Abstract
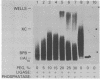
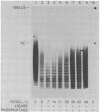
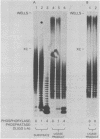
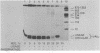
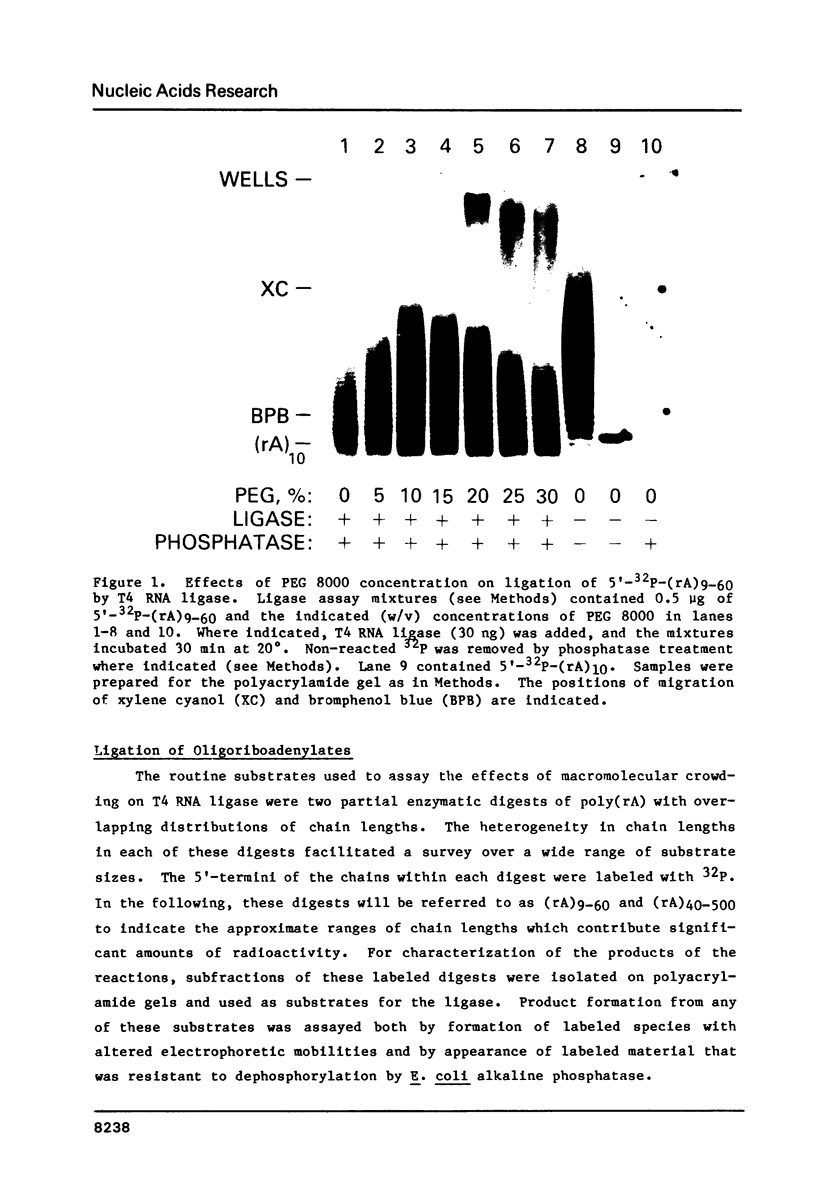
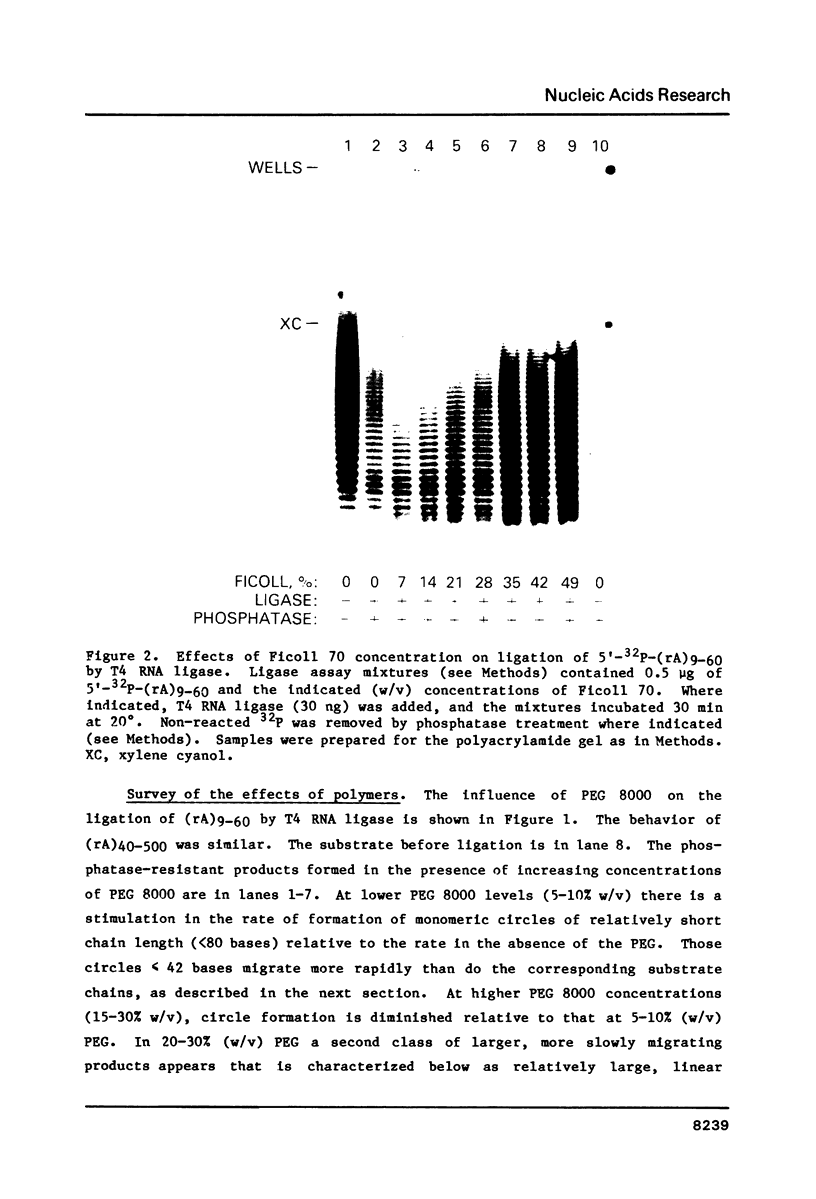
The effects of macromolecular crowding were tested on several reactions catalyzed by T4 RNA ligase. The rate of cyclization of oligoriboadenylates was stimulated up to 10-fold by relatively high concentrations of several polymers (polyethylene glycol (PEG) 8000 or 20,000; bovine plasma albumin; Ficoll 70). In addition, higher concentrations of PEG 8000 or PEG 20,000 allowed the novel formation of large linear products from the oligoriboadenylates. Also stimulated by high concentrations of PEG 8000 were the rate at which T4 RNA ligase joined p(dT)10 to oligoriboadenylates and the rate at which the enzyme activated p(dT)n by transfer of an adenylyl moiety from ATP to the oligonucleotides. These results with T4 RNA ligase are compared to earlier studies on the effects of crowding on DNA ligases.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dugaiczyk A., Boyer H. W., Goodman H. M. Ligation of EcoRI endonuclease-generated DNA fragments into linear and circular structures. J Mol Biol. 1975 Jul 25;96(1):171–184. doi: 10.1016/0022-2836(75)90189-8. [DOI] [PubMed] [Google Scholar]
- Eisenberg H., Felsenfeld G. Studies of the temperature-dependent conformation and phase separation of polyriboadenylic acid solutions at neutral pH. J Mol Biol. 1967 Nov 28;30(1):17–37. doi: 10.1016/0022-2836(67)90240-9. [DOI] [PubMed] [Google Scholar]
- Greer C. L., Javor B., Abelson J. RNA ligase in bacteria: formation of a 2',5' linkage by an E. coli extract. Cell. 1983 Jul;33(3):899–906. doi: 10.1016/0092-8674(83)90032-6. [DOI] [PubMed] [Google Scholar]
- Harvey C. L., Wright R. Ligase joining of oligodeoxythymidylates. Biochemistry. 1972 Jul 4;11(14):2667–2671. doi: 10.1021/bi00764a018. [DOI] [PubMed] [Google Scholar]
- Higgins N. P., Geballe A. P., Cozzarelli N. R. Addition of oligonucleotides to the 5'-terminus of DNA by T4 RNA ligase. Nucleic Acids Res. 1979 Mar;6(3):1013–1024. doi: 10.1093/nar/6.3.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann G., Klein T., Littauer U. Z. T4 RNA ligase: substrate chain length requirements. FEBS Lett. 1974 Sep 15;46(1):271–275. doi: 10.1016/0014-5793(74)80385-6. [DOI] [PubMed] [Google Scholar]
- Lerman L. S. A transition to a compact form of DNA in polymer solutions. Proc Natl Acad Sci U S A. 1971 Aug;68(8):1886–1890. doi: 10.1073/pnas.68.8.1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniatis T., Jeffrey A., van deSande H. Chain length determination of small double- and single-stranded DNA molecules by polyacrylamide gel electrophoresis. Biochemistry. 1975 Aug 26;14(17):3787–3794. doi: 10.1021/bi00688a010. [DOI] [PubMed] [Google Scholar]
- McCoy M. I., Gumport R. I. T4 ribonucleic acid ligase joins single-strand oligo(deoxyribonucleotides). Biochemistry. 1980 Feb 19;19(4):635–642. doi: 10.1021/bi00545a005. [DOI] [PubMed] [Google Scholar]
- Minton A. P. The effect of volume occupancy upon the thermodynamic activity of proteins: some biochemical consequences. Mol Cell Biochem. 1983;55(2):119–140. doi: 10.1007/BF00673707. [DOI] [PubMed] [Google Scholar]
- Pheiffer B. H., Zimmerman S. B. Polymer-stimulated ligation: enhanced blunt- or cohesive-end ligation of DNA or deoxyribooligonucleotides by T4 DNA ligase in polymer solutions. Nucleic Acids Res. 1983 Nov 25;11(22):7853–7871. doi: 10.1093/nar/11.22.7853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shore D., Baldwin R. L. Energetics of DNA twisting. I. Relation between twist and cyclization probability. J Mol Biol. 1983 Nov 15;170(4):957–981. doi: 10.1016/s0022-2836(83)80198-3. [DOI] [PubMed] [Google Scholar]
- Silber R., Malathi V. G., Hurwitz J. Purification and properties of bacteriophage T4-induced RNA ligase. Proc Natl Acad Sci U S A. 1972 Oct;69(10):3009–3013. doi: 10.1073/pnas.69.10.3009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snopek T. J., Sugino A., Agarwal K. L., Cozzarelli N. R. Catalysis of DNA joining by bacteriophage T4 RNA ligase. Biochem Biophys Res Commun. 1976 Jan 26;68(2):417–424. doi: 10.1016/0006-291x(76)91161-x. [DOI] [PubMed] [Google Scholar]
- Sugino A., Snoper T. J., Cozzarelli N. R. Bacteriophage T4 RNA ligase. Reaction intermediates and interaction of substrates. J Biol Chem. 1977 Mar 10;252(5):1732–1738. [PubMed] [Google Scholar]
- Zimmerman S. B., Pheiffer B. H. Macromolecular crowding allows blunt-end ligation by DNA ligases from rat liver or Escherichia coli. Proc Natl Acad Sci U S A. 1983 Oct;80(19):5852–5856. doi: 10.1073/pnas.80.19.5852. [DOI] [PMC free article] [PubMed] [Google Scholar]