Abstract
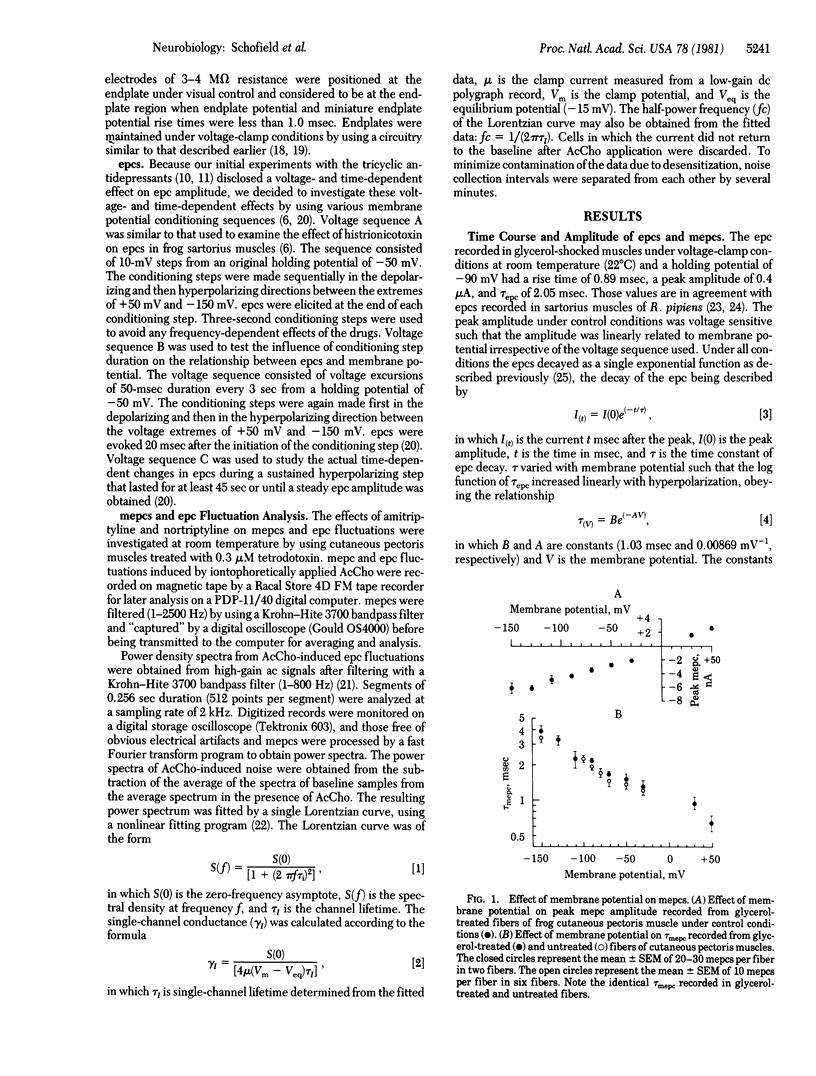
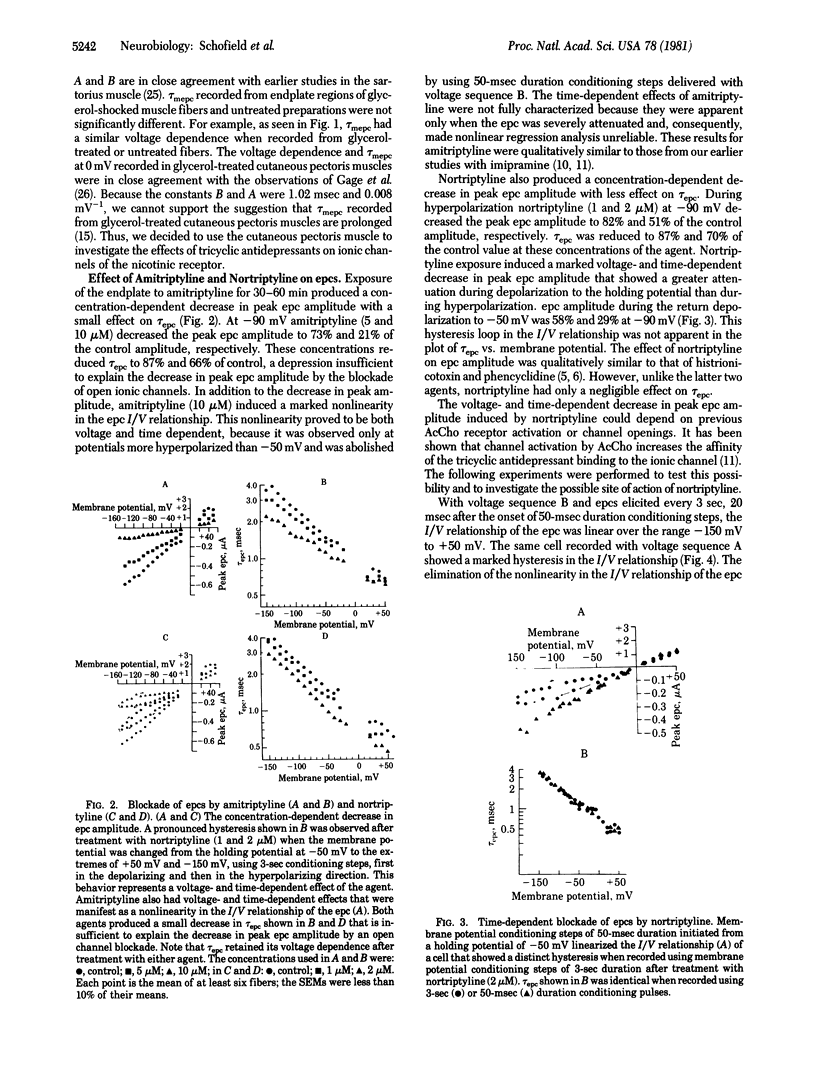
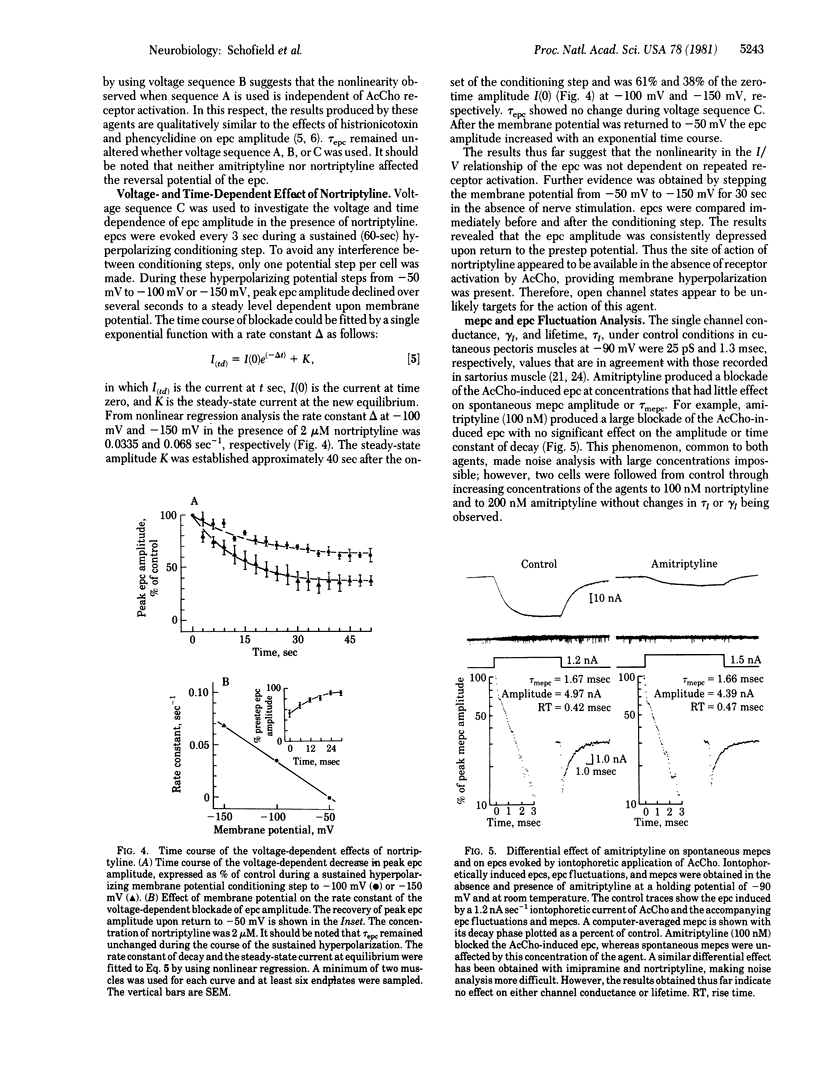
The actions of two clinically important dibenzocycloheptane antidepressant drugs, amitriptyline and nortriptyline, were studied on ionic channels of nicotinic acetylcholine (AcCho) receptors at the neuromuscular junction of frog skeletal muscle. Amitriptyline (5-10 microM) and nortriptyline (1-2 microM), like imipramine (5-10 microM), did not react with the nicotinic AcCho receptor but caused a voltage- and time-dependent decrease in the peak amplitude of the endplate current (epc). The time constant of epc decay, however, retained its voltage sensitivity. The voltage- and time-dependent effect of amitriptyline was nonlinear with regard to the current/voltage (I/V) relationship. Nortriptyline also had a more pronounced voltage- and time-dependent effect evidenced by a hysteresis loop in the I/V relationship of the epc was eliminated by the use of 50-msec stepwise changes of the membrane potential. The nonlinearity and hysteresis were due to a time-dependent phenomenon and did not involve previous AcCho receptor activation. The rate constant of the voltage- and time-dependent decrease in epc amplitude was sensitive to the membrane electric field and varied linearly with the membrane potential. Iontophoretically elicited epcs were much more depressed by both drugs than were spontaneous miniature epcs. There was no effect on the time constant of miniature epc decay, single-channel lifetime, or conductance. Thus (as we have pointed out in our histrionicotoxin studies) the primary site of action of these agents presumably is the activated but nonconducting species of the ionic channel of the nicotinic AcCho receptor. These agents, particularly nortriptyline, point to several different binding sites of the ionic channel and are suitable tools for the separation of the effects on peak current amplitude from its time constant of decay.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler M., Albuquerque E. X. An analysis of the action of atropine and scopolamine on the end-plate current of frog sartorius muscle. J Pharmacol Exp Ther. 1976 Feb;196(2):360–372. [PubMed] [Google Scholar]
- Adler M., Albuquerque E. X., Lebeda F. J. Kinetic analysis of end plate currents altered by atropine and scopolamine. Mol Pharmacol. 1978 May;14(3):514–529. [PubMed] [Google Scholar]
- Adler M., Oliveira A. C., Albuquerque E. X., Mansour N. A., Eldefrawi A. T. Reaction of tetraethylammonium with the open and closed conformations of the acetylcholine receptor ionic channel complex. J Gen Physiol. 1979 Jul;74(1):129–152. doi: 10.1085/jgp.74.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albuquerque E. X., Adler M., Spivak C. E., Aguayo L. Mechanism of nicotinic channel activation and blockade. Ann N Y Acad Sci. 1980;358:204–238. doi: 10.1111/j.1749-6632.1980.tb15397.x. [DOI] [PubMed] [Google Scholar]
- Albuquerque E. X., Kuba K., Daly J. Effect of histrionicotoxin on the ionic conductance modulator of the cholinergic receptor: a quantitative analysis of the end-plate current. J Pharmacol Exp Ther. 1974 May;189(2):513–524. [PubMed] [Google Scholar]
- Albuquerque E. X., Tsai M. C., Aronstam R. S., Eldefrawi A. T., Eldefrawi M. E. Sites of action of phencyclidine. II. Interaction with the ionic channel of the nicotinic receptor. Mol Pharmacol. 1980 Sep;18(2):167–178. [PubMed] [Google Scholar]
- Albuquerque E. X., Tsai M. C., Aronstam R. S., Witkop B., Eldefrawi A. T., Eldefrawi M. E. Phencyclidine interactions with the ionic channel of the acetylcholine receptor and electrogenic membrane. Proc Natl Acad Sci U S A. 1980 Feb;77(2):1224–1228. doi: 10.1073/pnas.77.2.1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson C. R., Stevens C. F. Voltage clamp analysis of acetylcholine produced end-plate current fluctuations at frog neuromuscular junction. J Physiol. 1973 Dec;235(3):655–691. doi: 10.1113/jphysiol.1973.sp010410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aronstam R. S. Interactions of tricyclic antidepressants with a synaptic ion channel. Life Sci. 1981 Jan 5;28(1):59–64. doi: 10.1016/0024-3205(81)90366-0. [DOI] [PubMed] [Google Scholar]
- Eldefrawi M. E., Aronstam R. S., Bakry N. M., Eldefrawi A. T., Albuquerque E. X. Activation, inactivation, and desensitization of acetylcholine receptor channel complex detected by binding of perhydrohistrionicotoxin. Proc Natl Acad Sci U S A. 1980 Apr;77(4):2309–2313. doi: 10.1073/pnas.77.4.2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldefrawi M. E., Warnick J. E., Schofield G. G., Albuquerque E. X., Eldefrawi A. T. Interaction of imipramine with the ionic channel of the acetylcholine receptor of motor endplate and electric organ. Biochem Pharmacol. 1981 Jun 1;30(11):1391–1394. doi: 10.1016/0006-2952(81)90331-2. [DOI] [PubMed] [Google Scholar]
- FUJINO M., YAMAGUCHI T., SUZUKI K. 'Glycerol effect' and the mechanism linking excitation of the plasma membrane with contraction. Nature. 1961 Dec 23;192:1159–1161. doi: 10.1038/1921159a0. [DOI] [PubMed] [Google Scholar]
- Gage P. W., Eisenberg R. S. Action potentials without contraction in frog skeletal muscle fibers with disrupted transverse tubules. Science. 1967 Dec 29;158(3809):1702–1703. doi: 10.1126/science.158.3809.1702. [DOI] [PubMed] [Google Scholar]
- Gage P. W., McBurney R. N., Schneider G. T. Effects of some aliphatic alcohols on the conductance change caused by a quantum of acetylcholine at the toad end-plate. J Physiol. 1975 Jan;244(2):409–429. doi: 10.1113/jphysiol.1975.sp010806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuba K., Albuquerque E. X., Daly J., Barnard E. A. A study of the irreversible cholinesterase inhibitor, diisopropylfluorophosphate, on time course of end-plate currents in frog sartorius muscle. J Pharmacol Exp Ther. 1974 May;189(2):499–512. [PubMed] [Google Scholar]
- Lambert J. J., Durant N. N., Reynolds L. S., Volle R. L., Henderson E. G. Characterization of end-plate conductance in transected frog muscle: modification by drugs. J Pharmacol Exp Ther. 1981 Jan;216(1):62–69. [PubMed] [Google Scholar]
- Magleby K. L., Stevens C. F. The effect of voltage on the time course of end-plate currents. J Physiol. 1972 May;223(1):151–171. doi: 10.1113/jphysiol.1972.sp009839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masukawa L. M., Albuquerque E. X. Voltage- and time-dependent action of histrionicotoxin on the endplate current of the frog muscle. J Gen Physiol. 1978 Sep;72(3):351–367. doi: 10.1085/jgp.72.3.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neher E., Sakmann B., Steinbach J. H. The extracellular patch clamp: a method for resolving currents through individual open channels in biological membranes. Pflugers Arch. 1978 Jul 18;375(2):219–228. doi: 10.1007/BF00584247. [DOI] [PubMed] [Google Scholar]
- Schofield G. G., Warnick J. E., Albuquerque E. X. Elucidation of the mechanism and site of action of quinuclidinyl benzilate (QNB) on the electrical excitability and chemosensitivity of the frog sartorius muscle. Cell Mol Neurobiol. 1981 Jun;1(2):209–230. doi: 10.1007/BF00710720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAKEUCHI A., TAKEUCHI N. Active phase of frog's end-plate potential. J Neurophysiol. 1959 Jul;22(4):395–411. doi: 10.1152/jn.1959.22.4.395. [DOI] [PubMed] [Google Scholar]
- Tiedt T. N., Albuquerque E. X., Bakry N. M., Eldefrawi M. E., Eldefrawi A. T. Voltage- and time-dependent actions of piperocaine on the ion channel of the acetylcholine receptor. Mol Pharmacol. 1979 Nov;16(3):909–921. [PubMed] [Google Scholar]
- Tsai M. C., Albuquerque E. X., Aronstam R. S., Eldefrawi A. T., Eldefrawi M. E., Triggle D. J. Sites of action of phencyclidine. I. Effects on the electrical excitability and chemosensitive properties of the neuromuscular junction of skeletal muscle. Mol Pharmacol. 1980 Sep;18(2):159–166. [PubMed] [Google Scholar]


