Abstract
BACKGROUND
Mutations in the type I ryanodine receptor gene (RYR1) result in malignant hyperthermia, a pharmacogenetic disorder typically triggered by administration of anesthetics. However, cases of sudden death during exertion, heat challenge, and febrile illness in the absence of triggering drugs have been reported. The underlying causes of such drug-free fatal “awake” episodes are unknown.
METHODS
De novo R3983C variant in RYR1 was identified in two unrelated children that experienced fatal, nonanesthetic awake episodes associated with febrile illness and heat stress. One of the children also possessed a second novel maternally-inherited D4505H variant located on a separate haplotype. Effects of all possible heterotypic expression conditions on RYR1 sensitivity to caffeine-induced Ca2+ release were determined in expressing RyR1-null myotubes.
RESULTS
Compared to wild-type RYR1 alone (EC50 = 2.85 ± 0.49 mM), average (±SEM) caffeine sensitivity of Ca2+ release was modestly increased following coexpression with either R3983C (EC50 = 2.00 ± 0.39 mM) or D4505H (EC50 = 1.64 ± 0.24 mM). Remarkably, coexpression of wild-type RYR1 with the double mutant in cis (R3983C-D4505H) produced a significantly stronger sensitization of caffeine-induced Ca2+ release (EC50 = 0.64 ± 0.17 mM) compared to that observed following coexpression of the two variants on separate subunits (EC50 = 1.53 ± 0.18 mM).
CONCLUSIONS
The R3983C mutation potentiates D4505H-mediated sensitization of caffeine-induced RYR1 Ca2+ release when the mutations are in cis (on the same subunit), but not when present on separate subunits. Nevertheless, coexpression of the two variants on separate subunits still resulted in a ~2-fold increase in caffeine sensitivity, consistent with the observed awake episodes and heat sensitivity.
Introduction
Malignant Hyperthermia (MH) manifests as a drug induced severe metabolic reaction in susceptible individuals that occurs with administration of potent inhalation anesthetics and/or depolarizing muscle relaxants.1 MH susceptibility (MHS) is a genetic predisposition that is usually inherited as an autosomal dominant trait. About 70% of MHS families carry mutations in the gene that encodes the type1 ryanodine receptor (RYR1),2 which functions as the Ca2+ release channel in the sarcoplasmic reticulum of skeletal muscle. With the exception of patients with RYR1 mutations associated with congenital myopathies, it is commonly thought that most MHS individuals are asymptomatic and lack clinical manifestations of muscle disease until challenged by anesthetic drugs.1
Increasing evidence indicates that a subset of MHS individuals develop MH-like symptoms during exercise, emotional stress, exposure to environmental heat stress, or a combination of these triggers. Indeed, several confirmed fulminant nonanesthetic or “awake” episodes that resulted in sudden death have been reported over the past decade.3–7 Such variability of MH expressivity has been attributed to a complex interaction between genetic, environmental, and other modulatory factors.8 However, the exact nature of the interaction between genetic MH predisposition with environmental triggers and modulators remains unknown. We report the clinical history and genetic analysis of two unrelated children who suffered fatal nonanesthetic awake episodes triggered by either a viral prodrome or exposure to environmental heat stress. Functional studies of the identified RYR1 variants homologously expressed in myotubes derived from RYR1-null mice demonstrate that allelic segregation and genetic background can be a critical, and heretofore unappreciated, modifying factor in the variable expressivity of MH.
Materials and Methods
Patients and Samples
Clinical histories and specimens from two children who died of nonanesthetic awake events were studied. The first patient (Case 1) experienced an anesthetic event suspicious for MH, followed by numerous non-anesthetic awake episodes. He was previously diagnosed as MH susceptible (MHS) by the caffeine-halothane contracture test.9 The second patient (Case 2) had not had anesthesia, but experienced a prior awake episode in the absence of anesthesia. Molecular genetic studies were approved for both cases by the Institutional Review Board of the Uniformed Services University of the Health Sciences, Bethesda, Maryland. After obtaining consents, family members of the two probands were also enrolled in genetic studies.
Case 1
A 7 month–old male underwent bilateral ptosis repair with general anesthesia. While breathing oxygen/halothane (0.5–1%)/nitrous oxide, he became “dusky and rigid.” Halothane was discontinued, 100% oxygen was given. Surgery was cancelled. Muscle rigidity resolved promptly, but tachycardia and tachypnea persisted. Rectal temperature peaked at 100.1°F (38°C). Creatine kinase was 1,883 IU/L 8 h later. Neurologic examination revealed mild hypotonia in the upper extremities without weakness. Histopathology was normal except for mild variation in fiber size with several atrophic Type 1 and 2 fibers. Electron microscopy detected no ultra-structural abnormalities.
At 20 months, he experienced his first nonanesthetic awake episode. On a warm day he awoke hot, flushed, and restless. Gradually his legs became extended and remained in extension for 60 – 80 min. At the local emergency room he was alert and oriented, but extremely warm and in respiratory distress. Vital signs were: heart rate = 176/min, respiratory rate = 62/min, temperature = 104°F (40°C). Laboratory results were normal, except bicarbonate of 18mEq/l. He received intravenous fluids, oxygen, external cooling and antipyretics. Later the same day, he experienced a recurrence with temperature of 105.1°F (40.6°C) and rigidity of his lower limbs. Resolution followed aggressive cooling and antipyretics (paracetamol) over 60–80 minutes. Electrolytes were normal and creatine kinase was 7,525 IU/L 24 h later. The presence of short stature, congenital ptosis, mild hypotonia of the upper extremities and malignant hyperthermia susceptibility suggested central core disease and King Denborough Syndrome be considered in the differential diagnosis. However, the child did not appear dysmorphic, a key feature of King Denborough Syndrome, to an experienced pediatric neurologist.10
Over the next 3 yr, he had numerous episodes starting with leg cramping, tachycardia and tachypnea, and elevated creatine kinase that varied in severity. Mild upper respiratory and/or gastrointestinal infections were often present, but some episodes occurred in the absence of infection. Mild episodes usually resolved within 30 min after prompt administration of acetaminophen and cooling. Severe episodes required hospitalization and intravenous dantrolene. One emergency room report noted that he was “profusely diaphoretic, with stiff extended limbs and exaggerated lordosis.” Episodes, marked by elevated temperature as high as 105°F (40.6°C), serum creatine kinase (100,000 IU/L), myoglobinuria, and serum potassium (8.0 mEq/L) required IV dantrolene. On occasion, muscle cramping involved the abdominal, neck, wrist and hand muscles. Since these episodes responded well to dantrolene, his mother requested that he be placed on low dose oral dantrolene as prophylaxis whenever he developed a febrile illness. This approach appeared to reduce the frequency of episodes.
At age 5 yr he exhibited marked muscle hypertrophy. Nerve conduction studies of the peroneal and sural nerves were within normal limits. Electromyography examination of the anterior tibialis muscle was consistent with a congenital myopathic pattern of muscle discharges. Lumbar lordosis with truncal weakness was noted.
When he underwent another ptosis surgery, muscle was obtained for caffeine-halothane contracture test and histopathological examination of the muscle samples. The caffeine-halothane contracture test was markedly positive with a mean response of 8.5 g contracture in the presence of 3% halothane and 2.4 g contracture in the presence of 2 mM caffeine. This second histological analysis revealed non-specific changes such as scattered type II atrophic fibers, a few angular fibers, increased internal nuclei and occasional subsarcolemmal crescents. There was no evidence of cores. Differential diagnoses included atypical carnitine palmitoyltransferase II deficiency and an incomplete form of King Denborough Syndrome. Muscle stiffness with high fever in conjunction with mild infections continued throughout his life. At 9 yr, he died enroute to the hospital during another episode. There was no family history of MH or other neuromuscular disorders.
Case 2:
A 6-yr-old girl presented to the hospital with fever (102.7°F or 39.3°C), muscular rigidity, masseter spasm and vomiting. Over a few hours, her symptoms worsened. Seizures appeared. Diazepam and ketorolac were given, but muscle rigidity persisted. Oxygen saturation decreased as her breathing became labored. Rectal temperature increased to 108°F (42.2°C). Asystole was treated with cardiopulmonary resuscitation. Dantrolene, 10 mg/kg IV, was given but did not reverse the rigidity. Acidosis (pH 6.6) and hyperkalemia (>10.0 mmol/L) were severe. She died after 2 h of cardiopulmonary resuscitation. Autopsy was unremarkable except for mild chronic upper airway inflammation, reflective of a recent viral illness.
Her medical history was notable for mild facial nerve palsy (Bell’s) in infancy. She experienced a spontaneous episode of high fever (>105°F or 40.6°C) at 4 yr of age, accompanied by total body and jaw muscle rigidity following a day playing at the beach. Symptoms were promptly reversed by immersion in ice bath, followed by oral antipyretics and IV fluids. In a local hospital that day, she experienced an explosive bout of diarrhea. Stool testing was positive for rotavirus. She had hypertrophy of the lower extremity muscles. There was no family history of MH or other neuromuscular disorders.
Candidate gene and haplotype analysis
All 106 RYR1 exons were analyzed in both cases with the use of genomic DNA extracted from blood lymphocytes using standard methods. In addition, exons 2 and 24 of the α1 subunit of voltage gated L-type calcium channel gene (CACNA1S) and exons 1, 3 and 4 of the carnitine palmitoyltransferase II gene (CPT2) were analyzed in Case 1. The primers used for amplifying and sequencing of 106 RYR1 exons (supplemental digital content 1, table) were designed using the Primer3 software (Broad Institute, Boston, MA). Amplified products were cleaned and sequenced. Genotyping of family members was conducted with the use of four microsatellite markers from 19q12-q13.2.11 Haplotypes were determined using marker allele segregation in the pedigrees of both families. In Case 2, a 1.8kb fragment spanning codons encoding the identified mutations sites (corresponding 3983 and 4505) was generated using skeletal muscle message RNA. The amplified sequences were subcloned and 30 different colonies were selected for sequencing to chromosomal origin of the R3983C and D4505H variants in Case 2 (supplemental digital content 2).
Identified RYR1 variants in subjects and population controls
A heterozygous nucleotide substitution c.11947C>T (NM_000540.2) resulting in p.Arg3983Cys (R3983C) substitution in exon 87 was identified in both cases. A second heterozygous nucleotide substitution c.13513G>C resulting in p.Asp4505His (D4505H) substitution in exon 92 was also identified in Case 2. The frequency of each RYR1 variant was estimated in 100 MHS subjects. Healthy, unrelated Caucasian controls (N = 150) were screened for the presence of the identified variants. All samples were previously collected for other studies and were made available for this study without personal information.12,13
Preparation and nuclear cDNA injections of RYR1-null myotubes
The two identified RYR1 variants (R3983C and D4505H) and a double variant (R3983C-D4505H) were introduced into a full-length rabbit RYR1 complementary DNA (cDNA) (accession #X15750) using standard two-step site-directed mutagenesis.14,15 All sequences generated and modified by polymerase chain reaction were checked for integrity by sequence analysis. Myotubes were prepared from primary cultures of myoblasts obtained from skeletal muscle of newborn RYR1-null (dyspedic) mice as described previously.14,15 All animals were housed in a pathogen-free area at the University of Rochester, Rochester, New York and experiments performed in accordance with procedures reviewed and approved by the local University Committees on Animal Resources. Expression of wild-type (WT) RYR1 or either variant was achieved by direct microinjection of myotube nuclei with cDNA mixtures including CD8 (0.1 µg/μl) plus the appropriate RYR1 expression plasmid (0.5 µg/µl).14,15 In coexpression experiments, nuclei of dyspedic myotubes were microinjected with a 1:1 cDNA mixture (0.25 µg/µl each) of two plasmids out of all four possible heterotypic combinations (WT+R3983C, WT+D4505H, R3983C+D4505H, and WT+R3983C-D4505H). Expressing myotubes were identified 2–4 days after nuclear microinjection by incubation with CD8 antibody-coated beads as described previously.14,15
Intracellular Ca2+ Measurements
Intracellular Ca2+ measurements were obtained from Indo-1 AM-loaded myotubes as described previously.14,15 Briefly, myotubes grown on glass bottom dishes were loaded with 6 µM Indo-1AM for 1 h at 37°C in a normal rodent Ringer’s solution consisting of (in mM): 145 NaCl, 5 KCl, 2 CaCl2, 1 MgCl2, 10 HEPES, pH 7.4. Cytosolic dye within a small rectangular region of the expressing myotube was excited at 350 ± 10 nm and fluorescence emission at 405 ± 30 nm (F405) and 485 ± 25 nm (F485) was recorded using a photomultiplier detection system with results presented as the ratio of F405 and F485 (R=F405/F485).
Caffeine concentration-response curves were obtained by sequential exposure of expressing myotubes to increasing concentrations of caffeine applied through a rapid (<5 s response time) local perfusion system. For these experiments, expressing myotubes were exposed for 30 seconds to different concentrations of caffeine (0.1, 0.3, 0.7, 1.0, 2.0, 3.0, 10, and 30 mM) with each concentration followed by a 30 s wash with control solution. Relative caffeine-induced changes in intracellular Ca2+ were expressed as changes in indo-1 ratio (ΔRatio = Rcaffeine − Rbaseline, where Rcaffeine is the peak indo-1 ratio observed during caffeine application and Rbaseline is the resting indo-1 ratio observed just prior to caffeine addition) and plotted against caffeine concentration.
Statistical Analyses
Caffeine concentration-response curves were fitted with the following 3-parameter Hill equation:
Y= Fmax/(1+[EC50/x]n)
where Fmax is the maximal change in indo-1 ratio, EC50 is the concentrations for half-maximal activation, and n is the Hill coefficient. EC50 values were obtained from a total of 128 myotube concentration-response experiments. Following log-transformation of the data, one EC50 value was determined an outlier based on the Grubbs’ test, and thus, discarded from the final statistical analyses. All results are given as means ± SEM with statistical significance (p < 0.05, two-tailed) determined using either a Student’s t test for comparison between two groups or a one-way analysis of variance (ANOVA) and post hoc Duncan’s test for multiple comparisons (identical results were also obtained using Student-Newman-Keuls and Fisher’s Least Significant Difference tests). All curve-fitting and statistical analyses were conducted using SigmaPlot10 and SigmaStat software suites (Systat Software Inc., San Jose, CA).
Results
Identification of RYR1 variants of highly conserved residues in patients
A heterozygous RYR1 nucleotide change c.11947C>T (NM_000540.2) in exon 87 resulting in a p.Arg3983Cys (R3983C) amino acid substitution was identified in both cases (supplemental digital content 3, figure). The R3983C substitution was further screened in 98 previously reported MHS individuals from North America and in 150 controls (the Caucasian population in the United States) with negative results. Identification of two index cases with R3983C mutation in 100 North American MHS individuals provides an estimated carrier frequency of ~2%. No additional nonsynonomous RYR1 variants were identified in Case 1 (supplemental digital content 4, table). However, a second heterozygous RYR1 nucleotide variation c.13513G>C resulting in a p.Asp4505His (D4505H) substitution in exon 92 was identified in Case 2 (supplemental digital content 2). The D4505H variant was not found in either 100 previously reported MHS individuals from North America or in 100 controls representing the Caucasian population in the United States.
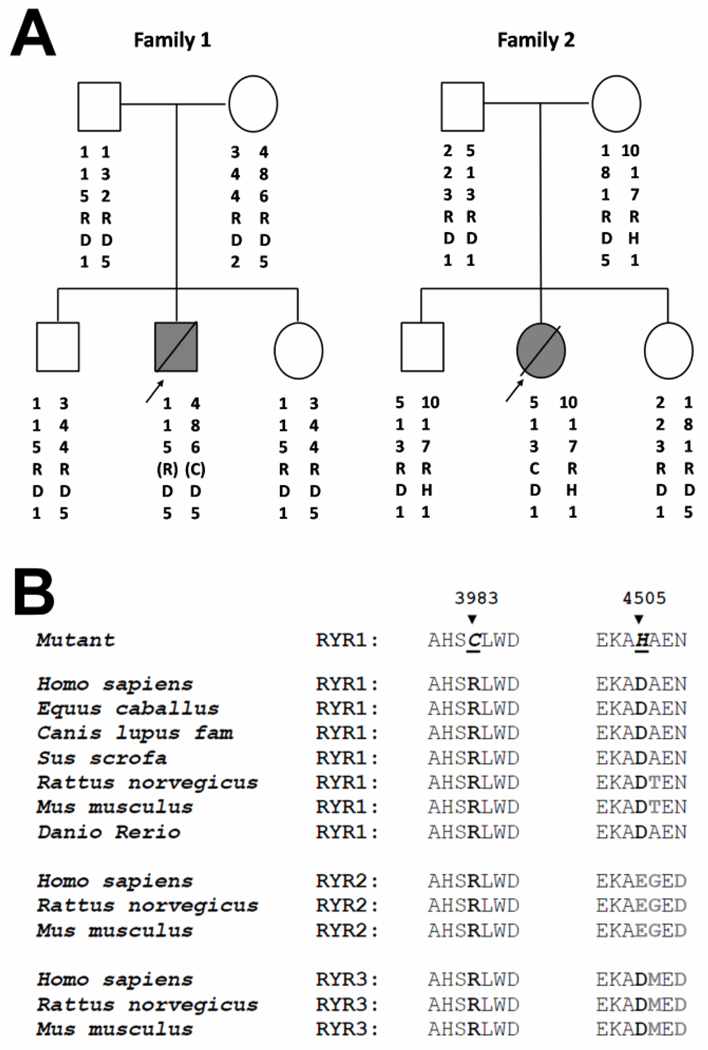
The R3983C variant was absent in the parents of both children (fig. 1A). Four microsatellite markers tightly linked to the RYR1 region confirmed paternity and maternity, as well as the inheritance of these markers in the two families (fig. 1A). In addition, genotyping showed segregation of different haplotypes in each family indicating that the two families are unrelated. These data demonstrate that the R3983C variant occurred de novo in both probands. In the second family, a novel D4505H variant was identified on a separate haplotype from the R3983C variant (supplemental digital content 2) and was also present in both the patient’s mother and brother. Segregation analysis of microsatellite markers and six single single nucleotide polymorphism within the RYR1 gene (data not shown) in the second family showed association of the 4505H variant with the maternal 10-1-7-1 haplotype (fig. 1A). The two identified RYR1 residues are highly conserved across a wide range of species and are also conserved in the type 2 and 3 ryanodine receptor isoforms (RYR2 and RYR3, respectively) (fig. 1B).
Figure 1.
A) Pedigrees of two unrelated families with a child that experienced fatal, nonanesthetic episodes. Filled symbols with a bisecting line indicate individuals that died from a nonanesthetic episode; empty symbols represent clinically healthy family members. R or C - denote arginine or cysteine residue at position 3983; D or H - denote an aspartic acid or histidine residue at position 4505. The haplotype was constructed on the basis of genotyping with microsatellite markers D19S191, D19S224, D19S220, and D19S47. The marker order is from centromere to telomere. The parentheses for the proband in family #1 indicate that the relative location of the 3983C haplotype is unknown. In family #2, the 3983C and 4505H variants were located on different alleles and the R4505H variant was associated with the maternal 10-1-7-1 haplotype. B) Alignment of the region of ryanodine receptor (RYR) variants and flanking residues across species and RYR isoforms. The mutated residues are shown in bold. Nonconserved residues across RYR isoforms are shaded.
Effects of R3983C and D4505H mutations on the sensitivity of caffeine-induced Ca2+ release following expression in RYR1-null myotubes
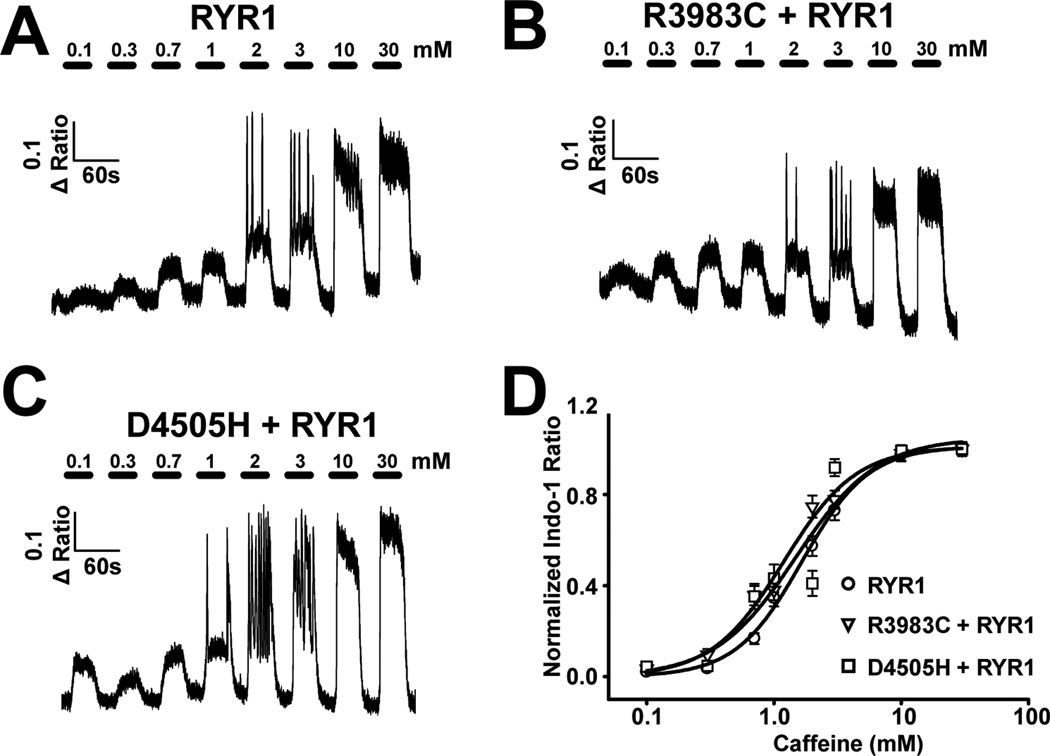
Increased sensitivity to activation of the RYR1 by caffeine is used as a primary diagnostic determinant of MH susceptibility. Moreover, an increase in caffeine sensitivity of Ca2+ release is recapitulated for MH mutations in RYR1 following expression in either Human Embryonic Kidney 293 cells16 or skeletal myotubes derived from RYR1-knockout mice.17 Since the proband in family one was heterozygous for the R3983C variant and the mother and sibling in family two was heterozygous for the D4505H variant, we determined whether the sensitivity of sarcoplasmic reticulum Ca2+ release to activation by caffeine was increased by co-expression of WT RYR1 with either the R3983C or D4505H variants (fig. 2). Dyspedic myotubes expressing either WT RYR1 (fig. 2A), WT RYR1 + R3983C (fig. 2B), or WT RYR1 + D4505H (fig. 2C) were exposed to sequential 30 s applications of increasing concentrations of caffeine (0.1, 0.3, 0.7, 1.0, 2.0, 3.0, 10, and 30 mM), with each application followed by a 30 s wash with control Ringer’s solution. Since naïve dyspedic myotubes lack robust caffeine-induced Ca2+ release,14 functional RYR1 expression was confirmed in each experiment by the presence of robust Ca2+ release when challenged with a high concentration of caffeine (30 mM). Average (± SEM) caffeine concentration-response curves are presented in figure 2D. The caffeine sensitivity of RYR1 Ca2+ release in WT RYR1-expressing myotubes (EC50 = 2.85 ± 0.49 mM) was modestly enhanced following coexpression of R3983C (EC50 = 2.00 ± 0.39 mM) and significantly increased ~2-fold following co-expression of D4505H (EC50 = 1.64 ± 0.24 mM).
Figure 2.
Effect of coexpressing either the R3893C or D4505H variants with wild-type (WT) type 1 ryanodine receptor (RYR1) in dyspedic myotubes on the caffeine sensitivity of sarcoplasmic reticulum Ca2+ release. A–C) Representative caffeine concentration responses in dyspedic myotubes expressing (A) WT RYR1 alone (n=34), (B) WT RYR1+R3893C (n=30), and (C) WT RYR1+D4505H (n=24). (D) Average (±SEM) caffeine concentration-response curves for the conditions shown in A–C.
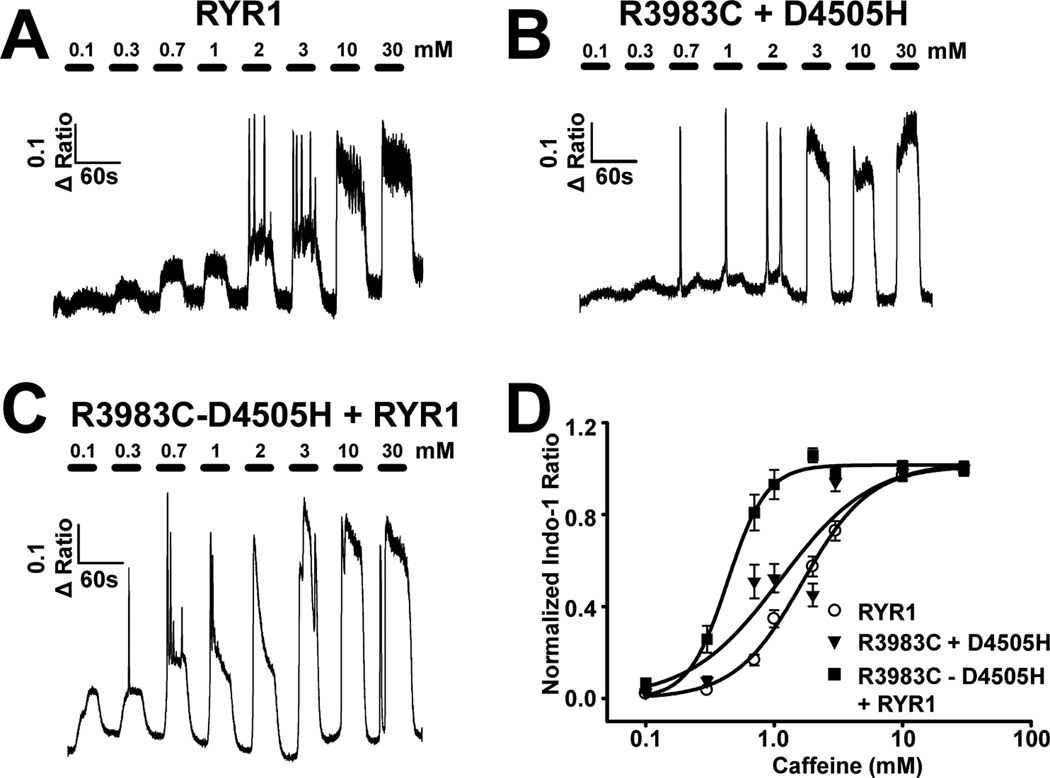
Genetic analysis revealed that the proband in family two was compound heterozygous for both the R3983C and D4505H variants and that the two variants localized to different haplotypes (fig. 1A). We directly compared the effect of the two potential compound heterotypic expression conditions (i.e., R3983C+D4505H and WT RYR1+R3983C-D4505H) on the sensitivity of sarcoplasmic reticulum Ca2+ release to activation by caffeine (fig. 3). For these experiments, dyspedic myotubes were injected with cDNAs encoding WT RYR1 alone (fig. 3A) or a 1:1 mixture of either R3983C+D4505H (fig. 3B; the two variants on separate cDNAs) or WT RYR1+R3983C-D4505H (fig. 3C; WT RYR1 plus the two variants engineered on the same cDNA). Average (± SEM) caffeine concentration-response curves are shown in figure 3D. The results indicate that coexpression of WT RYR1 with the two variants on the same subunit exhibited a significantly (p < 0.05) greater sensitivity to caffeine-induced Ca2+ release (EC50 = 0.64 ± 0.17 mM) compared to that for expression of either WT RYR1 alone (EC50 = 2.85 ± 0.49 mM) or the two variants on separate subunits (EC50 = 1.53 ± 0.18 mM). These results indicate a synergistic effect of the two variants on the sensitivity of caffeine-induced RYR1 Ca2+ release when the variants are present on the same subunit, but not when present on separate subunits.
Figure 3.
Effect of co-expressing both the R3893C and D4505H variants together with wild-type (WT) type 1 ryanodine receptor (RYR1) in dyspedic myotubes on the caffeine sensitivity of sarcoplasmic reticulum Ca2+ release. A–C) Representative caffeine concentration responses in dyspedic myotubes expressing (A) wild-type RYR1 alone (n=34), (B) R3893C+D4505H (n=25), and (C) WT RYR1+R3893C-D4505H (n=14). (D) Average (± SEM) caffeine concentration-response curves for the conditions shown in A–C.
Discussion
This report describes two cases in which novel RYR1 variants are associated with fatal nonanesthetic awake episodes in children. Like other confirmed MHS mutations in RYR1,16,17 the D4505H variant resulted in a ~2-fold increase in the sensitivity to activation by caffeine. Importantly, this increase in caffeine sensitivity occurred when the D4505H variant was coexpressed with WT RYR1, demonstrating a “gain-of-function” effect of D4505H subunits on RYR1 release channel sensitivity, consistent with the known autosomal dominant pattern of inheritance of MH. Coexpression of the R3983C variant with WT RYR1 produced a more modest enhancement in RYR1 caffeine sensitivity. Remarkably, caffeine sensitivity was greatly potentiated when the two mutations were incorporated into the same subunit, but not on separate subunits. Specifically, the EC50 for caffeine activation was essentially the same for the D4505H variant when co-expressed with either WT RYR1 (EC50 = 1.64 ± 0.24 mM) or R3983C (EC50 = 1.53 ± 0.18 mM), while the R3983C-D4505H double mutant coexpressed with WT RYR1 (EC50 = 0.64 ± 0.17 mM) resulted in a further 2.5-fold increase in caffeine sensitivity and a nearly 5-fold increase in sensitivity compared to WT RYR1 alone (EC50 = 2.85 ± 0.49 mM). Thus, while the R3983C mutation only modestly altered RYR1 sensitivity by itself, it is a potent enhancer of D4505H-induced sensitization when present on the same subunit. On the other hand, peak caffeine-induced responses were not significantly different between WT RYR1 (ΔRatio= 0.52 ± 0.02) and any of the different coexpression conditions (ΔRatio was 0.54 ± 0.03, 0.55 ± 0.02, 0.52 ± 0.03, and 0.54 ± 0.03 for WT+R39833C, WT+D4505H, R3983C+D4505H, and WT+R3983C-D4505H, respectively). Nevertheless, we cannot exclude the possibility that minor changes in the levels of RYR1 expression in our experiments might alter release channel caffeine release sensitivity, but not efficacy.
Although the R3983C variant was the only RYR1 alteration identified in Case 1, susceptibility to nonanesthetic, stress-induced hyperthermic reactions appeared to be more pronounced in this patient compared to that of the child in Case 2 as evident from his clinical history. Discordance between susceptibility of the child in family 1 and the lower caffeine sensitivity of the R3893C variant following coexpression with RYR1 in RYR1-null myotubes could reflect differences between effects of the mutation in human versus rabbit RyR1, effects in mature muscle fibers versus developing myotubes, or the influence of a modifying variable present only in the patient (e.g., a mutation in regulatory or intronic regions of the RYR1 gene, WT allele silencing, or a second mutation at another MH gene locus). However, extended genetic analyses for additional mutations including three MH-associated mutations in the CACNA1S gene and the most common mutations in the CPTII gene were negative. An RYR1 mutation previously associated with King Denborough Syndrome was also not found.18 Nevertheless, it remains possible that the nonanesthetic events observed in this individual could involve the R3983C variant potentiating effects of a second, yet unidentified mutation in either a noncoding region of RYR1 or another MH susceptible loci.1 Intronic mutations resulting in altered splicing of RYR1 exons in core myopathies have been reported19,20 and epigenetic gene silencing of the normal RYR1 allele in skeletal muscle has also been demonstrated in families presenting with apparent recessively-inherited core myopathies.21,22 However, the only study of epigenetic RYR1 allelic silencing in MH found no evidence in 14 discordant cases from 11 independent families.23 Similar potentiating effects could explain why the proband in Case 2 possessing both the R3983C and D4505H RYR1 variants exhibited a more severe awake phenotype than either her mother or brother who carried only the D4505H variant.
The increase in internal nuclei observed in Case 1 is consistent with recent studies showing an increase in nuclear internalization in RyR1-related myopathies20,24. Interestingly, Wilmshurst et al identified a nonconservative substitution of a negatively charged RyR1 residue (E4502G) in an individual with centronuclear myopathy that is only three amino acids upstream of a similar nonconservative substitution of a negatively charged RyR1 residue (D4505H) identified in the proband in Case 2.24 Together, these findings are consistent with a histopathological continuum between MH- and myopathy-related RYR1 phenotypes.
It is important to note that a different de novo missense mutation of the same RYR1 residue R3983 (R3983H) was recently described in a case report of a child with previous MH history that experienced a fatal non-anesthetic episode following ondansetron administration.4 This child also presented with clinical and histopathologic signs consistent with multi-minicore disease. Although multi-minicore disease is typically inherited as a recessive myopathy, neither a second RYR1 variant nor monoallelic RYR1 expression were reported in this child. In addition, this report did not determine whether the identified R3893H variant was causative of increased MH susceptibility by assessing its impact on RYR1 function. Nevertheless, the identification of de novo mutations to the identical RYR1 residue (R3893) in three independent families indicates that sporadic cases of MH due to de novo mutations in the RYR1 gene are likely to be more common than previously appreciated.
The R3983 residue is located within a putative ryanodine receptor and inositol 1,4,5-triphosphate receptor homology associated domain that spans RYR1 residues 3870–3992. While the homology associated domain is specific to RYR and IP3R, the function of this domain for this superfamily of intracellular Ca2+ release channels is unknown. The R3983 residue is conserved across species in all three RYR isoforms, while the D4505 residue is conserved only in RYR1 (fig. 1B). In fact, the D4505 residue is located within RYR1 divergent region 1 (D1; residues 4254–4631); one of three evolutionarily divergent regions of RYR isoforms.25 The D1 region maps to part of the “handle” domain on the RYR cryoelectron microscopy three dimensional structure.26 According to current RYR topological models,27 the D1 region includes at least one transmembrane domain and adjacent cytoplasmic and intraluminal sequences. Interestingly, deletion of the majority of the RYR1 D1 region (Δ4274–4535) potentiates voltage-gated Ca2+ release and enhances release channel sensitivity to activation by the dihydropyridine receptor.28 Based on these results, the D1 region functions as a negative regulatory module that increases the energy barrier for Ca2+ release channel opening. Thus, the D4505H mutation may enhance RYR1 release channel sensitivity to activation by disrupting the integrity of the D1 negative regulatory module.
Our results demonstrate that the functional impact of the two variants expressed in RyR1-nullmyotubes depends on whether the two variants are located on common or separate subunits. Genetic analysis of the second family revealed that the two variants are localized to separate subunits in Case 2. Although the caffeine sensitivity with the variants on separate subunits is not as high as when they localize to the same subunit, the allelic relationship in Case 2 does not necessarily imply similar a expression level of the two proteins. Furthermore, coexpression of the two variants on separate cDNAs resulted in a 2-fold increase in caffeine sensitivity, consistent with the child’s awake episodes and heat sensitivity. The unusually high caffeine sensitivity when the two variants localize to the same subunit demonstrates for the first time an allele-dependent synergism between two novel RYR1 gene variants. Our results are consistent with the two residues contributing to a negative regulatory module within the D1 region of each monomer. As a result, variants of both residues within the same subunit may lead to a synergistic antagonism of D1 function that potentiates RYR1 release channel sensitivity to activation. Together, these findings indicate that allelic segregation can be a critical, and heretofore unappreciated, pathogenic factor in MH individuals.
Summary.
R3983C and D4505H ryanodine receptor variants were identified in a child that experienced a fatal, nonanesthetic awake episode. The impact of the two variants depends on whether they are located on common or separate alleles.
What we already know about this topic
* A subset of malignant hyperthermia susceptible patients can develop malignant hyperthermia-like symptoms in response to nonanesthetic stimuli.
* The interactions between genetic risk factors and environmental triggers for such "awake episodes" are unclear.
What this article tells us that is new
* Examination of the type I ryanodine receptor gene in two unrelated children who experienced fatal, non-anesthetic awake episodes revealed the presence of an identical new variant in both and a second unique variant in one.
* Functional analyses of the two variants in myotubes demonstrate that allelic segregation and genetic background play a critical role in the expression of symptoms.
Supplementary Material
Acknowledgements
Funding: This work was supported by National Institutes of Health, Bethesda, Maryland, grants AR44657 (to RTD) and AR052354 (to RTD and SMM).
We thank Dr. P. D. Allen, M.D., Ph.D., Professor, Department of Anesthesia, Brigham and Women’s Hospital, Boston, Massachusetts, for providing access to the RYR1-null mice used in this study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Meeting Abstract: A preliminary account of these findings was presented at the Annual Meeting of the American Society of Anesthesiologists on 10/19/2008 /in San Diego, California.
References
- 1.Rosenberg H, Sambuughin N, Dirksen RT. GeneReviews at GeneTests: Medical Genetics Information Resource [database online]. Copyright. Seattle: University of Washington; 1997–2010. Malignant hyperthermia susceptibility. [PubMed] [Google Scholar]
- 2.Robinson RL, Carpenter D, Shaw M, Halsall J, Hopkins P. Mutations in RYR1 in malignant hyperthermia and central core disease. Hum Mutat. 2006;27:977–989. doi: 10.1002/humu.20356. [DOI] [PubMed] [Google Scholar]
- 3.Brown RL, Pollock AN, Couchman KG, Hodges M, Hutchinson DO, Waaka R, Lynch P, McCarthy TV, Stowell KM. A novel ryanodine receptor mutation and genotype-phenotype correlation in a large malignant hyperthermia New Zealand Maori pedigree. Hum Mol Genet. 2000;12:1515–1524. doi: 10.1093/hmg/9.10.1515. [DOI] [PubMed] [Google Scholar]
- 4.Gener B, Burns JM, Griffin S, Boyer EW. Administration of ondansetron is associated with lethal outcome. Pediatrics. 2010;125:E1514–E1517. doi: 10.1542/peds.2009-2795. [DOI] [PubMed] [Google Scholar]
- 5.Nishino H, Nishio H, Sato T, Fukunishi S, Tamura A, Iwata M, Tsuboi K, Suzuki K. Identification of malignant hyperthermia-susceptible ryanodine receptor type 1 gene (RYR1) mutations in a child who died in a car after exposure to a high environmental temperature. Leg Med. 2009;11:142–143. doi: 10.1016/j.legalmed.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 6.Tobin J, Jason DR, Challa VR, Nelson TE, Sambuughin N. Malignant hyperthermia and apparent heat stroke. JAMA. 2001;286:168–116. doi: 10.1001/jama.286.2.168. [DOI] [PubMed] [Google Scholar]
- 7.Wappler F, Fiege M, Steinfath M, Agarwal K, Scholz J, Singh S, Matschke J, Schulte Am Esch J. Evidence for susceptibility to malignant hyperthermia in patients with exercise-induced rhabdomyolysis. Anesthesiology. 2001;94:95–100. doi: 10.1097/00000542-200101000-00019. [DOI] [PubMed] [Google Scholar]
- 8.Capacchione JF, Muldoon SM. The relationship between exertional heat illness, exertional rhabdomyolysis, and malignant hyperthermia. Anesth Analg. 2009;109:1065–1069. doi: 10.1213/ane.0b013e3181a9d8d9. [DOI] [PubMed] [Google Scholar]
- 9.Larach MG. Standardization of the caffeine halothane muscle contracture test. North American Malignant Hyperthermia Group. Anesth Analg. 1989;69:511–515. [PubMed] [Google Scholar]
- 10.Isaacs H, Badenhorst ME. Dominantly inherited malignant hyperthermia in the King-Denborough syndrome. Muscle Nerve. 1992;15:740–742. doi: 10.1002/mus.880150619. [DOI] [PubMed] [Google Scholar]
- 11.Sambuughin N, Nelson TE, Jankovic J, Xin C, Meissner G, Mullakandov M, Ji J, Rosenberg H, Sivakumar K, Goldfarb L. Identification and functional characterization of a novel ryanodine receptor mutation causing malignant hyperthermia in North American and South American families. Neuromuscul Disord. 2001;11:530–537. doi: 10.1016/s0960-8966(01)00202-4. [DOI] [PubMed] [Google Scholar]
- 12.Sei Y, Sambuughin N, Davis EJ, Sachs D, Cuenca P, Brandom BW, Tautz T, Rosenberg H, Nelson TE, Muldoon SM. Malignant hyperthermia in North America: Screening the three hotspots in the type I ryanodine receptor gene. Anesthesiology. 2004;101:824–830. doi: 10.1097/00000542-200410000-00005. [DOI] [PubMed] [Google Scholar]
- 13.Sambuughin N, Holley H, Muldoon S, Brandom BW, de Bantel AM, Tobin JR, Nelson TE, Goldfarb LG. Screening of the ryanodine receptor type 1 coding region for sequence variants associated with malignant hyperthermia susceptibility in the North American population. Anesthesiology. 2005;102:515–521. doi: 10.1097/00000542-200503000-00007. [DOI] [PubMed] [Google Scholar]
- 14.Avila G, Dirksen RT. Functional effects of central core disease mutations in the cytoplasmic region of the skeletal muscle ryanodine receptor. J Gen Physiol. 2001;118:277–290. doi: 10.1085/jgp.118.3.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Avila G, O’Brien JJ, Dirksen RT. Excitation-contraction uncoupling by a human central core disease mutation in the ryanodine receptor. Proc Natl Acad Sci U S A. 2001;98:4215–4220. doi: 10.1073/pnas.071048198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tong J, Oyamada H, Demaurex N, Grinstein S, McCarthy TV, MacLennan DH. Caffeine and halothane sensitivity of intracellular Ca2+ release is altered by 15 calcium release channel (ryanodine receptor) mutations associated with malignant hyperthermia and/or central core disease. J Biol Chem. 1997;272:26332–26339. doi: 10.1074/jbc.272.42.26332. [DOI] [PubMed] [Google Scholar]
- 17.Yang T, Ta TA, Pessah IN, Allen PD. Functional defects in six ryanodine receptor isoform-1 (RyR1) mutations associated with malignant hyperthermia and their impact on skeletal excitation-contraction coupling. J Biol Chem. 2003;278:25722–25730. doi: 10.1074/jbc.M302165200. [DOI] [PubMed] [Google Scholar]
- 18.D’Arcy CE, Bjorksten A, Yiu EM, Bankler A, Gillies R, McLean CA, Shield LK, Ryan MM. King-Denborough syndrome cause by a novel mutation in the ryanodine receptor gene. Neurology. 2008;71:776–777. doi: 10.1212/01.wnl.0000324929.33780.2f. [DOI] [PubMed] [Google Scholar]
- 19.Monnier N, Ferreiro A, Marty I, Labarre-Vila A, Mezin P, Lunardi J. A homozygous splicing mutation causing a depletion of skeletal muscle RYR1 is associated with multi-minicore disease congenital myopathy with ophthalmoplegia. Hum Mol Genet. 2003;12:1171–1178. doi: 10.1093/hmg/ddg121. [DOI] [PubMed] [Google Scholar]
- 20.Bevilacqua JA, Monnier N, Bitoun M, Eymard B, Ferreiro A, Monges S, Lubieniecki F, Taratuto AL, Laquerrière A, Claeys KG, Marty I, Fardeau M, Guicheney P, Lunardi J, Romero NB. Recessive RYR1 mutations cause unusual congenital myopathy with prominent nuclear internalization and large areas of myofibrillar disorganization. Neuropathol Appl Neurobiol. 2011;37:271–284. doi: 10.1111/j.1365-2990.2010.01149.x. [DOI] [PubMed] [Google Scholar]
- 21.Zhou H, Yamaguchi N, Xu L, Wang Y, Sewry C, Junbluth H, Zorzato F, Bertini E, Muntoni F, Meissner G, Treves S. Characterization of recessive RYR1 mutations in core myopathies. Hum Mol Genet. 2006;15:2791–2803. doi: 10.1093/hmg/ddl221. [DOI] [PubMed] [Google Scholar]
- 22.Zhou H, Jungbluth H, Sewry CA, Feng L, Bertini E, Bushby K, Straub V, Roper H, Rose MR, Brockington M, Kinali M, Manzur A, Robb S, Appleton R, Messina S, D'Amico A, Quinlivan R, Swash M, Müller CR, Brown S, Treves S, Muntoni F. Molecular mechanisms and phenotypic variation in RYR1-related congenital myopathies. Brain. 2007;130:2024–2036. doi: 10.1093/brain/awm096. [DOI] [PubMed] [Google Scholar]
- 23.Robinson RL, Carpenter D, Halsall PJ, Iles DE, Booms P, Steele D, Hopkins PM, Shaw MA. Epigenetic allele silencing and variable penetrance of malignant hyperthermia susceptibility. Br J Anaesth. 2009;103:220–225. doi: 10.1093/bja/aep108. [DOI] [PubMed] [Google Scholar]
- 24.Wilmshurst JM, Lillis S, Zhou H, Pillay K, Henderson H, Kress W, Müller CR, Ndondo A, Cloke V, Cullup T, Bertini E, Boennemann C, Straub V, Quinlivan R, Dowling JJ, Al-Sarraj S, Treves S, Abbs S, Manzur AY, Sewry CA, Muntoni F, Jungbluth H. RYR1 mutations are a common cause of congenital myopathies with central nuclei. Ann Neurol. 2010;68:717–726. doi: 10.1002/ana.22119. [DOI] [PubMed] [Google Scholar]
- 25.Hamilton S, Serysheva II. Ryanodine Receptor Structure: progress and challenges. J Biol Chem. 2009;284:4047–4051. doi: 10.1074/jbc.R800054200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu Z, Zhang J, Li P, Chen SR, Wagenknecht T. Three-dimensional reconstruction of the recombinant type 2 ryanodine receptor and localization of its divergent region 1. J Biol Chem. 2002;277:46712–46719. doi: 10.1074/jbc.M208124200. [DOI] [PubMed] [Google Scholar]
- 27.Du GG, Avila G, Sharma P, Khanna VK, Dirksen RT, MacLennan DH. Role of the sequence surrounding predicted transmembrane helix M4 in membrane association and function of the Ca2+ release channel of skeletal muscle sarcoplasmic reticulum (ryanodine receptor isoform 1) J Biol Chem. 2004;279:37566–37574. doi: 10.1074/jbc.M406637200. [DOI] [PubMed] [Google Scholar]
- 28.Du GG, Sandhu B, Khanna VK, Guo XH, MacLennan DH. Topology of the Ca2+ release channel of skeletal muscle sarcoplasmic reticulum (RyR1) Proc Natl Acad Sci U S A. 2002;99:16725–16730. doi: 10.1073/pnas.012688999. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.