Abstract
Although best known for its ability to cause severe pneumonia in people whose immune defenses are weakened, Legionella pneumophila and Legionella longbeachae are two species of a large genus of bacteria that are ubiquitous in nature, where they parasitize protozoa. Adaptation to the host environment and exploitation of host cell functions are critical for the success of these intracellular pathogens. The establishment and publication of the complete genome sequences of L. pneumophila and L. longbeachae isolates paved the way for major breakthroughs in understanding the biology of these organisms. In this review we present the knowledge gained from the analyses and comparison of the complete genome sequences of different L. pneumophila and L. longbeachae strains. Emphasis is given on putative virulence and Legionella life cycle related functions, such as the identification of an extended array of eukaryotic like proteins, many of which have been shown to modulate host cell functions to the pathogen’s advantage. Surprisingly, many of the eukaryotic domain proteins identified in L. pneumophila as well as many substrates of the Dot/Icm type IV secretion system essential for intracellular replication are different between these two species, although they cause the same disease. Finally, evolutionary aspects regarding the eukaryotic like proteins in Legionella are discussed.
Keywords: Legionella pneumophila, Legionella longbeachae, evolution, comparative genomics, eukaryotic like proteins, virulence
Introduction
Genomics has the potential to provide an in depth understanding of the genetics, biochemistry, physiology, and pathogenesis of a microorganism. Furthermore comparative genomics, functional genomics, and related technologies, are helping to unravel the molecular basis of the pathogenesis, evolution, and phenotypic differences among different species, strains, or clones and to uncover potential virulence genes. Knowledge of the genomes provides the basis for the application of new powerful approaches for the understanding of the biology of the organisms studied.
Although Legionella are mainly environmental bacteria, several species are pathogenic to humans, in particular Legionella pneumophila (Fraser et al., 1977; Mcdade et al., 1977) and Legionella longbeachae (Mckinney et al., 1981). Legionnaires’ disease has emerged in the second half of the twentieth century partly due to human alterations of the environment. The development of artificial water systems in the last decades like air conditioning systems, cooling towers, showers, and other aerosolizing devices has allowed Legionella to gain access to the human respiratory system. When inhaled in contaminated aerosols, pathogenic Legionella can reach the alveoli of the lung where they are subsequently engulfed by macrophages. In contrast to most bacteria, which are destroyed, some Legionella species can multiply within the phagosome and eventually kill the macrophage, resulting in a severe, often fatal pneumonia called legionellosis or Legionnaires’ disease (mortality rate of 5–20%; up to 50% in nosocomial infections; Steinert et al., 2002; Marrie, 2008; Whiley and Bentham, 2011). To replicate intracellularly L. pneumophila manipulates host cellular processes using bacterial proteins that are delivered into the cytosolic compartment of the host cell by a specialized type IV secretion system called Dot/Icm. The proteins delivered by the Dot/Icm system target host factors implicated in controlling membrane transport in eukaryotic cells, which enables L. pneumophila to create an endoplasmic reticulum-like vacuole that supports intracellular replication in both protozoan and mammalian host cells (for a review see Hubber and Roy, 2010).
An interesting epidemiological observation is, that among the over 50 Legionella species described today, strains belonging to the species L. pneumophila are responsible for over 90% of the legionellosis cases worldwide and strains belonging to the species L. longbeachae are responsible for about 5% of human legionellosis cases worldwide (Yu et al., 2002). Surprisingly, this distribution is very different in Australia and New Zealand where L. pneumophila accounts for “only” 45.7% of the cases but L. longbeachae is implicated in 30.4% of the human cases. Furthermore, among the strains causing Legionnaires’ disease, L. pneumophila serogroup 1 (Sg1) alone is responsible for over 85% of cases (Yu et al., 2002; Doleans et al., 2004) despite the description of 15 different Sg within this species. In addition, the characterization of over 400 different L. pneumophila Sg1 strains has shown that only a minority among these is responsible for causing most of the human disease (Edelstein and Metlay, 2009). Some of these clones are distributed worldwide like L. pneumophila strain Paris (Cazalet et al., 2008) others have a more restricted geographical distribution, like the recently described endemic clone, prevalent in Ontario, Canada (Tijet et al., 2010). For the species L. longbeachae two serogroups are described to date (Bibb et al., 1981; Mckinney et al., 1981). L. longbeachae Sg1 is predominant in human disease as it causes up to 95% of the cases of legionellosis worldwide and most outbreaks and sporadic cases in Australia (Anonymous, 1997; Montanaro-Punzengruber et al., 1999). The two main human pathogenic Legionella species, L. pneumophila and L. longbeachae cause the same disease and symptoms in humans (Amodeo et al., 2009), however, there exist major differences between both species in niche adaptation and host susceptibility.
-
(i)
They are found in different environmental niches, as L. pneumophila is mainly found in natural and artificial water circuits and L. longbeachae is principally found in soil and therefore associated with gardening and use of potting compost (O’Connor et al., 2007). However, although less common, the isolation of L. pneumophila from potting soil in Europe has also been reported (Casati et al., 2009; Velonakis et al., 2009). Human infection due to L. longbeachae is particularly common in Australia but cases have been documented also in other countries like the USA, Japan, Spain, England, or Germany (MMWR, 2000; Garcia et al., 2004; Kubota et al., 2007; Kumpers et al., 2008; Pravinkumar et al., 2010).
-
(ii)
As described for other Legionella species, person to person transmission of L. longbeachae has not been documented, however, the primary transmission mode seems to be inhalation of dust from contaminated compost or soil that contains the organism (Steele et al., 1990; MMWR, 2000; O’Connor et al., 2007).
-
(iii)
Furthermore, for L. pneumophila a biphasic life cycle was observed in vitro and in vivo as exponential phase bacteria do not express virulence factors and are unable to replicate intracellularly. The ability of L. pneumophila to replicate intracellularly is triggered at the post-exponential phase by a complex regulatory cascade (Molofsky and Swanson, 2004; Sahr et al., 2009). In contrast, less is known on the L. longbeachae intracellular life cycle and its virulence factors. It was recently shown that unlike L. pneumophila the ability of L. longbeachae to replicate intracellularly is independent of the bacterial growth phase (Asare and Abu Kwaik, 2007) and that phagosome biogenesis is different. Like L. pneumophila, the L. longbeachae phagosome is surrounded by endoplasmic reticulum and does not mature to a phagolysosome; however it acquires early and late endosomal markers (Asare and Abu Kwaik, 2007).
-
(iv)
Another interesting difference between these two species is their ability to colonize the lungs of mice. While only A/J mice are permissive for replication of L. pneumophila, A/J, C57BL/6, and BALB/c mice are all permissive for replication of L. longbeachae (Asare et al., 2007; Gobin et al., 2009). Resistance of C57BL/6 and BALB/c mice to L. pneumophila has been attributed to polymorphisms in Nod-like receptor apoptosis inhibitory protein 5 (naip5) allele that recognizes the C-terminus of flagellin (Wright et al., 2003; Molofsky et al., 2006; Ren et al., 2006; Lightfield et al., 2008). The current model is that L. pneumophila replication is restricted due to flagellin dependent caspase-1 activation through Naip5-Ipaf and early macrophage cell death by pyroptosis. However, although depletion or inhibition of caspase-1 activity leads to decreased targeting of bacteria to lysosomes, the mechanism of caspase-1-dependent restriction of L. pneumophila replication in macrophages and in vivo is not fully understood (Schuelein et al., 2011).
In the last years, six genomes of different L. pneumophila strains (Paris, Lens, Philadelphia, Corby, Alcoy, and 130b (Cazalet et al., 2004; Chien et al., 2004; Steinert et al., 2007; D’Auria et al., 2010; Schroeder et al., 2010) have been published. The genome sequences of all but strain 130b were completely finished. Furthermore, the sequencing and analysis of four genomes of L. longbeachae have been carried out recently (Cazalet et al., 2010). L. longbeachae strain NSW150 of Sg1 isolated in Australia from a patient was sequenced completely, and for the remaining three strains (ATCC33462, Sg1 isolated from a human lung, C-4E7 and 98072, both of Sg2 isolated from patients) a draft genome sequence was reported. A fifth L. longbeachae strain (D-4968 of Sg1, isolated in the US from a patient) was recently sequenced and the analysis of the genome sequences assembled into 89 contigs was reported (Kozak et al., 2010).
Here we will describe what we learned from the analysis and comparison of the sequenced Legionella strains. We will discuss their general characteristics and then highlight the specific features or common traits with respect to the different ecological niches and the differences in host susceptibility of these two Legionella species. Emphasis will be put on putative virulence and Legionella life cycle related functions. In the last part we will analyze and discuss the possible evolution of the identified virulence factors. Finally, future perspectives in Legionella genomics are presented.
General Features of the L. pneumophila and L. longbeachae Genomes
Legionella pneumophila and L. longbeachae each have a single, circular chromosome with a size of 3.3–3.5 Mega bases (Mb) for L. pneumophila and 3.9–4.1 Mb for L. longbeachae. For both the average G + C content is 38% (Tables 1). The L. pneumophila strains Paris and Lens each contain different plasmids, 131.9 kb and 59.8 kb in size, respectively. In strain Philadelphia-1, 130b, Alcoy, and Corby no plasmid was identified. The L. longbeachae strains NSW10 and D-4986 carry highly similar plasmids of about 70 kb and DNA identity of 99%, strains C-4E7 and 98072 also contain each a highly similar plasmid of 133.8 kb in size. Thus similar plasmids circulate among L. longbeachae strains, but they seem to be different from those found in L. pneumophila.
Table 1.
General features of the sequenced Legionella genomes.
| A. Complete and draft genomes of L. pneumophila obtained by classical or new generation sequencing | ||||||
|---|---|---|---|---|---|---|
| L. pneumophila | ||||||
| Paris | Lens | Philadelphia | Corby | Alcoy | 130bc | |
| Chromosome size (kb)a | 3504 (131.9)b | 3345 (59.8) | 3397 | 3576 | 3516 | 3490 |
| G + C content (%) | 38.3 (37.4) | 38.4 (38) | 38.3 | 38 | 38.4 | 38.2 |
| No. of genesa | 3123 (142) | 2980 (60) | 3031 | 3237 | 3197 | 3288 |
| No. of protein coding genesa | 3078 (140) | 2921 (60) | 2999 | 3193 | 3097 | 3141 |
| Percentage of CDS (%) | 87.9 | 88.0 | 90.2 | 86.8 | 86.0 | 87.9 |
| No. of specific genes | 225 | 181 | 213 | 144 | 182 | 386c |
| No. of 16S/23S/5S | 03/03/03 | 03/03/03 | 03/03/03 | 03/03/03 | 03/03/03 | ND |
| No. transfer RNA | 44 | 43 | 43 | 43 | 43 | 42 |
| Plasmids | 1 | 1 | 0 | 0 | 0 | 0 |
| B. Complete and draft genomes of L. longbeachae obtained by classical or new generation sequencing | ||||||
| L. longbeachae | ||||||
| NSW 150 | D-4968 | ATCC33462 | 98072 | C-4E7 | ||
| Chromosome size (Kb) | 4077 (71) | 4016 (70) | 4096 | 4018 (133.8) | 3979 (133.8) | |
| G + C content (%) | 37.1 (38.2) | 37.0 | 37.0 | 37.0 (37.8) | 37 (37.8) | |
| No. of genes | 3660 (75) | 3557 (61) | – | – | – | |
| No. of 16S/23S/5S | 04/04/04 | 04/04/04 | 04/04/04 | 04/04/04 | 04/04/04 | |
| No. of contigs > 0.5–300 kb | Complete | 13 | 64 | 65 | 63 | |
| N50 contig size* | Complete | – | 138 kb | 129 kb | 134 kb | |
| Percentage of coverage** | 100% | 96.3 | 96.3 | 93.4 | 93.1 | |
| Number of SNP with NSW150 | 0 | 1900 | 1611 | 16 853 | 16 820 | |
| Plasmids | 1 | 1 | 0 | 1 | 1 | |
aUpdated annotation; CDS, coding sequence; bdata from plasmids in parenthesis; cThe 130b sequence is not a manually corrected and finished assembly, thus the high number of specific genes might be due to not corrected sequencing errors; ND, not determined; *N50 contig size, calculated by ordering all contig sizes and adding the lengths (starting from the longest contig) until the summed length exceeds 50% of the total length of all contigs (half of all bases reside in a contiguous sequence of the given size or more); SNP, single nucleotide polymorphism; **for SNP detection; – not determined.
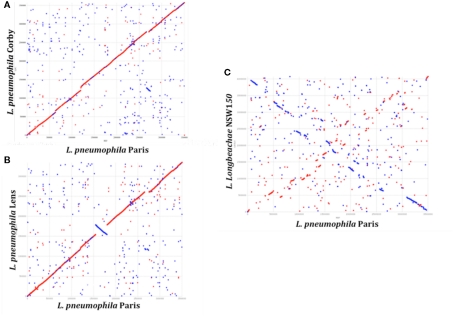
A total of ∼3000 and 3500 protein-encoding genes are predicted in the L. pneumophila and L. longbeachae genomes, respectively. No function could be predicted for about 40% of these genes and about 20% are unique to the genus Legionella. Comparative analysis of the genome structure of the L. pneumophila genomes showed high colinearity, with only few translocations, duplications, deletions, or inversions (Figures 1A,B) and identified between 6 and 11% of genes as specific to each L. pneumophila strain. Principally, the genomes contain three large plasticity zones, where the synteny is disrupted: a 260-kb inversion in strain Lens with respect to strains Paris and Philadelphia-1, a 130-kb fragment which is inserted in a different genomic location in strains Paris and Philadelphia-1 and the about 50 kb chromosomal region carrying the Lvh type IV secretion system, previously described in strain Philadelphia-1 (Segal et al., 1999). Furthermore, deletions and insertions of several smaller regions were identified in each strain, as well as regions with variable gene content. In contrast, comparison of the completed chromosome sequences of L. pneumophila and L. longbeachae shows that the two Legionella species have a significantly different genome organization (Figure 1C). Moreover only about 65% of the L. longbeachae genes are orthologous to L. pneumophila genes, whereas about 34% of all genes are specific to L. longbeachae with respect to L. pneumophila Paris, Lens, Philadelphia, and Corby (defined by less than 30% amino acid identity over 80% of the length of the smallest protein).
Figure 1.
Synteny plot of the chromosomes of L. pneumophila strains Paris, Lens, Corby, and L. longbeachae NSW150. The plot was created using the mummer software package. (A) Synteny plot of the chromosomes of strains L. pneumophila Paris and Corby (B) and strains L. pneumophila Paris and Lens and (C) strains L. pneumophila Paris and L. longbeachae NSW150. Inversions between the genomic sequences are represented in blue. Genome-wide synteny is disrupted by a 260 kb inversion (blue) and a 130 kb plasticity zone between strain L. pneumophila Paris and Lens. In contrast, synteny between L. pneumophila and L. longbeachae is highly conserved.
Analysis of single nucleotide polymorphisms (SNP) revealed a very low SNP number of less than 0.4% among the four L. longbeachae genomes, which is significantly lower than the polymorphism of about 2% between L. pneumophila Sg1 strains Paris and Philadelphia (Table 1). Comparison of the two L. longbeachae Sg1 genomes (NSW150, ATCC33462) identified 1611 SNPs of which 1426 are located in only seven chromosomal regions mainly encoding putative mobile elements, whereas the remaining 185 SNPs were evenly distributed around the chromosome. A similar number of about 1900 SNPs were identified when comparing strains NSW150 to strain D-4968 (Table 1). In contrast, the SNP number between two strains of different Sg was higher, with about 16000 SNPs present between Sg1 and Sg2 strains (Table 1). This low SNP number and relatively homogeneous distribution of the SNPs around the chromosome suggest recent expansion for the species L. longbeachae (Cazalet et al., 2010). The sequences and their analysis are accessible at http://genolist.pasteur.fr/LegioList/.
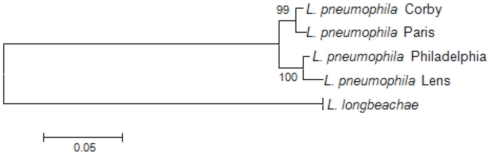
To investigate the phylogenetic relationship among the L. pneumophila and L. longbeachae strains we here used the nucleotide sequence of recN (recombination and repair protein-encoding gene) aligned based on the protein alignment. Based on an analysis of 32 protein-encoding genes widely distributed among bacterial genomes, RecN was described as the gene with the greatest potential for predicting genome relatedness at the genus or subgenus level (Zeigler, 2003). As depicted in Figure 2, the phylogenetic relationship among the four L. pneumophila strains is very high, and L. longbeachae is clearly more distant.
Figure 2.
Phylogenetic tree showing the relationship of the sequenced L. pneumophila and L. longbeachae strains based on the recN sequence. The tree was constructed using the recN sequences of each genome and the Neighbor joining method in MEGA. L. longbeachae is indicated without strain designation, as the RecN sequence of all sequenced strains is identical and thus only one representative strain is indicated on the tree. Numbers at branching nodes are percentages of 1000 bootstrap replicates.
Diversity in Secretion Systems and Their Substrates may Contribute to Differences in Intracellular Trafficking and Niche Adaptation
The capacity of pathogens like Legionella to infect eukaryotic cells is intimately linked to the ability to manipulate host cell functions to establish an intracellular niche for their replication. Essential for the ability of Legionella to subvert host functions are its different secretion systems. The two major ones, known to be involved in virulence of L. pneumophila are the Dot/Icm type IV secretion system (T4BSS) and the Lsp type II secretion system (T2SS; Marra et al., 1992; Berger and Isberg, 1993; Rossier and Cianciotto, 2001).
For L. pneumophila type II protein secretion is critical for infection of amebae, macrophages and mice. Analyses of the L. longbeachae genome sequences showed, that it contains all genes to encode a functional Lsp type II secretion machinery (Cazalet et al., 2010; Kozak et al., 2010). Several studies, including the analysis of the L. pneumophila type II secretome indicated that L. pneumophila encodes at least 25 type II secreted substrates (Debroy et al., 2006; Cianciotto, 2009). Although this experimentally defined repertoire of type II secretion-dependent proteins is the largest known in bacteria, it may contain even more than 60 proteins as 35 additional proteins with a signal sequence were identified by in silico analyses (Cianciotto, 2009). A search for homologs of these substrates in the L. longbeachae genome sequences revealed that 9 (36%) of the 25 type II secretion system substrates described for L. pneumophila are absent from L. longbeachae (Table 2). For example the phospholipase C encoded by plcA and the chiA-encoded chitinase, which was shown to promote L. pneumophila persistence in the lungs of A/J mice are not present in L. longbeachae (Debroy et al., 2006). Thus over a third of the T2SS substrates seem to differ between L. pneumophila and L. longbeachae, a feature probably related to the different ecological niches occupied, but also to different virulence properties in the hosts.
Table 2.
Distribution of type II secretion-dependent proteins of L. pneumophila in L. longbeachae.
| L. pneumophila | L. longbeachae | Name | Product | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Phila | Paris | Lens | Corby | Alcoy | 130b* | NSW | D-4968 | ||
| lpg0467 | lpp0532 | lpl0508 | lpc2877 | lpa00713 | lpw05741 | llo2721 | llb2607 | proA | Zinc metalloprotease, promotes amebal infection |
| lpg1119 | lpp1120 | lpl1124 | lpc0577 | lpa01742 | – | llo1016 | llb0700 | map | Tartrate-sensitive acid phosphatase |
| lpg2343 | lpp2291 | lpl2264 | lpc1811 | lpa03353 | lpw25361 | llo2819 | llb2504 | plaA | Lysophospholipase A |
| lpg2837 | lpp2894 | lpl2749 | lpc3121 | lpa04118 | lpw30971 | llo0210 | llb1661 | plaC | Glycerophospholipid:cholestrol transferase |
| lpg0502 | lpp0565 | lpl0541 | lpc2843 | lpa00759 | lpw05821 | – | – | plcA | Phospholipase C |
| lpg0745 | lpp0810 | lpl0781 | lpc2548 | lpa01148 | lpw08251 | llo2076 | llb3335 | lipA | Mono- and triacylglycerol lipase |
| lpg1157 | lpp1159 | lpl1164 | lpc0620 | lpa01801 | lpw12111 | llo2433 | llb2928 | lipB | Triacylglycerol lipase |
| lpg2848 | lpp2906 | lpl2760 | lpc3133 | lpa04141 | lpw31111 | llo0201 | llb1671 | srnA | Type 2 ribonuclease, promotes amebal infection |
| lpg1116 | lpp1117 | lpl1121 | lpc0574 | lpa01738 | lpw11641 | – | – | chiA | Chitinase, promotes lung infection |
| lpg2814 | lpp2866 | lpl2729 | lpc3100 | lpa04088 | lpw30701 | llo0255 | llb1611 | lapA | Leucine, phenylalanine, and tyrosine aminopeptidase |
| lpg0032 | lpp0031 | lpl0032 | lpc0032 | lpa00041 | lpw00321 | – | – | lapB | Lysine and arginine aminopeptidase |
| lpg0264 | lpp0335 | lpl0316 | lpc0340 | lpa00461 | lpw03521 | llo3103 | llb2271 | Weakly similar to bacterial amidase | |
| lpg2622 | lpp2675 | lpl2547 | lpc0519 | lpa03836 | lpw28341 | – | – | Weakly similar to bacterial cysteine protease | |
| lpg1918 | lpp1893 | lpl1882 | lpc1372 | lpa02774 | lpw19571 | llo3308 | llb2032 | celA | Endoglucanase |
| lpg2999 | lpp3071 | lpl2927 | lpc3315 | lpa04395 | lpw32851 | – | – | Predicted astacin-like zink endopeptidase | |
| lpg2644 | lpp2697 | lpl2569 | lpc0495 | lpa03870 | – | – | – | Some similarity to collagen like protein | |
| lpg1809 | lpp1772 | lpl1773 | lpc1253 | lpa02614 | lpw18401 | llo1104 | llb0603 | Unknown | |
| lpg1385 | lpp1340 | lpl1336 | lpc0801 | lpa02037 | lpw13951 | llo1474 | llb0177 | Unknown | |
| lpg0873 | lpp0936 | lpl0906 | lpc2419 | lpa01320 | lpw09571 | llo2475 | llb2883 | Unknown | |
| lpg0189 | lpp0250 | lpl0249 | lpc0269 | lpa00360 | lpw02811 | – | – | Unknown | |
| lpg0956 | lpp1018 | lpl0958 | lpc2331 | lpa01443 | lpw10421 | llo1935 | llb3498 | Unknown | |
| lpg2689 | lpp2743 | lpl2616 | lpc0447 | lpa03925 | lpw29431 | llo0361 | llb1497 | icmX | Linked to Dot/Icm type IV secretion genes |
| lpg1244 | lpp0181 | lpl0163 | – | – | lpw01541 | – | – | lvrE | Linked to Lvh type IV secretion genes |
| lpg1832 | lpp1795 | lpl1796 | lpc1276 | lpa02647 | lpw18641 | llo1152 | llb0546 | Weakly similar to VirK | |
| lpg1962 | lpp1946 | lpl1936 | lpc1440 | lpa02861 | lpw20131 | – | – | Putative peptidyl-prolyl cis-trans isomerase | |
| lpg0422 | lpp0489 | lpl0465 | lpc2921 | lpa0657 | lpw05041 | llo2801 | llb2523 | gamA | Glucoamylase |
Substrates in this list are according to Cianciotto (2009); *strain 130b is not a finished sequence and not manually curated. Thus absence of a substrate can also be due to gaps in the sequence; – means not present; NSW means L. longbeachae NSW150.
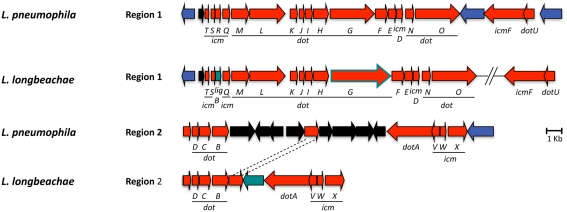
Indispensible for replication of L. pneumophila in the eukaryotic host cells is the Dot/Icm T4SS (Nagai and Kubori, 2011), which translocate a large repertoire of bacterial effectors into the host cell. These effectors modulate multiple host cell processes and in particular, redirect trafficking of the L. pneumophila phagosome and mediate its conversion into an ER-derived organelle competent for intracellular bacterial replication (Shin and Roy, 2008; Cianciotto, 2009). The Dot/Icm system is conserved in L. longbeachae with a similar gene organization and protein identities of 47–92% with respect to L. pneumophila (Figure 3). This is similar to what has been reported previously for other Legionella species (Morozova et al., 2004). The only major differences identified are that in L. longbeachae the icmR gene is replaced by the ligB gene, however, the encoded proteins have been shown to perform similar functions (Feldman and Segal, 2004; Feldman et al., 2005) and that the DotG/IcmE protein of L. longbeachae (1525 aa) is 477 amino acids larger than that of L. pneumophila (1048 aa; Cazalet et al., 2010). DotG of L. pneumophila is part of the core transmembrane complex of the secretion system and is composed of three domains: a transmembrane N-terminal domain, a central region composed of 42 repeats of 10 amino acid and a C-terminal region homologous to VirB10. In contrast, the central region of L. longbeachae DotG is composed of approximately 90 repeats. Among the many VirB10 homologs present in bacteria, the Coxiella DotG and the Helicobacter pylori Cag7 are the only ones, which also have multiple repeats of 10 aa (Segal et al., 2005). It will be challenging to understand the impact of this modification on the function of the type IV secretion system. A L. longbeachae T4SS mutant obtained by deleting the dotA gene is strongly attenuated for intracellular growth in Acanthamoeba castellanii and human macrophages (Cazalet et al., 2010, and unpublished data), is outcompeted by the wild type strain 24 and 72 h after infection of lungs of A/J mice and is also dramatically attenuated for replication in lungs of A/J mice upon single infections (Cazalet et al., 2010). Thus, similar to what is seen for L. pneumophila, the Dot/Icm T4SS of L. longbeachae is also central for its pathogenesis and the capacity to replicate in eukaryotic host cells.
Figure 3.
Alignment of the chromosomal regions of L. pneumophila and L. longbeachae coding the Dot/Icm type 4 secretion system genes. The comparison shows that all genes are highly conserved (47–92% identity) between L. pneumophila Paris and L. longbeachae. Red arrows, genes conserved between L. pneumophila and L. longbeachae (>47% identity); black arrows, L. pneumophila specific genes compared to L. longbeachae (<35% identity); blue arrows, genes conserved between L. pneumophila and L. longbeachae but located in different places of the genome; green arrows, L. longbeachae specific genes compared to L. pneumophila. Red arrow boxed in green depicts dotG. N-terminal and C-terminal parts of dotG are highly conserved while the central part composed of repeated sequences differs between L. pneumophila and L. longbeachae.
This T4SS is crucial for intracellular replication for Legionella as it secretes an exceptionally large number of proteins into the host cell. Using different methods, 275 substrates have been shown to be translocated in the host cell in a Dot/Icm T4SS dependent manner (Campodonico et al., 2005; De Felipe et al., 2005, 2008; Shohdy et al., 2005; Burstein et al., 2009; Heidtman et al., 2009; Zhu et al., 2011). Table 3 shows the distribution of the 275 Dot/Icm substrates identified in L. pneumophila strain Philadelphia and their distribution in the six L. pneumophila and five L. longbeachae genomes sequenced. Their conservation among different L. pneumophila strains is very high, as over 80% of the substrates are present in all L. pneumophila strains analyzed here. In contrast, the search for homologs of these L. pneumophila Dot/Icm substrates in L. longbeachae showed that even more pronounced differences are present than in the repertoire of type II secreted substrates. Only 98 of these 275 L. pneumophila Dot/Icm substrates have homologs in the L. longbeachae genomes (Table 3). However, the repertoire of L. longbeachae substrates seems also to be quite large, as a search for proteins that encode eukaryotic like domains and contain the secretion signal described by Nagai et al. (2005) and the additional criteria defined by Kubori et al. (2008) predicted 51 putative Dot/Icm substrates specific for L. longbeachae NSW150 (Cazalet et al., 2010) indicating that at least over 140 proteins might be secreted by the Dot/Icm T4SS of L. longbeachae. A similar number of L. longbeachae specific putative eukaryotic like proteins and effectors was predicted for strain D-4968 (Kozak et al., 2010). Examples of effector proteins conserved between the two species are RalF, VipA, VipF, SidC, SidE, SidJ, YlfA LepA, and LepB, which contribute to trafficking or recruitment and retention of vesicles to L. pneumophila (Nagai et al., 2002; Chen et al., 2004; Luo and Isberg, 2004; Campodonico et al., 2005; Shohdy et al., 2005; Liu and Luo, 2007). It is interesting to note that homologs of SidM/DrrA and SidD are absent from L. longbeachae but a homolog of LepB is present. For L. pneumophila it was shown that SidM/DrrA, SidD, and LepB act in cooperation to manipulate Rab1 activity in the host cell. DrrA/SidM possesses three domains, an N-terminal AMP-transfer domain (AT), a nucleotide exchange factor (GEF) domain in the central part and a phosphatidylinositol-4-Phosphate binding domain (P4M) in its C-terminal part. After association of DrrA/SidM with the membrane of the Legionella-containing vacuole (LCV) via P4M (Brombacher et al., 2009), it recruits Rab1 via the GEF domain and catalyzes the GDP–GTP exchange (Ingmundson et al., 2007; Machner and Isberg, 2007). Rab1 is then adenylated by the AT domain leading to inhibition of GAP-catalyzed Rab1-deactivation (Müller et al., 2010). LepB cannot bind AMPylated Rab1 (Ingmundson et al., 2007). Recently it was shown that SidD deAMPylates Rab1 and enables LepB to bind Rab1 to promote its GTP–GDP exchange (Neunuebel et al., 2011; Tan and Luo, 2011). One might assume that other proteins of L. longbeachae not yet identified may perform the functions of DrrA/SidM and SidD. Another interesting observation is, that all except four of the effector proteins of L. pneumophila that are conserved in L. longbeachae are also conserved in all sequenced L. pneumophila genomes (Table 3).
Table 3.
Distribution of 275 Dot/Icm substrates identified in strain L. pneumophila Philadelphia in the 5 sequenced L. pneumophila and 5 sequenced L. longbeachae strains.
| L. pneumophila | L. longbeachae | Name | Product | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Phila | Paris | Lens | Corby | Alcoy | 130b | NSW 150 | D-4968 | AT | 98072 | C-4E7 | ||
| lpg0008 | lpp0008 | lpl0008 | lpc0009 | lpa0011 | lpw00071 | – | – | − | − | − | ravA | Unknown |
| lpg0012 | lpp0012 | lpl0012 | lpc0013 | lpa0016 | lpw00111 | – | – | − | − | − | cegC1 | Ankyrin |
| lpg0021 | lpp0021 | lpl0022 | lpc0022 | lpa0030 | lpw00221 | llo0047 | llb1841 | + | + | + | – | Unknown |
| lpg0030 | lpp0030 | lpl0031 | lpc0031 | lpa0040 | lpw00311 | – | – | − | − | − | ravB | Unknown |
| lpg0038 | lpp0037 | lpl0038 | lpc0039 | lpa0049 | lpw00381 | – | – | − | − | − | ankQ/legA10 | Ankyrin repeat |
| lpg0041 | – | – | lpc0042 | lpa0056 | – | – | – | − | − | − | – | Putative metalloprotease |
| lpg0045 | lpp0046 | lpl0044 | lpc0047 | lpa0060 | lpw00441 | – | – | − | − | − | – | Unknown |
| lpg0046 | lpp0047 | lpl0045 | lpc0048 | lpa0062 | lpw00451 | – | – | − | − | − | – | Unknown |
| lpg0059 | lpp0062 | lpl0061 | lpc0068 | lpa0085 | lpw00621 | – | – | − | − | − | ceg2 | Unknown |
| lpg0080 | lpp0094 | – | – | lpa3018 | lpw00781 | – | – | − | − | − | ceg3 | Unknown |
| lpg0081 | lpp0095 | – | – | – | lpw00791 | – | – | − | − | − | – | Unknown |
| lpg0090 | lpp0104 | lpl0089 | lpc0109 | lpa0132 | lpw00881 | – | – | − | − | − | lem1 | Unknown |
| lpg0096 | lpp0110 | lpl0096 | lpc0115 | lpa0145 | lpw00961 | llo1322 | llb0347 | + | + | + | ceg4 | Unknown |
| lpg0103 | lpp0117 | lpl0103 | lpc0122 | lpa0152 | lpw01031 | llo3312 | llb2028 | + | + | + | vipF | N-terminal acetyl-transferase, GNAT |
| lpg0126 | lpp0140 | lpl0125 | lpc0146 | lpa0185 | lpw01261 | – | – | − | − | − | cegC2 | Ninein |
| lpg0130 | lpp0145 | lpl0130 | lpc0151 | lpa0194 | lpw01311 | llo3270 | llb2073 | + | + | + | – | Unknown |
| lpg0135 | lpp0150 | lpl0135 | lpc0156 | lpa0204 | lpw01361 | llo2439 | llb2921 | + | + | + | sdhB | Unknown |
| lpg0160 | lpp0224 | lpl0224 | lpc0242 | lpa0322 | lpw02541 | – | – | − | − | − | ravD | Unknown |
| lpg0170 | lpp0232 | lpl0233 | lpc0251 | lpa0335 | lpw02641 | llo1378 | llb0280 | + | + | + | ravC | Unknown |
| lpg0171 | lpp0233 | lpl0234 | – | – | lpw02651 | – | – | − | − | − | legU1 | F-box motif |
| lpg0172 | lpp0234 | – | lpc0253 | lpa0339 | lpw02661 | – | – | − | − | − | – | Unknown |
| lpg0181 | lpp0245 | lpl0244 | lpc0265 | lpa0388 | lpw02761 | llo2453 | llb2907 | + | + | + | – | Unknown |
| lpg0191 | lpp0251 | – | – | – | lpw02821 | – | – | − | − | − | ceg5 | Unknown |
| lpg0195 | lpp0253 | lpl0251 | lpc0272 | lpa0339 | lpw02851 | – | – | − | − | − | ravE | Unknown |
| lpg0196 | lpp0254 | lpl0252 | – | – | lpw02861 | llo2549 | llb2798 | + | + | + | ravF | Unknown |
| lpg0210 | lpp0269 | lpl0264 | lpc0285 | lpa0388 | lpw02981 | – | – | − | − | − | ravG | Unknown |
| lpg0227 | lpp0286 | lpl0281 | lpc0303 | lpa0412 | lpw03151 | llo2491 | llb2864 | + | + | + | ceg7 | Unknown |
| lpg0234 | lpp0304 | lpl0288 | lpc0309 | lpa0419 | lpw03221 | llo0425 | llb1431 | + | + | + | sidE/laiD | Unknown |
| lpg0240 | lpp0310 | lpl0294 | lpc0316 | lpa0428 | lpw03291 | llo1601 | llb0040 | + | + | + | ceg8 | Unknown |
| lpg0246 | lpp0316 | lpl0300 | lpc0323 | lpa0436 | lpw03361 | – | – | − | − | − | ceg9 | Unknown |
| lpg0257 | lpp0327 | lpl0310 | lpc0334 | lpa0450 | lpw03461 | llo2362 | llb3009 | + | + | + | sdeA | Multidrug resistance protein |
| lpg0260 | lpp0332 | lpl0313 | lpc0337 | lpa0456 | lpw03491 | – | – | − | − | − | – | Unknown |
| lpg0275 | lpp0349 | lpl0327 | lpc0351/3529 | lpa0477 | lpw03641 | – | – | − | − | − | sdbA | Unknown |
| lpg0276 | lpp0350 | lpl0328 | lpc0353 | lpa0479 | lpw03651 | llo0327 | llb1533 | + | + | + | legG2 | Ras guanine nucleotide exchange factor |
| lpg0284 | lpp0360 | lpl0336 | lpc0361 | lpa0490 | lpw03741 | – | – | − | − | − | ceg10 | Unknown |
| lpg0285 | lpp0361 | lpl0337 | lpc0362 | lpa0492 | lpw03751 | – | – | − | − | − | lem2 | Unknown |
| lpg0294 | lpp0372 | lpl0347 | lpc0373 | lpa0508 | lpw03861 | llo0464 | llb1386 | + | + | + | – | Unknown |
| lpg0364 | lpp0429 | lpl0405 | lpc2980 | lpa0578 | lpw04431 | – | – | − | − | − | – | Unknown |
| lpg0365 | lpp0430 | lpl0406 | lpc2979 | lpa0580 | lpw04441 | llo0525 | llb1334 | + | + | + | – | Unknown |
| lpg0375 | lpp0442 | lpl0418 | lpc2968 | lpa0596 | – | – | – | − | − | − | – | Unknown |
| lpg0376 | lpp0443 | lpl0419 | lpc2967 | lpa0597 | lpw04591 | llo0548 | llb1307 | + | + | + | sdhA | GRIP, coiled-coil |
| lpg0390 | lpp0457 | lpl0433 | lpc2954 | lpa0613 | lpw04721 | – | – | − | − | − | vipA | Unknown |
| lpg0401 | lpp0468 | lpl0444 | lpc2942 | lpa0629 | lpw04831 | llo2582 | llb2763 | + | + | + | legA7/ceg11 | Unknown |
| lpg0402 | – | – | – | – | – | – | – | − | − | − | ankY/legA9 | Ankyrin, STPK |
| lpg0403 | lpp0469 | lpl0445 | lpc2941 | lpa0630 | lpw04841 | – | – | − | − | − | ankG/ankZ/legA7 | Ankyrin |
| lpg0405 | lpp0471 | lpl0447 | lpc2939 | lpa0633 | lpw04861 | llo2845 | llb2472 | + | + | + | – | Spectrin domain |
| lpg0422 | lpp0489 | lpl0465 | lpc2921 | lpa0657 | lpw05041 | llo2801 | llb2523 | + | + | + | legY | Putative Glucan 1,4-alpha-glucosidase |
| lpg0436 | lpp0503 | lpl0479 | lpc2906 | lpa0673 | lpw05181 | – | – | − | − | − | ankJ/legA11 | Ankyrin |
| lpg0437 | lpp0504 | lpl0480 | lpc2905 | lpa0674 | lpw05191 | – | – | − | − | − | ceg14 | Unknown |
| lpg0439 | lpp0505 | lpl0481 | lpc2904 | lpa0678 | lpw05201 | llo2983 | llb2392 | + | + | + | ceg15 | Unknown |
| lpg0483 | lpp0547 | lpl0523 | lpc2861 | lpa0739 | lpw05631 | llo2705 | llb2623 | + | + | + | ankC/legA12 | Ankyrin |
| lpg0515 | lpp0578 | lpl0554 | lpc2829 | lpa0776 | lpw05951 | llo3224 | llb2129 | + | + | + | legD2 | Phytanoyl-CoA dioxygenase domain |
| lpg0518 | lpp0581 | lpl0557 | lpc2826 | lpa0781 | lpw05981 | – | – | − | − | − | – | Unknown |
| lpg0519 | – | – | – | – | – | – | – | − | − | − | ceg17 | Unknown |
| lpg0621 | lpp0675 | lpl0658 | lpc2673 | lpa0975 | lpw06951 | – | – | − | − | − | sidA | Unknown |
| lpg0634 | lpp0688 | lpl0671 | lpc2660 | lpa0996 | lpw07081 | llo2574 | llb2771 | + | + | + | – | Unknown |
| lpg0642 | lpp0696/97 | lpl0679 | lpc2651 | lpa1005 | lpw07161 | – | – | − | − | − | wipB | Unknown |
| lpg0695 | lpp0750 | lpl0732 | lpc2599 | lpa1082 | lpw07721 | – | – | − | − | − | ankN/ankX legA8 | Ankyrin |
| lpg0696 | lpp0751 | lpl0733 | lpc2598 | lpa1084 | lpw07731 | – | – | − | − | − | lem3 | Unknown |
| lpg0716 | lpp0782 | lpl0753 | lpc2577 | lpa1108 | lpw07931 | – | – | − | + | + | – | Unknown |
| lpg0733 | lpp0799 | lpl0770 | lpc2559 | lpa1135 | lpw08111 | llo0831 | llb0892 | + | + | + | ravH | Unknown |
| lpg0796 | lpp0859 | – | – | – | – | – | – | − | − | − | – | Unknown |
| lpg0898 | lpp0959 | lpl0929 | lpc2395 | lpa1360 | lpw09801 | – | – | − | − | − | ceg18 | Unknown |
| lpg0926 | lpp0988 | lpl0957 | lpc2365 | lpa1397 | lpw10111 | – | – | − | − | − | ravI | Unknown |
| lpg0940 | lpp1002 | lpl0971 | lpc2349 | lpa1415 | lpw10251 | – | – | − | − | − | lidA | Unknown |
| lpg0944 | lpp1006 | – | lpc2345 | lpa1421 | – | – | – | − | − | − | ravJ | Unknown |
| lpg0945 | lpp1007 | lpl1579 | lpc2344 | lpa1423 | lpw10311 | – | – | − | − | − | legL1 | LLR |
| lpg0963 | lpp1025 | lpl0992 | lpc2324 | lpa1453 | lpw10491 | llo0934 | llb0782 | + | + | + | – | Unknown |
| lpg0967 | lpp1029 | – | lpc2320 | lpa1459 | lpw10531 | – | – | − | − | − | – | Unknown |
| lpg0968 | lpp1030 | lpl0997 | lpc2319 | lpa1460 | lpw10541 | – | – | − | − | − | sidK | Unknown |
| lpg0969 | lpp1031 | lpl0998 | lpc2318 | lpa1461 | lpw10551 | llo3265 | llb2078 | + | + | + | ravK | Unknown |
| lpg1083 | – | – | – | – | – | – | – | − | − | − | – | Unknown |
| lpg1101 | lpp1101 | lpl1100 | lpc2154* | lpa1709 | lpw11451 | – | – | − | − | − | lem4 | Unknown |
| lpg1106 | lpp1105 | lpl1105 | lpc2149 | lpa1719 | lpw11501 | llo1414 | llb0239/40 | + | + | + | – | Unknown |
| lpg1108 | lpp1108 | lpl1108 | lpc2146 | lpa1724 | lpw11531 | llo3030 | llb2350 | + | + | + | ravL | Unknown |
| lpg1109 | lpp1109 | – | lpc2145 | lpa1725 | – | – | – | − | − | − | ravM | Unknown |
| lpg1110 | lpp1111 | lpl1114 | lpc2142 | lpa1728 | lpw11571 | – | – | − | − | − | lem5 | Unknown |
| lpg1111 | lpp1112 | lpl1115 | lpc2141 | lpa1730 | lpw11581 | llo3126 | llb2244 | + | + | + | ravN | Unknown |
| lpg1120 | – | – | – | – | lpw11681 | – | – | − | − | − | lem6 | Unknown |
| lpg1121 | lpp1121 | lpl1126 | lpc0578 | lpa1743 | lpw11691 | llo1321 | llb0348 | + | + | + | ceg19 | Unknown |
| lpg1124 | lpp1125 | lpl1129 | lpc0582 | lpa1748 | lpw11741 | llo3206 | llb2150 | + | + | + | – | Unknown |
| lpg1129 | lpp1130 | – | – | – | lpw11801 | – | – | − | − | − | ravO | Unknown |
| lpg1137 | lpp1139 | lpl1144 | lpc0601 | lpa1776 | lpw11901 | llo2404 | llb2962 | + | + | + | – | Unknown |
| lpg1144 | lpp1146 | lpl1150 | lpc0607 | lpa1785 | lpw11971 | – | – | − | − | − | cegC3 | Unknown |
| lpg1145 | lpp1147 | lpl1151 | lpc0608 | lpa1787 | lpw11981 | – | – | − | − | − | lem7 | Unknown |
| lpg1147 | lpp1149 | lpl1153 | lpc0610 | lpa1789 | lpw12001 | – | – | − | − | − | – | GCN5-related N-acetyltransferase |
| lpg1148 | lpp1150 | lpl1154 | lpc0611 | lpa1790 | lpw12011 | – | – | − | − | − | – | Unknown |
| lpg1152 | lpp1154 | lpl1159 | lpc0615 | lpa1795 | lpw12061 | – | – | − | − | − | ravP | Unknown |
| lpg1154 | lpp1156 | lpl1161 | lpc0617 | lpa1797 | lpw12081 | llo2487 | llb2868 | + | + | + | ravQ | Unknown |
| lpg1158 | lpp1160 | lpl1165* | lpc0621 | lpa1802 | lpw12121 | – | – | − | − | − | – | Unknown |
| lpg1166 | lpp1168 | lpl1174 | lpc0631 | lpa1819 | lpw12211 | llo1034 | llb0680 | + | + | + | ravR | Unknown |
| lpg1171 | lpp1173 | lpl1179 | lpc0637 | lpa1826 | – | – | – | − | − | − | – | Spectrin domain |
| lpg1183 | lpp1186 | lpl1192 | lpc0650 | lpa1839 | lpw12401 | llo2390 | llb2978 | + | + | + | ravS | Unknown |
| lpg1227 | lpp1235 | lpl1235 | lpc0696 | lpa1899 | lpw12861 | – | – | − | − | − | vpdB | Unknown |
| lpg1273 | lpp1236 | lpl1236 | lpc0698 | lpa1901 | lpw12871 | – | – | − | − | − | – | Unknown |
| lpg1290 | lpp1253 | – | – | – | – | – | – | − | − | − | lem8 | Unknown |
| lpg1312 | – | – | – | – | lpw13261 | – | – | − | − | − | legC1 | Unknown |
| lpg1316 | – | – | – | – | – | llo1389 | llb0269 | + | + | + | ravT | Unknown |
| lpg1317 | – | – | – | – | – | – | – | − | − | − | ravW | Unknown |
| lpg1328 | lpp1283 | lpl1282 | lpc0743 | lpa1958 | – | – | – | − | − | − | legT | Thaumatin domain |
| lpg1355 | lpp1309 | – | – | – | – | – | – | − | − | − | sidG | Coiled-coil |
| lpg1426 | lpp1381 | lpl1377 | lpc0842 | lpa2090 | lpw14431 | llo1791 | llb3606 | + | + | + | vpdC | Patatin domain |
| lpg1449 | lpp1404 | – | – | – | lpw14671 | – | – | − | − | − | – | Unknown |
| lpg1453 | lpp1409 | lpl1591 | lpc0868 | lpa2119 | lpw14711 | – | – | − | − | − | – | Unknown |
| lpg1483 | lpp1439 | lpl1545 | lpc0898 | lpa2161 | lpw15031 | llo1682 | llb3727 | + | + | + | legK1 | STPK |
| lpg1484 | lpp1440 | lpl1544 | lpc0899 | lpa2162 | lpw15041 | – | – | − | − | − | – | Unknown |
| lpg1488 | lpp1444 | lpl1540 | lpc0903* | lpa2168 | lpw15081 | – | – | − | − | − | lgt3/legc5 | Coiled-coil |
| lpg1489 | lpp1445 | lpl1539 | lpc0905 | lpa2169 | lpw15091 | – | – | − | − | − | ravX | Unknown |
| lpg1491 | lpp1447 | – | – | – | – | – | – | − | − | − | lem9 | Unknown |
| lpg1496 | lpp1453 | lpl1530 | lpc0915 | lpa2185 | lpw15181 | – | – | − | − | − | lem10 | Unknown |
| lpg1551 | lpp1508 | lpl1475 | lpc0972 | lpa2253 | – | – | – | − | − | − | ravY | Unknown |
| lpg1578 | lpp4178 | lpl4143 | lpc1002 | lpa2292 | lpw16011 | llo1503 | llb0148 | + | + | + | – | Unknown |
| lpg1588 | lpp1546 | lpl1437 | lpc1013 | lpa2305 | lpw16131 | – | – | − | − | − | legC6 | Coiled–coil |
| lpg1598 | lpp1556 | lpl1427 | lpc1025 | lpa2317 | lpw16231 | – | – | − | − | − | lem11 | Unknown |
| lpg1602 | lpp1567 | lpl1423/26* | lpc1028 | lpa2318 | lpw16241 | – | – | − | − | − | legL2 | LRR |
| lpg1621 | lpp1591 | lpl1402 | lpc1048 | lpa2346 | lpw16461 | llo1014 | llb0702 | + | + | + | ceg23 | Unknown |
| lpg1625 | lpp1595 | lpl1398 | lpc1052 | lpa2350 | lpw16511 | llo0719 | llb1016 | + | + | + | lem23 | Unknown |
| lpg1639 | lpp1609 | lpl1387 | lpc1068 | lpa2367 | lpw16651 | – | – | − | − | − | – | Unknown |
| lpg1642 | lpp1612a/b | lpl1384 | lpc1071 | lpa2371 | lpw16681 | – | – | − | − | − | sidB | Putative hydrolase |
| lpg1654 | lpp1625 | – | lpc1084 | lpa2390 | – | llo0791 | llb0935 | + | + | + | – | Unknown |
| lpg1660 | lpp1631 | lpl1625 | lpc1090 | lpa2398 | lpw16861 | – | – | − | − | − | legL3 | LRR |
| lpg1661 | lpp1632 | lpl1626 | lpc1091 | lpa2399 | lpw16871 | llo1691 | llb3715 | + | + | + | – | Putative N-acetyl transferase |
| lpg1666 | lpp1637 | lpl1631 | lpc1096 | lpa2408 | lpw16921 | – | – | − | − | − | – | Unknown |
| lpg1667 | lpp1638 | lpl1632 | lpc1097 | lpa2409 | lpw16931 | – | – | − | − | − | – | Unknown |
| lpg1670 | lpp1642 | lpl1635 | lpc1101 | lpa2413 | lpw16971 | – | – | − | − | − | – | Unknown |
| lpg1683 | – | – | lpc1114 | lpa2431 | – | llo2508 | llb2843 | + | + | + | ravZ | Unknown |
| lpg1684 | – | – | lpc1115 | lpa2432 | – | llo2267 | llb3113 | + | + | + | – | Unknown |
| lpg1685 | – | – | lpc1116 | lpa2433 | – | llo3208 | llb2147 | + | + | + | – | Unknown |
| lpg1687 | lpp1656 | lpl1650 | lpc1118 | lpa2437 | lpw17121 | – | – | − | − | − | mavA | Unknown |
| lpg1689 | lpp1658 | lpl1652 | lpc1120 | lpa2439 | lpw17141 | llo1697 | llb3708 | + | + | + | – | Unknown |
| lpg1692 | – | – | lpc1123 | lpa2442 | – | – | – | − | − | − | – | Unknown |
| lpg1701 | lpp1666 | lpl1660 | lpc1130 | lpa2455 | lpw17231 | – | – | − | − | − | ppeA/legC3 | Coiled-coil |
| lpg1702 | lpp1667 | lpl1661 | lpc1131 | lpa2456 | lpw17241 | – | – | − | − | − | ppeB | Unknown |
| lpg1716 | lpp1681 | lpl1675 | lpc1146 | lpa2474 | lpw17391 | – | – | − | − | − | – | Unknown |
| lpg1717 | lpp1682 | – | – | – | lpw17401 | – | – | − | − | − | – | Unknown |
| lpg1718 | lpp1683 | lpl1682 | lpc1152 | lpa2484 | lpw17411 | – | – | − | − | − | ankI/legAS4 | Ankyrin |
| lpg1751 | lpp1715 | lpl1715 | lpc1191 | lpa2538 | lpw17761 | llo2314 | llb3061 | + | + | + | – | Unknown |
| lpg1752 | lpp1716 | lpl1716 | lpc1192 | lpa2539 | lpw17771 | llo2315 | llb3060 | + | + | + | – | Unknown |
| lpg1776 | lpp1740 | lpl1740 | lpc1217 | lpa2570 | lpw18031 | llo1437 | llb0214* | + | + | + | – | Unknown |
| lpg1797 | – | – | lpc1239 | lpa2599 | lpw32931 | – | – | − | − | − | rvfA | Unknown |
| lpg1798 | lpp1761 | lpl1761 | lpc1241 | lpa2600 | lpw18281 | llo0991 | llb0731 | + | + | + | marB | Unknown |
| lpg1803 | lpp1766 | lpl1766 | lpc1246 | lpa2606 | lpw18331 | llo2611 | llb2729 | + | + | + | – | Unknown |
| lpg1836 | lpp1799 | lpl1800 | lpc1280 | lpa2652 | lpw18691 | – | – | − | − | − | ceg25 | Unknown |
| lpg1851 | lpp1818 | lpl1817 | lpc1296 | lpa2675 | lpw18871 | llo1047 | llb0666 | + | + | + | lem14 | Unknown |
| lpg1884 | lpp1848 | lpl1845 | lpc1331 | lpa2714 | lpw19161 | – | – | − | − | − | ylfB/legC2 | Coiled-coil |
| lpg1888 | lpp1855 | lpl1850 | lpc1336 | lpa2723 | lpw19211 | – | – | − | − | − | – | Unknown |
| lpg1890 | – | lpl1852 | lpc1338 | lpa2726 | lpw19231 | – | – | − | − | − | legLC8 | LRR, coiled-coil |
| lpg1907 | lpp1882 | lpl1871 | lpc1361 | lpa2762 | lpw19461 | llo1240 | llb0452 | + | + | + | – | Unknown |
| lpg1924 | lpp1899 | lpl1888 | lpc1378 | lpa2783 | lpw19631 | – | – | − | − | − | – | Unknown |
| lpg1933 | lpp1914 | lpl1903 | lpc1406 | lpa2811 | lpw19721 | – | – | − | − | − | lem15 | Unknown |
| lpg1947 | lpp1930 | lpl1917* | – | lpa2835 | lpw19951 | – | – | − | − | − | lem16 | Spectrin domain |
| lpg1948 | – | – | – | – | – | – | – | − | − | − | legLC4 | LRR, coiled-coil |
| lpg1949 | lpp1931 | lpl1918 | lpc1422 | lpa2837 | lpw19961 | – | – | − | − | − | lem17 | Unknown |
| lpg1950 | lpp1932 | lpl1919 | lpc1423 | lpa2838 | lpw19971 | llo1397 | llb0259 | + | + | + | ralF | Sec7 domain |
| lpg1953 | lpp1935 | lpl1922 | lpc1426 | lpa2842 | lpw20041 | – | – | − | − | − | legC4 | Coiled-coil |
| lpg1958 | lpp1940 | – | – | – | – | – | – | − | − | − | legL5 | LRR |
| lpg1959 | lpp1941 | – | – | lpa2857 | lpw20101 | – | – | − | − | − | – | Unknown |
| lpg1960 | lpp1942 | lpl1934* | lpc1437 | lpa2859 | lpw20111 | llo0565 | llb1288 | + | + | + | lirA | Unknown |
| lpg1962 | lpp1946 | lpl1936 | lpc1440 | lpa2861 | lpw20131 | – | – | − | − | − | lirB | Rotamase |
| lpg1963 | – | – | lpc1441/42 | lpa2863 | – | – | – | − | − | − | pieA/lirC | Unknown |
| lpg1964 | – | – | – | – | – | – | – | − | − | − | pieB/lirD | Unknown |
| lpg1965 | – | – | lpc1443/45 | lpa2865 | lpw20141 | – | – | − | − | − | pieC/lirE | Unknown |
| lpg1966 | lpp1947 | – | lpc1446 | lpa2867 | lpw20151 | – | – | − | − | − | pieD/lirF | Unknown |
| lpg1969 | lpp1952 | lpl1941 | lpc1452 | lpa2874 | lpw20201 | llo3131 | llb2239 | + | + | + | pieE | Unknown |
| lpg1972 | lpp1955 | lpl1950 | lpc1459 | lpa2884 | lpw20291 | – | – | − | − | − | pieF | Unknown |
| lpg1975 | lpp1959 | lpl1953 | lpc1462 | lpa2889(1) | lpw20351 | – | – | − | − | − | – | Unknown |
| lpg1976 | lpp1959 | lpl1953 | lpc1462 | lpa2889(2) | lpw20351 | – | – | − | − | − | pieG/legG1 | Regulator of chromosome condensation |
| lpg1978 | lpp1961 | lpl1955 | lpc1464 | lpa2892 | lpw20371 | – | – | − | − | − | setA | Putative Glyosyltransferase |
| lpg1986 | lpp1967 | lpl1961 | lpc1469 | lpa2898 | lpw20431 | – | – | − | − | − | – | Unknown |
| lpg2050 | lpp2033 | lpl2028 | lpc1536 | lpa2992 | lpw21141 | – | – | − | − | − | – | Unknown |
| lpg2131 | – | – | – | – | – | – | – | − | − | − | legA6 | Unknown |
| lpg2137 | lpp2076 | lpl2066 | lpc1586 | lpa3060 | lpw23101 | – | – | − | − | − | legK2 | STPK |
| lpg2144 | lpp2082 | lpl2072 | lpc1593 | lpa3071 | lpw23181 | – | – | − | − | − | ankB/legAU13/ceg27 | Ankyrin, F-box |
| lpg2147 | lpp2086 | lpl2075 | lpc1596 | lpa3076 | lpw23211 | – | – | − | − | − | mavC | Unknown |
| lpg2148 | lpp2087 | lpl2076 | lpc1597 | lpa3077 | lpw23221 | – | – | − | − | − | – | Unknown |
| lpg2149 | lpp2088 | lpl2077 | lpc1598 | lpa3078 | lpw23231 | – | – | − | − | − | – | Unknown |
| lpg2153 | lpp2092 | lpl2081 | lpc1602 | lpa3083 | lpw23271 | – | – | − | − | − | sdeC | Unknown |
| lpg2154 | lpp2093 | lpl2082 | lpc1603 | lpa3086 | lpw23281 | llo3097 | llb2278 | + | + | + | sdeC | Unknown |
| lpg2155 | lpp2094 | lpl2083 | lpc1604 | lpa3087 | lpw23291 | llo3096 | llb2279 | + | + | + | sidJ | Unknown |
| lpg2156 | lpp2095 | lpl2084 | lpc1605 | lpa3088 | lpw23301 | llo3095 | llb2280 | + | + | +? | sdeB | Unknown |
| lpg2157 | lpp2096 | lpl2085 | lpc1618 | lpa3037 | lpw23331 | – | – | − | − | − | sdeC | Unknown |
| lpg2166 | lpp2104 | lpl2093 | lpc1626 | lpa3107 | lpw23451 | llo2398 | llb2969 | + | + | + | lem19 | Unknown |
| lpg2160 | lpp2099 | lpl2088 | lpc1621 | lpa3100 | lpw23361 | llo2645 | llb2690 | + | + | + | – | Unknown |
| lpg2176 | lpp2128 | lpl2102 | lpc1635 | lpa3118 | lpw23561 | – | – | – | – | – | legS2 | Sphingosine-1-phosphate lyase |
| lpg2199 | lpp2149 | lpl2123 | lpc1663 | lpa3157 | lpw23811 | – | – | – | – | – | cegC4 | Unknown |
| lpg2200 | lpp2150 | lpl2124 | lpc1664 | lpa3158 | lpw23821 | – | – | – | – | – | cegC4 | Unknown |
| lpg2215 | lpp2166 | lpl2140 | lpc1680 | lpa3179 | lpw24011 | – | – | – | – | – | legA2 | Ankyrin |
| lpg2216 | lpp2167 | lpl2141 | lpc1681 | lpa3180 | lpw24021 | – | – | – | – | – | lem20 | Unknown |
| lpg2222 | lpp2174 | lpl2147 | lpc1689 | lpa3191 | lpw24081 | llo1443 | llb0208 | + | + | + | lpnE | Putative beta-lactamase (SEL1 domain) |
| lpg2223 | lpp2175 | lpl2149* | lpc1691 | lpa3196 | lpw24091 | – | – | – | – | – | – | Unknown |
| lpg2224 | – | – | – | – | – | – | – | – | – | – | ppgA | Regulator of chromosome condensation |
| lpg2239 | lpp2192 | – | – | – | lpw24261 | – | – | – | – | – | – | Unknown |
| lpg2248 | lpp2202 | lpl2174 | lpc1717 | lpa3237 | lpw24371 | – | – | – | – | – | lem21 | Unknown |
| lpg2271 | lpp2225 | lpl2197 | lpc1740 | lpa3268 | lpw24611 | llo2530 | llb2821 | + | + | + | – | Unknown |
| lpg2298 | lpp2246 | lpl2217 | lpc1763 | lpa3296 | lpw24841 | llo1707 | llb3696 | + | + | + | ylfA/legC7 | Coiled-coil |
| lpg2300 | lpp2248 | lpl2219 | lpc1765 | lpa3298 | lpw24871 | llo0584 | llb1266 | + | + | + | ankH/legA3, ankW | Ankyrin, NfkappaB inhibitor |
| lpg2311 | lpp2259 | lpl2230 | lpc1776 | lpa3312 | lpw24981 | – | – | − | − | − | ceg28 | Unknown |
| lpg2322 | lpp2270 | lpl2242 | lpc1789 | lpa3328 | lpw25121 | llo0570 | llb1282 | + | + | + | ankK/legA5 | Ankyrin |
| lpg2327 | lpp2275 | lpl2247 | lpc1794 | lpa3335 | lpw25181 | – | – | − | − | − | – | Unknown |
| lpg2328 | lpp2276 | lpl2248 | lpc1795 | lpa3336 | lpw25191 | – | – | − | − | − | lem22 | Unknown |
| lpg2344 | lpp2292 | lpl2265 | lpc1812 | lpa3355 | lpw25371 | – | – | − | − | − | mavE | Unknown |
| lpg2351 | lpp2300 | lpl2273 | lpc1820 | lpa3367 | lpw25461 | llo2850 | llb2466 | + | + | + | mavF | Unknown |
| lpg2359 | lpp2308 | lpl2281 | lpc1828 | lpa3376 | lpw25561 | llo2856 | llb2460 | + | + | + | – | Unknown |
| lpg2370 | – | – | – | – | – | – | – | − | − | − | – | HipA fragment |
| lpg2372 | lpp3009 | – | lpc3248 | lpa4300 | – | – | – | − | − | − | – | Unknown |
| lpg2382 | lpp2444 | lpl2300 | lpc2108 | lpa3446 | lpw25841 | llo1576 | llb0071 | + | + | + | – | Unknown |
| lpg2391 | lpp2458 | lpl2315 | lpc2086 | lpa3485 | lpw26021 | – | – | − | − | − | sdbC | Unknown |
| lpg2392 | lpp2459 | lpl2316 | lpc2085 | lpa3486 | lpw26041 | – | – | − | − | − | legL6 | LRR |
| lpg2400 | – | lpl2323 | – | – | lpw26121 | – | – | − | − | − | legL6 | LRR |
| lpg2406 | lpp2472 | lpl2329 | lpc2070 | lpa3506 | lpw26191 | llo2172 | llb3225 | + | + | + | lem23 | Unknown |
| lpg2407 | lpp2474 | – | lpc2069 | lpa3507 | – | – | – | − | − | − | – | Unknown |
| lpg2409 | lpp2476 | lpl2332 | lpc2067 | lpa3511 | lpw26241 | – | – | − | − | − | ceg29 | Unknown |
| lpg2410 | lpp2479 | lpl2334 | lpc2065 | lpa3513 | lpw26261 | – | – | − | − | − | vpdA | Patatin domain |
| lpg2411 | lpp2480 | lpl2335 | lpc2064 | lpa3515 | lpw26281 | llo2227 | llb3158 | + | + | + | lem24 | Unknown |
| – | lpp2486 | – | – | – | – | − | − | – | F-box | |||
| lpg2416 | – | lpl2339 | lpc2057 | lpa3527 | lpw26351 | – | – | − | − | − | legA1 | Unknown |
| lpg2420 | – | lpl2343 | lpc2056 | lpa3529 | lpw26391 | – | – | − | − | − | – | Unknown |
| lpg2422 | lpp2487 | lpl2345 | lpc2055 | lpa3530 | lpw26401 | llo1650 | llb3763/64 | + | + | + | lem25 | Unknown |
| lpg2424 | lpp2489 | lpl2347 | lpc2053 | lpa3532 | lpw26421 | – | – | − | − | − | mavG | Unknown |
| lpg2425 | lpp2491 | lpl2348 | lpc2051 | lpa3537 | lpw26431 | – | – | − | − | − | mavH | Unknown |
| lpg2433 | lpp2500 | lpl2353 | lpc2043 | lpa3548 | lpw26521 | – | – | − | − | − | ceg30 | Unknown |
| lpg2434 | lpp2501 | lpl2355 | lpc2042 | lpa3550 | lpw26531 | – | – | − | − | − | – | Unknown |
| lpg2443 | lpp2510 | lpl2363 | lpc2033 | lpa3562 | – | – | – | − | − | − | – | Unknown |
| lpg2444 | lpp2511 | lpl2364 | lpc2032 | lpa3563 | lpw26641 | – | – | − | − | − | mavI | Unknown |
| lpg2452 | lpp2517 | lpl2370 | lpc2026 | lpa3574 | lpw26701 | – | – | − | − | − | ankF/legA14/ceg31 | Ankyrin |
| lpg2456 | lpp2522 | lpl2375 | lpc2020 | lpa3583 | lpw26751 | llo0365 | llb1493 | + | + | + | ankD/legA15 | Ankyrin |
| lpg2461 | lpp2527 | lpl2380 | lpc2015 | lpa3589 | lpw26801 | llo1991 | llb3433 | + | + | + | – | Unknown |
| lpg2464 | – | lpl2384 | – | – | lpw26851 | – | – | − | − | − | sidM/drrA | Unknown |
| lpg2465 | – | lpl2385 | – | – | lpw26861 | – | – | − | − | − | sidD | Unknown |
| lpg2490 | lpp2555 | lpl2411 | lpc1987 | lpa3628 | lpw27131 | – | – | − | − | − | lepB | Coiled-coil, Rab1 GAP |
| lpg2482 | lpp2546 | lpl2402 | lpc1996 | lpa3615 | lpw27041 | – | – | − | − | − | sdbB | Unknown |
| lpg2498 | lpp2566 | lpl2420 | lpc1975 | lpa3646 | lpw27241 | – | – | − | − | − | mavJ | Unknown |
| lpg2504 | lpp2572 | lpl2426 | lpc1967 | lpa3658 | lpw27301 | llo2525 | llb2826 | + | + | + | sidI/ceg32 | Unknown |
| lpg2505 | lpp2573 | lpl2427 | lpc1966 | lpa3659 | lpw27311 | llo2526 | llb2825 | + | + | + | – | Unknown |
| lpg2508 | lpp2576 | lpl2430 | lpc1962/63* | lpa3666 | lpw27341 | – | – | − | − | − | sdjA | Unknown |
| lpg2509 | lpp2577 | lpl2431 | lpc1961 | lpa3667 | lpw27351 | llo3097 | llb2278 | + | + | + | sdeD | Unknown |
| lpg2510 | lpp2578 | lpl2432 | lpc1960 | lpa3668 | – | llo3098 | llb2276 | + | + | + | sdcA | Unknown |
| lpg2511 | lpp2579 | lpl2433 | lpc1959 | lpa3669 | lpw27371 | – | – | − | − | − | sidC | PI(4)P binding domain |
| lpg2523 | – | – | – | – | lpw27501 | – | – | − | − | − | lem26 | Unknown |
| lpg2525 | – | – | – | – | – | – | – | − | − | − | mavK | Unknown |
| lpg2526 | lpp2591 | lpl2446 | lpc1946 | lpa3687 | lpw27521 | – | – | − | − | − | mavL | Unknown |
| lpg2527 | lpp2592 | lpl2447 | lpc1944 | lpa3688 | lpw27531 | llo3335 | llb2002 | + | + | + | – | Unknown |
| lpg2529 | lpp2594 | lpl2449 | lpc1942 | lpa3692 | lpw27551 | llo2238 | llb3146 | + | + | + | lem27 | Unknown |
| lpg2538 | lpp2604 | lpl2459 | lpc1930 | lpa3706 | lpw27671 | – | – | − | − | − | – | Unknown |
| lpg2539 | lpp2605 | lpl2460 | lpc1929 | lpa3707 | lpw27681 | llo1348 | llb0317 | + | + | + | – | Unknown |
| lpg2541 | lpp2607 | lpl2462 | lpc1927 | lpa3710 | lpw27701 | – | – | − | − | − | – | Unknown |
| lpg2546 | lpp2615 | – | lpc1919 | lpa3727 | lpw27791 | – | – | − | − | − | – | Unknown |
| lpg2552 | lpp2622 | lpl2473 | lpc1911 | lpa3738 | lpw27871 | llo1062 | llb0648 | + | + | + | – | Unknown |
| lpg2555 | lpp2625 | lpl2480 | lpc1908 | lpa3743 | lpw27901 | llo2220 | llb3170 | + | + | + | – | Unknown |
| lpg2556 | lpp2626 | lpl2481 | lpc1906 | lpa3745 | lpw27911 | llo2218 | llb3172 | + | + | + | legK3 | STPK |
| lpg2577 | lpp2629 | lpl2499 | lpc0570 | lpa3768 | lpw28241 | – | – | − | − | − | mavM | Unknown |
| lpg2584 | lpp2637 | lpl2507 | lpc0561 | lpa3779 | lpw28321 | – | – | − | − | − | sidF | Unknown |
| lpg2588 | lpp2641 | lpl2511 | lpc0557 | lpa3784 | lpw28361 | llo2622 | llb2718 | + | + | + | legS1 | Unknown |
| lpg2591 | lpp2644 | lpl2514 | lpc0551 | lpa3790 | lpw28391 | llo0626 | llb1219 | + | + | + | ceg33 | Unknown |
| lpg2603 | lpp2656 | lpl2526 | lpc0539 | lpa3807 | lpw28521 | – | – | − | − | − | lem28 | Unknown |
| lpg2628 | lpp2681 | lpl2553 | lpc0513 | lpa3846 | lpw28781 | – | – | − | − | − | – | Unknown |
| lpg2637 | lpp2690 | lpl2562 | lpc0503 | lpa3859 | lpw28871 | – | – | − | − | − | – | Unknown |
| lpg2638 | lpp2691 | lpl2563 | lpc0502 | lpa3861 | lpw28891 | llo2645 | llb2690 | + | + | + | mavV | Unknown |
| lpg2692 | lpp2746 | lpl2619 | lpc0444 | lpa3929 | lpw29461 | – | – | − | − | − | – | Unknown |
| lpg2694 | lpp2748 | lpl2621 | lpc0442 | lpa3931 | lpw29481 | – | – | − | − | − | legD1 | Phyhd1 protein |
| lpg2718 | lpp2775 | lpl2646 | lpc0415 | lpa3966 | lpw29771 | – | – | − | − | − | wipA | Unknown |
| lpg2720 | lpp2777 | lpl2648 | lpc0413 | lpa3968 | lpw29791 | – | – | − | − | − | legN | cAMP-binding protein |
| lpg2744 | lpp2800 | lpl2669 | lpc0386 | lpa4004 | lpw30031 | – | – | − | − | − | – | Unknown |
| lpg2745 | lpp2801 | lpl2670 | lpc0385 | lpa4005 | lpw30041 | llo0308 | llb1553 | + | + | + | – | Unknown |
| lpg2793 | lpp2839 | lpl2708 | lpc3079 | lpa4063 | lpw30471 | – | – | − | − | − | lepA | Effector protein A |
| lpg2804 | lpp2850 | lpl2719 | lpc3090 | lpa4076 | lpw30591 | llo0267 | llb1598 | + | + | + | lem29 | Unknown |
| lpg2815 | lpp2867 | lpl2730 | lpc3101 | lpa4089 | lpw30711 | llo0254 | llb1612 | + | + | + | mavN | Unknown |
| lpg2826 | – | lpl2741 | lpc3113 | lpa4104 | lpw30831 | – | – | − | − | − | ceg34 | Unknown |
| lpg2828 | lpp2882 | lpl2743 | lpc3115 | lpa4109 | lpw30851 | llo0783 | llb0944 | + | + | + | – | Unknown |
| lpg2829 | lpp2883/86* | – | – | – | lpw30861 | – | – | − | − | − | sidH | Unknown |
| lpg2830 | lpp2887 | – | – | – | lpw30881 | – | – | − | − | − | lubX/legU2 | U-box motif |
| lpg2831 | lpp2888 | – | – | – | lpw30891 | – | – | − | − | − | VipD | Patatin-like phopholipase |
| lpg2832 | lpp2889 | lpl2744 | lpc3116 | lpa4110 | lpw30921 | llo0214 | llb1656 | + | + | + | – | Putative hydrolase |
| lpg2844 | lpp2903 | lpl2756 | lpc3128 | lpa4133 | – | – | – | − | − | − | – | Unknown |
| lpg2862 | – | – | – | – | – | – | – | − | − | − | Lgt2/legC8 | Coiled-coil |
| lpg2874 | lpp2933 | lpl2787 | lpc3160 | lpa4176 | lpw31411 | – | – | – | – | – | – | Unknown |
| lpg2879 | lpp2938 | lpl2792 | lpc3165 | lpa4186 | lpw31471 | llo0192 | llb1681 | + | + | + | – | Unknown |
| lpg2884 | lpp2943 | lpl2797 | lpc3170 | lpa4193 | lpw31531 | llo0197 | llb1676 | + | + | + | – | Unknown |
| lpg2885 | lpp2944 | lpl2798 | lpc3171 | – | lpw31541 | – | – | − | − | − | – | Unknown |
| lpg2888 | lpp2947 | lpl2801 | lpc3174 | lpa4199 | lpw31571 | llo0200 | llb1672 | + | + | + | – | Unknown |
| lpg2912 | lpp2980 | lpl2830 | lpc3214 | lpa4255 | lpw31931 | – | – | − | − | − | – | Unknown |
| lpg2936 | lpp3004 | lpl2865 | lpc3243 | lpa4293 | lpw32251 | llo0081 | llb1804 | + | + | + | – | rRNA small subunit methyltransferase E |
| lpg2975 | lpp3047 | lpl2904 | lpc3290 | lpa4358 | −? | llo3405 | llb1930 | + | + | + | – | Unknown |
| lpg2999 | lpp3071 | lpl2927 | lpc3315 | lpa4395 | lpw32851 | – | – | − | − | − | legP | Astacin protease |
| lpg3000 | lpp3072 | lpl2928 | lpc3316 | lpa4397 | lpw32861 | llo3444 | llb1887 | + | + | + | – | Unknown |
List of substrates is based on Isberg et al. (2009), De Felipe et al. (2008), Ninio et al. (2009), Zhu et al. (2011); AT = ATCC33462; *pseudogene, +? or −? strains 130b, C-4E7 and 98072 are not a finished sequence and not manually curated. Thus absence of a substrate can also be due to gaps in the sequence; shaded in gray, substrates conserved in all L. pneumophila and L. longbeachae genomes.
Taken together the T2SS Lsp and the T4SS Dot/Icm are highly conserved between L. pneumophila and L. longbeachae. However, more than a third of the known L. pneumophila type II- and over 70% of type IV-dependent substrates differ between both species. These species specific, secreted effectors might be implicated in the different niche adaptations and host susceptibilities. Most interestingly, of the 98 L. pneumophila substrates conserved in L. longbeachae 87 are also present in all L. pneumophila strains sequenced to date. Thus, these 87 Dot/Icm substrates might be essential for intracellular replication of Legionella and represent a minimal toolkit for intracellular replication that has been acquired before the divergence of the two species.
Molecular Mimicry is a Major Virulence Strategy of L. pneumophila and L. longbeachae
The L. pneumophila genome sequence analysis has revealed that many of the predicted or experimentally verified Dot/Icm secreted substrates are proteins similar to eukaryotic proteins or contain motifs mainly or only found in eukaryotic proteins (Cazalet et al., 2004; De Felipe et al., 2005). Thus comparative genomics suggested that L. pneumophila encodes specific virulence factors that have evolved during its evolution with eukaryotic host cells such as fresh-water ameba (Cazalet et al., 2004). The protein-motifs predominantly found in eukaryotes, which were identified in the L. pneumophila genomes are ankyrin repeats, SEL1 (TPR), Set domain, Sec7, serine threonine kinase domains (STPK), U-box, and F-box motifs. Examples for eukaryotic like proteins of L. pneumophila are two secreted apyrases, a sphingosine-1-phosphate lyase and sphingosine kinase, eukaryotic like glycoamylase, cytokinin oxidase, zinc metalloprotease, or an RNA binding precursor (Cazalet et al., 2004; De Felipe et al., 2005; Bruggemann et al., 2006). Function prediction based on similarity searches suggested that many of these proteins are implicated in modulating host cell functions to the pathogens advantage (Cazalet et al., 2004). Recent functional studies confirm these predictions.
As a first example, it was shown that L. pneumophila is able to interfere with the host ubiquitination pathway. The L. pneumophila U-box containing protein LubX was shown to be a secreted effector of the Dot/Icm secretion system that mediates polyubiquitination of a host kinase Clk1 (Kubori et al., 2008). Recently, LubX was described as the first example of an effector protein, which targets and regulates another effector within host cells, as it functions as an E3 ubiquitin ligase that hijacks the host proteasome to specifically target the bacterial effector protein SidH for degradation. Delayed delivery of LubX to the host cytoplasm leads to the shutdown of SidH within the host cells at later stages of infection. This demonstrates a sophisticated level of co-evolution between eukaryotic cells and L. pneumophila involving an effector that functions as a key regulator to temporally coordinate the function of a cognate effector protein (Kubori et al., 2010; Luo, 2011). Furthermore, AnkB/Lpp2028, one of the three F-box proteins of L. pneumophila, was shown to be a T4SS effector that is implicated in virulence of L. pneumophila and in recruiting ubiquitinated proteins to the LCV (Al-Khodor et al., 2008; Price et al., 2009; Habyarimana et al., 2010; Lomma et al., 2010).
A second example is the apyrases (Lpg1905 and Lpg0971) encoded in the L. pneumophila genomes. Indeed, both are secreted enzymes important for intracellular replication of L. pneumophila. Lpg1905 is a novel prokaryotic ecto-NTPDase, similar to CD39/NTPDase1, which is characterized by the presence of five apyrase-conserved regions and enhances the replication of L. pneumophila in eukaryotic cells (Sansom et al., 2007). Apart from ATP and ADP, Lpg1905 also cleaves GTP and GDP with similar efficiency to ATP and ADP, respectively (Sansom et al., 2008). A third example is a L. pneumophila homolog of the highly conserved eukaryotic enzyme sphingosine-1-phosphate lyase (Spl). In eukaryotes, SPL is an enzyme that catalyzes the irreversible cleavage of sphingosine-1-phosphate (S1P). S1P is implicated in various physiological processes like cell survival, apoptosis, proliferation, migration, differentiation, platelet aggregation, angiogenesis, lymphocyte trafficking and development. Despite the fact that the function of the L. pneumophila Spl remains actually unknown, the hypothesis is that it plays a role in autophagy and/or apoptosis (Cazalet et al., 2004; Bruggemann et al., 2006). Recently it has been shown that the L. pneumophila Spl is a secreted effector of the Dot/Icm T4SS, that it is able to complement the sphingosine-sensitive phenotype of Saccharomyces cerevisiae. Moreover, L. pneumophila Spl co-localizes to the host cell mitochondria (Degtyar et al., 2009).
Taken together, the many different functional studies undertaken based on the results of the genome sequence analyses deciphering the roles of the eukaryotic like proteins have clearly established that they are secreted virulence factors that are involved in host cell adhesion, formation of the LCV, modulation of host cell functions, induction of apoptosis and egress of Legionella (Nora et al., 2009; Hubber and Roy, 2010). Most of these effector proteins are expressed at different stages of the intracellular life cycle of L. pneumophila (Bruggemann et al., 2006) and are delivered to the host cell by the Dot/Icm T4SS. Thus molecular mimicry of eukaryotic proteins is a major virulence strategy of L. pneumophila.
As expected, eukaryotic like proteins and proteins encoding domains mainly found in eukaryotic proteins are also present in the L. longbeachae genomes. However, between the two species a considerable diversity in the repertoire of these proteins exists. For example Spl, LubX, the three L. pneumophila F-box proteins, and the homolog of one (Lpg1905) of the two apyrases are missing in all sequenced L. longbeachae genomes. In contrast a glycoamylase (Herrmann et al., 2011) and an uridine kinase homolog are present also in L. longbeachae (Cazalet et al., 2010; Kozak et al., 2010; Table 3). However, other proteins encoded by the L. longbeachae genome contain U-box and F-box domains and might therefore fulfill similar functions. Thus, although the specific proteins may not be conserved, the eukaryotic like protein–protein interaction domains found in L. pneumophila are also present in L. longbeachae.
The differences in trafficking between L. longbeachae and L. pneumophila mentioned above might be related to specific effectors encoded by L. longbeachae. A search for such specific putative effectors of L. longbeachae identified several proteins that might contribute to these differences like a family of Ras-related small GTPases (Cazalet et al., 2010; Kozak et al., 2010). These proteins may be involved in vesicular trafficking and thus may account at least partly for the specificities of the L. longbeachae life cycle. L. pneumophila is also known to exploit monophosphorylated host phosphoinositides (PI) to anchor the effector proteins SidC, SidM/DrrA, LpnE, and LidA to the membrane of the replication vacuole (Machner and Isberg, 2006; Murata et al., 2006; Weber et al., 2006, 2009; Newton et al., 2007; Brombacher et al., 2009). L. longbeachae may employ an additional strategy to interfere with the host PI as a homolog of the mammalian PI metabolizing enzyme phosphatidylinositol-4-phosphate 5-kinase was identified in its genome. One could speculate that this protein allows direct modulation of the host cell PI levels.
Interestingly, although 23 of the 29 ankyrin proteins identified in the L. pneumophila strains are absent from the L. longbeachae genome, L. longbeachae encodes a total of 23 specific ankyrin repeat proteins (Table 3). For example, L. pneumophila AnkX/AnkN that was shown to interfere with microtubule-dependent vesicular transport is missing in L. longbeachae (Pan et al., 2008). However, L. longbeachae encodes a putative tubulin–tyrosine ligase (TTL). TTL catalyzes the ATP-dependent post-translational addition of a tyrosine to the carboxy terminal end of detyrosinated alpha-tubulin. Although the exact physiological function of alpha-tubulin has so far not been established, it has been linked to altered microtubule structure and function (Eiserich et al., 1999). Thus this protein might take over this function in L. longbeachae.
Legionella longbeachae is the first bacterial genome encoding a protein containing an Src Homology 2 (SH2) domain. SH2 domains, in eukaryotes, have regulatory functions in various intracellular signaling cascades. Furthermore, L. longbeachae encodes two proteins with pentatricopeptide repeat (PPR) domains. This family seems to be greatly expanded in plants, where they appear to play essential roles in organellar RNA metabolism (Lurin et al., 2004; Nakamura et al., 2004; Schmitz-Linneweber and Small, 2008). Only 12 bacterial PPR domain proteins have been identified to date, all encoded by two species, the plant pathogens Ralstonia solanacearum and the facultative photosynthetic bacterium Rhodobacter sphaeroides. Thus, genome analysis revealed a particular feature of the Legionella genomes, the presence of many eukaryotic like proteins and protein domains, some of which are common to the two Legionella species, others which are specific and may thus account for the species specific features in intracellular trafficking and niche adaptation in the environment.
Surface Structures – A Clue to Mouse Susceptibility to Infection with Legionella
Despite the presence of many different species of Legionella in aquatic reservoirs, the vast majority of human disease is caused by a single serogroup (Sg) of a single species, namely L. pneumophila Sg1, which is responsible for about 84% of all cases worldwide (Yu et al., 2002). Similar results are obtained for L. longbeachae. Two serogroups are described, but L. longbeachae Sg1 is predominant in human disease. Lipopolysaccharide (LPS) is the basis for the classification of serogroups but it is also a major immunodominant antigen of L. pneumophila and L. longbeachae. Interestingly, it has also been shown that membrane vesicles shed by virulent L. pneumophila containing LPS are sufficient to inhibit phagosome–lysosome fusion (Fernandez-Moreira et al., 2006). Results obtained from large-scale genome comparisons of L. pneumophila suggested that LPS of Sg1 itself might be implicated in the predominance of Sg1 strains in human disease compared to other serogroups of L. pneumophila and other Legionella species (Cazalet et al., 2008). A comparative search for LPS coding regions in the genome of L. longbeachae NSW 150 identified two gene clusters encoding proteins that could be involved in production of lipopolysaccharide (LPS) and/or capsule. Neither shared homology with the L. pneumophila LPS biosynthesis gene cluster suggesting considerable differences in this major immunodominant antigen between the two Legionella species. However, homologs of L. pneumophila lipidA biosynthesis genes (LpxA, LpxB, LpxD, and WaaM) are present. Electron microscopy also demonstrated that, in contrast to L. pneumophila, L. longbeachae produces a capsule-like structure, suggesting that one of the aforementioned gene cluster encodes LPS and the other the capsule (Cazalet et al., 2010).
As mentioned in the introduction, only A/J mice are permissive for replication of L. pneumophila, in contrast A/J, C57BL/6, and BALB/c mice are all permissive for replication of L. longbeachae. In C57BL/6 mice cytosolic flagellin of L. pneumophila triggers Naip5-dependent caspase-1 activation and subsequent proinflammatory cell death by pyroptosis rendering them resistant to infection (Diez et al., 2003; Wright et al., 2003; Molofsky et al., 2006; Ren et al., 2006; Zamboni et al., 2006; Lamkanfi et al., 2007; Lightfield et al., 2008). Genome analysis shed light on the reasons for these differences. L. longbeachae does not carry any flagellar biosynthesis genes except the sigma factor FliA, the regulator FleN, the two-component system FleR/FleS and the flagellar basal body rod modification protein FlgD (Cazalet et al., 2010; Kozak et al., 2010). Analysis of the genome sequences of strains L. longbeachae D-4968, ATCC33642, 98072, and C-4E7 as well as a PCR-based screening of 50 L. longbeachae isolates belonging to both serogroups by Kozak et al. (2010) and of 15 additional isolates by Cazalet et al. (2010) did not detect flagellar genes in any isolate confirming that L. longbeachae, in contrast to L. pneumophila does not synthesize flagella. Interestingly, all genes bordering flagellar gene clusters are conserved between L. longbeachae and L. pneumophila, suggesting deletion of these regions from the L. longbeachae genome. This result suggests, that L. longbeachae fails to activate caspase-1 due to the lack of flagellin, which may also partly explain the differences in mouse susceptibility to L. pneumophila and L. longbeachae infection. The putative L. longbeachae capsule may also contribute to this difference.
Quite interestingly, although L. longbeachae does not encode flagella, it encodes a putative chemotaxis system. Chemotaxis enables bacteria to find favorable conditions by migrating toward higher concentrations of attractants. In many bacteria, the chemotactic response is mediated by a two-component signal transduction pathway, comprising a histidine kinase CheA and a response regulator CheY. Homologs of this regulatory system are present in the L. longbeachae genomes sequenced (Cazalet et al., 2010; Kozak et al., 2010). Furthermore, two homologs of the “adaptor” protein CheW that associate with CheA or cytoplasmic chemosensory receptors are present. Ligand-binding to receptors regulates the autophosphorylation activity of CheA in these complexes. The CheA phosphoryl group is subsequently transferred to CheY, which then diffuses away to the flagellum where it modulates motor rotation. Adaptation to continuous stimulation is mediated by a methyltransferase CheR. Together, these proteins represent an evolutionarily conserved core of the chemotaxis pathway, common to many bacteria and archea (Kentner and Sourjik, 2006; Hazelbauer et al., 2008). Homologs of all these proteins are present in the L. longbeachae genomes (Cazalet et al., 2010; Kozak et al., 2010) and a similar chemotaxis system is present in Legionella drancourtii LLAP12 (La Scola et al., 2004) but it is absent from L. pneumophila. The flanking genomic regions are highly conserved among L. longbeachae and all L. pneumophila strains sequenced, suggesting that L. pneumophila, although it encodes flagella has lost the chemotaxis system encoding genes by deletion events.
Thus these two species differ markedly in their surface structures. L. longbeachae encodes a capsule-like structure, synthesizes a very different LPS, does not synthesize flagella but encodes a chemotaxis system. These differences in surface structures seem to be due to deletion events leading to the loss of flagella in L. longbeachae and the loss of chemotaxis in L. pneumophila leading in part to the adaptation to their different main niches, soil, and water.
Evolution of Eukaryotic Effectors – Acquisition by Horizontal Gene Transfer from Eukaryotes?
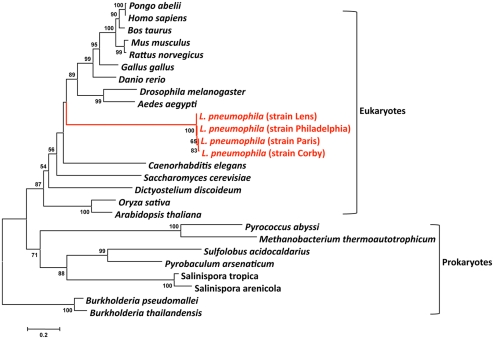
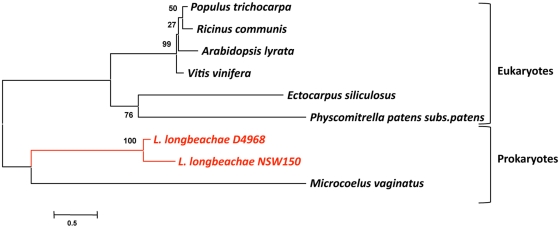
Human to human transmission of Legionella has never been reported. Thus humans have been inconsequential in the evolution of these bacteria. However, Legionella have co-evolved with fresh-water protozoa allowing the adaptation to eukaryotic cells. The idea that protozoa are training grounds for intracellular pathogens was born with the finding by Rowbotham (1980) that Legionella has the ability to multiply intracellularly. This lead to a new percept in microbiology: bacteria parasitize protozoa and can utilize the same process to infect humans. Indeed, the long co-evolution of Legionella with protozoa is reflected in its genome by the presence of eukaryotic like genes, many of which are clearly virulence factors used by L. pneumophila to subvert host functions. These genes may have been acquired either through horizontal gene transfer (HGT) from the host cells (e.g., aquatic protozoa) or from bacteria or may have evolved by convergent evolution. Recently it has been reported that L. drancourtii a relative of L. pneumophila has acquired a sterol reductase gene from the Acanthamoeba polyphaga Mimivirus genome, a virus that grows in ameba (Moliner et al., 2009). Thus, the acquisition of some of the eukaryotic like genes of L. pneumophila by HGT from protozoa is plausible. ralF was the first gene suggested to have been acquired by L. pneumophila from eukaryotes by HGT, as RalF carries a eukaryotic Sec 7 domain (Nagai et al., 2002). In order to study the evolutionary origin of eukaryotic L. pneumophila genes, we have undertaken a phylogenetic analysis of the eukaryote-like sphingosine-1-phosphate lyase of L. pneumophila that is encoded by lpp2128 described earlier. The phylogenetic analyses shown in Figure 4 revealed that it was most likely acquired from a eukaryotic organism early during Legionella evolution (Degtyar et al., 2009; Nora et al., 2009) as the Lpp2128 protein sequence of L. pneumophila clearly falls into the eukaryotic clade of SPL sequences.
Figure 4.
Phylogenetic tree of a multiple sequence comparison of sphingosine-phosphate lyase proteins present in eukaryotic and prokaryotic genomes. Phylogenetic reconstruction was done with MEGA using the Neighbor-Joining method. Numbers indicate bootstrap values after 1000 bootstrap replicates. The red lines indicate the L. pneumophila sequences that are embedded in the eukaryotic clade. The bar at the bottom represents the estimated evolutionary distance.
We then tested the hypothesis that L. longbeachae might have acquired genes also from plants, which is conceivable as it is found in soil. We thus undertook here a phylogenetic analysis similar to that described above for the L. longbeachae protein Llo2643 that contains PPR repeats, a protein family typically present in plants. A Blast search in the database revealed that homologs of Llo2643 are only found in eukaryotes, in particular in plants and algae. The only prokaryotes encoding this protein are the cyanobacteria Microcoelus vaginatus and Cylindrospermopsis rasiborskii. This rare presence in bacteria is suggestive of a horizontal transfer event from eukaryotes to these bacteria. Figure 5 shows the phylogenetic tree we obtained. The fact that the bacterial proteins group together may also be due to a phenomenon of long branch attraction. Thus, the Llo2643 protein of L. longbeachae appears closer to plant proteins than prokaryotic ones. Once more plant proteins, perhaps from algae, will be in the database, it might become possible to evaluate whether L. longbeachae indeed acquired genes from plants.
Figure 5.
Phylogenetic tree of the protein Llo2643 and their homologs after blastp search. The tree was constructed by the Neighbor-joining method using the program MEGA. The red lines indicate the L. longbeachae sequences that are close to sequences derived from plant genomes. Numbers indicate bootstrap support for nodes from 1000 NJ bootstrap replicates. The bar at the bottom represents the estimated evolutionary distance.
Legionella is not the only prokaryote whose genome shows an enrichment of proteins with eukaryotic domains. Another example is the genome of “Ca. Amoebophilus asiaticus” a Gram-negative, obligate intracellular ameba symbiont belonging to the Bacteroidetes, which has been discovered within an ameba isolated from lake sediment (Schmitz-Esser et al., 2008) has been reported (Schmitz-Esser et al., 2010). In a recent report Schmitz-Esser et al. (2010) show that the genome of this organism also encodes an arsenal of proteins with eukaryotic domains. To further investigate the distribution of these protein domains in other bacteria the authors have undertaken an enrichment analysis comparing the fraction of all functional protein domains among 514 bacterial proteomes (Schmitz-Esser et al., 2010). This showed that the genomes of bacteria for which the replication in ameba has been demonstrated were enriched in protein domains that are predominantly found in eukaryotic proteins. Interestingly, the domains potentially involved in host cell interaction described above, such as ANK repeats, LRR, SEL1 repeats, and F- and U-box domains, are among the most highly enriched domains in proteomes of ameba-associated bacteria. Bacteria that can exploit amebae as hosts thus share a set of eukaryotic domains important for host cell interaction despite their different lifestyles and their large phylogenetic diversity. This suggests that bacteria thriving within ameba use similar mechanisms for host cell interaction to facilitate survival in the host cell. Due to the phylogenetic diversity of these bacteria, it is most likely that these traits were acquired independently during evolutionary early interaction with ancient protozoa.
Conclusion
Legionella pneumophila and L. longbeachae are two human pathogens that are able to modulate, manipulate, and subvert many eukaryotic host cell functions to their advantage, in order to enter, replicate, and evade protozoa or human alveolar macrophages during disease. In the last years genome analyses, as well as comparative and functional genomics have demonstrated that genome plasticity plays a major role in differences in host cell exploitation and niche adaptation of Legionella. The genomes of these environmental pathogens are shaped by HGT between eukaryotes and prokaryotes, allowing them to mimic host cell functions and to exploit host cell pathways. Genome plasticity and HGT lead in each strain and species to a different repertoire of secreted effectors that may allow subtle adaptations to, e.g., different protozoan hosts. Plasmids can be exchanged among strains and phages and deletions of surface structures like flagella or chemotaxis systems has taken place. Thus genome plasticity is major mechanism by which Legionella may adapt to different niches and hosts.
Access to genomic data has revealed many potential virulence factors of L. pneumophila and L. longbeachae as well as metabolic capacities of these bacteria. The increasing information in the genomic database will allow a better identification of the origin and similarity of eukaryotic like proteins or eukaryotic protein domains and other virulence factors. New eukaryotic genomes like that of the natural host of Legionella, A. castellanii are in progress. These additional data will allow studying possible transfer events of genes from the eukaryotic host to Legionella more in depth. Taken together, the progressive increase of information on Legionella as well as on protozoa will allow more complete comparative and phylogenetic studies to shed light on the evolution of virulence in Legionella. However, much work remains to be done to translate the basic findings from genomics research into improved understanding of the biology of this organism. As data are accumulating, new fields of investigation will emerge. Without doubt the investigation and characterization of regulatory ncRNAs will be one such field. Manipulation of host-epigenetic information and investigating host susceptibility to disease will be another. In particular development of high throughput techniques for comparative and functional genomics as well as more and more powerful imaging techniques will accelerate the pace of knowledge acquisition.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We would like to thank many of our colleagues who have contributed in different ways to this research. This work received financial support from the Institut Pasteur, the Centre National de la Recherche (CNRS) and the Institut Carnot. Laura Gomez-Valero is holder of a FRM (Fondation pour la Recherché Médicale) postdoctoral research fellowship.
References
- Al-Khodor S., Price C. T., Habyarimana F., Kalia A., Abu Kwaik Y. (2008). A Dot/Icm-translocated ankyrin protein of Legionella pneumophila is required for intracellular proliferation within human macrophages and protozoa. Mol. Microbiol. 70, 908–923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amodeo M. R., Murdoch D. R., Pithie A. D. (2009). Legionnaires’ disease caused by Legionella longbeachae and Legionella pneumophila: comparison of clinical features, host-related risk factors, and outcomes. Clin. Microbiol. Infect. 16, 1405–1407 10.1111/j.1469-0691.2009.03125.x [DOI] [PubMed] [Google Scholar]
- Anonymous (1997). Legionellosis. Commun. Dis. Intell. 21, 137. 10.1111/j.1469-0691.2009.03125.x [DOI] [PubMed] [Google Scholar]
- Asare R., Abu Kwaik Y. (2007). Early trafficking and intracellular replication of Legionella longbeachaea within an ER-derived late endosome-like phagosome. Cell. Microbiol. 9, 1571–1587 10.1111/j.1462-5822.2007.00894.x [DOI] [PubMed] [Google Scholar]
- Asare R., Santic M., Gobin I., Doric M., Suttles J., Graham J. E., Price C. D., Abu Kwaik Y. (2007). Genetic susceptibility and caspase activation in mouse and human macrophages are distinct for Legionella longbeachae and L. pneumophila. Infect. Immun. 75, 1933–1945 10.1128/IAI.00025-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger K. H., Isberg R. R. (1993). Two distinct defects in intracellular growth complemented by a single genetic locus in Legionella pneumophila. Mol. Microbiol. 7, 7–19 10.1111/j.1365-2958.1993.tb01092.x [DOI] [PubMed] [Google Scholar]
- Bibb W. F., Sorg R. J., Thomason B. M., Hicklin M. D., Steigerwalt A. G., Brenner D. J., Wulf M. R. (1981). Recognition of a second serogroup of Legionella longbeachae. J. Clin. Microbiol. 14, 674–677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brombacher E., Urwyler S., Ragaz C., Weber S. S., Kami K., Overduin M., Hilbi H. (2009). Rab1 guanine nucleotide exchange factor SidM is a major phosphatidylinositol 4-phosphate-binding effector protein of Legionella pneumophila. J. Biol. Chem. 284, 4846–4856 10.1074/jbc.M807505200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruggemann H., Cazalet C., Buchrieser C. (2006). Adaptation of Legionella pneumophila to the host environment: role of protein secretion, effectors and eukaryotic-like proteins. Curr. Opin. Microbiol. 9, 86–94 10.1016/j.mib.2005.12.009 [DOI] [PubMed] [Google Scholar]
- Burstein D., Zusman T., Degtyar E., Viner R., Segal G., Pupko T. (2009). Genome-scale identification of Legionella pneumophila effectors using a machine learning approach. PLoS Pathog. 5, e1000508. 10.1371/journal.ppat.1000508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campodonico E. M., Chesnel L., Roy C. R. (2005). A yeast genetic system for the identification and characterization of substrate proteins transferred into host cells by the Legionella pneumophila Dot/Icm system. Mol. Microbiol. 56, 918–933 10.1111/j.1365-2958.2005.04595.x [DOI] [PubMed] [Google Scholar]
- Casati S., Gioria-Martinoni A., Gaia V. (2009). Commercial potting soils as an alternative infection source of Legionella pneumophila and other Legionella species in Switzerland. Clin. Microbiol. Infect. 15, 571–575 10.1111/j.1469-0691.2009.02742.x [DOI] [PubMed] [Google Scholar]
- Cazalet C., Gomez-Valero L., Rusniok C., Lomma M., Dervins-Ravault D., Newton H. J., Sansom F. M., Jarraud S., Zidane N., Ma L., Bouchier C., Etienne J., Hartland E. L., Buchrieser C. (2010). Analysis of the Legionella longbeachae genome and transcriptome uncovers unique strategies to cause Legionnaires’ disease. PLoS Genet. 6, e1000851. 10.1371/journal.pgen.1000851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cazalet C., Jarraud S., Ghavi-Helm Y., Kunst F., Glaser P., Etienne J., Buchrieser C. (2008). Multigenome analysis identifies a worldwide distributed epidemic Legionella pneumophila clone that emerged within a highly diverse species. Genome Res. 18, 431–441 10.1101/gr.7229808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cazalet C., Rusniok C., Bruggemann H., Zidane N., Magnier A., Ma L., Tichit M., Jarraud S., Bouchier C., Vandenesch F., Kunst F., Etienne J., Glaser P., Buchrieser C. (2004). Evidence in the Legionella pneumophila genome for exploitation of host cell functions and high genome plasticity. Nat. Genet. 36, 1165–1173 10.1038/ng1447 [DOI] [PubMed] [Google Scholar]
- Chen J., De Felipe K. S., Clarke M., Lu H., Anderson O. R., Segal G., Shuman H. A. (2004). Legionella effectors that promote nonlytic release from protozoa. Science 303, 1358–1361 10.1126/science.1094226 [DOI] [PubMed] [Google Scholar]
- Chien M., Morozova I., Shi S., Sheng H., Chen J., Gomez S. M., Asamani G., Hill K., Nuara J., Feder M., Rineer J., Greenberg J. J., Steshenko V., Park S. H., Zhao B., Teplitskaya E., Edwards J. R., Pampou S., Georghiou A., Chou I. C., Iannuccilli W., Ulz M. E., Kim D. H., Geringer-Sameth A., Goldsberry C., Morozov P., Fischer S. G., Segal G., Qu X., Rzhetsky A., Zhang P., Cayanis E., De Jong P. J., Ju J., Kalachikov S., Shuman H. A., Russo J. J. (2004). The genomic sequence of the accidental pathogen Legionella pneumophila. Science 305, 1966–1968 10.1126/science.1099776 [DOI] [PubMed] [Google Scholar]
- Cianciotto N. P. (2009). Many substrates and functions of type II secretion: lessons learned from Legionella pneumophila. Future Microbiol. 4, 797–805 10.2217/fmb.09.53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Auria G., Jimenez-Hernandez N., Peris-Bondia F., Moya A., Latorre A. (2010). Legionella pneumophila pangenome reveals strain-specific virulence factors. BMC Genomics 11, 181. 10.1186/1471-2164-11-181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Felipe K. S., Glover R. T., Charpentier X., Anderson O. R., Reyes M., Pericone C. D., Shuman H. A. (2008). Legionella eukaryotic-like type IV substrates interfere with organelle trafficking. PLoS Pathog. 4, e1000117. 10.1371/journal.ppat.1000117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Felipe K. S., Pampou S., Jovanovic O. S., Pericone C. D., Ye S. F., Kalachikov S., Shuman H. A. (2005). Evidence for acquisition of Legionella type IV secretion substrates via interdomain horizontal gene transfer. J. Bacteriol. 187, 7716–7726 10.1128/JB.187.22.7716-7726.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debroy S., Dao J., Soderberg M., Rossier O., Cianciotto N. P. (2006). Legionella pneumophila type II secretome reveals unique exoproteins and a chitinase that promotes bacterial persistence in the lung. Proc. Natl. Acad. Sci. U.S.A. 103, 19146–19151 10.1073/pnas.0608279103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degtyar E., Zusman T., Ehrlich M., Segal G. (2009). A Legionella effector acquired from protozoa is involved in sphingolipids metabolism and is targeted to the host cell mitochondria. Cell. Microbiol. 11, 1219–1235 10.1111/j.1462-5822.2009.01328.x [DOI] [PubMed] [Google Scholar]
- Diez E., Lee S. H., Gauthier S., Yaraghi Z., Tremblay M., Vidal S., Gros P. (2003). Birc1e is the gene within the Lgn1 locus associated with resistance to Legionella pneumophila. Nat. Genet. 33, 55–60 10.1038/ng1065 [DOI] [PubMed] [Google Scholar]
- Doleans A., Aurell H., Reyrolle M., Lina G., Freney J., Vandenesch F., Etienne J., Jarraud S. (2004). Clinical and environmental distributions of Legionella strains in France are different. J. Clin. Microbiol. 42, 458–460 10.1128/JCM.42.1.458-460.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelstein P. H., Metlay J. P. (2009). Legionella pneumophila goes clonal – Paris and Lorraine strain-specific risk factors. Clin. Infect. Dis. 49, 192–194 10.1086/599826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eiserich J. P., Estévez A. G., Bamberg T. V., Ye Y. Z., Chumley P. H., Beckman J. S., Freeman B. A. (1999). Microtubule dysfunction by posttranslational nitrotyrosination of alpha-tubulin: a nitric oxide-dependent mechanism of cellular injury. Proc. Natl. Acad. Sci. U.S.A. 96, 6365–6370 10.1073/pnas.96.11.6365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman M., Segal G. (2004). A specific genomic location within the icm/dot pathogenesis region of different Legionella species encodes functionally similar but nonhomologous virulence proteins. Infect. Immun. 72, 4503–4511 10.1128/IAI.72.8.4503-4511.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman M., Zusman T., Hagag S., Segal G. (2005). Coevolution between nonhomologous but functionally similar proteins and their conserved partners in the Legionella pathogenesis system. Proc. Natl. Acad. Sci. U.S.A. 102, 12206–12211 10.1073/pnas.0500044102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Moreira E., Helbig J. H., Swanson M. S. (2006). Membrane vesicles shed by Legionella pneumophila inhibit fusion of phagosomes with lysosomes. Infect. Immun. 74, 3285–3295 10.1128/IAI.01382-05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser D. W., Tsai T. R., Orenstein W., Parkin W. E., Beecham H. J., Sharrar R. G., Harris J., Mallison G. F., Martin S. M., Mcdade J. E., Shepard C. C., Brachman P. S. (1977). Legionnaires’ disease: description of an epidemic of pneumonia. N. Engl. J. Med. 297, 1189–1197 10.1056/NEJM197712012972201 [DOI] [PubMed] [Google Scholar]
- Garcia C., Ugalde E., Campo A. B., Minambres E., Kovacs N. (2004). Fatal case of community-acquired pneumonia caused by Legionella longbeachae in a patient with systemic lupus erythematosus. Eur. J. Clin. Microbiol. Infect. Dis. 23, 116–118 10.1007/s10096-003-1071-7 [DOI] [PubMed] [Google Scholar]
- Gobin I., Susa M., Begic G., Hartland E. L., Doric M. (2009). Experimental Legionella longbeachae infection in intratracheally inoculated mice. J. Med. Microbiol. 58, 723–730 10.1099/jmm.0.007476-0 [DOI] [PubMed] [Google Scholar]
- Habyarimana F., Price C. T., Santic M., Al-Khodor S., Kwaik Y. A. (2010). Molecular characterization of the Dot/Icm-translocated AnkH and AnkJ eukaryotic-like effectors of Legionella pneumophila. Infect. Immun. 78, 1123–1134 10.1128/IAI.00913-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazelbauer G. L., Falke J. J., Parkinson J. S. (2008). Bacterial chemoreceptors: high-performance signaling in networked arrays. Trends Biochem. Sci. 33, 9–19 10.1016/j.tibs.2007.09.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidtman M., Chen E. J., Moy M. Y., Isberg R. R. (2009). Large-scale identification of Legionella pneumophila Dot/Icm substrates that modulate host cell vesicle trafficking pathways. Cell. Microbiol. 11, 230–248 10.1111/j.1462-5822.2008.01249.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann V., Eidner A., Rydzewski K., Bladel I., Jules M., Buchrieser C., Eisenreich W., Heuner K. (2011). GamA is a eukaryotic-like glucoamylase responsible for glycogen- and starch-degrading activity of Legionella pneumophila. Int. J. Med. Microbiol. 301, 133–139 10.1016/j.ijmm.2010.08.016 [DOI] [PubMed] [Google Scholar]
- Hubber A., Roy C. R. (2010). Modulation of host cell function by Legionella pneumophila type IV effectors. Annu. Rev. Cell Dev. Biol. 26, 261–283 10.1146/annurev-cellbio-100109-104034 [DOI] [PubMed] [Google Scholar]
- Ingmundson A., Delprato A., Lambright D. G., Roy C. R. (2007). Legionella pneumophila proteins that regulate Rab1 membrane cycling. Nature 450, 365–369 10.1038/nature06336 [DOI] [PubMed] [Google Scholar]
- Isberg R. R., O’connor T. J., Heidtman M. (2009). The Legionella pneumophila replication vacuole: making a cosy niche inside host cells. Nat. Rev. Microbiol. 7, 13–24 10.1038/nrmicro1967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kentner D., Sourjik V. (2006). Spatial organization of the bacterial chemotaxis system. Curr. Opin. Microbiol. 9, 619–624 10.1016/j.mib.2006.10.012 [DOI] [PubMed] [Google Scholar]
- Kozak N. A., Buss M., Lucas C. E., Frace M., Govil D., Travis T., Olsen-Rasmussen M., Benson R. F., Fields B. S. (2010). Virulence factors encoded by Legionella longbeachae identified on the basis of the genome sequence analysis of clinical isolate D-4968. J. Bacteriol. 192, 1030–1044 10.1128/JB.01272-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubori T., Hyakutake A., Nagai H. (2008). Legionella translocates an E3 ubiquitin ligase that has multiple U-boxes with distinct functions. Mol. Microbiol. 67, 1307–1319 10.1111/j.1365-2958.2008.06124.x [DOI] [PubMed] [Google Scholar]
- Kubori T., Shinzawa N., Kanuka H., Nagai H. (2010). Legionella metaeffector exploits host proteasome to temporally regulate cognate effector. PLoS Pathog. 6, e1001216. 10.1371/journal.ppat.1001216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubota M., Tomii K., Tachikawa R., Harada Y., Seo R., Kaji R., Takeshima Y., Hayashi M., Nishimura T., Ishihara K. (2007). Legionella longbeachae pneumonia infection from home garden soil. Nihon Kokyuki Gakkai Zasshi 45, 698–703 [PubMed] [Google Scholar]
- Kumpers P., Tiede A., Kirschner P., Girke J., Ganser A., Peest D. (2008). Legionnaires’ disease in immunocompromised patients: a case report of Legionella longbeachae pneumonia and review of the literature. J. Med. Microbiol. 57, 384–387 10.1099/jmm.0.47556-0 [DOI] [PubMed] [Google Scholar]
- La Scola B., Birtles R. J., Greub G., Harrison T. J., Ratcliff R. M., Raoult D. (2004). Legionella drancourtii sp. nov., a strictly intracellular amoebal pathogen. Int. J. Syst. Evol. Microbiol. 54, 699–703 10.1099/ijs.0.02455-0 [DOI] [PubMed] [Google Scholar]
- Lamkanfi M., Amer A., Kanneganti T. D., Munoz-Planillo R., Chen G., Vandenabeele P., Fortier A., Gros P., Nunez G. (2007). The Nod-like receptor family member Naip5/Birc1e restricts Legionella pneumophila growth independently of caspase-1 activation. J. Immunol. 178, 8022–8027 [DOI] [PubMed] [Google Scholar]
- Lightfield K. L., Persson J., Brubaker S. W., Witte C. E., Von Moltke J., Dunipace E. A., Henry T., Sun Y. H., Cado D., Dietrich W. F., Monack D. M., Tsolis R. M., Vance R. E. (2008). Critical function for Naip5 in inflammasome activation by a conserved carboxy-terminal domain of flagellin. Nat. Immunol. 9, 1171–1178 10.1038/ni.1646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Luo Z. Q. (2007). The Legionella pneumophila effector SidJ is required for efficient recruitment of endoplasmic reticulum proteins to the bacterial phagosome. Infect. Immun. 75, 592–603 10.1128/IAI.00045-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomma M., Dervins-Ravault D., Rolando M., Nora T., Newton H. J., Sansom F. M., Sahr T., Gomez-Valero L., Jules M., Hartland E. L., Buchrieser C. (2010). The Legionella pneumophila F-box protein Lpp2082 (AnkB) modulates ubiquitination of the host protein parvin B and promotes intracellular replication. Cell. Microbiol. 12, 1272–1291 10.1111/j.1462-5822.2010.01467.x [DOI] [PubMed] [Google Scholar]
- Luo Z. Q. (2011). Targeting one of its own: expanding roles of substrates of the Legionella pneumophila Dot/Icm type IV secretion system. Front. Microbiol. 2:31. 10.3389/fmicb.2011.00031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Z. Q., Isberg R. R. (2004). Multiple substrates of the Legionella pneumophila Dot/Icm system identified by interbacterial protein transfer. Proc. Natl. Acad. Sci. U.S.A. 101, 841–846 10.1073/pnas.0304482101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lurin C., Andrés C., Aubourg S., Bellaoui M., Bitton F., Bruyère C., Caboche M., Debast C., Gualberto J., Hoffmann B., Lecharny A., Le Ret M., Martin-Magniette M. L., Mireau H., Peeters N., Renou J. P., Szurek B., Taconnat L., Small I. (2004). Genome-wide analysis of Arabidopsis pentatricopeptide repeat proteins reveals their essential role in organelle biogenesis. Plant Cell 16, 2089–2103 10.1105/tpc.104.022236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machner M. P., Isberg R. R. (2006). Targeting of host Rab GTPase function by the intravacuolar pathogen Legionella pneumophila. Dev. Cell 11, 47–56 10.1016/j.devcel.2006.05.013 [DOI] [PubMed] [Google Scholar]
- Machner M. P., Isberg R. R. (2007). A bifunctional bacterial protein links GDI displacement to Rab1 activation. Science 318, 974–977 10.1126/science.1149121 [DOI] [PubMed] [Google Scholar]
- Marra A., Blander S. J., Horwitz M. A., Shuman H. A. (1992). Identification of a Legionella pneumophila locus required for intracellular multiplication in human macrophages. Proc. Natl. Acad. Sci. U.S.A. 89, 9607–9611 10.1073/pnas.89.20.9607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrie T. J. (2008). “Legionnaires’ disease – clinical picture,” in Legionella pneumophila: Pathogenesis and Immunity, eds Hoffmann P., Friedman H., Bendinelli M. (New York: Springer; ), 133–150 [Google Scholar]
- Mcdade J. E., Shepard C. C., Fraser D. W., Tsai T. R., Redus M. A., Dowdle W. R. (1977). Legionnaires’ disease: isolation of a bacterium and demonstration of its role in other respiratory disease. N. Engl. J. Med. 297, 1197–1203 10.1056/NEJM197712012972202 [DOI] [PubMed] [Google Scholar]
- Mckinney R. M., Porschen R. K., Edelstein P. H., Bissett M. L., Harris P. P., Bondell S. P., Steigerwalt A. G., Weaver R. E., Ein M. E., Lindquist D. S., Kops R. S., Brenner D. J. (1981). Legionella longbeachae species nova, another etiologic agent of human pneumonia. Ann. Intern. Med. 94, 739–743 [DOI] [PubMed] [Google Scholar]
- MMWR (2000). Legionnaires’ disease associated with potting soil – California, Oregon, and Washington, May-June 2000. Morb. Mortal. Wkly. Rep. 49, 777–778 [PubMed] [Google Scholar]
- Moliner C., Ginevra C., Jarraud S., Flaudrops C., Bedotto M., Couderc C., Etienne J., Fournier P. E. (2009). Rapid identification of Legionella species by mass spectrometry. J. Med. Microbiol. 59(Pt 3), 273–284 10.1099/jmm.0.014100-0 [DOI] [PubMed] [Google Scholar]
- Molofsky A. B., Byrne B. G., Whitfield N. N., Madigan C. A., Fuse E. T., Tateda K., Swanson M. S. (2006). Cytosolic recognition of flagellin by mouse macrophages restricts Legionella pneumophila infection. J. Exp. Med. 17, 1093–1104 10.1084/jem.20051659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molofsky A. B., Swanson M. S. (2004). Differentiate to thrive: lessons from the Legionella pneumophila life cycle. Mol. Microbiol. 53, 29–40 10.1111/j.1365-2958.2004.04129.x [DOI] [PubMed] [Google Scholar]
- Montanaro-Punzengruber J. C., Hicks L., Meyer W., Gilbert G. L. (1999). Australian isolates of Legionella longbeachae are not a clonal population. J. Clin. Microbiol. 37, 3249–3254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morozova I., Qu X., Shi S., Asamani G., Greenberg J. E., Shuman H. A., Russo J. J. (2004). Comparative sequence analysis of the icm/dot genes in Legionella. Plasmid 51, 127–147 10.1016/j.plasmid.2003.12.004 [DOI] [PubMed] [Google Scholar]
- Müller M. P., Peters H., Blümer J., Blankenfeldt W., Goody R. S., Itzen A. (2010). The Legionella effector protein DrrA AMPylates the membrane traffic regulator Rab1b. Science 329, 946–949 10.1126/science.1194560 [DOI] [PubMed] [Google Scholar]
- Murata T., Delprato A., Ingmundson A., Toomre D. K., Lambright D. G., Roy C. R. (2006). The Legionella pneumophila effector protein DrrA is a Rab1 guanine nucleotide-exchange factor. Nat. Cell Biol. 8, 971–977 10.1038/ncb1463 [DOI] [PubMed] [Google Scholar]
- Nagai H., Cambronne E. D., Kagan J. C., Amor J. C., Kahn R. A., Roy C. R. (2005). A C-terminal translocation signal required for Dot/Icm-dependent delivery of the Legionella RalF protein to host cells. Proc. Natl. Acad. Sci. U.S.A. 102, 826–831 10.1073/pnas.0406239101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai H., Kagan J. C., Zhu X., Kahn R. A., Roy C. R. (2002). A bacterial guanine nucleotide exchange factor activates ARF on Legionella phagosomes. Science 295, 679–682 10.1126/science.1067025 [DOI] [PubMed] [Google Scholar]
- Nagai H., Kubori T. (2011). Type IVB secretion systems of Legionella and other Gram-negative bacteria. Front. Microbiol. 2:136. 10.3389/fmicb.2011.00136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura T., Schuster G., Sugiura M., Sugita M. (2004). Chloroplast RNA-binding and pentatricopeptide repeat proteins. Biochem. Soc. Trans. 32, 571–574 10.1042/BST0320571 [DOI] [PubMed] [Google Scholar]
- Neunuebel M. R., Chen Y., Gaspar A. H., Backlund P. S., Jr., Yergey A., Machner M. P. (2011). De-AMPylation of the small GTPase Rab1 by the pathogen Legionella pneumophila. Science 333, 453–456 10.1126/science.1207193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton H. J., Sansom F. M., Dao J., Mcalister A. D., Sloan J., Cianciotto N. P., Hartland E. L. (2007). Sel1 repeat protein LpnE is a Legionella pneumophila virulence determinant that influences vacuolar trafficking. Infect. Immun. 75, 5575–5585 10.1128/IAI.00443-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ninio S., Celli J., Roy C. R. (2009). A Legionella pneumophila effector protein encoded in a region of genomic plasticity binds to Dot/Icm-modified vacuoles. PLoS Pathog 5, e1000278. 10.1371/journal.ppat.1000278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nora T., Lomma M., Gomez-Valero L., Buchrieser C. (2009). Molecular mimicry: an important virulence strategy employed by Legionella pneumophila to subvert host functions. Future Microbiol. 4, 691–701 10.2217/fmb.09.47 [DOI] [PubMed] [Google Scholar]
- O’Connor B. A., Carman J., Eckert K., Tucker G., Givney R., Cameron S. (2007). Does using potting mix make you sick? Results from a Legionella longbeachae case-control study in South Australia. Epidemiol. Infect. 135, 34–39 10.1017/S095026880600656X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan X., Lührmann A., Satoh A., Laskowski-Arce M. A., Roy C. R. (2008). Ankyrin repeat proteins comprise a diverse family of bacterial type IV effectors. Science 320, 1651–1654 10.1126/science.1158160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pravinkumar S., Edwards G., Lindsay D., Redmond S., Stirling J., House R., Kerr J., Anderson E., Breen D., Blatchford O., Mcdonald E., Brown A. (2010). A cluster of Legionnaires’ disease caused by Legionella longbeachae linked to potting compost in Scotland, 2008-2009. Euro Surveill. 15, 19496. [DOI] [PubMed] [Google Scholar]
- Price C. T., Al-Khodor S., Al-Quadan T., Santic M., Habyarimana F., Kalia A., Kwaik Y. A. (2009). Molecular mimicry by an F-Box Effector of Legionella pneumophila hijacks a conserved polyubiquitination machinery within macrophages and protozoa. PLoS Pathog. 5, e1000704. 10.1371/journal.ppat.1000704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren T., Zamboni D. S., Roy C. R., Dietrich W. F., Vance R. E. (2006). Flagellin-deficient Legionella mutants evade caspase-1- and Naip5-mediated macrophage immunity. PLoS Pathog. 2, e18. 10.1371/journal.ppat.0020018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossier O., Cianciotto N. P. (2001). Type II protein secretion is a subset of the PilD-dependent processes that facilitate intracellular infection by Legionella pneumophila. Infect. Immun. 69, 2092–2098 10.1128/IAI.69.4.2092-2098.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowbotham T. J. (1980). Preliminary report on the pathogenicity of Legionella pneumophila for freshwater and soil amoebae. J. Clin. Pathol. 33, 1179–1183 10.1136/jcp.33.12.1179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahr T., Bruggemann H., Jules M., Lomma M., Albert-Weissenberger C., Cazalet C., Buchrieser C. (2009). Two small ncRNAs jointly govern virulence and transmission in Legionella pneumophila. Mol. Microbiol. 72, 741–762 10.1111/j.1365-2958.2009.06677.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sansom F. M., Newton H. J., Crikis S., Cianciotto N. P., Cowan P. J., D’Apice A. J., Hartland E. L. (2007). A bacterial ecto-triphosphate diphosphohydrolase similar to human CD39 is essential for intracellular multiplication of Legionella pneumophila. Cell. Microbiol. 9, 1922–1935 10.1111/j.1462-5822.2007.00924.x [DOI] [PubMed] [Google Scholar]
- Sansom F. M., Riedmaier P., Newton H. J., Dunstone M. A., Müller C. E., Stephan H., Byres E., Beddoe T., Rossjohn J., Cowan P. J., D’Apice A. J., Robson S. C., Hartland E. L. (2008). Enzymatic properties of an ecto-nucleoside triphosphate diphosphohydrolase from Legionella pneumophila: substrate specificity and requirement for virulence. J. Biol. Chem. 283, 12909–12918 10.1074/jbc.M801006200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz-Esser S., Tischler P., Arnold R., Montanaro J., Wagner M., Rattei T., Horn M. (2010). The genome of the amoeba symbiont “Candidatus Amoebophilus asiaticus” reveals common mechanisms for host cell interaction among amoeba-associated bacteria. J. Bacteriol. 192, 1045–1057 10.1128/JB.01379-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz-Esser S., Toenshoff E. R., Haider S., Heinz E., Hoenninger V. M., Wagner M., Horn M. (2008). Diversity of bacterial endosymbionts of environmental Acanthamoeba isolates. Appl. Environ. Microbiol. 74, 5822–5831 10.1128/AEM.01093-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz-Linneweber C., Small I. (2008). Pentatricopeptide repeat proteins: a socket set for organelle gene expression. Trends Plant Sci. 13, 663–670 10.1016/j.tplants.2008.10.001 [DOI] [PubMed] [Google Scholar]
- Schroeder G. N., Petty N. K., Mousnier A., Harding C. R., Vogrin A. J., Wee B., Fry N. K., Harrison T. G., Newton H. J., Thomson N. R., Beatson S. A., Dougan G., Hartland E. L., Frankel G. (2010). Legionella pneumophila strain 130b possesses a unique combination of type IV secretion systems and novel Dot/Icm secretion system effector proteins. J. Bacteriol. 192, 6001–6016 10.1128/JB.00778-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuelein R., Ang D. K., Van Driel I. R., Hartland E. L. (2011). Immune control of Legionella infection: an in vivo perspective. Front. Microbiol. 2:126. 10.3389/fmicb.2011.00126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal G., Feldman M., Zusman T. (2005). The Icm/Dot type-IV secretion systems of Legionella pneumophila and Coxiella burnetii. FEMS Microbiol. Rev. 29, 65–81 10.1016/j.femsre.2004.07.001 [DOI] [PubMed] [Google Scholar]
- Segal G., Russo J. J., Shuman H. A. (1999). Relationships between a new type IV secretion system and the icm/dot virulence system of Legionella pneumophila. Mol. Microbiol. 34, 799–809 10.1046/j.1365-2958.1999.01642.x [DOI] [PubMed] [Google Scholar]
- Shin S., Roy C. R. (2008). Host cell processes that influence the intracellular survival of Legionella pneumophila. Cell. Microbiol. 10, 1209–1220 10.1111/j.1462-5822.2008.01151.x [DOI] [PubMed] [Google Scholar]
- Shohdy N., Efe J. A., Emr S. D., Shuman H. A. (2005). Pathogen effector protein screening in yeast identifies Legionella factors that interfere with membrane trafficking. Proc. Natl. Acad. Sci. U.S.A. 102, 4866–4871 10.1073/pnas.0501315102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele T. W., Moore C. V., Sangster N. (1990). Distribution of Legionella longbeachae serogroup 1 and other legionellae in potting soils in Australia. Appl. Environ. Microbiol. 56, 2984–2988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinert M., Hentschel U., Hacker J. (2002). Legionella pneumophila: an aquatic microbe goes astray. FEMS Microbiol. Rev. 26, 149–162 10.1111/j.1574-6976.2002.tb00607.x [DOI] [PubMed] [Google Scholar]
- Steinert M., Heuner K., Buchrieser C., Albert-Weissenberger C., Glockner G. (2007). Legionella pathogenicity: genome structure, regulatory networks and the host cell response. Int. J. Med. Microbiol. 297, 577–587 10.1016/j.ijmm.2007.03.009 [DOI] [PubMed] [Google Scholar]
- Tan Y., Luo Z. Q. (2011). Legionella pneumophila SidD is a deAMPylase that modifies Rab1. Nature 475, 506–509 10.1038/nature10307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tijet N., Tang P., Romilowych M., Duncan C., Ng V., Fisman D. N., Jamieson F., Low D. E., Guyard C. (2010). New endemic Legionella pneumophila serogroup I clones, Ontario, Canada. Emerging Infect. Dis. 16, 447–454 10.3201/eid1603.081689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velonakis E. N., Kiousi I. M., Koutis C., Papadogiannakis E., Babatsikou F., Vatopoulos A. (2009). First isolation of Legionella species, including L. pneumophila serogroup 1, in Greek potting soils: possible importance for public health. Clin. Microbiol. Infect. 16, 763–766 10.1111/j.1469-0691.2009.02957.x [DOI] [PubMed] [Google Scholar]
- Weber S. S., Ragaz C., Hilbi H. (2009). The inositol polyphosphate 5-phosphatase OCRL1 restricts intracellular growth of Legionella, localizes to the replicative vacuole and binds to the bacterial effector LpnE. Cell. Microbiol. 11, 442–460 10.1111/j.1462-5822.2008.01266.x [DOI] [PubMed] [Google Scholar]
- Weber S. S., Ragaz C., Reus K., Nyfeler Y., Hilbi H. (2006). Legionella pneumophila exploits PI(4)P to anchor secreted effector proteins to the replicative vacuole. PLoS Pathog. 2, e46. 10.1371/journal.ppat.0020046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiley H., Bentham R. (2011). Legionella longbeachae and legionellosis. Emerging Infect. Dis. 17, 579–583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright E. K., Goodart S. A., Growney J. D., Hadinoto V., Endrizzi M. G., Long E. M., Sadigh K., Abney A. L., Bernstein-Hanley I., Dietrich W. F. (2003). Naip5 affects host susceptibility to the intracellular pathogen Legionella pneumophila. Curr. Biol. 13, 27–36 10.1016/S0960-9822(02)01359-3 [DOI] [PubMed] [Google Scholar]
- Yu V. L., Plouffe J. F., Pastoris M. C., Stout J. E., Schousboe M., Widmer A., Summersgill J., File T., Heath C. M., Paterson D. L., Chereshsky A. (2002). Distribution of Legionella species and serogroups isolated by culture in patients with sporadic community-acquired legionellosis: an international collaborative survey. J. Infect. Dis. 186, 127–128 10.1086/341087 [DOI] [PubMed] [Google Scholar]
- Zamboni D. S., Kobayashi K. S., Kohlsdorf T., Ogura Y., Long E. M., Vance R. E., Kuida K., Mariathasan S., Dixit V. M., Flavell R. A., Dietrich W. F., Roy C. R. (2006). The Birc1e cytosolic pattern-recognition receptor contributes to the detection and control of Legionella pneumophila infection. Nat. Immunol. 7, 318–325 10.1038/ni1305 [DOI] [PubMed] [Google Scholar]
- Zeigler D. R. (2003). Gene sequences useful for predicting relatedness of whole genomes in bacteria. Int. J. Syst. Evol. Microbiol. 53, 1893–1900 10.1099/ijs.0.02713-0 [DOI] [PubMed] [Google Scholar]
- Zhu W., Banga S., Tan Y., Zheng C., Stephenson R., Gately J., Luo Z. Q. (2011). Comprehensive identification of protein substrates of the Dot/Icm type IV transporter of Legionella pneumophila. PLoS ONE 6, e17638. 10.1371/journal.pone.0017638 [DOI] [PMC free article] [PubMed] [Google Scholar]