Abstract
We investigated the cell cycle modulation of dihydrofolate reductase (DHFR; tetrahydrofolate dehydrogenase, 7,8-dihydroxyfolate:NADP+ oxidoreductase, EC 1.5.1.3) levels in methotrexate-resistant Chinese hamster ovary cells synchronized by mitotic selection. DNA content and DHFR concentration were analyzed throughout the cell cycle by standard biochemical techniques and by double fluorescence staining utilizing the fluorescence-activated cell sorter. We found an S phase-specific period of DHFR biosynthetic activity. Commencing within hour 2 of S phase and continuing throughout the duration of S phase, there is a 90% increase in DHFR specific activity. This results from an approximately 2.5-fold increase in the level of DHFR, while total soluble protein increases 50% during the same period. This increase is the result of new synthesis of DHFR molecules initiated after the cell is physiologically committed to DNA replication. This increase in DHFR activity through S phage parallels the increasing rate of [3H]thymidine incorporation during the same interval. The maximum peak of DHFR activity is coincident with the maximum rate of DNA synthesis, both activities occurring during the bulk of DNA replication within the last stages of the 6.5-hr S phase.
Full text
PDF

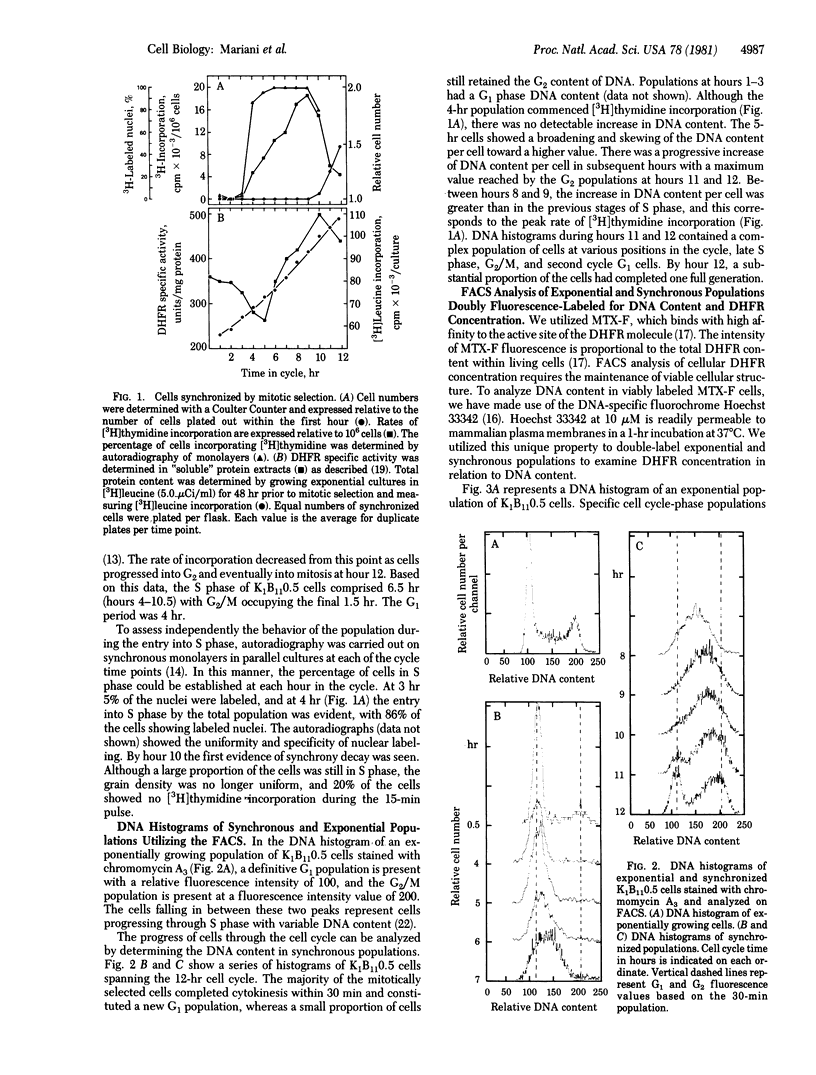
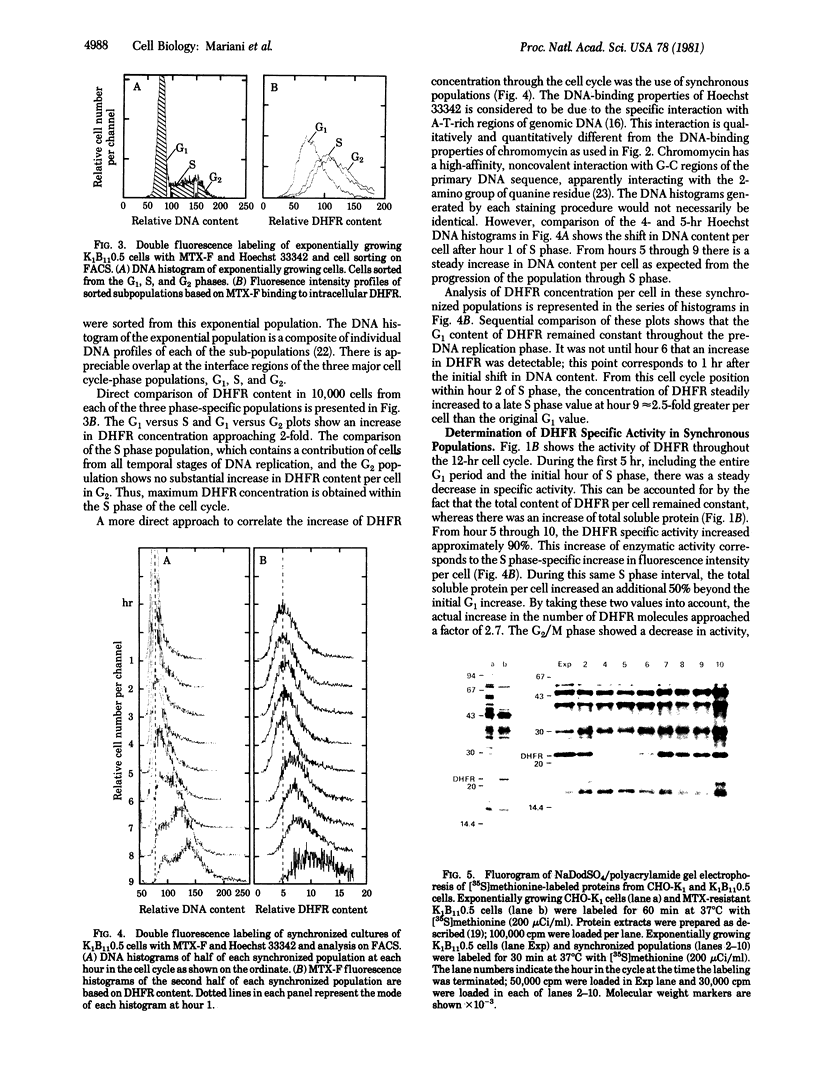

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alt F. W., Kellems R. E., Bertino J. R., Schimke R. T. Selective multiplication of dihydrofolate reductase genes in methotrexate-resistant variants of cultured murine cells. J Biol Chem. 1978 Mar 10;253(5):1357–1370. [PubMed] [Google Scholar]
- Alt F. W., Kellems R. E., Schimke R. T. Synthesis and degradation of folate reductase in sensitive and methotrexate-resistant lines of S-180 cells. J Biol Chem. 1976 May 25;251(10):3063–3074. [PubMed] [Google Scholar]
- Arndt-Jovin D. J., Jovin T. M. Analysis and sorting of living cells according to deoxyribonucleic acid content. J Histochem Cytochem. 1977 Jul;25(7):585–589. doi: 10.1177/25.7.70450. [DOI] [PubMed] [Google Scholar]
- Bonner W. A., Hulett H. R., Sweet R. G., Herzenberg L. A. Fluorescence activated cell sorting. Rev Sci Instrum. 1972 Mar;43(3):404–409. doi: 10.1063/1.1685647. [DOI] [PubMed] [Google Scholar]
- Brent T. P., Butler J. A., Crathorn A. R. Variations in phosphokinase activities during the cell cycle in synchronous populations of HeLa cells. Nature. 1965 Jul 10;207(993):176–177. doi: 10.1038/207176a0. [DOI] [PubMed] [Google Scholar]
- Conrad A. H. Thymidylate synthetase activity in cultured mammalian cells. J Biol Chem. 1971 Mar 10;246(5):1318–1323. [PubMed] [Google Scholar]
- Fried J. Analysis of deoxyribonucleic acid histograms from flow cytofluorometry. Estimation of the distribution of cells within S phase. J Histochem Cytochem. 1977 Jul;25(7):942–951. doi: 10.1177/25.7.894010. [DOI] [PubMed] [Google Scholar]
- Gelbard A. S., Kim J. H., Perez A. G. Fluctuations in deoxycytidine monophosphate deaminase activity during the cell cycle in synchronous populations of HeLa cells. Biochim Biophys Acta. 1969 Jun 17;182(2):564–566. doi: 10.1016/0005-2787(69)90209-3. [DOI] [PubMed] [Google Scholar]
- Gray J. W., Coffino P. Cell cycle analysis by flow cytometry. Methods Enzymol. 1979;58:233–248. doi: 10.1016/s0076-6879(79)58140-3. [DOI] [PubMed] [Google Scholar]
- Kaufman R. J., Bertino J. R., Schimke R. T. Quantitation of dihydrofolate reductase in individual parental and methotrexate-resistant murine cells. Use of a fluorescence activated cell sorter. J Biol Chem. 1978 Aug 25;253(16):5852–5860. [PubMed] [Google Scholar]
- Kellems R. E., Morhenn V. B., Pfendt E. A., Alt F. W., Schimke R. T. Polyoma virus and cyclic AMP-mediated control of dihydrofolate reductase mRNA abundance in methotrexate-resistant mouse fibroblasts. J Biol Chem. 1979 Jan 25;254(2):309–318. [PubMed] [Google Scholar]
- Kersten W., Kersten H., Szybalski W. Physicochemical properties of complexes between deoxyribonucleic acid and antibiotics which affect ribonucleic acid synthesis (actinomycin, daunomycin, cinerubin, nogalamycin, chormomycin, mithramycin, and olivomycin). Biochemistry. 1966 Jan;5(1):236–244. doi: 10.1021/bi00865a031. [DOI] [PubMed] [Google Scholar]
- Klevecz R. R., Keniston B. A. The temporal structure of S phase. Cell. 1975 Jun;5(2):195–203. doi: 10.1016/0092-8674(75)90027-6. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Littlefield J. W. The periodic synthesis of thymidine kinase in mouse fibroblasts. Biochim Biophys Acta. 1966 Feb 21;114(2):398–403. doi: 10.1016/0005-2787(66)90319-4. [DOI] [PubMed] [Google Scholar]
- Murphree S., Stubblefield E., Moore E. C. Synchronized mammalian cell cultures. 3. Variation of ribonucleotide reductase activity during the replication cycle of Chinese hamster fibroblasts. Exp Cell Res. 1969 Nov;58(1):118–124. doi: 10.1016/0014-4827(69)90121-9. [DOI] [PubMed] [Google Scholar]
- Navalgund L. G., Rossana C., Muench A. J., Johnson L. F. Cell cycle regulation of thymidylate synthetase gene expression in cultured mouse fibroblasts. J Biol Chem. 1980 Aug 10;255(15):7386–7390. [PubMed] [Google Scholar]
- Nunberg J. H., Kaufman R. J., Schimke R. T., Urlaub G., Chasin L. A. Amplified dihydrofolate reductase genes are localized to a homogeneously staining region of a single chromosome in a methotrexate-resistant Chinese hamster ovary cell line. Proc Natl Acad Sci U S A. 1978 Nov;75(11):5553–5556. doi: 10.1073/pnas.75.11.5553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardee A. B., Dubrow R., Hamlin J. L., Kletzien R. F. Animal cell cycle. Annu Rev Biochem. 1978;47:715–750. doi: 10.1146/annurev.bi.47.070178.003435. [DOI] [PubMed] [Google Scholar]
- Rothenberg S. P. A rapid radioassay for folic acid reductase and amethopterin. Anal Biochem. 1966 Jul;16(1):176–178. doi: 10.1016/0003-2697(66)90095-9. [DOI] [PubMed] [Google Scholar]
- Rønning O. W., Pettersen E. O., Seglen P. O. Protein synthesis and protein degradation through the cell cycle of human NHIK 3025 cells in vitro. Exp Cell Res. 1979 Oct 1;123(1):63–72. doi: 10.1016/0014-4827(79)90421-x. [DOI] [PubMed] [Google Scholar]
- Stein G. H., Yanishevsky R. Autoradiography. Methods Enzymol. 1979;58:279–292. doi: 10.1016/s0076-6879(79)58143-9. [DOI] [PubMed] [Google Scholar]
- Wiedemann L. M., Johnson L. F. Regulation of dihydrofolate reductase synthesis in an overproducing 3T6 cell line during transition from resting to growing state. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2818–2822. doi: 10.1073/pnas.76.6.2818. [DOI] [PMC free article] [PubMed] [Google Scholar]