,13,16,23,24,27–30 Radiation retinopathy has been reported to be more common after external beam radiation, but abnormalities after brachytherapy can be significant.9,31 Pathologic studies of radiation retinopathy have demonstrated that the primary vascular event is endothelial cell loss and capillary closure, with vitreoretinal neovascularization occurring when retinal ischemia becomes widespread.32
Although retinal changes in the posterior pole observed after brachytherapy are attributed primarily to the effects of radiation, intrinsic tumor angiogenic and inflammatory factors released during growth and regression may be just as important in causing ocular morbidity.30,33 With relatively few studies systematically characterizing the angiographic and funduscopic features of tumor-affected eyes before radiation treatment in the current literature, it can be difficult to separate tumor and radiation effects. Most studies have focused on describing incidence of radiation retinopathy, radiation maculopathy, and/or radiation optic neuropathy without describing the incidence of their specific components.7,13,16–18,21,23,27
With the completion of the COMS and the conclusion that survival was similar among patients with medium-sized choroidal melanoma treated with enucleation and a standard protocol for I125 brachytherapy, this method of radiotherapy has become widely used. The purpose of this article is to report photographic and angiographic baseline features and abnormalities during the first 8 years after I125 brachytherapy for medium sized choroidal melanoma to better characterize the prevalence of specific features of radiation retinopathy and optic neuropathy in eyes treated with I125 brachytherapy. Baseline patient, tumor, and treatment characteristics as they related to posterior pole abnormalities also were examined.
METHODS
The COMS design has been described previously.34
Patient selection and follow-up
Patients whose eyes are the subject of this analysis had medium-sized choroidal melanoma 22.5 mm but not >10.0 mm in height (not >8.0 mm in height when the tumor was near the disc), and not >16 mm in greatest diameter. Patients who had peripapillary tumors that subtended an angle of 290°, as measured from the center of the optic disc, were ineligible. Detailed information from a patient interview and ophthalmic examination was recorded on standard forms at baseline, every 6 months for 5 years, and yearly thereafter. The COMS was conducted with institutional review board approval at all participating institutions, and a written consent was provided by all participating patients.
The first patient enrolled in January 1987 and the last in July 1998. A total of 1317 were enrolled; 657 were randomly assigned to I125 brachytherapy. Of these patients, 7 (1.1%) declined their assigned treatment and were excluded from this analysis. The remaining 650 patients are the subject of this report.
Patients with the baseline diagnosis of systemic conditions including diabetes and hypertension were identified from patient questionnaires.
Treatment Protocol
The size of the radioactive plaque was chosen so that the tumor base and a tumor-free margin of 2 to 3 mm on all borders were completely covered by the plaque. The absorbed dose at the prescription point was 85 Gy delivered at a rate of 20.42 Gy/hr, but not >1.05 Gy/hr. For tumors 25 mm in apical height, the tumor dose was prescribed at the apex of the tumor. For tumors from 2.5 to 5 mm in apical height, the prescription point was 5 mm from the inner surface of the sclera. All radiation doses in this paper are reported according to the recommendations of the American Association of Physics in Medicine Task Group 43 for dosimetry of interstitial sources.35,36
Funduscopic and Angiographic Grading Protocol
At baseline and follow-up examinations, fundus photography and fluorescein angiography were performed according to COMS protocol. Photographs and angiograms were sent to the COMS Photograph Reading Center at the University of Iowa Hospitals and Clinics for standard interpretation, and coding of selected features of the eye and tumor. Photographic quality and, at baseline, consistency with the diagnosis of choroidal melanoma were assessed. For this analysis, photographs and angiograms at baseline and 2, 5, and 8 years were selected for analysis because fluorescein angiography was specified in the protocol at these times.
Grading Scheme
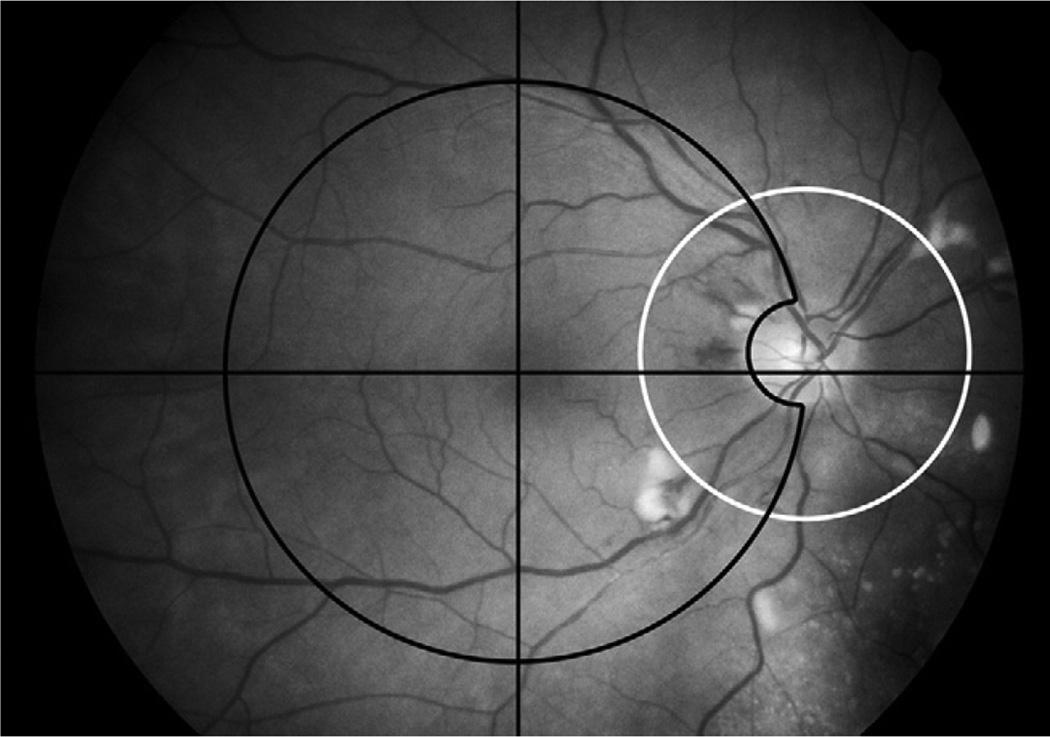
Thirty selected photographic and angiographic features were graded categorically (either present or absent) and on a severity scale by 3 graders. A list of features graded, their definitions, the numeric grading scheme and standard photographs may be accessed at http://webeye.ophth.uiowa.edu/dept/coms (last accessed May 5, 2008). Only the posterior pole photographs were graded for this analysis. Both color photographs and angiograms were utilized for grading features and were divided into 2 zones (Fig 1). The macular zone included a circle with a radius that extended from the center of the fovea to the center of the optic disc, but did not include the optic disc itself. The peripapillary zone was a 2250 micron radius circle centered on the optic nerve. There was some overlap in the temporal peripapillary region. Gradings included lesions of the retina and vitreous overlying the tumor when the tumor was within the macular or peripapillary zone.
Figure 1.
Macular zone (outlined with black line) and peripapillary zone (outlined with white line) as defined in the Collaborative Ocular Melanoma Study Photographic Reading Center grading scheme. There is overlap of these two zones temporal to the optic disc.
Grading was performed for both the peripapillary and macular zones separately for each feature unless the feature was specific to the macula or optic nerve. “Questionable” responses were permitted and accounted for 49 (0.1%) of a total of 44,486 coded items. To determine the relationship of the features of retinopathy with visual acuity, features also were graded as to whether they were within 750 microns of the center of the fovea or involved the center of the fovea. Neovascularization of the retina was not a separately graded feature in the macular field. If it was present, graders recorded neovascularization of the retina in the field named “other” features. The only gradings that were performed on the fellow eyes was a fluorescein angiographic assessment of disc hyperfluorescence to use as a comparison to the study eye to evaluate for optic disc hyperfluorescence. To assess reliability between graders, 5% of graded photographs and angiograms were randomly selected for review. Intergrader reliability was assessed using the K statistic.
Statistical Methods
This analysis was based on data received at the COMS Coordinating Center as of February 9, 2004, the date of final closure of the database derived from active COMS clinical follow-up. For each of the 30 photographic and angiographic characteristics of interest, frequency distributions of each characteristic at each follow-up time were generated for all patients (n = 650), as well as patients with (n = 65) and without (n = 583) diabetes at enrollment, and with (n = 185) and without hypertension (n = 464) at enrollment. Two patients had an unknown diabetes status and 1 patient had an unknown hypertension status. For each follow-up time, we also computed the mean and median number of features that were gradable, and the mean and median number of features that were graded as present for all patients and for patients who had 21 feature graded as present. For purposes of analysis, a grading of “questionable” was counted as present. A nonparametric test for trend, the Jonckheere test, was used to identify trends in median number of features over follow-up time.37 This test was used because, unlike possible parametric alternatives, it makes no specific assumptions regarding the form of the trend. To compare prevalence of each feature across follow-up times, we fit a logistic regression model with each feature coded as present or absent, using generalized estimating equations to account for correlation between time points.38 Prevalence at each time point was then compared with all other time points using linear contrasts; P<0.01 was considered as evidence of a significant difference in prevalence. To compare severity of each feature across follow-up times, we performed an analogous analysis of the severity scores using linear regression. In both types of models, time was treated as a categorical variable so as not to impose any assumptions regarding trends over time. We chose to analyze the prevalence of features rather than cumulative incidence of features (using an actuarial method) because some of the features, such as cotton wool spots and retinal detachment, were more commonly seen in early follow-up and decreased over time.
Exploratory analyses were performed to determine whether the baseline demographic, medical, tumor, and treatment characteristics were associated with prevalence and/or severity of features: age, gender, smoking, diabetes, hypertension, tumor height and largest basal dimension, distance of tumor from the fovea and edge of the optic nerve, tumor shape, and radiation dose to the foveola, optic nerve head, lens, and prescription point. Given the exploratory nature of the analyses and the number of baseline characteristics tested, it is likely that some statistically significant associations were found owing to chance. To determine whether baseline characteristics, such as diabetes or hypertension, were associated with prevalence and severity of features, including proliferative retinopathy and optic neuropathy, we compared patients with versus without the condition at baseline with respect to prevalence and severity of each feature at each follow-up time using the Fisher exact test to compare prevalences and the exact Wilcoxon rank-sum test to compare severity scores. For continuous baseline characteristics, such as tumor apical height and basal dimension, and radiation doses to critical structures, patients were divided into 2 groups using a median split and the 2 groups were compared. To control for multiple comparisons at the 3 follow-up times, P<0.0125 was considered evidence of a statistically significant difference between groups. All statistical analysis was performed using SAS software, version 9.0 (SAS Inc., Cary, NC).
RESULTS
Photographs and angiograms were coded for 21 features for 648 (99.7%) of the 650 subjects at baseline, 493 (75.8%) subjects during the second year, 318 (48.9%) subjects at year 5, and 162 (31.5%) subjects at 8 years (Table 1; available online at http://aaojournal.org). Intergrader reliability ranged from 0.6 to 0.8 for most features. Table 1 also includes the number of patients with no photographs and reasons that follow-up photographs were not available to be graded. Death of the patient was the primary reason for unavailability of photographs at later follow-up times, although the number of patients who missed a COMS examination also increased with follow-up time, as did the number of examinations at which photographs could not be taken because the eye had been removed. One hundred thirty-six patients did not enroll early enough to be eligible for an 8-year follow-up examination at the time COMS clinical follow-up ended.
Table 1.
Availability of Photograph Gradings and Reasons for No Photograph Grading by Year of Follow-up for 650 Patients in the Collaborative Ocular Melanoma Study (COMS), Randomized to and Treated by Iodine-125 Brachytherapy
| Reason | Baseline | 2 Years | 5 Years | 8 Years |
|---|---|---|---|---|
| Photos were taken and gradable for at least some features | 648 (99.7%) | 493 (75.8%) | 318 (48.9%) | 162 (24.9%) |
| Patient not eligible for photos* | 0 (0%) | 0 (0%) | 0 (0%) | 136 (20.9%) |
| Patient had died | 0 (0%) | 24 (3.7%) | 116 (17.8%) | 136 (20.9%) |
| Eye enucleated | 0 (0%) | 30 (4.6%) | 60 (9.2%) | 51 (7.8%) |
| Patient missed clinic examination | 0 (0%) | 51 (7.8%) | 65 (10.0%) | 98 (15.1%) |
| Patient had clinic examination, but photos were not taken | 0 (0%) | 29 (4.5%) | 65 (10.0%) | 50 (7.7%) |
| Photos were taken but not gradable for any feature | 2 (0.3%) | 23 (3.5%) | 26 (4.0%) | 17 (2.6%) |
| Total | 650 | 650 | 650 | 650 |
Patient had not reached the follow-up time in question by the time of close of clinical follow-up in the COMS.
For patients who had a clinical examination, but did not have photographs taken, the reason was recorded on the ophthalmic examination form. A large proportion of patients had documented media opacity (cataract, vitreous hemorrhage, or other vitreous opacity) at each follow-up time: at year 2, 15 of 29 patients (52%); at year 5, 40 of 65 patients (62%); and at year 8, 32 of 50 patients (64%). For the remaining patients, the reason is unknown.
Of subjects who had photographs taken at baseline, 90% also had a baseline angiogram. At follow-up, the percentages were 81.7% at 2 years, 78.3% at 5 years, and 82.1% at 8 years. There was an increase in fluorescein allergies with time.
Prevalence of Features
Baseline
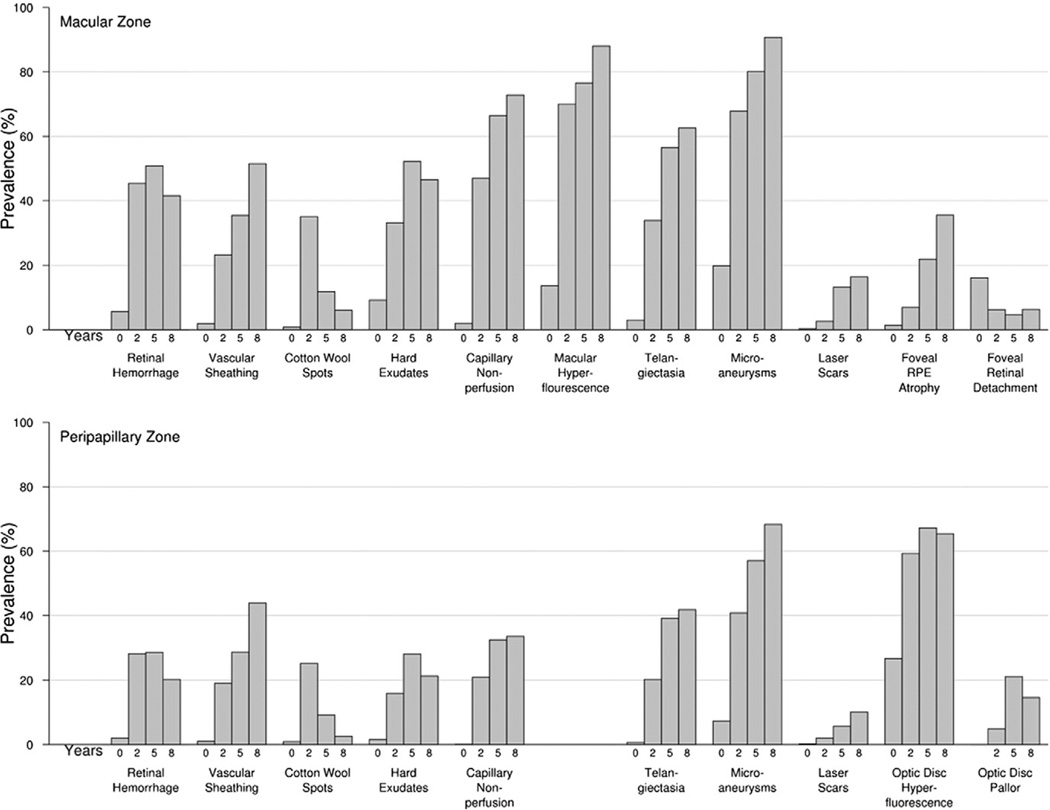
In COMS patients, some features often attributed to radiation retinopathy were already present in the eyes of some patients at baseline, before the initiation of radiation treatment. The most common findings at baseline in the macular zone were microaneurysms (19.8%), foveal retinal detachment (15.9%), and macular angiographic leakage (13.5%). In the peripapillary zone, they were optic disc hyperfluorescence (26.7%), microaneurysms (7.3%), and retinal hemorrhages (2%; Fig 2). A total of 81 patients had macular microaneurysms noted on baseline photographs or angiograms. Of these, 12 (15%) had a history of diabetes and 69 (85%) did not. A total of 29 patients had peripapillary microaneurysms. Of these, 12 (41%) had diabetes and 17 (59%) did not.
Figure 2.
Prevalence of selected macular and peripapillary zone photographic features at baseline and during follow-up. RPE = Retinal Pigment Epithelium
Follow-up
Overall, the most frequent findings across all follow-up visits were macular microaneurysms (75.6%), macular angiographic leakage (75.1%), and optic disc hyperfluorescence (62.8%). Macular and peripapillary preretinal hemorrhage were among the least common features (Table 2). The feature with the greatest prevalence at a given time point was macular microaneurysms (90.7%) at the 8-year follow-up.
Table 2.
Overall prevalence of photographic features across all follow-up examinations, in order of decreasing prevalence
| Feature | No. of examinations at which feature was graded* |
No. of examinations at which feature was present |
Overall prevalence (%) |
|---|---|---|---|
| Macular microaneurysms | 742 | 561 | 75.6 |
| Macular angiographic leakage | 728 | 547 | 75.1 |
| Optic disc hyperfluorescence | 748 | 470 | 62.8 |
| Macular capillary nonperfusion | 658 | 378 | 57.4 |
| Peripapillary microaneurysms | 701 | 355 | 50.6 |
| Macular retinal hemorrhage | 935 | 435 | 46.5 |
| Macular telangiectasia | 863 | 394 | 45.6 |
| Macular hard exudates | 933 | 387 | 41.5 |
| Macular vascular sheathing | 870 | 274 | 31.5 |
| Peripapillary telangiectasia | 877 | 260 | 29.6 |
| Peripapillary retinal hemorrhage | 940 | 254 | 27.0 |
| Peripapillary capillary nonperfusion | 664 | 178 | 26.8 |
| Peripapillary vascular sheathing | 882 | 229 | 26.0 |
| Macular cotton wool spots | 922 | 211 | 22.9 |
| Peripapillary hard exudates | 937 | 194 | 20.7 |
| Macular RPE atrophy | 918 | 151 | 16.4 |
| Peripapillary cotton wool spots | 930 | 152 | 16.3 |
| Optic disc pallor | 911 | 104 | 11.4 |
| Macular laser scars | 945 | 77 | 8.2 |
| Optic disc hemorrhage | 912 | 65 | 7.1 |
| Macular retinal detachment | 944 | 54 | 5.7 |
| Peripapillary laser scars | 954 | 43 | 4.5 |
| Neovascularization of the disc | 911 | 34 | 3.7 |
| Macular vitreous hemorrhage | 954 | 27 | 2.8 |
| Optic disc edema | 919 | 25 | 2.7 |
| Macular subretinal hemorrhage | 947 | 24 | 2.5 |
| Peripapillary vitreous hemorrhage | 954 | 22 | 2.3 |
| Macular preretinal hemorrhage | 949 | 19 | 2.0 |
| Peripapillary subretinal hemorrhage | 951 | 7 | 0.7 |
| Peripapillary preretinal hemorrhage | 951 | 5 | 0.5 |
Not all features could be graded at every examination depending on photograph quality and availability of a fluorescein angiogram.
RPE = Retinal pigment epithelium
Trends over Time
The percentage of patients with 21 feature was 49.2% at baseline, 84.4% at 2 years, 91.2% at 5 years, and 90.7% at 8 years. Retinal hemorrhage in the macular and peripapillary zone, optic disc hemorrhage, and optic disc hyperfluorescence all were significantly more prevalent and more severe at follow-up compared with baseline (P<0.01 for baseline versus each follow-up time). However, there were no statistically significant differences among posttreatment follow-up times in prevalence or severity of features (P>0.01 for all pair-wise comparisons of posttreatment follow-up times; Fig 2; severity data not shown).
Macular zone vascular sheathing, microaneurysms, foveal retinal pigment epithelium atrophy, and peripapillary zone vascular sheathing had significantly increased prevalence and severity with each successive follow-up time (P<0.01 for comparison of each pair of successive follow-up times). Macular angiographic leakage showed significantly greater severity, but not prevalence, with each successive follow-up time.
Only cotton wool spots had a significant peak in prevalence and severity at an interim follow-up time (2 years) after brachytherapy (P<0.01 for comparison of 2 years versus each other follow-up time for both zones; Fig 2); the only feature that was significantly more prevalent and more severe at baseline than at follow-up was foveal retinal detachment. Other features had observed peaks at an interim follow-up time (retinal hemorrhage, hard exudates, optic disc hyper-fluorescence, optic disc pallor), but the observed peak in prevalence was not statistically greater than prevalence at 21 other follow-up times.
Gradable Features Per Patient
For patients with 21 set of photographs of sufficient quality to grade 21 feature of interest, the median number of features that were present was 0 at baseline, 4 at 2 years, 6 at 5 years, and 7 at 8 years of follow-up. For patients with 21 feature present, the median number of features was 1 at baseline, 5 at 2 years, 7 at 5 years, and 8 at 8 years of follow-up. These trends reflect a statistically significant increase over time in number of findings per patient both overall and among patients with 21 finding (P<0.0001; Jonckheere test for trend).
Proliferative Retinopathy
Proliferative retinopathy, which is typically defined as neovascularization of the disc or retina, was defined for this study strictly as neovascularization of the disc. The prevalences were 0.3% at baseline, 3.4% at 2 years, 5.2% at 5 years, and 2.0% at 8 years. There were no patients in whom neovascularization of the retina was detected in the macular field, unless it actually represented neovascularization of the disc and was already included in the overlapping peripapillary field.
Optic Neuropathy
The following features were considered to be indicative of optic neuropathy: optic disc edema, optic disc hemorrhage, and/or optic disc pallor. The prevalence of optic neuropathy was 0.5% at baseline, 11.4% at 2 years, 27.4% at 5 years, and 18.9% at 8 years.
Patient Factors
Diabetes
Diabetics had significantly more prevalent and more severe features in the macular zone at baseline (retinal hemorrhage, cotton wool spots, hard exudates, microaneurysms, capillary nonperfusion, and hyperfluorescence) as compared with non-diabetics (Table 3). Diabetics also had significantly more prevalent and more severe features at 2 years of follow-up compared with nondiabetics with respect to macular zone retinal hemorrhage, preretinal hemorrhage, vascular sheathing, cotton wool spots, capillary nonperfusion, peripapillary zone retinal hemorrhage, disc neovascularization, optic disc pallor, microaneurysms, and capillary nonperfusion (Table 3). Diabetics had significantly more proliferative retinopathy at the 2-year follow-up examination (P = 0.008); however, diabetes was not associated with optic neuropathy. By years 5 and 8, diabetics did not differ significantly from nondiabetics on prevalence or severity of any of the coded features; however, diabetics had higher mortality compared with nondiabetics (40% vs 16% at 5 years and 56% vs 24% at 8 years), and there were few diabetics still alive at later follow-up times. Lower survivorship may account for the lack of significant differences between the surviving diabetics and nondiabetics.
Table 3.
Prevalence (%) of photographic features in patients with versus without diabetes at baseline
| Photographic feature | Follow-up time (years)* | |||||
|---|---|---|---|---|---|---|
| 0 | 2 | 5 | ||||
| DM N=64 |
No DM N=582 |
DM N=46 |
No DM N=445 |
DM N=22 |
No DM N=294 |
|
| Macular zone: | ||||||
| Retinal hemorrhage | 22 % | 4c % | 74 % | 42c % | 50 % | 51 % |
| Subretinal hemorrhage | 6 | 2 | 9 | 2 | 5 | 2 |
| Preretinal hemorrhage | 2 | <1 | 11 | <1c | 14 | 3 |
| Vitreous hemorrhage | 2 | 2 | 7 | 3 | 9 | 3 |
| Vascular sheathing | 6 | 1 | 66 | 19c | 57 | 34 |
| Cotton wool spots | 6 | <1b | 60 | 33a | 5 | 12 |
| Hard exudates | 25 | 7c | 40 | 32 | 55 | 52 |
| Microaneurysms | 56 | 16c | 84 | 66 | 79 | 80 |
| Capillary nonperfusion | 14 | <1b | 81 | 43c | 77 | 66 |
| Macular angiographic leakage | 36 | 11c | 74 | 70 | 64 | 78 |
| Telangiectasia | 8 | 2 | 49 | 33 | 56 | 57 |
| Laser scars | 0 | <1 | 11 | 2a | 25 | 11 |
| Foveal RPE atrophy | 3 | 1 | 14 | 6 | 22 | 22 |
| Foveal retinal detachment | 19 | 16 | 9 | 6 | 11 | 4 |
| Peripapillary zone: | ||||||
| Retinal hemorrhage | 11 | 1 | 61 | 25c | 40 | 28 |
| Subretinal hemorrhage | 2 | <1 | 2 | <1 | 0 | 0 |
| Preretinal hemorrhage | 0 | <1 | 7 | 0b | 0 | <1 |
| Vitreous hemorrhage | 2 | 1 | 7 | 2 | 5 | 3 |
| Optic disc hemorrhage | 0 | <1 | 7 | 7 | 17 | 8 |
| Vascular sheathing | 2 | <1 | 50 | 16c | 47 | 27 |
| Cotton wool spots | 6 | <1 | 56 | 22c | 5 | 10 |
| Hard exudates | 5 | 1 | 14 | 16 | 30 | 28 |
| Telangiectasia | 0 | <1 | 30 | 19 | 24 | 40 |
| NVD | 2 | <1 | 12 | 2a | 16 | 4 |
| Optic disc edema | 2 | <1 | 2 | 4 | 0 | 3 |
| Optic disc pallor | 0 | 0 | 14 | 4a | 25 | 21 |
| Optic disc hyperfluorescence | 12 | 28 | 47 | 60 | 69 | 67 |
| Microaneurysms | 32 | 5 | 77 | 37c | 71 | 56 |
| Capillary nonperfusion | 0 | 0 | 55 | 17c | 38 | 32 |
| Laser scars | 0 | <1 | 11 | 1a | 21 | 5 |
8 year data is not shown as there were only 7 diabetic patients with 8 year follow-up. Features and follow-up times for which prevalence differed significantly between diabetics versus non-diabetics appear in bold.
0.001<p≤0.0125
0.0001<p≤0.001
Other Patient Factors
Patient age, gender, hypertension, and smoking were not associated with prevalence or severity of features at baseline or follow-up (data not shown).
Tumor Factors
Tumor Height and Largest Basal Dimension
Tumor height (>4.1 vs ≤4.1 mm) and largest basal dimension (>11.5 vs ≤11.5 mm) did not display any consistent association with prevalence or severity of features at baseline or follow-up (data not shown).
Tumor Distance From Fovea and Edge of Optic Nerve
Most tumors were either close to both the foveal avascular zone (FAZ; <3.0 mm) and optic nerve (<4.5 mm) or far from both (23.0 and 24.5 mm, respectively). Eyes with tumors in the former group had significantly higher prevalence and severity of macular and peripapillary zone vascular sheathing, hard exudates, and capillary nonperfusion. They also had greater prevalence and severity of foveal retinal pigment epithelium atrophy, macular retinal detachment, peripapillary telangiectasia, and optic disc pallor (Table 4; severity data not shown). Eyes with tumors farther from the FAZ (>3.0 mm) but closer to the optic nerve were significantly less likely to have some of the macular findings (vascular sheathing, capillary nonperfusion, and retinal detachment) than eyes with tumors close to both the FAZ and nerve (data not shown). Eyes with tumors close to the FAZ but not to the nerve did not differ from eyes with tumors close to both the FAZ and nerve. However, there were few eyes in the former group (data not shown).
Table 4.
Prevalence (%) of selected features by tumor distance and radiation dose to the fovea and optic disc
| Photographic feature | Follow-up time (years) | |||||||
|---|---|---|---|---|---|---|---|---|
| 0 | 2 | 5 | 8 | |||||
| Distance from tumor to fovea and optic disc | ||||||||
| Macular: | Close N=211 | Far N=280 | Close N=158 | Far N=208 | Close N=89 | Far N=138 | Close N=51 | Far N=70 |
| Retinal hemorrhage | 9 | 4 | 59 | 28c | 58 | 42 | 52 | 33 |
| Vascular sheathing | 4 % | 1 % | 39 % | 9c% | 62 % | 15c % | 83 % | 27c% |
| Hard exudates | 15 | 6b | 47 | 16c | 62 | 43a | 53 | 37 |
| Capillary nonperfusion | 3 | 1 | 61 | 33c | 82 | 55b | 86 | 61 |
| Telangiectasia | 3 | 3 | 44 | 23c | 62 | 50 | 71 | 58 |
| Microaneurysms | 23 | 15 | 71 | 62 | 78 | 78 | 91 | 89 |
| RPE atrophy | 3 | <1 | 15 | 1c | 37 | 9c | 55 | 21b |
| Retinal detachment | 38 | 4c | 13 | 1c | 10 | 1a | 16 | 3 |
| Peripapillary: | ||||||||
| Retinal hemorrhage | 1 | 3 | 40 | 13c | 37 | 19a | 28 | 10 |
| Vascular sheathing | 2 | <1 | 30 | 7c | 40 | 14c | 65 | 24c |
| Hard exudates | 2 | 2 | 27 | 6c | 39 | 18b | 33 | 9b |
| Capillary nonperfusion | 0 | 0 | 34 | 6c | 45 | 19b | 50 | 16a |
| Telangiectasia | <1 | <1 | 29 | 10b | 53 | 21c | 65 | 24c |
| Microaneurysms | 3 | 7 | 54 | 26c | 66 | 43a | 78 | 52 |
| Optic disc pallor | 0 | 0 | 10 | 1c | 37 | 7c | 24 | 5a |
| Optic disc hyperfluor. | 27 | 24 | 67 | 47b | 86 | 54c | 75 | 59 |
| Dose to fovea and optic nerve | ||||||||
| Macular: | Low N=236 | High N=234 | Low N=171 | High N=177 | Low N=122 | High N=109 | Low N=61 | High N=59 |
| Retinal hemorrhage | 4 | 7 | 25 | 59c | 42 | 58 | 39 | 46 |
| Vascular sheathing | 0 % | 3 % | 9 % | 36c % | 11 % | 57c % | 22 % | 78c % |
| Hard exudates | 6 | 11 | 15 | 50c | 44 | 60 | 34 | 54 |
| Capillary nonperfusion | 0 | 2 | 32 | 60c | 52 | 78b | 62 | 83 |
| Telangiectasia | 1 | 4 | 23 | 41b | 46 | 63 | 54 | 69 |
| Microaneurysms | 17 | 19 | 59 | 71 | 80 | 78 | 87 | 90 |
| RPE atrophy | <1 | 3 | 3 | 13b | 10 | 32c | 17 | 48b |
| Retinal detachment | 2 | 34c | 1 | 14c | 2 | 9 | 3 | 14 |
| Peripapillary: | ||||||||
| Retinal hemorrhage | 3 | <1 | 10 | 39c | 19 | 35a | 12 | 25 |
| Vascular sheathing | <1 | 1 | 7 | 29c | 11 | 38c | 20 | 62c |
| Hard exudates | 2 | 2 | 6 | 28c | 16 | 37 | 9 | 30a |
| Capillary nonperfusion | 0 | 0 | 6 | 34c | 18 | 41a | 13 | 46a |
| Telangiectasia | <1 | <1 | 12 | 27b | 19 | 52c | 22 | 60c |
| Microaneurysms | 9 | 4 | 22 | 50c | 39 | 63a | 49 | 73 |
| Optic disc pallor | 0 | 0 | 1 | 10b | 7 | 35c | 2 | 24b |
| Optic disc hyperfluor. | 25 | 26 | 45 | 65b | 51 | 80c | 60 | 67 |
For distance, close corresponds to <3.0 mm from the closest border of the tumor to the center of the fovea and <4.5 mm to the center of the optic disc, and far corresponds to ≥3.0 mm to the center of the fovea and ≥4.5 mm to the center of the optic disc. For dose, low corresponds to ≤80 Gy to the center of the foveola and ≤51 Gy to the center of the optic disc, and high corresponds to >80 Gy to the center of the fovea and >51 Gy to the center of the optic disc. Significant differences in prevalence for close versus far distance groups and low versus high dose groups appear in bold.
0.001<p≤0.0125
0.0001<p≤0.001
p≤0.0001
RPE = Retinal pigment epithelium
hyperfluor. = hyperfluorescence
Tumor Shape
Fewer dome-shaped tumors than tumors of other configurations had subretinal and vitreous hemorrhage at baseline and 2 years of follow-up (data not shown). No other association was found between tumor shape and prevalence or severity of features of interest.
Treatment Factors
Radiation Dose to Foveola and Optic Nerve Head
Lower dose of radiation to the foveola (≤80 Gy) and optic nerve head (≤51 Gy) was associated with lower prevalence (Table 4) and severity (data not shown) of retinal hemorrhage, vascular sheathing, hard exudates, and capillary nonperfusion in both the macular and peripapillary zones as compared to higher radiation doses to these structures (>80 Gy to foveola and >51 Gy to optic nerve head). It also was associated with lower prevalence (Table 4) and severity (data not shown) of foveal retinal pigment epithelium atrophy, foveal retinal detachment, peripapillary telangiectasia, peripapillary microaneurysms, optic disc pallor, and optic disc hyperfluorescence. Patients who received a high dose of radiation to the optic nerve head (>51 Gy), but lower dose to the foveola (defined as the center of the fovea; ≤80 Gy) had more prevalent and severe features in the peripapillary zone than patients receiving lower doses to both structures (data not shown).
Radiation Dose to Other Sites
Radiation dose to the prescription point was not related to prevalence or severity of features in either the macular or peripapillary zones. Postradiation incidence of cataract was related to the dose to the lens, as reported elsewhere.39
DISCUSSION
This article presents a grading protocol for describing baseline and posttreatment fundus photographic and fluorescein angiographic abnormalities in the posterior pole of patients with choroidal melanoma in a systematic and reliable manner, and describes the results of this grading protocol as applied to the COMS database. There is no consensus on the definition of radiation retinopathy in the ophthalmic literature, and it is not the purpose of this manuscript to establish a minimum threshold for nonproliferative radiation retinopathy. A strength of the COMS database is the inclusion of a grading of photographs taken before initiation of any treatment, and defined grading standards for the features of interest, obtained at standardized intervals.
Features typically attributed to radiation were already present at baseline in a significant proportion of patients (49.2% had 21 feature documented photographically before treatment commenced). Features found at baseline in a significant number of patients (including nondiabetics) included macular microaneurysms, macular angiographic leakage, optic disc hyperfluorescence, and macular hard exudates. This finding supports earlier reports that factors other than radiation treatment, such as tumor effects, may contribute to posterior pole abnormalities.30,33
Features typically attributed to radiation retinopathy increased in prevalence during follow-up. Up to 91.2% of patients had 21 feature documented photographically by 5 years of follow-up. The most common features overall were macular microaneurysms, macular angiographic leakage, and macular capillary nonperfusion. Each of these features is significant for its sight-threatening potential. Overall, the number of features present increased with time. However, foveal retinal detachment and cotton wool spots had significant prevalence peaks at baseline and at 2 years, respectively, reflecting the transient nature of these features. Foveal retinal detachment likely is related more to tumor than radiation effects.
Prevalence rates for nonproliferative radiation retinopathy and radiation maculopathy have been reported previously21,23,24,27 in retrospective studies to range from 42% to 94%. The definition of retinopathy varies for these studies, as does the size and location of tumors, the source of radiation, the methods used for determining retinopathy (fundus photographs, clinical examination, medical records, fluorescein angiography), the timing and duration of follow-up, and the statistical methods for accounting for censored data. Grading standards were not used.
Gündüz et al23 reported on a retrospective review of 1300 patients with uveal melanoma treated with plaque radiotherapy. Retinopathy was graded using results of indirect ophthalmoscope, fundus photography and fluorescein angiography. Nonproliferative radiation retinopathy was diagnosed if 22 of the following were present: microaneurysm, capillary dilation, capillary nonperfusion, retinal hemorrhage, retinal exudation, retinal edema, nerve fiber layer infarction or vascular sheathing. Radiation maculopathy was diagnosed when radiation retinopathy occurred within 3 mm of the foveola. Proliferative radiation retinopathy was diagnosed when there was retinal or optic disc neovascularization.
Gündüz et al found that 5% of patients had nonproliferative radiation retinopathy at 1 year and 42% at 5 years. Proliferative radiation retinopathy was found in 1% at 1 year and 8% at 5 years. Multivariate analyses showed variables related to the development of nonproliferative radiation retinopathy included tumor margin <4 mm from the foveola, tumor limited to choroid, and radiation dose rate of >260 cGy/hr to the tumor base. The most important predictors for the development of proliferative radiation retinopathy included diabetes, use of iridium-192 (a higher energy isotope than iodine-125), and a tumor base >10 mm.
For melanomas located within 3 mm of the foveola and treated with brachytherapy, Gündüz et al24 reported visually significant maculopathy in 40% of the patients at 5 years. Guyer et al33 reported on radiation maculopathy after proton beam irradiation for melanomas located within 4 disc diameters of the foveola. Microvascular changes (microaneurysms and/or telangiectasia), retinal hemorrhages, and capillary non-perfusion were seen in 76%, 70%, and 64% of eyes, respectively, by 3 years after treatment.33 Gragoudas et al22 reported a 5-year rate of maculopathy of approximately 75% for tumors within 1 disc diameter of the macula and 40% for tumors >1 disc diameter of the macula. As noted, comparison of prevalence rates for retinopathy among studies is complicated because of differences in the definition of radiation retinopathy and other differences in the study protocols.
In the COMS, posterior pole abnormalities were more prevalent and severe in patients with preexisting diabetes. Diabetics more often developed proliferative retinopathy at 2 years than nondiabetics. Diabetes causes a retinal vasculopathy that preferentially damages endothelial cells more than pericytes,40 resulting in a clinical picture very similar to radiation retinopathy. These changes may be additive to the vasculopathic effects of radiation, leading to retinal changes that are often more severe than those seen in nondiabetics.
Other researchers have found an association between diabetes and radiation retinopathy, both nonproliferative9,23,41,42 and proliferative.9 One smaller study failed to detect an increase in proliferative radiation retinopathy in diabetes.43 Certain chemotherapies may also exacerbate radiation retinopathy.9 Preexisting systemic cancer was an exclusion criterion in the COMS, so this association cannot be evaluated through the COMS.
The maximum prevalence of neovascularization of the disc was observed at 5 years and was 5.2%. The prevalence for neovascularization of the disc only is similar to prevalence of 8% at 5 years seen by Gündüz et al,23 which included both retinal and disc neovascularization.
The maximum prevalence of optic neuropathy was 27.4% at 5 years. The range of estimates reported in the literature is 11% to 57% for follow-up times ranging from 37 months to 5 years.14,16,19,21,27 Neither diabetics nor hypertensives were more likely to develop optic neuropathy than individuals without these conditions.
Optic disc hyperfluorescence was present in 23.3% of study eyes at baseline, indicating that this abnormality can be present before radiation. Optic disc hyperfluorescence was only graded as present when there was a clear increase in the hyperfluorescence of the optic disc in the late phase of the fluorescein angiogram when compared with the fellow eye. Similar to the retinal abnormalities that can be detected in eyes of patients with melanoma before radiation treatment, the tumor itself may play some role in causing optic disc hyperfluorescence. Although optic disc hyperfluorescence was a common feature at baseline and increased after brachytherapy, its clinical significance is uncertain.
Tumor factors associated with more prevalent or more severe features were decreased distance to both the FAZ and optic nerve. Dome-shaped tumors were less likely to be associated with subretinal and vitreous hemorrhage than tumors of other configurations. Treatment factors associated with more prevalent and severe features include greater dose of radiation to the foveola and optic nerve.
There are several limitations to this analysis. It was inevitable that the ability to document lesions in the posterior pole photographically over time declined owing to death, loss or removal of the eye, patient refusal to return to the clinic for examinations or photography, and inability to photograph the eye owing to media opacities. Patients who have posterior pole photographs at extended follow-up times may no longer be representative of the initial population. As reported, diabetics had higher mortality compared with nondiabetics and were relatively underrepresented at 8-year follow-up compared with baseline. Also, because eyes in patients with large tumors received higher doses of radiation, and patients with larger tumors had higher mortality rates,44 many of the patients who died early during follow-up had received higher doses of radiation. It is reasonable to assume these patients had more retinopathy than the baseline population or the population still alive at any given point in follow-up. Thus, findings apply only to survivors at the time points presented.
When the COMS brachytherapy protocol was designed, there were concerns regarding the accuracy of calculating dosimetry for smaller tumors. Therefore, the radiation dose for all tumors ≤5 mm in height was prescribed to a point 5 mm from the inner surface of the sclera. This protocol resulted in eyes with smaller tumors in the COMS receiving a larger dose of radiation than was often administered to tumors treated outside of the COMS. This protocol would be expected to result in a higher prevalence of radiation-induced retinal and optic nerve abnormalities in these smaller tumors than has been reported in other series.
Proliferative retinopathy after radiation is typically defined by the presence of neovascularization of the disc or retina.23 Because the image grading for this study was restricted to the macular and peripapillary fields, neovascularization of the midperipheral retina would not be included. Therefore, defining proliferative retinopathy as neovascularization of the disc only should underestimate the prevalence of proliferative retinopathy.
Macular angiographic leakage and optic disc hyperfluorescence were graded features in this study. Macular angiographic leakage is a finding that is suggestive of macular edema, but it is likely that equating macular angiographic leakage with macular edema will both overcall and under-call eyes with macular edema. Because this study predominantly used 60° fluorescein angiography, grading for macular edema using the definition of stereoscopic thickening of the macula usually was not possible.
Another limitation to this study is the difficulty in separating tumor effects from radiation effects. It is not possible to have a control group for ethical reasons; it is the strong belief of the ophthalmic community that treatment is preferable to the natural course of the disease. An attempt was made to address this issue through standardized coding of baseline angiographic and funduscopic features attributable to potential tumor effects, to serve as a comparison for postradiation changes.
In the analysis, there was no evaluation of the photographic or angiographic abnormalities that occurred between baseline and 2 years. Although retinopathy is often minimal at 1 year after brachytherapy, it is possible that the development of certain features at 1 year might predict a more severe course of retinopathy. It would also be interesting to evaluate the interactions between photographic and angiographic features and best-corrected visual acuity, but that is beyond the scope of this paper. In addition, there are also radiation changes in the choroid that are likely to play a major role in fundus changes and visual outcomes after brachytherapy.45 However, the COMS imaging protocol did not allow for detailed evaluation of changes in choroidal circulation.
We have described the changing prevalence of retinal and optic disc abnormalities in a very large population of patients treated with brachytherapy using standard protocols for follow-up, photography, and photograph grading. Some of these abnormalities predate brachytherapy, implying that some of the changes may be due to the tumor. Prognostic factors for more prevalent and severe posterior pole lesions have been identified. The COMS grading protocol permits the identification of angiographic and funduscopic abnormalities that increase after radiation and are likely to be associated with poor visual outcomes.
Acknowledgements
The authors thank Cheryl J. Hiner and Rosemary Brothers for grading the fundus photographs and fluorescein angiograms in this study.
This work was supported by the National Eye Institute and National Cancer Institute through cooperative agreements EY06253, EY06257, EY06258, EY06259, EY06260, EY06264, EY06265, EY06266, EY06268, EY06269, Ey06270, EY06274, EY06275, EY06276, EY06279, EY06280, EY06282, EY06283, EY06284, EY06287, EY06288, EY06289, EY06291, EY06839, EY06843, EY06844, EY06848, EY06858, and EY06899 with the National Institutes of Health, Bethesda, Maryland.
Footnotes
The authors have no conflicts of interest related to this article.
This article contains online-only material. The following should appear online-only: Table 1.
Financial Disclosure(s): The authors have no proprietary or commercial interest in any materials discussed in this article.
REFERENCES
- 1.Bosworth JL, Packer S, Rotman M, et al. Choroidal melanoma: I-125 plaque therapy. Radiology. 1988;169:249–251. doi: 10.1148/radiology.169.1.3420267. [DOI] [PubMed] [Google Scholar]
- 2.Brady LW, Hernandez JC. Brachytherapy of choroidal melanomas. Strahlenter Onkol. 1992;168:61–65. [PubMed] [Google Scholar]
- 3.Jones R, Gore E, Mieler W, et al. Posttreatment visual acuity in patients treated with episcleral plaque therapy for choroidal melanomas: dose and dose rate effects. Int J Radiat Oncol Biol Phys. 2002;52:989–995. doi: 10.1016/s0360-3016(01)02723-7. [DOI] [PubMed] [Google Scholar]
- 4.Moore RF. Choroidal sarcoma treated by the intraocular insertion of radon seeds. Br J Ophthalmol. 1930;14:145–152. doi: 10.1136/bjo.14.4.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stallard HB. Radiant energy as (a) a pathogenic and (b) a therapeutic agent in ophthalmic disorders: Gifford Edmonds prize essay for 1932. Br J Ophthalmol Monograph Suppl. 1933;6:1–126. [Google Scholar]
- 6.Stallard HB. Radiotherapy for malignant melanoma of the choroids. Br J Ophthalmol. 1966;50:147–155. doi: 10.1136/bjo.50.3.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fontanesi J, Meyer D, Xu S, Tai D. Treatment of choroidal melanoma with I-125 plaque. Int J Radiat Oncol Biol Phys. 1993;26:619–623. doi: 10.1016/0360-3016(93)90278-4. [DOI] [PubMed] [Google Scholar]
- 8.Hayreh SS. Post-radiation retinopathy: a fluorescence fundus angiographic study. Br J Ophthalmol. 1970;54:705–714. doi: 10.1136/bjo.54.11.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brown GC, Shields JA, Sanborn G, et al. Radiation retinopathy. Ophthalmology. 1982;89:1494–1501. doi: 10.1016/s0161-6420(82)34611-4. [DOI] [PubMed] [Google Scholar]
- 10.Sealy R, le Roux LM, Rapley F, et al. The treatment of ophthalmic tumours with low-energy sources. Br J Radiol. 1976;49:551–554. doi: 10.1259/0007-1285-49-582-551. [DOI] [PubMed] [Google Scholar]
- 11.Packer S, Rotman M. Radiotherapy of choroidal melanoma with iodine-125. Ophthalmology. 1980;87:582–590. doi: 10.1016/s0161-6420(80)35194-4. [DOI] [PubMed] [Google Scholar]
- 12.Packer S, Fairchild RG, Salanitro P. New techniques for iodine-125 radiotherapy of intraocular tumors. Ann Ophthalmol. 1987;19:26–30. [PubMed] [Google Scholar]
- 13.Packer S, Stoller S, Lesser ML, et al. Long-term results of iodine 125 irradiation of uveal melanoma. Ophthalmology. 1992;99:767–773. doi: 10.1016/s0161-6420(92)31899-8. [DOI] [PubMed] [Google Scholar]
- 14.Lean EK, Cohen DM, Liggett PE, et al. Episcleral radioactive plaque therapy: initial clinical experience with 56 patients. Am J Clin Oncol. 1990;13:185–190. doi: 10.1097/00000421-199006000-00001. [DOI] [PubMed] [Google Scholar]
- 15.Hill JC, Sealy R, Shackleton D, et al. Improved iodine-125 plaque design in the treatment of choroidal malignant melanoma. Br J Ophthalmol. 1992;76:91–94. doi: 10.1136/bjo.76.2.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mameghan H, Karolis C, Fisher R, et al. Iodine-125 irradiation of choroidal melanoma: clinical experience from the Prince of Wales and Sydney Eye Hospitals. Australas Radiol. 1992;36:249–252. doi: 10.1111/j.1440-1673.1992.tb03161.x. [DOI] [PubMed] [Google Scholar]
- 17.Char DH, Quivey JM, Castro JR, et al. Helium ions versus iodine 125 brachytherapy in the management of uveal melanomas: a prospective, randomized, dynamically balanced trial. Ophthalmology. 1993;100:1547–1554. doi: 10.1016/s0161-6420(93)31446-6. [DOI] [PubMed] [Google Scholar]
- 18.Kreissig I, Rose D, Jost B. Long-term follow-up of iodine-125 brachytherapy for choroidal melanomas. Part 1: anatomical results and life expectancy. Eur J Ophthalmol. 1993;3:121–126. doi: 10.1177/112067219300300303. [DOI] [PubMed] [Google Scholar]
- 19.Quivey JM, Char DH, Phillips TL, et al. High intensity 125iodine (125I) plaque treatment of uveal melanoma. Int J Radiat Oncol Biol Phys. 1993;26:613–618. doi: 10.1016/0360-3016(93)90277-3. [DOI] [PubMed] [Google Scholar]
- 20.Quivey JM, Augsburger J, Snelling L, Brady LW. 125I plaque therapy for uveal melanoma: analysis of the impact of time and dose factors on local control. Cancer. 1996;77:2356–2362. doi: 10.1002/(SICI)1097-0142(19960601)77:11<2356::AID-CNCR26>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 21.De Potter P, Shields CL, Shields JA, et al. Plaque radiotherapy for juxtapapillary choroidal melanoma: visual acuity and survival outcome. Arch Ophthalmol. 1996;114:1357–1365. doi: 10.1001/archopht.1996.01100140557006. [DOI] [PubMed] [Google Scholar]
- 22.Gragoudas ES, Li W, Lane AM, et al. Risk factors for radiation maculopathy and papillopathy after intraocular irradiation. Ophthalmology. 1999;106:1571–1577. doi: 10.1016/S0161-6420(99)90455-4. [DOI] [PubMed] [Google Scholar]
- 23.Gündüz K, Shields CL, Shields JA, et al. Radiation retinopathy following plaque radiotherapy for posterior uveal melanoma. Arch Ophthalmol. 1999;117:609–614. doi: 10.1001/archopht.117.5.609. [DOI] [PubMed] [Google Scholar]
- 24.Gündüz K, Shields CL, Shields JA, et al. Radiation complications and tumor control after plaque radiotherapy of choroidal melanoma with macular involvement. Am J Ophthalmol. 1999;127:579–589. doi: 10.1016/s0002-9394(98)00445-0. [DOI] [PubMed] [Google Scholar]
- 25.Finger PT, Berson A, Ng T, Szechter A. Palladium-103 plaque radiotherapy for choroidal melanoma: an 11-year study. Int J Radiat Oncol Biol Phys. 2002;54:1438–1445. doi: 10.1016/s0360-3016(02)03751-3. [DOI] [PubMed] [Google Scholar]
- 26.Lommatzsch PK, Alberti W, Lommatzsch R, Rohrwacher F. Radiation effects on the optic nerve observed after brachytherapy of choroidal melanomas with 106Ru/106Rh plaques. Graefes Arch Clin Exp Ophthalmol. 1994;232:482–487. doi: 10.1007/BF00195358. [DOI] [PubMed] [Google Scholar]
- 27.Jensen AW, Petersen IA, Kline RW, et al. Radiation complications and tumor control after 125I plaque brachytherapy for ocular melanoma. Int J Radiat Oncol Biol Phys. 2005;63:101–108. doi: 10.1016/j.ijrobp.2005.01.022. [DOI] [PubMed] [Google Scholar]
- 28.Earle J, Kline RK, Robertson DM. Selection of iodine 125 for the Collaborative Ocular Melamona Study. Arch Ophthalmol. 1987;105:763–764. doi: 10.1001/archopht.1987.01060060049030. [DOI] [PubMed] [Google Scholar]
- 29.Garretson BR, Robertson DM, Earle JD. Choroidal melanoma treatment with iodine 125 brachytherapy. Arch Ophthalmol. 1987;105:1394–1397. doi: 10.1001/archopht.1987.01060100096035. [DOI] [PubMed] [Google Scholar]
- 30.Char DH, Castro JR, Quivey JM, et al. Uveal melanoma radiation: 125I brachytherapy versus helium ion irradiation. Ophthalmology. 1989;96:1708–1715. [PubMed] [Google Scholar]
- 31.Wilson MW, Hungerford JL. Comparison of episcleral plaque and proton beam radiation therapy for the treatment of choroidal melanoma. Ophthalmology. 1999;106:1579–1587. doi: 10.1016/S0161-6420(99)90456-6. [DOI] [PubMed] [Google Scholar]
- 32.Archer DB, Amoaku WM, Gardiner TA. Radiation retinopathy: clinical, histopathological, ultrastructural and experimental correlations. Eye. 1991;5:239–251. doi: 10.1038/eye.1991.39. [DOI] [PubMed] [Google Scholar]
- 33.Guyer DR, Mukai S, Egan KM, et al. Radiation maculopathy after proton beam irradiation for choroidal melanoma. Ophthalmology. 1992;99:1278–1285. doi: 10.1016/s0161-6420(92)31832-9. [DOI] [PubMed] [Google Scholar]
- 34.Design and methods of a clinical trial for a rare condition: the Collaborative Ocular Melanoma Study. COMS report number 3. Control Clin Trials. 1993;14:362–391. doi: 10.1016/0197-2456(93)90052-f. [DOI] [PubMed] [Google Scholar]
- 35.Nath R, Anderson LL, Luxton G, et al. Dosimetry of interstitial brachytherapy sources: recommendations of the AAPM Radiation Therapy Committee Task Group No. 43. Med Phys. 1995;22:209–234. doi: 10.1118/1.597458. [DOI] [PubMed] [Google Scholar]
- 36.Ray SK, Bhatnagar R, Hartsell WF, Desai GR. Review of eye plaque dosimetry based on AAPM Task Group 43 recommendations. Int J Radiat Oncol Biol Phys. 1998;41:701–706. doi: 10.1016/s0360-3016(97)00568-3. [DOI] [PubMed] [Google Scholar]
- 37.Jonckheere AR. A distribution-free K-sample test against ordered alternatives. Biometrika. 1954;41:133–145. [Google Scholar]
- 38.Zeger SL, Liang KY, Albert PS. Models for longitudinal data: a generalized estimating equation approach. Biometrics. 1988;44:1049–1060. [PubMed] [Google Scholar]
- 39.Collaborative Ocular Melanoma Study Group. Incidence of cataract and outcomes after cataract surgery in the first 5 years after iodine 125 brachytherapy in the Collaborative Ocular Melanoma Study. COMS report no. 27. Ophthalmology. 2007;114:1363–1371. doi: 10.1016/j.ophtha.2006.10.039. [DOI] [PubMed] [Google Scholar]
- 40.Irvine AR, Wood IS. Radiation retinopathy as an experimental model for ischemic proliferative retinopathy and rubeosis iridis. Am J Ophthalmol. 1987;103:790–797. doi: 10.1016/s0002-9394(14)74395-8. [DOI] [PubMed] [Google Scholar]
- 41.Amoaku WM, Archer DB. Cephalic radiation and retinal vasculopathy. Eye. 1990;4:195–203. doi: 10.1038/eye.1990.26. [DOI] [PubMed] [Google Scholar]
- 42.Viebahn M, Barricks ME, Osterloh MD. Synergism between diabetic and radiation retinopathy: case report and review. Br J Ophthalmol. 1991;75:629–632. doi: 10.1136/bjo.75.10.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kinyoun JL, Lawrence BS, Barlow WE. Proliferative radiation retinopathy. Arch Ophthalmol. 1996;114:1097–1100. doi: 10.1001/archopht.1996.01100140299007. [DOI] [PubMed] [Google Scholar]
- 44.Collaborative Ocular Melanoma Study (COMS) Group. The COMS randomized trial of iodine 125 brachytherapy for choroidal melanoma: V. Twelve-year mortality rates and prognostic factors: COMS report no. 28. Arch Ophthalmol. 2006;124:1684–1693. doi: 10.1001/archopht.124.12.1684. [DOI] [PubMed] [Google Scholar]
- 45.Amoaku WM, Lafaut B, Sallet G, De Laey JJ. Radiation choroidal vasculopathy: an indocyanine green angiography study. Eye. 1995;9:738–744. doi: 10.1038/eye.1995.187. [DOI] [PubMed] [Google Scholar]