Abstract
Objective
Because hormone therapy use benefits sleep, sleep problems may occur following suspension. We tested effects of short-term hormone therapy suspension on sleep problems.
Methods
A total of 1704 women aged 45–80 years at Group Health were randomized to stop hormone therapy for 1 month, 2 months, or continue use. This study included 1405 women willing to suspend hormone therapy use who returned both baseline and follow-up questionnaires, administered within approximately 3 months of randomization. We used generalized linear models to examine the relationships between hormone therapy suspension and nine individual items from a modified General Sleep Disturbance Scale (number of days experienced in the past week) and an overall sleep quality index at follow-up. We tested whether age, HT type, or duration of use modified these relationships.
Results
Suspension of hormone therapy for 1 or 2 months was associated with greater frequency of sleep problems for the overall sleep quality index and most individual sleep items. For example, the incident rate ratios for waking too early (95% confidence interval) for were 1.23 (1.10, 1.38) for 1-month suspension and 1.30 (1.17, 1.45) for 2-month suspension, compared with women who continued use. Age, type of and duration of hormone therapy use did not modify these relationships.
Conclusion
Short-term hormone therapy suspension was related to moderately greater frequency of sleep problems. Alternative forms of sleep management may benefit women who elect to discontinue hormone therapy use.
Keywords: hormone therapy, sleep, women’s health
Introduction
Current guidelines recommend use of hormone therapy (HT: estrogen with or without progestogen) for short-term relief of menopause-related symptoms.1 However, it is unclear whether menopausal symptoms return following HT discontinuation.2–4 As HT use may alleviate sleep problems,5–7 a resurgence of sleep problems may occur following HT discontinuation. Sleep problems could have negative implications for immediate daytime functioning8 and long-term health, such as development of the metabolic syndrome.9
Few studies have examined sleep problems following HT discontinuation, and results have been inconsistent. In a study of 377 women at Kaiser Permanente Northern California who voluntarily attempted to discontinue HT use, 16% reported difficulty sleeping.2 However, in the Women’s Health Initiative.3 sleep problems were equally common after discontinuation of estrogen and progestogen therapy (EPT) and placebo. In both cases women may have been using HT for menopause-related symptoms as well as for prevention. Symptoms experienced following HT suspension in women using HT for prevention purposes may differ from those among women using HT to address menopausal symptoms.10 As both of these studies used only a single question to assess sleep problems, a fuller understanding of which aspects of sleep are related to HT discontinuation may assist in specific management.
Previous studies did not provide any detail regarding which charateristics may place women at greater risk for sleep problems following HT discontinuation. For example, EPT may be more effective than estrogen alone therapy (ET) in helping women sleep because of the additional benefits of progestogens.11 Therefore, women discontinuing EPT may have a greater resurgence of sleep problems than those discontinuing ET. Women who are younger (and who are likely to have taken HT for fewer years) may benefit more from HT because their sleep problems may be related to the menopausal transition rather than to other age-related factors.12 Therefore, age and menopausal stage may affect the return of sleep problems following HT discontinuation than older women. Understanding these more detailed relationships are important for targeted therapies of sleep problems in midlife.
To examine the relationship between short term HT suspension and sleep problems, we performed a secondary analysis using data from the Radiological Evaluation and Breast Density (READ) randomized controlled trial. In this trial women were randomized to continue using HT or suspend for 1 or 2 months prior to an upcoming mammogram. At the time of the mammogram, women answered questions about sleep problems, allowing us to examine the association between HT suspension and subsequent sleep problems. Our primary hypothesis was that women randomized to HT suspension would have more frequent sleep problems within the two months following HT suspension than women randomized to continue HT. We also tested the following secondary hypotheses: 1) HT suspension would have a stronger effect on the frequency of sleep problems among younger than older women; 2) EPT suspension would result in more frequent sleep problems than suspension of ET; and 3) women who had used HT for short duration would experience more frequent sleep problems following HT suspension than women who had used HT for a long duration.
Methods
Study setting
The details of the READ trial have been described previously. 13,14 Briefly, we recruited women aged 45 to 80 years from Group Health, an integrated health plan in Western Washington state, to participate in a clinical trial to examine whether brief HT suspension before screening mammography decreased mammographic breast density and the need for additional mammographic imaging. Eligibility for the READ trial was based on mammography history and use of HT within the past 2 years and at the time of study enrollment, in 2004 to 2007. Women were randomly assigned (block random assignment by breast density and hormone therapy type) to one of the following HT use groups: continuation, 1-month suspension, or 2-month suspension. Approximately 3 months prior to their scheduled mammograms, all study participants received a mailed baseline questionnaire and instructions to suspend or continue HT personalized to randomization group. Women also received a follow-up questionnaire to be completed at the time of the mammogram. The institutional review boards at Group Health and the U.S. Department of Defense reviewed and approved this study.
Predictor and outcome assessment
Our main outcome variables, sleep problems, were assessed using a modified General Sleep Disturbance Scale,15 administered in the follow-up questionnaire. This scale assessed the number of days in the past week (0 to 7) the respondent had experienced the following: trouble falling asleep, waking while sleeping, waking too early, sleeping poorly, unsatisfied with sleep, waking not rested, too little sleep, using sleep aids, and falling asleep at unscheduled times. The sleep quality index was an average of the number of days the participant experienced each item, excluding using sleep aids.15 We used each of the individual sleep problems, as well as the sleep quality index, as outcome measures for analysis. Women were excluded if they responded to fewer than 50% of sleep questions.
Age, body mass index, and race were collected from Group Health automated data records. Other characteristics were assessed in the baseline questionnaire, including education, type and duration of HT use, smoking status, alcohol consumption, and whether a doctor had ever told the respondent she had high blood pressure, bone fracture after age 44 years, or depression. Menopausal symptoms in the past month were also assessed on the baseline questionnaire, using a summary index from the modified Wiklund Menopause Symptom Checklist16, 17, which measures the average severity scores across a wide range of symptoms including sleep disturbance, mood swings, vaginal bleeding, hot flashes, and night sweats. A separate summary score for the severity of vasomotor symptoms was also computed. A baseline assessment of sleep problems was also collected on the baseline questionnaire.
Statistical analysis
We used generalized linear regression models with robust sandwich variance estimators to test for differences in the frequency of sleep problems (individual items and the sleep quality index) between randomization groups. As the distribution of the sleep quality index was approximately normal, we used linear regression to estimate the difference and 95% confidence intervals (CIs) in the mean sleep quality index score at follow-up for the 1- and 2-month suspension groups relative to the mean for the continuation group. The distributions of the individual sleep items were heavily skewed, so we used a negative binomial model with a log link to calculate incident rate ratios (IRR) and CIs to compare the randomization groups for trouble falling asleep, waking while sleeping, waking too early, sleeping poorly, unsatisfied with sleep, waking not rested, and too little sleep. Because of the bimodal distribution of the number of days women used sleep aids or fell asleep at unexpected times, we created binary indicators for these two sleep items (any difficulty versus none) and used regression models with a log link and binomial error distribution to calculate relative risks (RRs) and CIs. Baseline demographic, general health, HT type and duration, menopausal symptoms, and reports of sleep problems did not differ by randomization group. Therefore, we present only unadjusted results.
We tested whether age (split at the sample median age of 58 years), HT type (ET vs. EPT), and HT duration prior to baseline (split at the median duration of use of 13 years) modified the relationships between HT suspension and sleep problems at follow-up. We included an interaction term between each of these stratification variables and an indicator for randomization group. Since results were similar for the 1-month and 2-month suspension groups, we combined these groups into a single suspension group in the models assessing effect modification. We conducted all analyses in Stata/SE version 10.1 (Stata Corp. LP, College Station, Texas).
Results
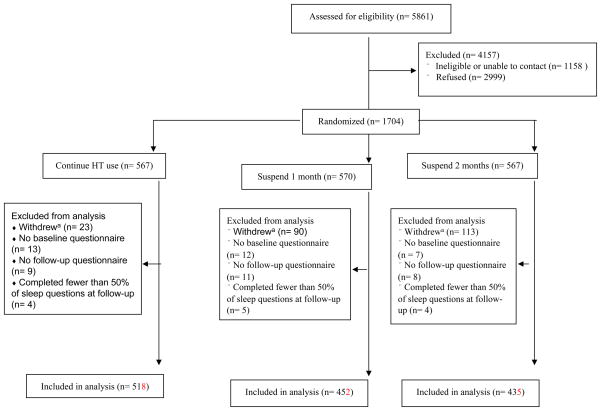
From the 1704 randomized participants in the READ trial14, 230 withdrew before the conclusion of the trial (Figure 1). We excluded 32 participants who did not complete a baseline questionnaire and 28 participants who did not return the follow-up questionnaire and 13 participants who returned the follow-up questionnaire but responded to fewer than 50% of the sleep questions. The analytic sample (n = 1405) comprised 518 women randomized to continue HT use, 452 women randomized to 1-month suspension, and 435 women randomized to 2-month suspension. Because nearly 60% of women who did not enroll in the study or withdrew cited an unwillingness to stop HT,14 our analysis sample largely represents women who were willing to stop HT.
Fig. 1.
Recruitment and participation for the READ trial. READ, Radiological Evaluation and Breast Density; HT, hormone therapy. aBefore the end of the trial.
Baseline sociodemographic, health, and menopausal symptom characteristics were similar by randomization group (Table 1). The average age across all randomization groups was 60 years, and most women in the trial were white, had an educational attainment beyond high school, and did not report a history of high blood pressure, bone fracture, or depression. Approximately half of women in the sample had never smoked, and about 80% of women consumed alcohol a maximum of 1–2 times per week. Across randomization groups, around 40% of women used EPT, and the median duration of HT use was 13 years (Table 2).
Table 1.
Distribution of Demographic and General Health Characteristics by HT Suspension Group Prior to Suspension (at Randomization)
| Characteristic | Continuation Group (n = 518) | 1-Month Suspension Group (n = 452) | 2-Month Suspension Group (n = 435) | p valuea |
|---|---|---|---|---|
| n (%) | n (%) | n (%) | ||
| Age (mean (SD)) | 60.4 (7.3) | 60.4 (7.2) | 60.5 (7.2) | 1.0 |
| Body Mass Index (kg/m2) | 0.2 | |||
| < 25 | 194 (38.0) | 174 (39.3) | 147 (34.1) | |
| 25–29.9 | 179 (35.1) | 138 (31.2) | 144 (33.4) | |
| ≥ 30 | 137 (26.9) | 131 (29.6) | 140 (32.5) | |
| Race and Ethnicity | 0.2 | |||
| White non-Hispanic | 466 (92.5) | 397 (90.2) | 379 (90.0) | |
| Hispanic | 5 (1.0) | 13 (3.0) | 10 (2.4) | |
| Other non-Hispanic | 33 (6.5) | 30 (6.8) | 32 (7.6) | |
| Education | 0.6 | |||
| High school or less | 72 (14.0) | 62 (14.0) | 69 (16.1) | |
| Some college | 221 (43.1) | 173 (39.0) | 170 (39.5) | |
| College graduate | 90 (17.5) | 93 (21.0) | 85 (19.8) | |
| Graduate school | 130 (25.3) | 115 (26.0) | 106 (24.7) | |
| High blood pressure | 0.7 | |||
| yes | 197 (38.8) | 178 (40.2) | 158 (37.4) | |
| no | 311 (61.2) | 265 (59.8) | 265 (62.7) | |
| Bone fractureb | 0.8 | |||
| yes | 71 (14.0) | 55 (12.6) | 59 (14.0) | |
| no | 435 (86.0) | 381 (87.4) | 362 (86.0) | |
| Depressionb | 0.9 | |||
| yes | 183 (36.9) | 157 (35.8) | 147 (35.1) | |
| no | 313 (63.1) | 281 (64.2) | 273 (64.9) | |
| Smoking Status | 0.4 | |||
| Never | 277 (53.9) | 246 (54.7) | 254 (58.8) | |
| Former | 206 (40.1) | 169 (37.6) | 154 (35.7) | |
| Current | 31 (6.0) | 35 (7.8) | 24 (5.6) | |
| Alcohol consumption | 1.0 | |||
| never | 147 (28.6) | 134 (29.8) | 125 (29.0) | |
| 1–3 times/month | 182 (35.4) | 150 (33.3) | 152 (35.3) | |
| 1–2 times/week | 66 (12.8) | 64 (14.2) | 52 (12.1) | |
| 3–4 times/week | 47 (9.1) | 35 (7.8) | 51 (11.8) | |
| almost daily | 72 (14.0) | 67 (14.9) | 51 (11.8) | |
We use a chi-squared test was used for binary exposure variables, a Kruskal-Wallis test for ordered categorical exposure variables, and ANOVA for continuous exposure variables.
self-reported
Table 2.
Distribution of HT, Menopausal, and Sleep Disturbance Characteristics by HT Suspension Group Prior to Suspension (at Randomization)
| Characteristic | Continuation Group (n = 517) a | 1-Month Suspension Group (n = 450 ) a | 2-Month Suspension Group (n = 434) a | p valueb |
|---|---|---|---|---|
| n (%) | n (%) | n (%) | ||
| Type of HT | 0.59 | |||
| ET | 283 (58.5) | 235 (57.0) | 241 (60.6) | |
| EPT | 201 (41.5) | 177 (43.0) | 157 (39.5) | |
| Duration of HT use (years) | 0.79 | |||
| <5 | 67 (13.4) | 57 (13.1) | 46 (11.1) | |
| 5 – 9 | 108 (21.5) | 100 (23.0) | 97 (23.4) | |
| 10–14 | 107 (21.3) | 103 (23.7) | 99 (23.9) | |
| 15+ | 220 (43.8) | 174 (40.1) | 172 (41.6) | |
|
| ||||
| Median (IQR) | Median (IQR) | Median (IQR) | ||
|
| ||||
| Intensity of menopausal symptoms (range 0 – 10) | 1.7 (0.9, 2.6) | 1.7 (0.8, 3.9) | 1.7 (0.8, 2.9) | 0.83 |
| Intensity vasomotor symptoms (range 0 – 10) | 1.0 (0, 2.5) | 1.0 (0, 5.5) | 1.0 (0, 3) | 0.62 |
| Sleep characteristics (number of days; range 0 – 7) | ||||
| Sleep quality index | 2.5 (1.6, 3.6) | 2.5 (1.4, 3.8) | 2.6 (1.5, 3.9) | 0.96 |
| Trouble falling asleep | 1 (0, 2) | 1 (0, 2) | 1 (0. 2) | 0.47 |
| Waking while sleeping | 5 (2, 7) | 5 (2, 7) | 5 (2, 7) | 0.49 |
| Waking too early | 2 (1, 5) | 2 (0, 5) | 2 (0, 4) | 0.73 |
| Sleeping Poorly | 2 (1, 4) | 2 (0, 4) | 2 (1, 4) | 0.89 |
| Unsatisfied with sleep | 3 (1, 6) | 3 (1, 6) | 4 (2, 6) | 0.57 |
| Not rested when woke up | 3 (1, 5) | 3 (1, 5) | 4 (2, 6) | 0.22 |
| Too little sleep | 2 (1, 4) | 2 (1, 4) | 2 (0, 4) | 0.99 |
|
| ||||
| n (%) | n (%) | n (%) | ||
|
| ||||
| Sleep characteristics (any days) | ||||
| Using sleep aids | 0.11 | |||
| yes | 176 (34.8) | 132 (29.9) | 124 (28.8) | |
| no | 330 (65.2) | 309 (70.1) | 306 (71.2) | |
| Falling asleep at unscheduled times | 0.93 | |||
| yes | 248 (49.4) | 223 (50.7) | 215 (49.9) | |
| no | 254 (50.6) | 217 (49.3) | 216 (50.1) | |
Number of women who responded to ≥ 50% of sleep questions
We used a chi-squared test was used for binary exposure variables, a Kruskal-Wallis test for ordered categorical exposure variables, and ANOVA for continuous exposure variables.
At baseline women reported relatively low severity of menopausal symptoms, with a mean of 1.7 for overall menopausal symptoms and 1.0 for vasomotor symptoms on a scale of 0–10, and there were no differences by randomization group (Table 2). Sleep difficulties reported at baseline also were similar across randomization groups. The sleep item with greatest frequency at baseline was “waking while sleeping”, which happened, on average 4.5 days per week. The majority of women did not use sleep aids, and about half reported falling asleep at unscheduled times.
At follow-up women randomized to the HT suspension groups reported significantly more frequent sleep problems than women who continued HT use, across nearly all measures of sleep difficulty (Table 3). The mean sleep quality index score was 0.49 (95% CI 0.29, 0.69) and 0.70 (95% CI 0.50, 0.91) higher for the 1-month and 2-month suspension groups, respectively, compared to the continuation group. Among the individual sleep items, the greatest differences between the women randomized to suspend HT compared to the women randomized to continue HT were for the outcomes of trouble falling asleep, sleeping poorly, and waking too early. Compared to the continuation group, the 2-month suspension group reported 46% more days with trouble falling asleep (IRR 1.46, 95% CI 1.24, 1.73), 46% days sleeping poorly (IRR 1.46, 95% CI 1.31, 1.62) more, and 31% more days waking too early (IRR 1.31, 95% CI 1.16, 1.48). The effects of 1-month suspension were less pronounced, but were statistically significant. Similarly, the differences in the sleep index and the estimated relative incidence rates for waking while sleeping, being unsatisfied with sleep, not rested when woke up, and too little sleep were stronger for 2-month than 1-month suspension. The frequency of use of sleep aids and falling asleep at unscheduled times did not vary according to randomization group. Approximately one-third of all women used sleep aids and approximately half of all participants fell asleep at unscheduled times in the past week.
Table 3.
Sleep Problems at Follow-Up by HT Suspension Group
| Continuation Group (n = 518) | 1-Month Suspension Group (n = 452) | 2-Month Suspension Group (n = 435) | 1-Month versus Continuation | 2-Months versus Continuation | |
|---|---|---|---|---|---|
| mean (SD) | mean (SD) | mean (SD) | Difference (95% CI) | Difference (95% CI) | |
| Sleep quality index (average number of days; range 0 – 7) | 2.7 (1.5) | 3.1 (1.6) | 3.4 (1.6) | 0.49 (0.29, 0.69) | 0.70 (0.50, 0.91) |
|
| |||||
| mean (SD) | mean (SD) | mean (SD) | IRR a (95% CI) | IRR a (95% CI) | |
|
| |||||
| Sleep characteristics (average number of days; range 0 – 7) | |||||
| Trouble falling asleep | 1.3 (1.7) | 1.6 (2.0) | 2.0 (2.1) | 1.23 (1.05, 1.46) | 1.46 (1.24, 1.73) |
| Waking while sleeping | 4.4 (2.4) | 5.0 (2.3) | 5.2 (2.3 ) | 1.14 (1.07, 1.21) | 1.17 (1.10, 1.25) |
| Waking too early | 2.5 (2.4) | 3.1 (2.5) | 3.3 (2.5) | 1.24 (1.10, 1.40) | 1.31 (1.16, 1.48) |
| Sleeping Poorly | 2.3 (2.2) | 3.1 (2.5) | 3.4 (2.4) | 1.36 (1.22, 1.52) | 1.46 (1.31, 1.62) |
| Unsatisfied with sleep | 3.6 (2.4) | 4.0 (2.4) | 4.3 (2.2) | 1.12 (1.03, 1.22) | 1.20 (1.10, 1.30) |
| Not rested when woke up | 3.4 (2.3) | 3.9 (2.3) | 4.1 (2.2) | 1.15 (1.06, 1.24) | 1.20 (1.10, 1.30) |
| Too little sleep | 2.5 (2.2) | 2.9 (2.5) | 3.0 (2.4) | 1.17 (1.04, 1.32) | 1.21 (1.07, 1.37) |
|
| |||||
| n (%) | n (%) | n (%) | RR b (95% CI) | RRb (95% CI) | |
|
| |||||
| Sleep characteristics (reporting any days) | |||||
| Using sleep aids | |||||
| yes | 170 (33.0) | 136 (30.4) | 137 (31.6) | 0.89 (0.68, 1.17) | 0.94 (0.71, 1.23) |
| no | 345 (67.0) | 311 (69.6) | 296 (68.4) | ||
| Falling asleep at unscheduled times | |||||
| yes | 254 (49.7) | 234 (52.4) | 244 (56.4) | 1.11 (0.86, 1.41) | 1.31 (1.01,1.69) |
| no | 257 (50.3) | 213 (47.7) | 189 (43.7) | ||
IRR=Incidence rate ratio, for sleep outcomes measured in the number of days
RR=Relative risk, for binary sleep outcomes
In secondary analyses to assess effect modification, the relationships between HT suspension and sleep problems did not differ significantly by age, HT type, or HT duration. The estimated effects of HT suspension on the overall sleep quality index score at follow-up within strata of these potential effect modifiers are summarized in Table 4 (data for other sleep items not shown). Although the point estimates suggest that the effect of HT suspension on the frequency of sleep problems may be greater in older women and women who used EPT, these differences were not statistically significant.
Table 4.
The Effect of HT Suspension versus HT Continuation1 on Sleep Quality Index, by Age, Type of HT, and Duration of HT Use
| Factor | Difference (95% CI) | p value3 |
|---|---|---|
| Age | ||
| < 57 years (n =683) 2 | 0.46 (0.22, 0.71) | 0.13 |
| ≥ 57 years (n = 722) 2 | 0.73 (0.48, 0.97) | |
| HT type | ||
| ET (n = 762) 2 | 0.48 (0.24, 0.73) | 0.24 |
| EPT (n = 535) 2 | 0.70 (0.44, 0.96) | |
| Duration of HT use | ||
| < 13 years (n = 710) 2 | 0.61 (0.38, 0.85) | 0.73 |
| ≥ 13 years (n = 695) 2 | 0.55 (0.31, 0.80) |
We combined 1-month and 2-month suspension groups into one group
Number of women who responded to ≥ 50% of sleep questions
From an interaction term
Discussion
In a large randomized controlled trial of short-term HT suspension, women randomized to 1- or 2-month suspension reported more frequent sleep problems than women randomized to continue HT use. Although differences between groups were moderate, HT suspension was associated with higher frequency in trouble falling asleep, waking while sleeping, waking too early, sleeping poorly, being unsatisfied with sleep, not rested when waking, and too little sleep. The aggregate effect of pervasive moderate sleep problems over time may be substantial.
Our results are consistent with those of a previous small study of voluntary HT suspension at Kaiser Permanente Northern California, which found a moderate increase in sleep problems following HT suspension after a median follow-up of 5.7 months.2 However, the Women’s Health Initiative (WHI) did not detect more sleep problems following HT discontinuation among women randomized to HT than those randomized to placebo.3 Differences in follow-up time and study population characteristics may explain inconsistencies between our results and those of the WHI. First, the follow-up period in the READ trial was only a few months, compared to 8 to 12 months in the other trial. It is possible that sleep problems dissipate with time after HT discontinuation, as both the READ trial and the previous observational study with abbreviated follow-up found moderate increases in sleep problems following HT suspension. Second, over 80% of women in the READ trial were taking HT for symptom management.14 Women in the WHI were randomized to HT for chronic disease prevention, and only 18% reported sleeping difficulty at baseline.3 Because a greater proportion of women in the WHI may not have had sleep problems prior to taking HT, one would not anticipate a worsening of symptoms after HT suspension.10
The high level of follow-up sleep problems in the READ trial may have been related to a return of vasomotor symptoms. Previous studies of menopausal symptoms following HT discontinuation have found vasomotor symptom resurgence to be a frequent problem within one year, although the association with sleep was not studied.2, 3, 18 Increased vasomotor symptoms following HT suspension may mediate the relationships between HT suspension and subsequent sleep problems,12 or a common underlying factor such as sympathetic activation may influence both vasomotor symptoms and sleep problems.19 Adjusting for a resurgence of menopausal symptoms in our regression models would lead to a model that would be difficult to interpret. We explored the possible relationship between vasomotor symptoms and sleep problems by comparing changes in vasomotor symptom scores and sleep quality index scores from baseline to follow-up. Overall, women who experienced a significant resurgence of vasomotor symptoms at follow-up, defined as having a change from baseline to in the highest quarter, were more likely to also experience increased sleep quality index scores.
Another explanation for the higher frequency of sleep problems after HT suspension that we observed may relate the effect of progesterone and estrogen on sleep in humans. Progesterone has an overall sedative effect,11 and exogenous estrogen has been shown to decrease latency to sleep onset,20, 21, decrease awakening after sleep onset,12,20 and increase total sleep time.21 Exogenous estrogen may also benefit sleep by reducing stress reactivity.22 While estrogen and progesterone may directly influence specific sleep processes,23 sex steroids may also influence sleep through effects on general central nervous system arousal24 and/or thermoregulation.12
Although our sample represents women willing to suspend HT, the use of a randomized controlled trial was a strength of the analysis. The population-based approach of random sampling from among midlife women Group Health who using HT and were due for a mammogram lessened sampling biases. For example, recruiting only women at the time of a treatment visit for menopausal symptoms might result in sampling women with more severe symptoms
The major limitation of our trial was that women were not blinded to HT suspension. Approximately 51 % of recruited women did not enroll, with around half of these women citing an unwillingness to stop HT as the reason for not participating. However, non-participants had similar key baseline characteristics asparticipants.14 Non-participants may have been more concerned about menopausal symptom resurgence than participants.14, 25, 26 a factor we did not measure. In addition, around 18% of women randomized to HT suspension dropped out prior to the completion of the trial. These women probably re-started HT use because of the return of symptoms. Therefore, our results are likely conservative estimates. Given that women will have different propensities for discontinuing HT use, our findings only described sleep problems following suspension among those willing to stop HT. Asmall proportion of women (9%) could not adhere to HT suspension following randomization. However, excluding non-adherent women (n = 82) and women who did not respond to the adherence question (n = 13) did not change our results. We were unable to record whether women used medications for other reasons that might have improved sleep, including selective serotonin reuptake inhibitors (SSRIs) or serotonin norepinephrine reuptake inhibitor (SNRIs).27 However, insomnia symptoms are also common side effects of SSRIs and SNRIs. Our study did not capture any laboratory measures of sleep, which may have different associations with HT suspension than self-reported sleep measures, 28,29 or severity of sleep problems.
Several characteristics of our sample may have masked differences in the relationships between HT suspension and sleep problems by age, HT type, or HT duration. Because eligible women must have been using HT for the two years prior to recruitment, our sample could not have detected differences related to recent initiation of HT. Similarly, 75% of the women in our sample were aged 53 years and over, which may have obscured any differences related to younger age, in particular the menopausal transition.
Conclusion
In conclusion, in a group of women willing to suspend HT use in the short term, women randomized to suspend HT had more frequent problems across sleep domains measured within a few months after suspension, compared to women randomized to continue HT. Women who elect to discontinue HT use may need specific assistance to manage sleep problems following HT discontinuation.
Acknowledgments
The authors thank Tammy Dodd, Linda Palmer, and Melissa Rabelhofer, as well as members of their advisory board: Hermien Watkins, Paula Hoffman, Deb Schiro, and Margrit Schubiger; members of the Data Safety and Monitoring Board: Susan Heckbert, Ben Anderson, Mary Anne Rossing, Robert D. Rosenberg, and Thomas Lumley; and Elizabeth Lin, the medical monitor. The authors also thank Robert Karl, Donna White, and Jo Ellen Callahan for their support for implementing this trial at Group Health. Finally, the authors thank Stephen Taplin, for his collaboration in getting this study funded when he was an investigator at Group Health Cooperative, and Jeannette Beasley, Louise Carter, and Carol Cahill for providing valuable comments on drafts.
Funding sources: The READ Trial was funded by the Department of Defense (DAMD17-03-1-0447). Study participants were recruited from the Group Health Breast Cancer Surveillance Project, funded by the National Cancer Institute (U01CA63731). This project was also supported by the National Institute on Aging (T32 AG027677). Dr. Tom, a UTMB BIRCWH Scholar, is supported by a research career development award (K12HD052023, PI: Berenson), that is co-funded by the Eunice Kennedy Shriver National Institute of Child Health & Human Development (NICHD), the Office of Research on Women’s Health, and the National Institute of Allergy & Infectious Diseases (NIAID). The content is solely the responsibility of the authors and does not necessarily represent the official views of the Eunice Kennedy Shriver National Institute of Child Health & Human Development or the National Institutes of Health.
Footnotes
ClinicalTrials.gov registration number: NCT00117663.
Conflicts of interest: none
References
- 1.North American Menopause Society. Estrogen and progestogen use in postmenopausal women: 2010 position statement of The North American Menopause Society. Menopause. 2010;17:242–55. doi: 10.1097/gme.0b013e3181d0f6b9. [DOI] [PubMed] [Google Scholar]
- 2.Grady D, Ettinger B, Tosteson AN, Pressman A, Macer JL. Predictors of difficulty when discontinuing postmenopausal hormone therapy. Obstet Gynecol. 2003;102:1233–9. doi: 10.1016/j.obstetgynecol.2003.09.025. [DOI] [PubMed] [Google Scholar]
- 3.Ockene JK, Barad DH, Cochrane BB, et al. Symptom experience after discontinuing use of estrogen plus progestin. JAMA. 2005;294:183–93. doi: 10.1001/jama.294.2.183. [DOI] [PubMed] [Google Scholar]
- 4.Haskell SG, Bean-Mayberry B, Gordon K. Discontinuing postmenopausal hormone therapy: an observational study of tapering versus quitting cold turkey: is there a difference in recurrence of menopausal symptoms? Menopause. 2009;16:494–9. doi: 10.1097/gme.0b013e31818fbff5. [DOI] [PubMed] [Google Scholar]
- 5.Kravitz HM, Zhao X, Bromberger JT, et al. Sleep disturbance during the menopausal transition in a multi-ethnic community sample of women. Sleep. 2008;31:979–90. [PMC free article] [PubMed] [Google Scholar]
- 6.Tom SE, Kuh D, Guralnik JM, Mishra GD. Self-reported sleep difficulty during the menopausal transition: results from a prospective cohort study. Menopause. 2010;17:1128–35. doi: 10.1097/gme.0b013e3181dd55b0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barnabei VM, Grady D, Stovall DW, et al. Menopausal symptoms in older women and the effects of treatment with hormone therapy. Obstet Gynecol. 2002;100:1209–18. doi: 10.1016/s0029-7844(02)02369-4. [DOI] [PubMed] [Google Scholar]
- 8.National Sleep Foundation. Sleep in America. [accessed February 15, 2011];Poll, Women and Sleep: Summary of Findings. http://www.sleepfoundation.org/sites/default/files/Summary_Of_Findings%20-%20FINAL.pdf.
- 9.Troxel WM, Buysse DJ, Matthews KA, et al. Sleep symptoms predict the development of the metabolic syndrome. Sleep. 2010;33:1633–40. doi: 10.1093/sleep/33.12.1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Welton AJ, Vickers MR, Kim J, et al. Health related quality of life after combined hormone replacement therapy: randomised controlled trial. BMJ. 2008;337:a1190. doi: 10.1136/bmj.a1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Manber R, Armitage R. Sex, steroids, and sleep: a review. Sleep. 1999;22:540–55. [PubMed] [Google Scholar]
- 12.Landis CA, Moe KE. Sleep and menopause. Nurs Clin North Am. 2004;39:97–115. doi: 10.1016/j.cnur.2003.11.006. [DOI] [PubMed] [Google Scholar]
- 13.Buist DS, Anderson ML, Reed SD, et al. Short-term hormone therapy suspension and mammography recall: a randomized trial. Ann Intern Med. 2009;150:752–65. doi: 10.7326/0003-4819-150-11-200906020-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reed SD, Buist DS, Anderson ML, et al. Short-term (1–2 mo) hormone therapy cessation before mammography. Menopause. 2009;16:1125–31. doi: 10.1097/gme.0b013e3181a5ce60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee KA. Self-reported sleep disturbances in employed women. Sleep. 1992;15:493–8. doi: 10.1093/sleep/15.6.493. [DOI] [PubMed] [Google Scholar]
- 16.Wiklund I, Karlberg J. Evaluation of quality of life in clinical trials. Selecting quality-of-life measures. Control Clin Trials. 1991;12:204S–16S. doi: 10.1016/s0197-2456(05)80024-8. [DOI] [PubMed] [Google Scholar]
- 17.Wiklund I, Holst J, Karlberg J, et al. A new methodological approach to the evaluation of quality of life in postmenopausal women. Maturitas. 1992;14:211–24. doi: 10.1016/0378-5122(92)90116-l. [DOI] [PubMed] [Google Scholar]
- 18.Brunner RL, Gass M, Aragaki A, et al. Effects of conjugated equine estrogen on health-related quality of life in postmenopausal women with hysterectomy: results from the Women’s Health Initiative Randomized Clinical Trial. Arch Intern Med. 2005;165:1976–86. doi: 10.1001/archinte.165.17.1976. [DOI] [PubMed] [Google Scholar]
- 19.Freedman RR, Roehrs TA. Effects of REM sleep and ambient temperature on hot flash-induced sleep disturbance. Menopause. 2006;13:576–83. doi: 10.1097/01.gme.0000227398.53192.bc. [DOI] [PubMed] [Google Scholar]
- 20.Polo-Kantola P, Erkkola R, Helenius H, Irjala K, Polo O. When does estrogen replacement therapy improve sleep quality? Am J Obstet Gynecol. 1998;178:1002–9. doi: 10.1016/s0002-9378(98)70539-3. [DOI] [PubMed] [Google Scholar]
- 21.Schiff I, Regestein Q, Tulchinsky D, Ryan KJ. Effects of estrogens on sleep and psychological state of hypogonadal women. JAMA. 1979;242:2405–4. [PubMed] [Google Scholar]
- 22.Lindheim SR, Legro RS, Bernstein L, et al. Behavioral stress responses in premenopausal and postmenopausal women and the effects of estrogen. Am J Obstet Gynecol. 1992;167:1831–6. doi: 10.1016/0002-9378(92)91783-7. [DOI] [PubMed] [Google Scholar]
- 23.Hadjimarkou MM, Benham R, Schwarz JM, Holder MK, Mong JA. Estradiol suppresses rapid eye movement sleep and activation of sleep-active neurons in the ventrolateral preoptic area. Eur J Neurosci. 2008;27:1780–92. doi: 10.1111/j.1460-9568.2008.06142.x. [DOI] [PubMed] [Google Scholar]
- 24.Goldstein JM, Jerram M, Poldrack R, et al. Hormonal cycle modulates arousal circuitry in women using functional magnetic resonance imaging. J Neurosci. 2005;25:9309–16. doi: 10.1523/JNEUROSCI.2239-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bosworth HB, Bastian LA, Grambow SC, et al. Initiation and discontinuation of hormone therapy for menopausal symptoms: results from a community sample. J Behav Med. 2005;28:105–14. doi: 10.1007/s10865-005-2721-2. [DOI] [PubMed] [Google Scholar]
- 26.Ness J, Aronow WS. Prevalence and causes of persistent use of hormone replacement therapy among postmenopausal women: a follow-up study. Am J Ther. 2006;13:109–12. doi: 10.1097/00045391-200603000-00004. [DOI] [PubMed] [Google Scholar]
- 27.Nelson HD, Vesco KK, Haney E, et al. Nonhormonal therapies for menopausal hot flashes: systematic review and meta-analysis. JAMA. 2006;295:2057–71. doi: 10.1001/jama.295.17.2057. [DOI] [PubMed] [Google Scholar]
- 28.Baker A, Simpson S, Dawson D. Sleep disruption and mood changes associated with menopause. J Psychosom Res. 1997;43:359–69. doi: 10.1016/s0022-3999(97)00126-8. [DOI] [PubMed] [Google Scholar]
- 29.Shaver JL, Giblin E, Paulsen V. Sleep quality subtypes in midlife women. Sleep. 1991;14:18–23. doi: 10.1093/sleep/14.1.18. [DOI] [PubMed] [Google Scholar]