Abstract
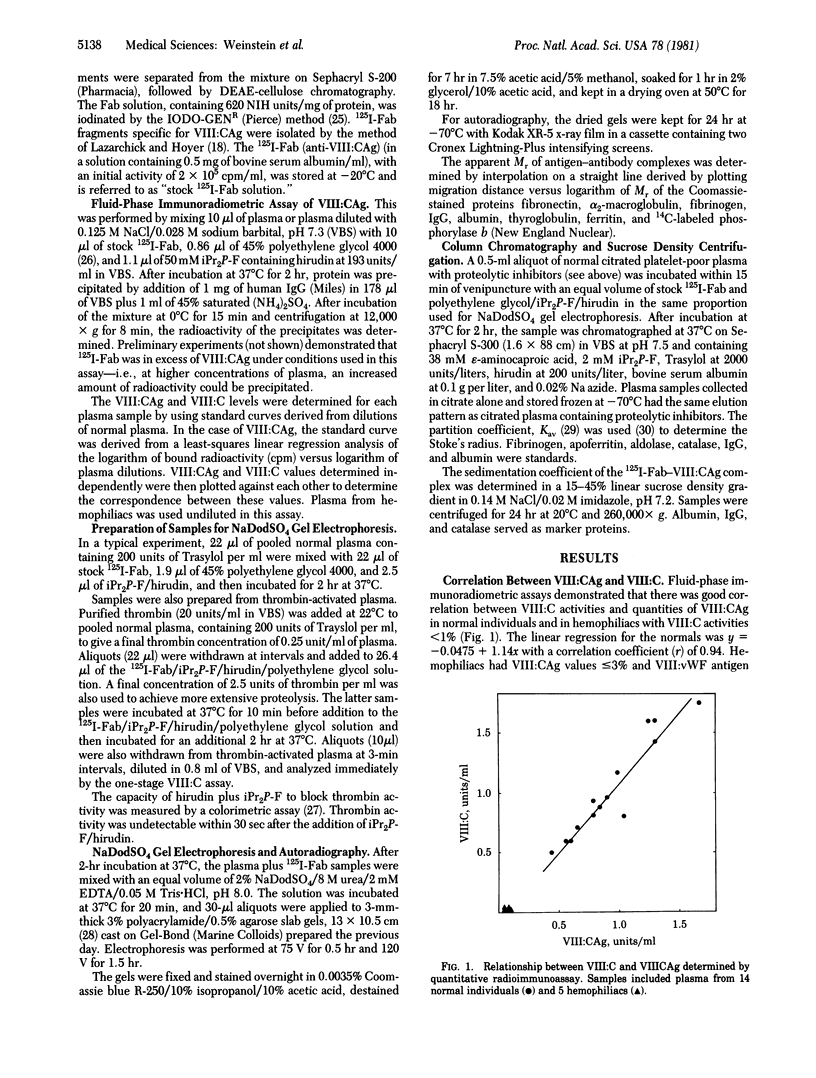
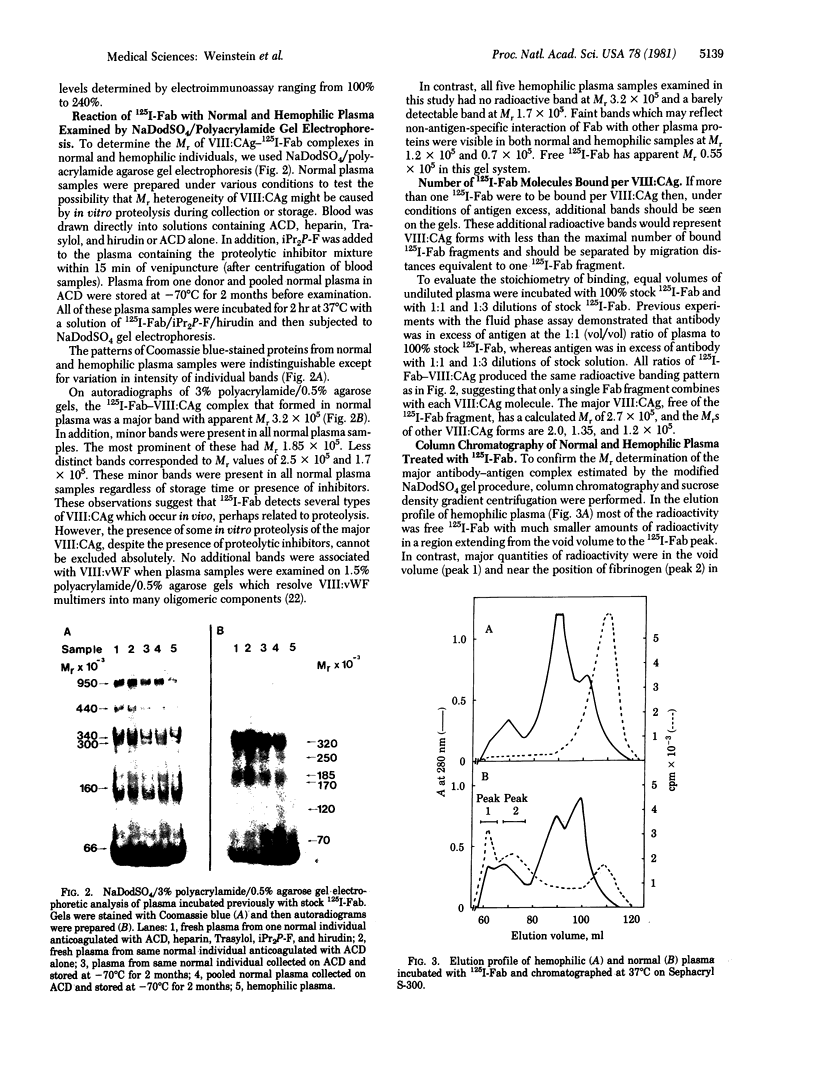
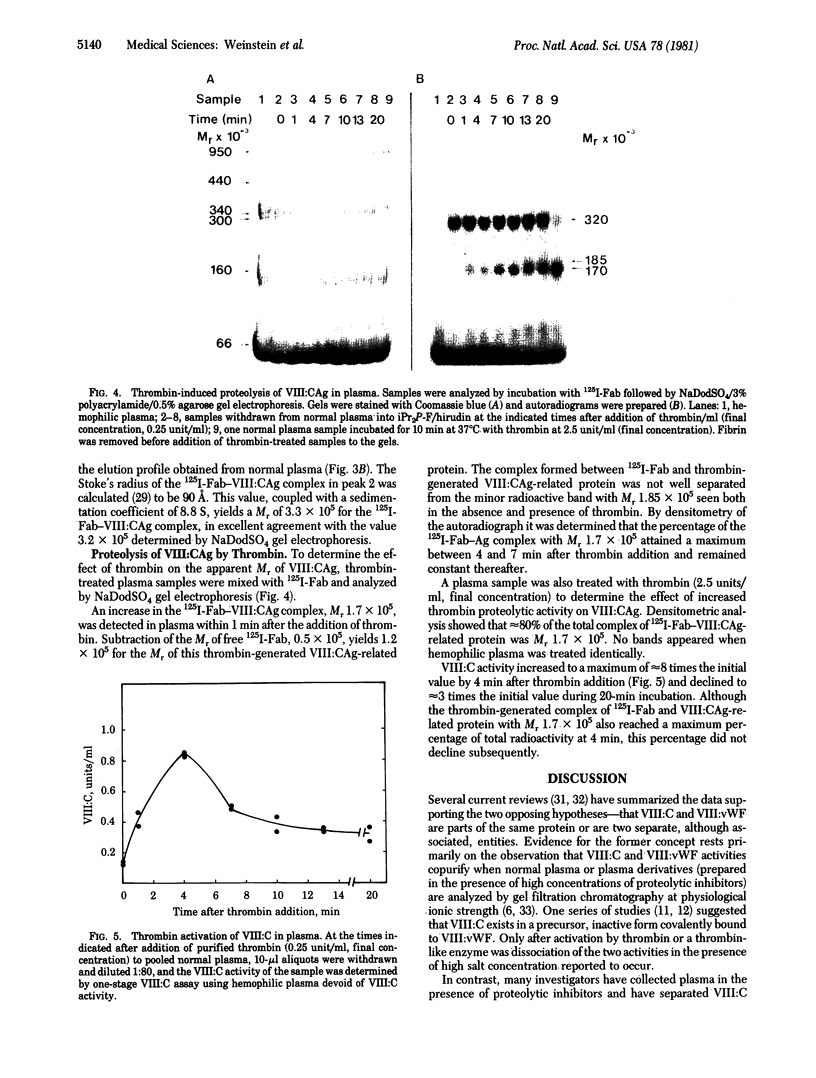
The relationship between Factor VIII coagulant antigen (VIII:CAg) and Factor VIII-associated von Willebrand factor (VIII:vWF), and the effect of thrombin on VIII:CAg have been determined in plasma by using complexes of VIII:CAg and 125I-labeled human anti-VIII:CAg-Fab. Antibody-treated plasma samples were electrophoresed on NaDodSO4/polyacrylamide agarose gels and analyzed by autoradiography. The major VIII:CAg-125I-labeled Fab complex that persisted in NaDodSO4 had Mr 3.2 x 10(5). This Mr value was confirmed by column chromatography and sucrose density centrifugation and is presumed to reflect a free VIII:CAg of Mr 2.7 x 10(5). Minor bands were also present on autoradiograms of normal plasma corresponding to Mr values of 2.5, 1.85, and 1.7 x 10(5) (free VIII:CAg related proteins with Mr values of 2.0, 1.35, and 1.2 x 10(5), respectively). None of the VIII:CAg bands was present in plasma samples from five patients with severe hemophilia A. No radioactivity was associated with VIII:vWF multimers on NaDodSO4 gels. Thrombin treatment of normal plasma eliminated the radioactive band at 3.2 x 10(5) and increased the intensity of a band of Mr 1.7 x 10(5). Generation of this presumed VIII:CAg fragment of Mr is approximately equal to 1.2 x 10(5) coincided with a thrombin-induced increase in Factor VIII coagulant activity. These data demonstrate that the form of VIII:CAg detected in normal plasma is not covalently linked to VIII:vWF multimers and is absent in plasma from five hemophilia A patients. Thrombin-induced proteolysis of VIII:CAg can be detected in microliter quantities of normal plasma.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ASTER R. H., JANDL J. H. PLATELET SEQUESTRATION IN MAN. I. METHODS. J Clin Invest. 1964 May;43:843–855. doi: 10.1172/JCI104970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper H. A., Griggs T. R., Wagner R. H. Factor VIII recombination after dissociation by CaCl12. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2326–2329. doi: 10.1073/pnas.70.8.2326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Counts R. B., Paskell S. L., Elgee S. K. Disulfide bonds and the quaternary structure of factor VIII/von Willebrand factor. J Clin Invest. 1978 Sep;62(3):702–709. doi: 10.1172/JCI109178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Counts R. B. Solid-phase immunoradiometric assay of factor-VIII protein. Br J Haematol. 1975 Dec;31(4):429–436. doi: 10.1111/j.1365-2141.1975.tb00878.x. [DOI] [PubMed] [Google Scholar]
- Fass D. N., Knutson G. J., Bowie E. J. Porcine Willebrand factor: a population of multimers. J Lab Clin Med. 1978 Feb;91(2):307–320. [PubMed] [Google Scholar]
- Green G. D., Shaw E. Thiobenzyl benzyloxycarbonyl-L-lysinate, substrate for a sensitive colorimetric assay for trypsin-like enzymes. Anal Biochem. 1979 Mar;93(2):223–226. doi: 10.1016/s0003-2697(79)80141-4. [DOI] [PubMed] [Google Scholar]
- HSIAO S., PUTNAM F. W. The cleavage of human gamma-globulin by papain. J Biol Chem. 1961 Jan;236:122–135. [PubMed] [Google Scholar]
- Hoyer L. W., Trabold N. C. The effect of thrombin on human factor VIII. Cleavage of the factor VIII procoagulant protein during activation. J Lab Clin Med. 1981 Jan;97(1):50–64. [PubMed] [Google Scholar]
- Lazarchick J., Hoyer L. W. Immunoradiometric measurement of the factor VIII procoagulant antigen. J Clin Invest. 1978 Nov;62(5):1048–1052. doi: 10.1172/JCI109209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legaz M. E., Schmer G., Counts R. B., Davie E. W. Isolation and characterization of human Factor VIII (antihemophilic factor). J Biol Chem. 1973 Jun 10;248(11):3946–3955. [PubMed] [Google Scholar]
- McKee P. A., Andersen J. C., Switzer M. E. Molecular structural studies of human factor VIII. Ann N Y Acad Sci. 1975 Jan 20;240:8–33. doi: 10.1111/j.1749-6632.1975.tb53319.x. [DOI] [PubMed] [Google Scholar]
- Newman J., Harris R. B., Johnson A. J. Molecular weights of antihaemophilic factor and von Willebrand factor proteins in human plasma. Nature. 1976 Oct 14;263(5578):612–613. doi: 10.1038/263612a0. [DOI] [PubMed] [Google Scholar]
- Owen W. G., Wagner R. H. Antihemophilic factor: separation of an active fragment following dissociation by salts or detergents. Thromb Diath Haemorrh. 1972 Jul 31;27(3):502–515. [PubMed] [Google Scholar]
- Peake I. R., Bloom A. L., Giddings J. C., Ludlam C. A. An immunoradiometric assay for procoagulant factor VIII antigen: results in haemophilia, von Willebrand's disease and fetal plasma and serum. Br J Haematol. 1979 Jun;42(2):269–281. doi: 10.1111/j.1365-2141.1979.tb01131.x. [DOI] [PubMed] [Google Scholar]
- Peake I. R., Bloom A. L. Immunoradiometric assay of procoagulant factor-VIII antigen in plasma and serum and its reduction in haemophilia. Preliminary studies on adult and fetal blood. Lancet. 1978 Mar 4;1(8062):473–475. doi: 10.1016/s0140-6736(78)90137-x. [DOI] [PubMed] [Google Scholar]
- Poon M. C., Ratnoff O. D. Evidence that functional subunits of antihemophilic factor (Factor VIII) are linked by noncovalent bonds. Blood. 1976 Jul;48(1):87–94. [PubMed] [Google Scholar]
- RAPAPORT S. I., SCHIFFMAN S., PATCH M. J., AMES S. B. The importance of activation of antihemophilic globulin and proaccelerin by traces of thrombin in the generation of intrinsic prothrombinase activity. Blood. 1963 Feb;21:221–236. [PubMed] [Google Scholar]
- Raney J. P., McLennan B. D. A modified quantitative precipitin test which is rapid, sensitive and reproducible. J Immunol Methods. 1979;29(1):65–70. doi: 10.1016/0022-1759(79)90126-1. [DOI] [PubMed] [Google Scholar]
- Reisner H. M., Barrow E. S., Graham J. B. Radioimmunoassay for coagulant factor VIII-related antigen (VIII:CAg). Thromb Res. 1979 Jan;14(1):235–239. doi: 10.1016/0049-3848(79)90042-2. [DOI] [PubMed] [Google Scholar]
- Rick M. E., Hoyer L. W. Immunologic studies of antihemophilic factor (AHF, factor VIII). V. Immunologic properties of AHF subunits produced by salt dissociation. Blood. 1973 Nov;42(5):737–747. [PubMed] [Google Scholar]
- Rock G. A., Palmer D. S., Tackaberry E. S., Cruickshank W. H. The presence of high and low molecular weight forms of factor VIII in heparinized plasma. Thromb Res. 1978 Jul;13(1):85–96. doi: 10.1016/0049-3848(78)90113-5. [DOI] [PubMed] [Google Scholar]
- Siegel L. M., Monty K. J. Determination of molecular weights and frictional ratios of proteins in impure systems by use of gel filtration and density gradient centrifugation. Application to crude preparations of sulfite and hydroxylamine reductases. Biochim Biophys Acta. 1966 Feb 7;112(2):346–362. doi: 10.1016/0926-6585(66)90333-5. [DOI] [PubMed] [Google Scholar]
- Switzer M. E., McKee P. A. Studies on human antihemophilic factor. Evidence for a covalently linked subunit structure. J Clin Invest. 1976 Apr;57(4):925–937. doi: 10.1172/JCI108369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Switzer M. E., Pizzo S. V., McKee P. A. Is there a precursive, relatively procoagulant-inactive form of normal antihemophilic factor (factor VIII)? Blood. 1979 Oct;54(4):916–927. [PubMed] [Google Scholar]
- Vehar G. A., Davie E. W. Preparation and properties of bovine factor VIII (antihemophilic factor). Biochemistry. 1980 Feb 5;19(3):401–410. doi: 10.1021/bi00544a001. [DOI] [PubMed] [Google Scholar]
- Weinstein M. J., Deykin D., Davie E. W. Quantitative determination of factor-VIII protein by two-stage gel electrophoresis. Br J Haematol. 1976 Jul;33(3):343–355. doi: 10.1111/j.1365-2141.1976.tb03550.x. [DOI] [PubMed] [Google Scholar]
- Weinstein M., Deykin D. Comparison of factor VIII-related von Willebrand factor proteins prepared from human cryoprecipitate and factor VIII concentrate. Blood. 1979 Jun;53(6):1095–1105. [PubMed] [Google Scholar]
- Weiss H. J., Baumgartner H. R., Tschopp T. B., Turitto V. T., Cohen D. Correction by factor VIII of the impaired platelet adhesion to subendothelium in von Willebrand disease. Blood. 1978 Feb;51(2):267–279. [PubMed] [Google Scholar]
- Weiss H. J., Hoyer I. W. Von Willebrand factor: dissociation from antihemophilic factor procoagulant activity. Science. 1973 Dec 14;182(4117):1149–1151. doi: 10.1126/science.182.4117.1149. [DOI] [PubMed] [Google Scholar]
- Weiss H. J., Kochwa S. Molecular forms of antihaemophilic globulin in plasma, cryoprecipitate and after thrombin activation. Br J Haematol. 1970 Jan;18(1):89–100. doi: 10.1111/j.1365-2141.1970.tb01421.x. [DOI] [PubMed] [Google Scholar]