Abstract
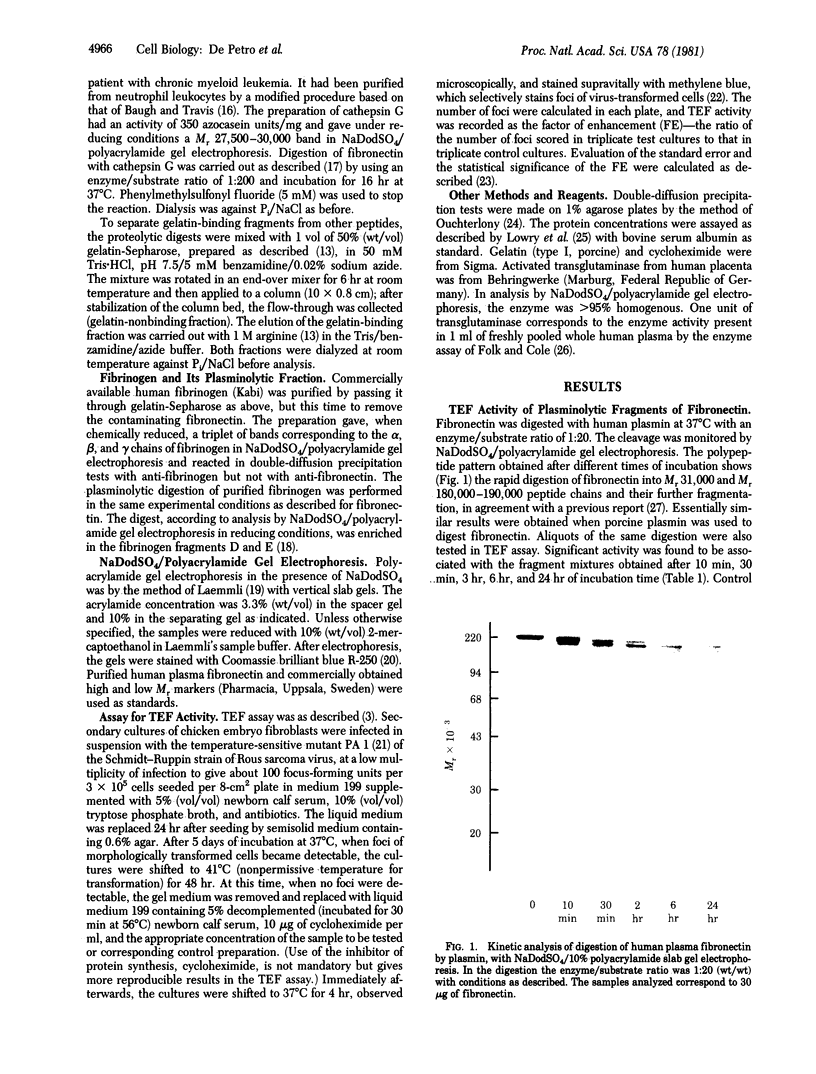
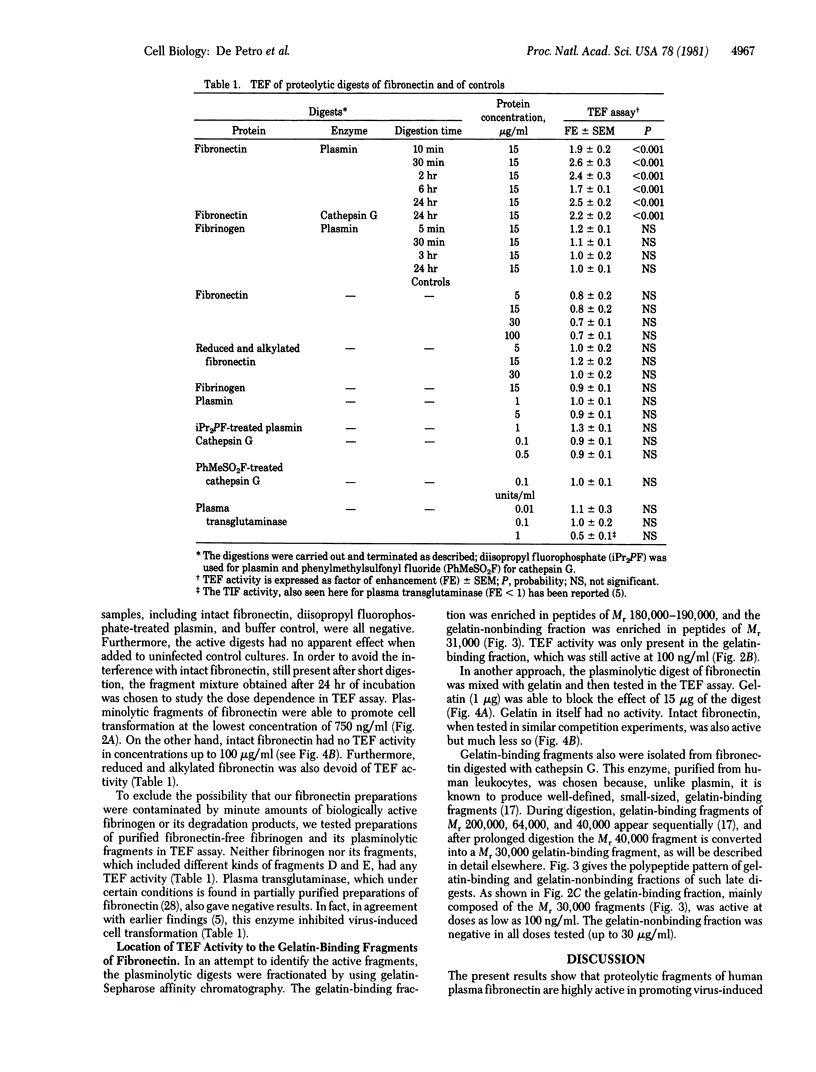
Studies have established that cryoprecipitates of the plasma of tumor patients contain a biological activity enhancing morphological cell transformation (transformation-enhancing factor; TEF) in cultures of chicken embryo fibroblasts infected with temperature-sensitive mutants of Rous sarcoma virus. We report here that similar TEF activity is effected by defined fragments of human plasma fibronectin obtained by limited digestion with major humoral or tissue proteinases. TEF activity was obtained from plasminolytic fragments of fibronectin and from cathepsin G-treated fibronectin. No activity was recorded from intact dimeric fibronectin or its reduced and alkylated subunits, from fibrinogen or its plasminolytic fragments, or from plasmin (EC 3.4.21.7) or cathepsin G (EC 3.4.21.20) treated or untreated with proteinase inhibitors. All of the TEF activity of the proteolytic fragments of fibronectin was located on the gelatin-binding peptides. The minimum effective doses in the TEF assay were 750 ng/ml of plasmin-treated fibronectin, 100 ng/ml of gelatin-binding plasminolytic fibronectin (enriched in Mr 180,000--190,000 polypeptides), and 100 ng/ml of gelatin-binding fragments of cathepsin G-treated fibronectin (enriched in a Mr 30,000 fragment). TEF activity of proteinase-treated fibronectin was inhibited by gelatin and by intact dimeric fibronectin. The potent TEF activity of proteolytic fragments of fibronectin raises the possibility that they may have a role in malignant transformation.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barlati S., Mignatti P., Kryceve C., Vigier P. Properties and kinetics of development of Rous sarcoma virus-infected cells evidenced by methylene blue staining. Intervirology. 1975;6(1):25–31. doi: 10.1159/000149450. [DOI] [PubMed] [Google Scholar]
- Baugh R. J., Travis J. Human leukocyte granule elastase: rapid isolation and characterization. Biochemistry. 1976 Feb 24;15(4):836–841. doi: 10.1021/bi00649a017. [DOI] [PubMed] [Google Scholar]
- Biquard J. M., Vigier P. Characteristics of a conditional mutant of Rous sarcoma virus defective in ability to transform cells at high temperature. Virology. 1972 Feb;47(2):444–455. doi: 10.1016/0042-6822(72)90280-2. [DOI] [PubMed] [Google Scholar]
- Blackett N. M. Statistical accuracy to be expected from cell colony assay; with special reference to the spleen colony assay. Cell Tissue Kinet. 1974 Jul;7(4):407–412. doi: 10.1111/j.1365-2184.1974.tb00423.x. [DOI] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Ferguson E. W., Fretto L. J., McKee P. A. A re-examination of the cleavage of fibrinogen and fibrin by plasmin. J Biol Chem. 1975 Sep 25;250(18):7210–7218. [PubMed] [Google Scholar]
- Folk J. E., Cole P. W. Transglutaminase: mechanistic features of the active site as determined by kinetic and inhibitor studies. Biochim Biophys Acta. 1966 Aug 10;122(2):244–264. doi: 10.1016/0926-6593(66)90066-x. [DOI] [PubMed] [Google Scholar]
- Gropp C., Egbring R., Havemann K. Fibrinogen split products, antiproteases and granulocytic elastase in patients with lung cancer. Eur J Cancer. 1980 May;16(5):679–685. doi: 10.1016/0014-2964(80)90209-1. [DOI] [PubMed] [Google Scholar]
- Jilek F., Hörmann H. Cold-insoluble globulin, II[1,2]. Cyanogen bromide and plasminolysis fragments containing a label introduced by transamidation. Hoppe Seylers Z Physiol Chem. 1977 Sep;358(9):1165–1168. [PubMed] [Google Scholar]
- Keski-Oja J., Mosher D. F., Vaheri A. Cross-linking of a major fibroblast surface-associated glycoprotein (fibronectin) catalyzed by blood coagulation factor XIII. Cell. 1976 Sep;9(1):29–35. doi: 10.1016/0092-8674(76)90049-0. [DOI] [PubMed] [Google Scholar]
- Krycéve C., Vigier P., Barlati S. Transformation-enhancing factor(s) produced by virus-transformed and established cells. Int J Cancer. 1976 Mar 15;17(3):370–379. doi: 10.1002/ijc.2910170314. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mignatti P., Ascari E., Barlati S. Potential diagnostic and prognostic significance of the transformation-enhancing factor(s) in the plasma cryoprecipitate of tumor patients. Int J Cancer. 1980 Jun 15;25(6):727–734. doi: 10.1002/ijc.2910250607. [DOI] [PubMed] [Google Scholar]
- Mosesson M. W., Umfleet R. A. The cold-insoluble globulin of human plasma. I. Purification, primary characterization, and relationship to fibrinogen and other cold-insoluble fraction components. J Biol Chem. 1970 Nov 10;245(21):5728–5736. [PubMed] [Google Scholar]
- Mosher D. F. Cross-linking of cold-insoluble globulin by fibrin-stabilizing factor. J Biol Chem. 1975 Aug 25;250(16):6614–6621. [PubMed] [Google Scholar]
- OUCHTERLONY O. Diffusion-in-gel methods for immunological analysis. Prog Allergy. 1958;5:1–78. [PubMed] [Google Scholar]
- Parsons R. G., Todd H. D., Kowal R. Isolation and identification of a human serum fibronectin-like protein elevated during malignant disease. Cancer Res. 1979 Nov;39(11):4341–4345. [PubMed] [Google Scholar]
- Peuscher F. W., Cleton F. J., Armstrong L., Stoepman-van Dalen E. A., van Mourik J. A., van Aken W. G. Significance of plasma fibrinopeptide A (fpA) in patients with malignancy. J Lab Clin Med. 1980 Jul;96(1):5–14. [PubMed] [Google Scholar]
- Quigley J. P. Phorbol ester-induced morphological changes in transformed chick fibroblasts: evidence for direct catalytic involvement of plasminogen activator. Cell. 1979 May;17(1):131–141. doi: 10.1016/0092-8674(79)90301-5. [DOI] [PubMed] [Google Scholar]
- Ruoslahti E., Vaheri A. Interaction of soluble fibroblast surface antigen with fribrinogen and fibrin. J Exp Med. 1975 Feb 1;141(2):497–501. doi: 10.1084/jem.141.2.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vartio T., Seppä H., Vaheri A. Susceptibility of soluble and matrix fibronectins to degradation by tissue proteinases, mast cell chymase and cathepsin G. J Biol Chem. 1981 Jan 10;256(1):471–477. [PubMed] [Google Scholar]
- Vuento M., Vaheri A. Purification of fibronectin from human plasma by affinity chromatography under non-denaturing conditions. Biochem J. 1979 Nov 1;183(2):331–337. doi: 10.1042/bj1830331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Petro G., Brega A., Mignatti P., Barlati S. Inhibition of Rous sarcoma-virus-induced transformation by proteins involved in blood coagulation. Int J Cancer. 1980 May 15;25(5):557–560. doi: 10.1002/ijc.2910250502. [DOI] [PubMed] [Google Scholar]