Abstract
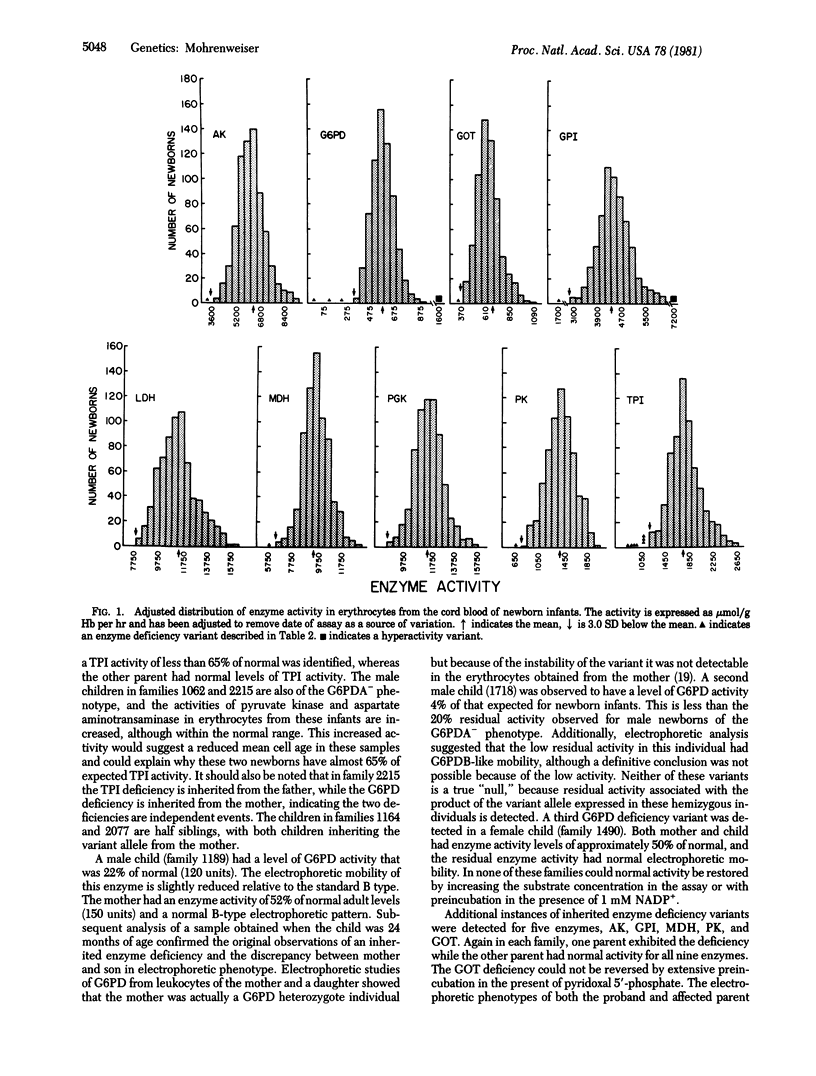
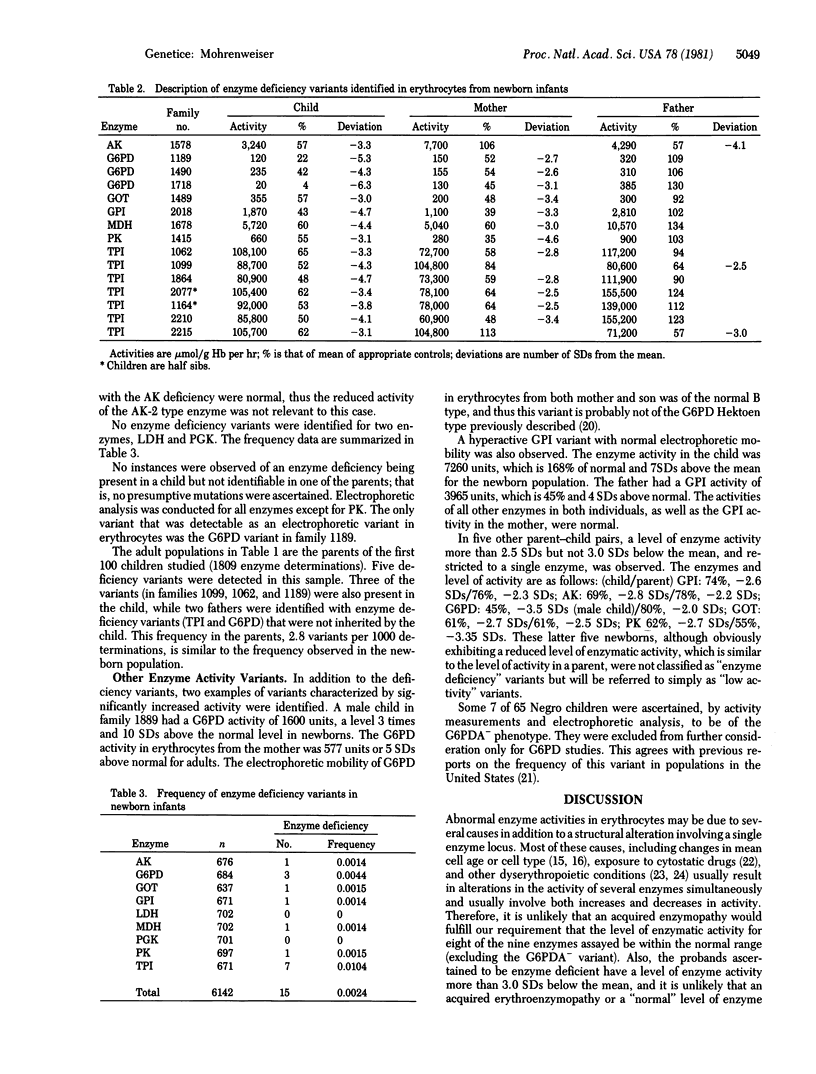
The frequency of enzyme deficiency variants, defined as alleles whose products are either absent or almost devoid of normal activity in erythrocytes, was determined for nine erythrocyte enzymes in some 675 newborn infants and in approximately 200 adults. Examples of this type of genetic abnormality, which in the homozygous condition are often associated with significant health consequences, were detected for seven of the nine enzymes studied. Fifteen inherited enzyme deficiency variants in 6142 determinations from the newborn population and 5 variants in 1809 determinations from adults were identified. Seven of the deficiency variants involved triosephosphate isomerase, a frequency of 0.01 in the newborn population. The average frequency of 2.4/1000 is 2-3 times the frequency observed for rare electrophoretic variants of erythrocyte enzymes in this same population.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burtis C. A., Mailen J. C., Johnson W. F., Scott C. D., Tiffany T. O., Anderson N. G. Development of a miniature fast analyzer. Clin Chem. 1972 Aug;18(8):753–761. [PubMed] [Google Scholar]
- Cohen P. T., Omenn G. S., Motulsky A. G., Chen S. H., Giblett E. R. Restricted variation in the glycolytic enzymes of human brain and erythrocytes. Nat New Biol. 1973 Feb 21;241(112):229–233. doi: 10.1038/newbio241229a0. [DOI] [PubMed] [Google Scholar]
- Dern R. J., McCurdy P. R., Yoshida A. A new structural variant of glucose-6-phosphate dehydrogenase with a high production rate (G6PD Hektoen). J Lab Clin Med. 1969 Feb;73(2):283–290. [PubMed] [Google Scholar]
- Etiemble J., Bernard J. F., Picat C., Belpomme D., Boivin P. Red blood cell enzyme abnormalities in patients treated with chemotherapy. Br J Haematol. 1979 Jul;42(3):391–398. doi: 10.1111/j.1365-2141.1979.tb01147.x. [DOI] [PubMed] [Google Scholar]
- Fielek S., Mohrenweiser H. W. Erythrocyte enzyme deficiencies assessed with a miniature centrifugal analyzer. Clin Chem. 1979 Mar;25(3):384–388. [PubMed] [Google Scholar]
- Glatzle D., Vuilleumier J. P., Weber F., Decker K. Glutathione reductase test with whole blood, a convenient procedure for the assessment of the riboflavin status in humans. Experientia. 1974 Jun 15;30(6):665–667. doi: 10.1007/BF01921531. [DOI] [PubMed] [Google Scholar]
- HAMILTON H. B., NEEL J. V., KOBARA T. Y., OZAKI K. The frequency in Japan of carriers of the rare "recessive" gene causing acatalasemia. J Clin Invest. 1961 Dec;40:2199–2208. doi: 10.1172/JCI104446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson F. M., Lewis S. E. Electrophoretically detected germinal mutations induced in the mouse by ethylnitrosourea. Proc Natl Acad Sci U S A. 1981 May;78(5):3138–3141. doi: 10.1073/pnas.78.5.3138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn A., Cottreau D., Boyer C., Marie J., Galand C., Boivin P. Causal mechanisms of multiple acquired red cell enzyme defects in a patient with acquired dyserythropoiesis. Blood. 1976 Nov;48(5):653–662. [PubMed] [Google Scholar]
- Komazawa M., Oski F. A. Biochemical characteristics of "young" and "old" erythrocytes of the newborn infant. J Pediatr. 1975 Jul;87(1):102–106. doi: 10.1016/s0022-3476(75)80082-5. [DOI] [PubMed] [Google Scholar]
- Modiano G., Scozzari R., Gigiani F., Santolamazza C., Spennati G. F., Saini P. Enzyme activity in two red cell adenylate kinase phenotypes. Am J Hum Genet. 1970 May;22(3):292–297. [PMC free article] [PubMed] [Google Scholar]
- Mukai T., Cockerham C. C. Spontaneous mutation rates at enzyme loci in Drosophila melanogaster. Proc Natl Acad Sci U S A. 1977 Jun;74(6):2514–2517. doi: 10.1073/pnas.74.6.2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neel J. V., Mohrenweiser H. W., Meisler M. H. Rate of spontaneous mutation at human loci encoding protein structure. Proc Natl Acad Sci U S A. 1980 Oct;77(10):6037–6041. doi: 10.1073/pnas.77.10.6037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racine R. R., Langley C. H., Voelker R. A. Enzyme mutants induced by low-dose-rate gamma-irradiation in Drosophila: frequency and characterization. Environ Mutagen. 1980;2(2):167–177. doi: 10.1002/em.2860020209. [DOI] [PubMed] [Google Scholar]
- Raivio K. O., Seegmiller J. E. Genetic diseases of metabolism. Annu Rev Biochem. 1972;41(10):543–576. doi: 10.1146/annurev.bi.41.070172.002551. [DOI] [PubMed] [Google Scholar]
- Scriver C. R. Inborn errors of amino-acid metabolism. Br Med Bull. 1969 Jan;25(1):35–41. doi: 10.1093/oxfordjournals.bmb.a070667. [DOI] [PubMed] [Google Scholar]
- Voelker R. A., Langley C. H., Brown A. J., Ohnishi S., Dickson B., Montgomery E., Smith S. C. Enzyme null alleles in natural populations of Drosophila melanogaster: Frequencies in a North Carolina population. Proc Natl Acad Sci U S A. 1980 Feb;77(2):1091–1095. doi: 10.1073/pnas.77.2.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voelker R. A., Schaffer H. E., Mukai T. Spontaneous Allozyme Mutations in DROSOPHILA MELANOGASTER: Rate of Occurrence and Nature of the Mutants. Genetics. 1980 Apr;94(4):961–968. doi: 10.1093/genetics/94.4.961. [DOI] [PMC free article] [PubMed] [Google Scholar]


