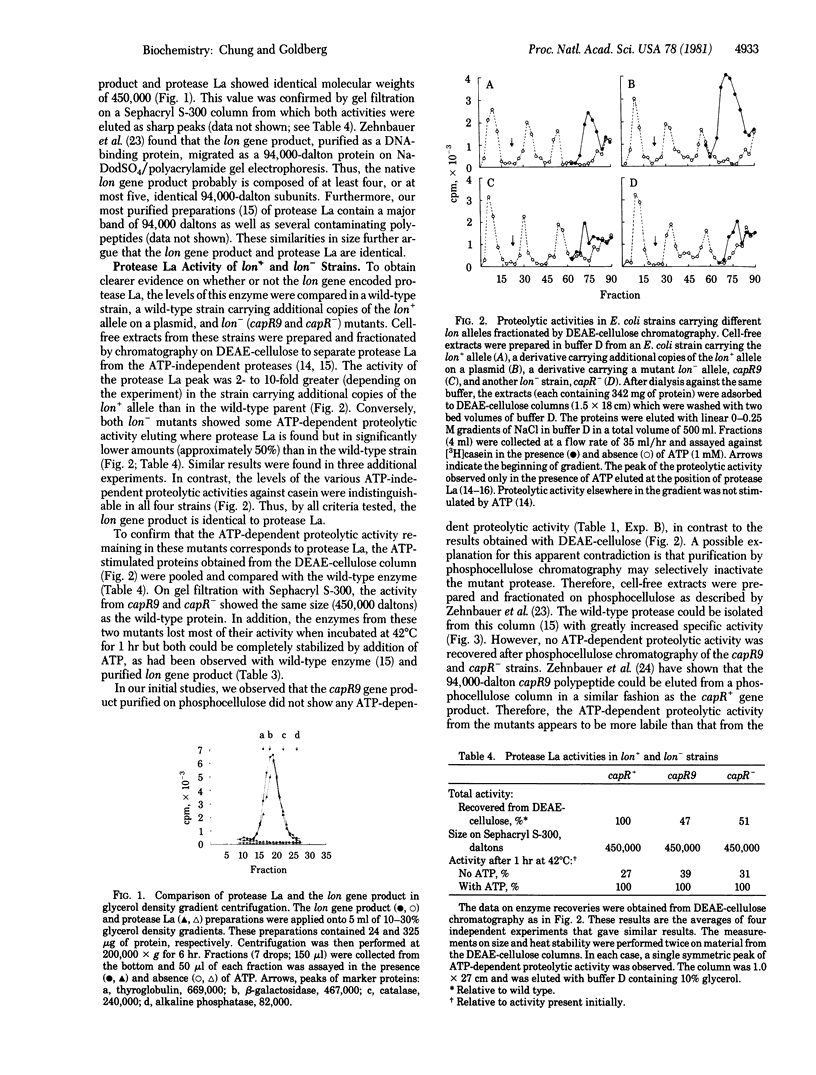
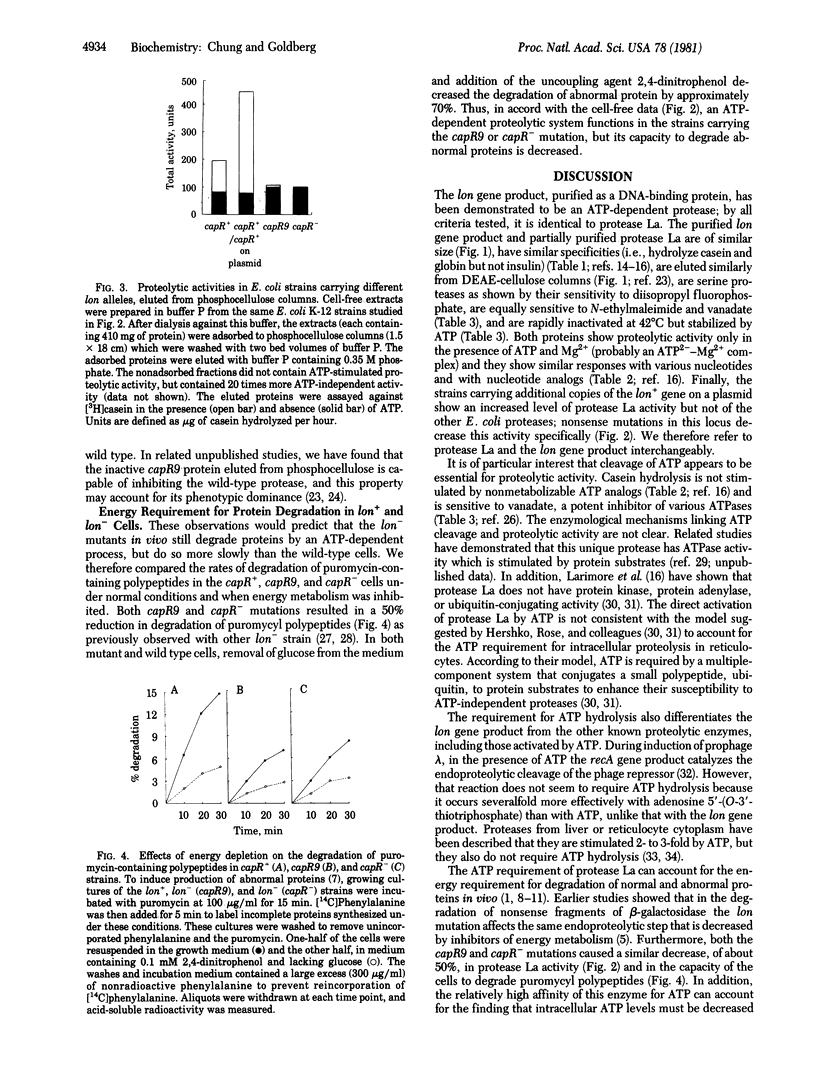
Abstract
In Escherichia coli, degradation of abnormal proteins is an energy-requiring process; it is decreased in mutants in the lon (capR or deg) gene. We find that the protein encoded by the lon gene is an ATP-dependent protease and is identical to protease La, recently described in E. coli. Both proteins are serine proteases that hydrolyze casein and globin, but not insulin, in the presence of ATP and Mg2+. Both respond to ATP, less well to other nucleoside triphosphates, and not to nonhydrolyzable ATP analogs. The purified lon protein has an apparent Mr of 450,000 and appears to be composed of four identical subunits. Its size, chromatographic behavior, and sensitivity to various inhibitors and heat are indistinguishable from those of protease La. Moreover, in a strain that carries additional copies of the lon+ allele on a plasmid, the content of protease La, but not of other proteases, is 2- to 10-fold greater than in the lon+ parent strain. Strains carrying the nonsense mutations capR9 and capR- also contain this ATP-dependent proteolytic activity, but it is present in substantially lower amounts and is inactivated by phosphocellulose chromatography, unlike the wild-type enzyme. Degradation of abnormal proteins in these lon- strains, which is slower than in the wild type, still requires ATP. Alterations in the ATP-dependent protease in the lon- mutants can account for the defect in intracellular proteolysis and perhaps also for the other phenotypic effects of this pleiotropic gene.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ADLER H. I., HARDIGREE A. A. ANALYSIS OF A GENE CONTROLLING CELL DIVISION AND SENSITIVITY TO RADIATION IN ESCHERICHIA COLI. J Bacteriol. 1964 Mar;87:720–726. doi: 10.1128/jb.87.3.720-726.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukhari A. I., Zipser D. Mutants of Escherichia coli with a defect in the degradation of nonsense fragments. Nat New Biol. 1973 Jun 20;243(129):238–241. doi: 10.1038/newbio243238a0. [DOI] [PubMed] [Google Scholar]
- Cantley L. C., Jr, Aisen P. The fate of cytoplasmic vanadium. Implications on (NA,K)-ATPase inhibition. J Biol Chem. 1979 Mar 25;254(6):1781–1784. [PubMed] [Google Scholar]
- Charette M. F., Henderson G. W., Markovitz A. ATP hydrolysis-dependent protease activity of the lon (capR) protein of Escherichia coli K-12. Proc Natl Acad Sci U S A. 1981 Aug;78(8):4728–4732. doi: 10.1073/pnas.78.8.4728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciechanover A., Heller H., Elias S., Haas A. L., Hershko A. ATP-dependent conjugation of reticulocyte proteins with the polypeptide required for protein degradation. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1365–1368. doi: 10.1073/pnas.77.3.1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig N. L., Roberts J. W. E. coli recA protein-directed cleavage of phage lambda repressor requires polynucleotide. Nature. 1980 Jan 3;283(5742):26–30. doi: 10.1038/283026a0. [DOI] [PubMed] [Google Scholar]
- DeMartino G. N., Goldberg A. L. Identification and partial purification of an ATP-stimulated alkaline protease in rat liver. J Biol Chem. 1979 May 25;254(10):3712–3715. [PubMed] [Google Scholar]
- Donch J., Greenberg J. Genetic analysis of lon mutants of strain K-12 of Escherichia coli. Mol Gen Genet. 1968;103(2):105–115. doi: 10.1007/BF00427138. [DOI] [PubMed] [Google Scholar]
- Goldberg A. L. Correlation between rates of degradation of bacterial proteins in vivo and their sensitivity to proteases. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2640–2644. doi: 10.1073/pnas.69.9.2640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg A. L., Dice J. F. Intracellular protein degradation in mammalian and bacterial cells. Annu Rev Biochem. 1974;43(0):835–869. doi: 10.1146/annurev.bi.43.070174.004155. [DOI] [PubMed] [Google Scholar]
- Goldberg A. L., St John A. C. Intracellular protein degradation in mammalian and bacterial cells: Part 2. Annu Rev Biochem. 1976;45:747–803. doi: 10.1146/annurev.bi.45.070176.003531. [DOI] [PubMed] [Google Scholar]
- Goldschmidt R. In vivo degradation of nonsense fragments in E. coli. Nature. 1970 Dec 19;228(5277):1151–1154. doi: 10.1038/2281151a0. [DOI] [PubMed] [Google Scholar]
- Gottesman S., Gottesman M., Shaw J. E., Pearson M. L. Protein degradation in E. coli: the lon mutation and bacteriophage lambda N and cII protein stability. Cell. 1981 Apr;24(1):225–233. doi: 10.1016/0092-8674(81)90518-3. [DOI] [PubMed] [Google Scholar]
- Gottesman S., Zipser D. Deg phenotype of Escherichia coli lon mutants. J Bacteriol. 1978 Feb;133(2):844–851. doi: 10.1128/jb.133.2.844-851.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOWARD-FLANDERS P., SIMSON E., THERIOT L. A LOCUS THAT CONTROLS FILAMENT FORMATION AND SENSITIVITY TO RADIATION IN ESCHERICHIA COLI K-12. Genetics. 1964 Feb;49:237–246. doi: 10.1093/genetics/49.2.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hershko A., Ciechanover A., Heller H., Haas A. L., Rose I. A. Proposed role of ATP in protein breakdown: conjugation of protein with multiple chains of the polypeptide of ATP-dependent proteolysis. Proc Natl Acad Sci U S A. 1980 Apr;77(4):1783–1786. doi: 10.1073/pnas.77.4.1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn J. P., Dodd J. Monosynaptic muscarinic activation of K+ conductance underlies the slow inhibitory postsynaptic potential in sympathetic ganglia. Nature. 1981 Aug 13;292(5824):625–627. doi: 10.1038/292625a0. [DOI] [PubMed] [Google Scholar]
- Kirby E. P., Ruff W. L., Goldthwait D. A. Cell division and prophage induction in Escherichia coli: effects of pantoyl lactone and various furan derivatives. J Bacteriol. 1972 Aug;111(2):447–453. doi: 10.1128/jb.111.2.447-453.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowit J. D., Goldberg A. L. Intermediate steps in the degradation of a specific abnormal protein in Escherichia coli. J Biol Chem. 1977 Dec 10;252(23):8350–8357. [PubMed] [Google Scholar]
- Murakami K., Voellmy R., Goldberg A. L. Protein degradation is stimulated by ATP in extracts of Escherichia coli. J Biol Chem. 1979 Sep 10;254(17):8194–8200. [PubMed] [Google Scholar]
- Pine M. J. Turnover of intracellular proteins. Annu Rev Microbiol. 1972;26:103–126. doi: 10.1146/annurev.mi.26.100172.000535. [DOI] [PubMed] [Google Scholar]
- Rice R. H., Means G. E. Radioactive labeling of proteins in vitro. J Biol Chem. 1971 Feb 10;246(3):831–832. [PubMed] [Google Scholar]
- Shineberg B., Zipser D. The ion gene and degradation of beta-galactosidase nonsense fragments. J Bacteriol. 1973 Dec;116(3):1469–1471. doi: 10.1128/jb.116.3.1469-1471.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon L. D., Gottesman M., Tomczak K., Gottesman S. Hyperdegradation of proteins in Escherichia coli rho mutants. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1623–1627. doi: 10.1073/pnas.76.4.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voellmy R. W., Goldberg A. L. ATP-stimulated endoprotease is associated with the cell membrane of E. coli. Nature. 1981 Apr 2;290(5805):419–421. doi: 10.1038/290419a0. [DOI] [PubMed] [Google Scholar]
- Zehnbauer B. A., Foley E. C., Henderson G. W., Markovitz A. Identification and purification of the Lon+ (capR+) gene product, a DNA-binding protein. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2043–2047. doi: 10.1073/pnas.78.4.2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zehnbauer B. A., Markovitz A. Cloning of gene lon (capR) of Escherichia coli K-12 and identification of polypeptides specified by the cloned deoxyribonucleic acid fragment. J Bacteriol. 1980 Aug;143(2):852–863. doi: 10.1128/jb.143.2.852-863.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]


