Abstract
Skin wound healing is mediated by inflammatory cell infiltration of the wound site. Inducible costimulator (ICOS), expressed on activated T cells, and its ligand, ICOS ligand (ICOSL), expressed on antigen-presenting cells, have been considered a single receptor–ligand pair. Although the ICOS-ICOSL pathway participates in adaptive immunity, its roles in skin wound healing, which is mediated by innate immune responses, remain unknown. To clarify these roles, repair of excisional wounds was examined in ICOS−/− mice, ICOSL−/− mice, and ICOS−/−ICOSL−/− mice. Each mutant strain showed similar, dramatic delays in wound healing, especially at early times. Knockout mice showed suppressed keratinocyte migration, angiogenesis, and granulation tissue formation, and diminished T-cell, macrophage, and neutrophil infiltration. The loss of ICOS and/or ICOSL resulted in marked suppression of cytokine expression in wounds, especially the Th2 cytokines interleukin (IL)-4, IL-6, and IL-10. T-cell transfer experiments and T-cell depletion therapy further clarified the important roles of ICOS expressed on T cells and its interaction with ICOSL. Application of IL-6, but not IL-4, to the wounds significantly increased the onset of early wound healing in mutant mice. Thus, our results indicate that ICOS-ICOSL costimulatory signaling has critical roles during wound healing, most likely by inducing IL-6 production.
Skin wound healing starts immediately after an injury and consists of three general stages: i) an inflammatory stage that consists of platelet aggregation and recruitment of inflammatory cells to the wound site; ii) a proliferative phase that involves the migration and proliferation of keratinocytes, fibroblasts, and endothelial cells, leading to re-epithelialization and granulation tissue formation; and iii) a long remodeling phase.1, 2 Migration of inflammatory cells to the wound site is important during wound repair.1, 2, 3, 4 Numerous studies have demonstrated that many cytokines, chemokines, and growth factors produced by infiltrating cells or resident cells contribute extensively to the wound healing process. These factors stimulate the synthesis of extracellular matrix by local fibroblasts, generate new blood vessels, promote granulation tissue formation, and enhance the re-epithelialization that takes place by the migration of keratinocytes from the edges of the wound toward the center.1, 2
Inducible costimulator (ICOS) is the third member of the CD28 family of costimulatory molecules, and is induced on the cell surface following T-cell activation.5, 6, 7 ICOSL (also called B7-H2, B7RP-1, LICOS, and GL50), the ligand of ICOS, is weakly expressed on antigen-presenting cells in the steady state and is up-regulated after activation of these cells.6, 7, 8 The ICOS-ICOSL pathway promotes T-cell activation, differentiation, and effector responses, and T cell–dependent B-cell responses. ICOS-mediated costimulation of T cells leads predominantly to the production of effector cytokines such as IL-4 and IL-10 and, to a lesser extent, IL-2, interferon (IFN)-γ, and tumor necrosis factor (TNF)-α,9 thereby playing a more important role in Th2 responses than Th1 responses.5, 10, 11, 12 However, recent studies demonstrated that ICOS influences the expansion of follicular helper T cells, Th17 cells, and regulatory T cells, indicating the complex roles of ICOS in several disease models.13, 14, 15 Because ICOS−/− mice and ICOSL−/− mice display similar defects in humoral immunity, ICOS and ICOSL have been considered a single receptor–ligand pair.16, 17, 18
It is generally accepted that ICOS-ICOSL signaling is critical for adaptive immunity, including autoimmunity, allergy, infectious diseases, and transplantation.18, 19, 20, 21 However, ICOSL is constitutively expressed, not only on B cells, but also on macrophages and dendritic cells that contribute to innate immunity.6, 8 Therefore, it is possible that ICOS-ICOSL signaling may be contributing to innate immunity as well as adaptive immunity. In fact, recent studies suggest involvement of ICOS and ICOSL in innate immune disease models.22, 23 As skin wound healing involves a strong innate immune component, we applied this model to mice deficient for ICOS and ICOSL to investigate the roles of these molecules in modulating innate immunity.
Materials and Methods
Mice
ICOS−/− mice and ICOSL−/− mice were purchased from Jackson Laboratory (Bar Harbor, ME). ICOS−/− mice and ICOSL−/− mice were backcrossed 10 and 8 generations onto the C57BL/6 genetic background, respectively. Mating these ICOS−/− mice with ICOSL−/− mice generated ICOS+/− ICOSL+/− mice, which were crossed to generate ICOS−/− ICOSL−/−. To verify the ICOS or ICOSL genotype, PCR amplification of each gene was conducted using genomic DNA from each mouse. All mice were housed in a specific pathogen-free barrier facility and screened regularly for pathogens. Eight- to 10-week-old male mice were used. The Committee on Animal Experimentation of Kanazawa University Graduate School of Medical Science approved all studies and procedures, including the use of diethyl ether for anesthesia.
Wounding and Macroscopic Examination
Mice were anesthetized and their backs shaved and cleaned with 70% alcohol. Four full-thickness excisional wounds per mouse were made using a disposable, sterile 6-mm biopsy punch (Kai, Tokyo, Japan), as described elsewhere.24 After surgery, mice were caged individually. Photos of the wounds were taken each day, and the open wound area was calculated and compared to that of the previous day using the free-hand tool of Photoshop Elements (Adobe Systems, Tokyo, Japan). No signs suggestive of local infection were detected in the wounded skin.
Histological Examination and Immunohistochemistry
After the mice were euthanized, wounds were harvested with a 2-mm rim of unwounded skin tissue. The wounds were cut into halves laterally, fixed in 3.5% paraformaldehyde, and were then paraffin embedded. Six-micrometer sections were stained with hematoxylin and eosin (H&E), or were immunostained. For immunohistochemistry, deparafinized sections were treated with endogenous peroxidase blocking reagent (DAKO Cytomation A/S, Copenhagen, Denmark) and proteinase K (DAKO Cytomation A/S) for 6 minutes at room temperature. Sections were then incubated with rat monoclonal antibodies specific for myeloperoxidase (Neomarkers, Fremont, CA), macrophages (clone F4/80; Abcam, Cambridge, UK), CD3 (Serotec, Kidlington, UK), CD31 (BD Pharmingen, Franklin Lakes, NJ), and α-smooth muscle actin (α-SMA; Sigma-Aldrich, St. Louis, MO). Rat IgG (Southern Biotechnology Associates Inc., Birmingham, AL) was used as a control for nonspecific staining. Sections were then incubated sequentially (20 minutes, 37°C) with a biotinylated rabbit anti-rat IgG secondary antibody (Ab) (Vectastain ABC method, Vector Laboratories, Burlingame, CA), then horseradish peroxidase–conjugated avidin-biotin complexes. Sections were washed three times with PBS between incubations. Sections were developed with 3,3′-diaminobenzidine tetrahydrochloride and hydrogen peroxide, and then counterstained with methyl green. Numbers of myeloperoxidase-positive neutrophils, F4/80-positive macrophages, and CD3-positive T cells were determined by counting in nine high-power fields (0.07 mm2, magnification, ×400) in the wound bed per section. Among the nine fields, six fields were selected from both edges of the wound bed, and the remaining three fields were chosen from the middle of the wound bed. The epithelial gap, which represents the distance between the leading edge of migrating keratinocytes, was measured in H&E-stained sections of wounds. We identified the area that consisted of newly formed capillaries and the collection of fibroblasts and macrophages as granulation tissue. Wound sections were visualized by microscopy (BX50; Olympus, Tokyo, Japan) with images collected using a digital camera (DP70; Olympus). After this, the area of granulation tissue was gated and measured using the same system. Vessel density, defined as CD31-positive regions, was measured in the whole wound bed areas using Photoshop Elements and was expressed as a percentage of this whole.
T-Cell Depletion
For T-cell depletion studies, 100 μg of anti-mouse Thy 1.2, monoclonal Ab (mAb) (BD Pharmingen) was injected intravenously 24 hours before wounding, as described previously.25
T-Cell Transfer
Single-cell splenic leukocyte suspensions were generated by gentle homogenization. Immunomicrobeads (Miltenyi Biotec, Bergisch Gladbach, Germany) were used to purify T-cell populations according to the manufacturer's instructions. 2.0 × 106 CD3+ T cells, CD4+ T cells, or CD8+ T cells/mouse were transferred intravenously to indicated recipient mice.
Real-Time Reverse-Transcription Polymerase Chain Reaction
Total RNAs were extracted from injured skin samples using Qiagen RNeasy spin columns (Qiagen, Crawley, UK) and digested with DNase I (Qiagen) to remove chromosomal DNA in accordance with the manufacturer's protocols. Total RNA was reverse-transcribed to cDNA using Reverse Transcription System with random hexamers (Promega, Madison, WI). Real-time quantitative RT-PCR was performed using the TaqMan system (Applied Biosystems, Foster City, CA) on an ABI Prism 7000 Sequence Detector (Applied Biosystems) according to the manufacturer's instructions. TaqMan probes and primers for IL-4, IL-6, IL-10, TNF-α, connective tissue growth factor (CTGF), platelet-derived growth factor (PDGF), transforming growth factor (TGF)-β, vascular endothelial growth factor (VEGF), and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were purchased from Applied Biosystems. Relative expression of real-time PCR products was determined using the ΔΔCT technique. Briefly, each set of samples was normalized using the difference in threshold cycle (CT) between the target gene and housekeeping gene (GAPDH): ΔCT = (CT target gene − CT GAPDH). Relative mRNA levels were calculated by the expression 2−ΔΔCT, where ΔΔCT = ΔCT sample − ΔCT calibrator. Each reaction was performed a minimum of three times.
Cytokine Concentration in Tissues
Samples of whole wounds were homogenized in 600 μL of lysis buffer [10 mmol/L PBS, 0.1% sodium dodecyl sulfate, 1% Nonidet P-40, 5 mmol/L ethylenediaminetetraacetic acid (EDTA)] containing a complete protease inhibitor mixture (Roche Diagnostics GmbH, Mannheim, Germany) to extract proteins. Homogenates were centrifuged at 22,000 × g for 15 minutes at 4°C to remove debris.24
The concentrations of IL-6, IL-10, IFN-γ, TNF-α, and monocyte chemotactic protein (MCP)-1 in supernatants were determined using the BD Cytometric Bead Array mouse inflammation kit (BD Biosciences, San Jose, CA) according to the manufacturer's protocol. Flow cytometric analysis was performed using a FACSCalibur flow cytometer (BD Biosciences). Total protein in the supernatant was measured with the Bicinchoninic Acid Protein Assay kit (Thermo Fischer Scientific Inc., Waltham, MA).
Application of Cytokines
An optimal concentration of cytokines was applied to each wound in 20 μL of phosphate-buffered saline. Cytokines were applied to wounds immediately after wounding and every 24 hours thereafter. Recombinant cytokines used in this study were as follows: 50 pg/20 μL IL-4 (R & D systems, Minneapolis, MN) and 50 pg/20 μL IL-6 (R & D systems). Skin samples from at least three mice for each genotype were harvested at 7 days after wounding.
Statistical Analysis
The Mann-Whitney U-test was used for determining the level of significance of differences between samples, and Bonferroni's test was used for multiple comparisons. A P value of <0.05 was considered statistically significant.
Results
Open Wound Areas
The open wound areas were measured each day after wounding to assess macroscopic healing defects (Figure 1). From day 1 to day 10, open wound areas were significantly larger in ICOS−/−, ICOSL−/−, and ICOS−/− ICOSL−/− mice (P < 0.01) than in wild-type mice. Each mutant mouse strain showed an equivalent delay in wound healing. In wild-type mice, wounds closed completely by day 10, but wounds of mutant mice required 15 days or more. Thus, the loss of ICOS and/or ICOSL dramatically disrupted skin wound healing at an early date.
Figure 1.
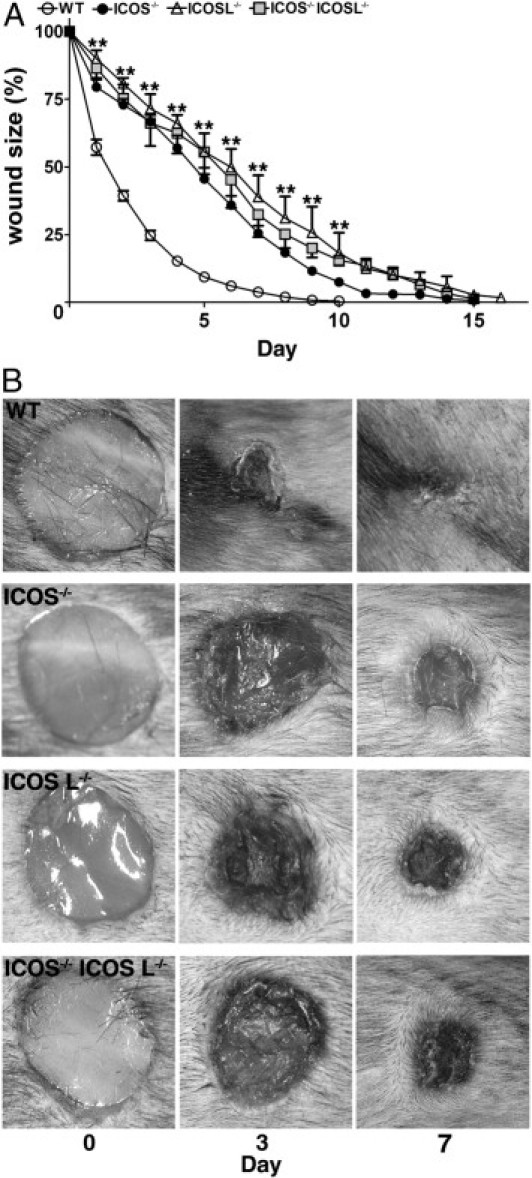
Cutaneous wound healing in wild-type and mutant mice. A: Full-thickness cutaneous wounds were made using a 6-mm punch biopsy. The open wound area was determined by tracing the wound photos using Adobe Photoshop. Each line graph shows the mean (+SEM) results obtained from 14 mice of each group. **P < 0.01, wild-type (WT) versus mutant mice (ICOS−/− mice, ICOSL−/− mice, and ICOS−/−ICOSL−/− mice). B: Representative wound closure in wild-type mice, ICOS−/− mice, ICOSL−/− mice, and ICOS−/−ICOSL−/− mice.
Epithelial Gap
Migration of keratinocytes under the eschar was assessed by microscopically measuring the epithelial gap (the distance between the migrating edges of keratinocytes) (Figure 2A). Keratinocyte migration was significantly inhibited in ICOS−/−, ICOSL−/−, and ICOS−/− ICOSL−/− mice relative to wild-type mice at days 3 and 7 after wounding (P < 0.01 for both) after wounding, consistent with the defects observed in macroscopic healing (Figure 1).
Figure 2.
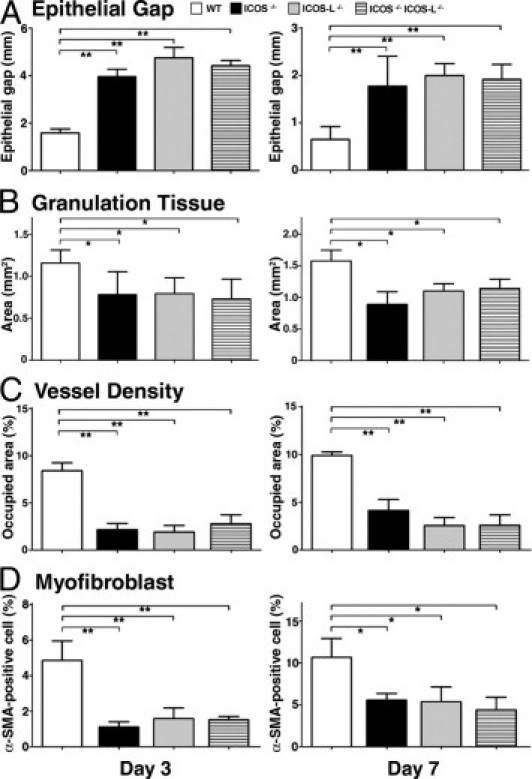
Keratinocyte migration, granulation tissue formation, angiogenesis, and myofibroblast proliferation in wild-type (WT) and mutant mice. A: The distance between the migrating edges of keratinocytes under the eschar (epithelial gap) and (B) the area of granulation tissue, were both measured in tissue sections. C: Vessel density was determined as CD31-positive areas, and (D) the frequency of myofibroblasts was identified as the percentage of α-SMA–positive cells among fibroblastic cells. Each histogram shows the mean (+SEM) results obtained from at least 10 mice of each group. *P < 0.05, **P < 0.01.
Granulation Tissue Formation
Granulation tissue formation is one of the most important components in wound repair; therefore, the area of granulation tissue was measured microscopically (Figure 2B). At 3 days after wounding, granulation tissue formation was reduced in ICOS−/−, ICOSL−/−, and ICOS−/− ICOSL−/− mice (P < 0.05) relative to wild-type controls. Deficiencies in granulation tissue formation were still present at day 7 in all knockout mice as compared to wild-type controls (P < 0.05 for ICOS−/−, ICOSL−/−, and ICOS−/− ICOSL−/− mice).
Angiogenesis
Angiogenesis is an important event observed in the proliferative phase of wound healing. To assess the extent of angiogenesis, we performed immunohistochemical staining with anti-CD31 mAb (Figure 2C). At 3 days after wounding, the vascular density in the wound bed was significantly reduced in ICOS−/−, ICOSL−/−, and ICOS−/− ICOSL−/− mice relative to wild-type controls (P < 0.01). At 7 days after injury, vessel density formation was also inhibited in ICOS−/−, ICOSL−/−, and ICOS−/− ICOSL−/− mice (P < 0.01).
Proliferation of Myofibroblasts
The proliferation of myofibroblasts is also an important event in wound healing; therefore, we performed immunohistochemical staining of myofibroblasts with anti–α-SMA mAb (Figure 2D). The percentage of cells staining positively for α-SMA among fibroblastic cells was significantly reduced in ICOS−/−, ICOSL−/−, and ICOS−/− ICOSL−/− mice (P < 0.01) at day 3 after wounding, as compared with wild-type controls. Similar trends were also observed at day 7 after wounding (P < 0.05 for ICOS−/−, ICOSL−/−, and ICOS−/− ICOSL−/− mice).
Infiltration of Neutrophils
The numbers of neutrophils that migrated outside of blood vessels were assessed in wound tissue by immunohistochemical analysis using anti-myeloperoxidase mAb (Figure 3A). At 1 hour after wounding, extravasated neutrophil numbers were significantly reduced in ICOS−/−, ICOSL−/−, and ICOS−/− ICOSL−/− mice (P < 0.05) relative to wild-type controls. Neutrophil numbers remained lower in ICOS−/−, ICOSL−/−, and ICOS−/− ICOSL−/− mice at 4 hours after wounding (P < 0.05) relative to wild-type controls.
Figure 3.
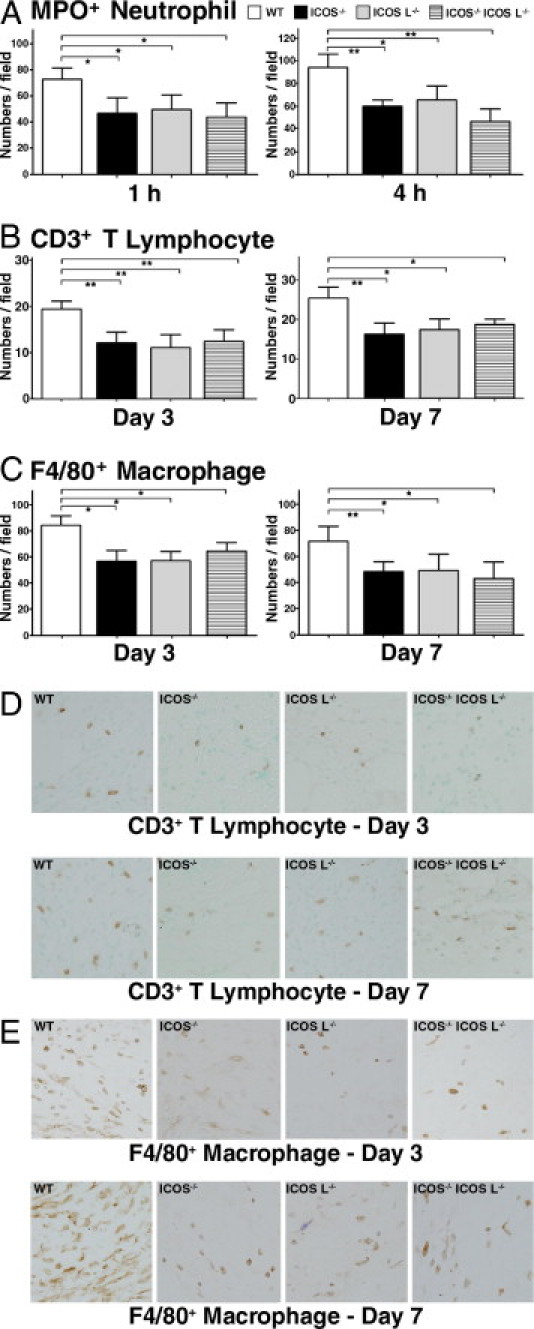
Representative histological sections showing inflammatory cell infiltration into wounded skin of wild-type (WT) mice, ICOS−/− mice, ICOSL−/− mice, and ICOS−/−ICOSL−/− mice. A: Number of neutrophils was determined by counting in myeloperoxidase-stained cells per high-power field (0.07 mm2) at 1 and 4 hours after injury. B: Numbers of CD3-positive T cells per high-power field (0.07 mm2) at 3 and 7 days after wounding. C: Numbers of F4/80-positive macrophages per high-power field (0.07 mm2) at 3 and 7 days after wounding. D: Sections were stained with mAbs specific for T cells (CD3). Original magnification, ×200. E: Sections were stained with mAbs specific for macrophages (F4/80). Original magnification, ×200. Each histogram shows the mean (+SEM) results obtained from at least 10 mice of each group. *P < 0.05, **P < 0.01.
Infiltration of CD3-Positive T Cells
T-cell infiltration was assessed using immunohistochemical staining for CD3 (Figure 3, B and D). CD3-positive T-cell numbers were significantly reduced in ICOS−/−, ICOSL−/−, and ICOS−/− ICOSL−/− mice (P < 0.01) relative to wild-type controls on day 3. At 7 days after wounding, the numbers of CD3-positive T cells in ICOS−/−, ICOSL−/−, and ICOS−/− ICOSL−/− mice had increased relative to corresponding day 3 values, but remained lower than the wild-type controls (P < 0.05 for all groups).
Infiltration of Macrophages
Macrophage infiltration was assessed by immunohistochemistry using the F4/80 mAb (Figure 3, C and E). Macrophage numbers were significantly reduced in ICOS−/−, ICOSL−/−, and ICOS−/− ICOSL−/− mice compared with wild-type mice at 3 days (P < 0.05) and 7 days (P < 0.05) after injury.
T Cell–Depleted Wild-Type Mice Show Equivalent Delays in Wound Healing Compared with Mutant Mice
To assess whether impaired T-cell function contributed to the delayed wound healing in ICOS−/−, ICOSL−/−, and ICOS−/− ICOSL−/− mice; a T cell–depleting anti-Thy1.2 mAb was administered to wild-type mice 24 hours before wounding (Figure 4A). Depletion of more than 80% of CD3+ T cells was confirmed by flow cytometry. Wild-type mice treated with anti-Thy1.2 mAb showed a significant delay in wound healing relative to untreated wild-type mice, with wound closure by day 10 in untreated mice and day 14 in treated mice (Figure 4A). ICOS−/− and ICOSL−/− mice injected with anti-Thy1.2 mAb showed no significant changes compared with untreated ICOS−/− and ICOSL−/− mice. Therefore, depletion of CD3+ T cells from wild-type mice results in an equivalent delay in wound healing compared to both ICOS−/− and ICOSL−/− mice. These findings indicate that the loss of ICOS or ICOSL may be affecting wound healing via inhibition of ICOS signaling in T cells.
Figure 4.
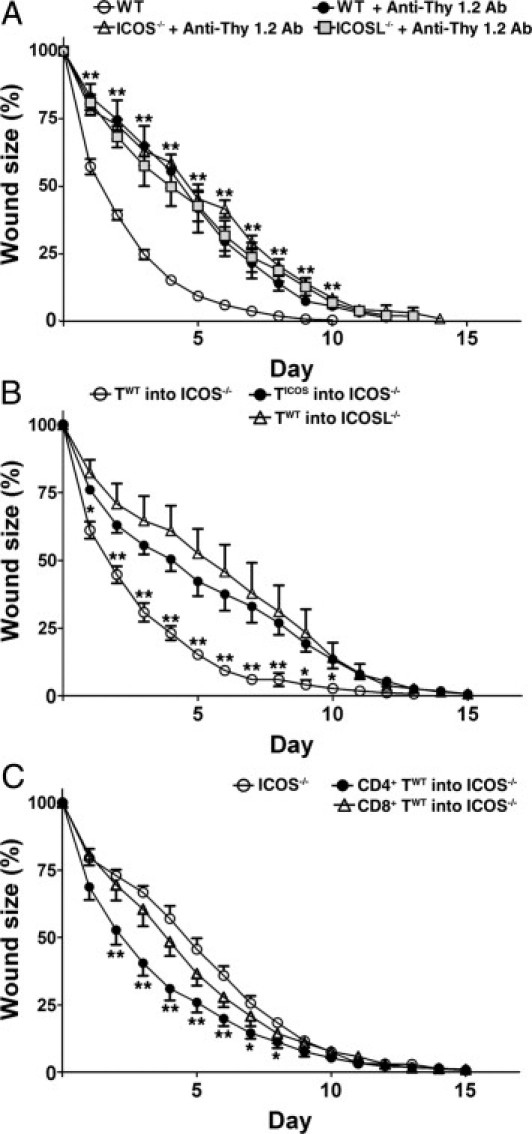
T cell–dependent effects on wound healing in wild-type and mutant mice. A: Wound healing in wild-type and mutant mice 24 hours after anti-Thy1.2 mAb treatment. **P < 0.01, mAb-treated wild-type or mutant mice versus untreated wild-type mice. B: Wound healing in wild-type and mutant mice 24 hours after purified T cell transfer. TWT and TICOS stand for T cells of wild-type and ICOS−/− mice, respectively. TWT were transferred into ICOS−/− mice and ICOSL−/− mice. TICOS were transferred into other ICOS−/− mice. *P < 0.05, **P < 0.01; TWT into ICOS−/− mice versus both TICOS into ICOS−/− mice, and TWT into ICOSL−/− mice. C: CD4-positve TWT and CD8-positve TWT were transferred into ICOS−/− mice. *P < 0.05, **P < 0.01 versus unmanipulated ICOS−/− mice. Each line graph shows the mean (+SEM) results obtained from at least five mice of each group.
Adoptively Transferred T Cells Improve Wound Healing
The role of ICOS-ICOSL signaling in wound healing was assessed using adoptive transfer experiments. Splenic T cells were purified from wild-type mice or ICOS−/− mice, and 2 × 106 cells were transferred into ICOS−/− and ICOSL−/− mice. ICOS−/− mice that received T cells from wild-type mice showed improved wound healing, with wound closure by day 13 (Figure 4B). There were no significant changes in the wound-healing process in ICOS−/− recipient mice that received ICOS-negative T cells, or in ICOSL−/− mice that received wild-type T cells. Furthermore, wound sizes were significantly smaller during days 1 to 10 in ICOS−/− recipient mice that received T cells from wild-type mice compared with ICOS−/− recipient mice that received T cells from ICOS−/− mice, or ICOSL−/− recipient mice that received wild-type T cells (P < 0.05). These findings, together with the results of our T-cell depletion studies, indicate that ICOS-ICOSL signaling is critical for wound healing.
ICOS Expression on CD4-Positive T Cells Has a Dominant Role in Wound Healing
Next, we assessed whether ICOS expression on either CD4+ or CD8+ T cells is important for wound healing. CD4+ T cells and CD8+ T cells were purified from wild-type mice, and 2 × 106 cells of each cell type were transferred into ICOS−/− mice. Although CD4+ T cell transfer significantly reduced the wound size relative to unmanipulated ICOS−/− mice during day 2 to 7, wound closure did not occur until day 15, similar to what was seen in unmanipulated ICOS−/− mice. CD8+ T-cell transfer did not significantly improve wound healing in ICOS−/− mice. Thus, ICOS expression on CD4+ T cells rather than CD8+ T cells is important for wound healing.
Cytokine Expression in the Wounds of Mutant Mice
To assess the effects of ICOS and/or ICOSL loss on cytokine expression, mRNA expression levels of IL-4, IL-6, IL-10, TNF-α, CTGF, TGF-β, PDGF, and VEGF in the wounds were assessed by real-time RT-PCR on day 0, 1, 3, and 7 (Figure 5A). In addition, the concentrations of IL-6, IL-10, IFN-γ, TNF-α, and MCP-1 in wound lysates were measured by cytometric bead array analysis, and were reported as a ratio of the total protein concentration (Figure 5B). Total protein concentrations (mean ± SEM) in the wound lysates were comparable between strains (wild type: 12.9 ± 6.1 mg/mL; ICOS−/−: 11.3 ± 6.7 mg/mL; ICOSL−/−: 12.7 ± 6.4 mg/mL; ICOS−/− ICOSL−/−:11.3 ± 5.5 mg/mL). In wild-type mice, mRNA levels or protein concentrations of these cytokines and growth factors tended to increase during the process of wound healing, although mRNA levels of IL-6 and IL-10 did not increase and the protein concentration of IFN-γ actually decreased. The mRNA levels of IL-4, IL-6, and IL-10 were significantly lower in mutant mice relative to wild-type mice from day 0 to day 7 (P < 0.05, Figure 5A). mRNA of TNF-α and CTGF tended to be reduced in mutant mice relative to wild-type mice from day 1 to day 7. Expression levels of PDGF, TGF-β, and VEGF were not generally different between strains. The relative concentration (cytokine concentration/total proteins concentration in tissue lysates) of IL-6 (from day 0 to day 7) and IL-10 (from day 1 to day 3) in the wounds from mutant mice were lower than those of wild-type mice (P < 0.05, Figure 5B). The relative concentrations of IFN-γ, TNF-α, and MCP-1 were not significantly different between strains (Figure 5B). Thus, reduced IL-4, IL-6, and IL-10 expression may be associated with the impaired wound healing in mutant mice.
Figure 5.
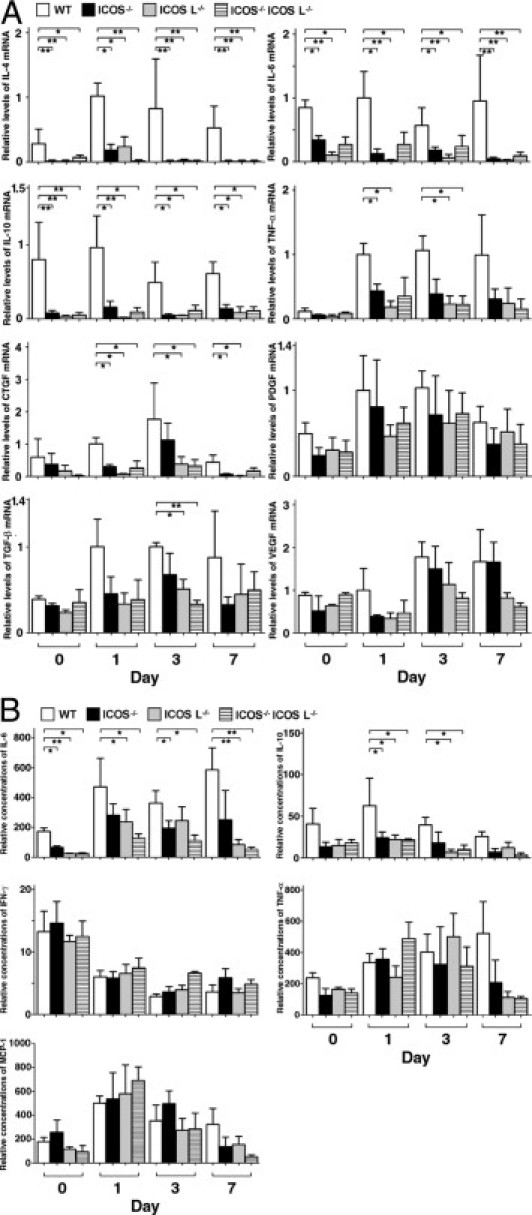
Cytokines, chemokines, and growth factors in wounds from wild-type (WT) and mutant mice. A: Expression of IL-4, IL-6, IL-10, TNF-α, CTGF, PDGF, TGF-β, and VEGF mRNAs measured by quantitative RT-PCR. B: Concentrations of IL-6, IL-10, TNF-α, IFN-γ, and MCP-1 were measured by Cytometric Bead Array (BD Biosciences) analysis in supernatants of wound homogenates. Total protein in the supernatant was measured by bicinchoninic acid protein assay. Relative concentrations of each cytokine [cytokine concentration (in picograms per milliliter) divided by total protein concentration (in milligrams per milliliter)] in tissue lysates were shown. Each histogram shows the mean (+SEM) results obtained from at least six mice of each group. *P < 0.05, **P < 0.01.
Effect of Cytokines on Delayed Wound Healing
Cytokines can promote wound repair, and are produced during the initial inflammatory phase by neutrophils, macrophages, and T cells. For this reason, the effect of cytokines on the delayed wound healing observed in mutant mice was examined. As mentioned above, mutant mice demonstrated a dramatic reduction of IL-4, IL-6, and IL-10 in the wound (Figure 5). Among these cytokines, IL-4 and IL-6 have been reported as important for wound healing.26, 27, 28 Therefore, we assessed whether supplement of IL-4 or IL-6 could improve the delayed wound healing in mutant mice (Figure 6). In both ICOS−/− mice and ICOSL−/− mice supplemented with exogenous IL-6, at day 1 after injury, the gross appearance of open wound areas was significantly improved, and the wound area was reduced to a level similar to that of wild-type mice. However, this effect was not maintained, and marked differences were present by day 5, possibly due to blocking of cytokine infiltration by the wound-fixed eschar. On the other hand, application of IL-4 did not show any positive effect on early wound healing in mutant mice. Thus, a loss of ICOS-ICOSL signaling may delay the early phase of wound healing by reducing IL-6 expression in the wounded skin.
Figure 6.
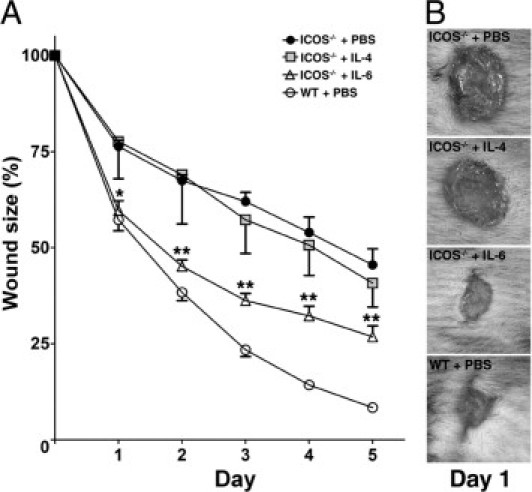
A: Effect of cytokines (IL-4 and IL-6) on delayed wound repair in mice deficient for ICOS or ICOSL. Cytokines were applied to each wound in 20 μL of PBS immediately after wounding and every 24 hours after wounding. Each line graph shows the mean (+SEM) results obtained from at least three mice of each group. *P < 0.05, **P < 0.01; ICOS−/− mice + PBS versus ICOS−/− mice + IL-6. B: Representative wound closure in each group. WT, wild type.
Discussion
To our knowledge, this is the first report that has shown significant roles for T-cell costimulatory molecules in wound healing. Disruption of the ICOS-ICOSL pathway dramatically delayed skin wound healing in mice. The loss of ICOS and/or ICOSL resulted in markedly reduced numbers of infiltrating T cells, macrophages, and neutrophils into wounds. The delayed wound healing observed in mutant mice was similar to that seen in wild-type mice that were depleted of T cells by anti-Thy1.2 mAb. T-cell transfer, specifically transfer of CD4+ T cells rather than CD8+ T cells, improved wound healing in ICOS−/− mice. Furthermore, all three types of knockout mice showed dramatic reductions in cytokines, especially Th2 cytokines such as IL-4, IL-6, and IL-10, in the skin during wound healing. Application of IL-6, but not IL-4, significantly improved the early wound healing in mutant mice. Together, these findings indicate that ICOS-ICOSL signaling and subsequent production of cytokines, such as IL-6, are critical for wound healing.
In this study, we observed a surprising delay in wound healing in mice deficient for ICOS, ICOSL, or both ICOS and ICOSL. Transfer of wild-type T cells reversed the delay in wound healing observed in ICOS−/−mice, resulting in responses that were almost equivalent to those of wild-type mice. This finding confirmed the critical roles played by T cell–expressed ICOS during wound healing. Furthermore, ICOS-ICOSL signaling is required for the function of ICOS in this process, since transfer of wild-type T cells did not improve wound healing in ICOSL−/− mice. T-cell depletion in wild-type mice using anti-Thy1.2 Ab led to delayed wound healing that was comparable to that observed in ICOS−/− and/or ICOSL−/− mice. Previous studies demonstrated that global T-cell depletion using anti-Thy1.2 antibody inhibited wound healing in wild-type mice29, 30 is consistent with our findings. There is a controversy regarding the roles of CD4+ T cells in wound healing. One group reported that CD4+ T-cell depletion did not significantly affect the wound healing in mice,31 whereas another group showed a significantly impaired wound healing by CD4+ T-cell depletion in rats.32 On the other hand, the depletion of CD8+ T cells improved wound healing in both groups' studies.31, 32 Although we did not perform depletion studies of CD4+ T cells or CD8+ T cells, our adoptively transferred experiment of CD4+ T cells or CD8+ T cells indicated that ICOS expressed on CD4+ T cells rather than CD8+ T cells are important for wound healing. This finding is not surprising, since ICOS is predominantly expressed on CD4+ T cells relative to CD8+ T cells, especially before activation.9 These results suggest that ICOS has a central role in the positive function of T cells during wound healing.
A recent study demonstrated that augmenting ICOS costimulation enhances the migration of lymphocytes, including B cells and non–antigen-specific T cells, by increasing the expression of chemokines in the lymph nodes.33 Another study showed that transfer of ICOS-enriched T cells followed by allergen airway challenge induced infiltration of recipient lymphocytes.34 Therefore, ICOS-ICOSL signaling is likely important for lymphocyte migration, although direct effects of ICOS on lymphocyte chemotaxis have not yet been examined. Consistent with this idea, mutant mice exhibited a marked reduction in inflammatory cell infiltration including neutrophils, lymphocytes, and macrophages during wound healing. The inflammatory response is believed to be instrumental in supplying cytokines, chemokines, and growth factors that orchestrate the cell movement necessary for wound repair.1, 2, 3, 4 An absence or a decrease in macrophage number at wound sites impairs tissue repair,35 and transfer of macrophages into aged mice accelerates wound healing.36 In addition to our current findings, it has been reported previously that T-cell depletion using anti-Thy1.2 Ab significantly inhibits wound healing in mice.37 Thus, inflammatory cell recruitment is considered to be important for wound healing, and one possible explanation for the delayed wound repair in ICOS−/− and ICOSL−/− mice could be impaired inflammatory cell infiltration. Unfortunately, Abs against ICOS or ICOSL that can clearly stain positive cells in the skin were not available.
All components necessary for wound healing, such as keratinocyte migration (epithelial gap), granulation tissue formation, angiogenesis, and myofibroblast proliferation were suppressed in ICOS−/−, ICOSL−/−, and double-knockout mice. These defects may be due to the reduction of various cytokines produced directly or indirectly by infiltrating inflammatory cells in the wound. Interestingly, Th2 cytokines such as IL-4, IL-6, and IL-10 were markedly suppressed in the skin of mutant mice before and after wounding. It is well known that ICOS-ICOSL signaling has a pivotal role in cytokine production by T cells, and this is particularly true for the induction of Th2 cytokines rather than Th1 cytokines.5, 10, 11, 12 Our findings support a role for ICOS in Th2 cytokine production during skin wound healing.
Importantly, application of IL-6, but not IL-4, significantly improved wound closure to the levels of wild-type mice during early wound healing. Prior studies had shown that IL-6–deficient mice have a marked delay in skin wound healing,27, 28 and that IL-6 deficiency reduced inflammatory cell infiltration, collagen deposition, and angiogenesis at wound sites.28 Furthermore, it has been demonstrated that IL-6 induces keratinocyte migration via signal transducer and activator of transcription 3 (STAT3) activation.38 In fact, STAT3 is essential for keratinocyte migration, and STAT3-disrupted mice exhibit significantly delayed wound healing.39 Therefore, the markedly suppressed IL-6 in our study may explain, at least in part, the delayed wound healing in ICOS−/− and ICOSL−/− mice. A previous report demonstrated that topical administration of IL-4 significantly accelerated wound healing, whereas IL-4 antisense oligonucleotides significantly inhibited healing.26 By contrast, IL-10 is an inhibitory factor for the remodeling of extracellular matrix during wound healing.40 Addition of a neutralizing anti–IL-10 Ab inhibits the infiltration of neutrophils and macrophages toward the wound.41 Therefore, reduced IL-10 expression is not likely contributing to the delayed wound healing seen in mutant mice in the current study, although reduced cell infiltration may be partially induced by decreased IL-10 expression. Although IFN-γ has been known to enhance wound healing,42 the concentration of IFN-γ in mutant mice was not significantly different from that seen in wild-type mice. Therefore, our findings suggest that loss of ICOS-ICOSL signaling reduces expression of cytokines such as IL-6, and thereby delays wound healing. Interestingly, Th2 cytokines, including IL-4, IL-6, and IL-10, were remarkably reduced even in the unwounded homeostatic skin (day 0) of knockout mice compared with wild-type mice. This may explain the dramatic early delay in wound healing similar to that of IL-6–deficient mice.27, 28
It is currently thought that ICOS and ICOSL are a single receptor–ligand pair with no other known binding partners.17 Previous studies have shown that ICOS−/− mice and ICOSL−/− mice have similar phenotypes.16, 17 Consistent with this idea, in the wound-healing model used in our current study, ICOS−/− mice and/or ICOSL−/− mice exhibited similar delays in wound healing. Most studies have investigated the roles of ICOS or ICOSL in disease models related to antigen-specific adaptive immunity.18, 19, 20, 21 Therefore, it is unexpected that T-cell costimulatory signals, including ICOS-ICOSL, have roles in wound healing. Although ICOS is expressed on effector and memory T cells, ICOSL is constitutively expressed on macrophages and dendritic cells that contribute to innate immunity, in addition to expression on B cells.5, 6, 7 Several studies have reported that ICOSL is expressed on subsets of epidermal cells, mesenchymal cells, and endothelial cells,7, 43, 44 although we could not detect expression on these cell types, at least by immunohistochemical staining (data not shown). Several recent studies, including our bleomycin model, showed the roles of ICOS and ICOSL in innate immune responses, including inflammation.22, 23 Our findings also suggest that ICOS-ICOSL signaling contributes to innate immunity during wound healing. Alternatively, ICOS-ICOSL signaling may affect cytokine balance or the production of cytokines in organs such as the skin under homeostatic and pathological conditions. Further investigation will be needed to clarify the multiple, robust, and complicated functions of ICOS-ICOSL signaling for clinical application of wound healing.
Footnotes
Supported by grants-in-aid from the Ministry of Education, Culture, Sports, Science, and Technology of Japan.
References
- 1.Singer A.J., Clark R.A.F. Cutaneous wound healing. N Engl J Med. 1999;341:738–746. doi: 10.1056/NEJM199909023411006. [DOI] [PubMed] [Google Scholar]
- 2.Martin P. Wound healing-aiming for perfect skin regeneration. Science. 1997;276:75–81. doi: 10.1126/science.276.5309.75. [DOI] [PubMed] [Google Scholar]
- 3.Kondo T., Ishida Y. Molecular pathology of wound healing. Forensic Sci Int. 2010;203:93–98. doi: 10.1016/j.forsciint.2010.07.004. [DOI] [PubMed] [Google Scholar]
- 4.Brancato S.K., Albina J.E. Wound macrophages as key regulators of repair: origin, phenotype, and function. Am J Pathol. 2011;178:19–25. doi: 10.1016/j.ajpath.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hutloff A., Dittrich A.M., Beier K.C., Eljaschewitsch B., Kraft R., Anagnostopoulos I., Kroczek R.A. ICOS is an inducible T-cell co-stimulator structurally and functionally related to CD28. Nature. 1999;397:263–266. doi: 10.1038/16717. [DOI] [PubMed] [Google Scholar]
- 6.Yoshinaga S.K., Whoriskey J.S., Khare S.D., Sarmiento U., Guo J., Horan T., Shih G., Zhang M., Coccia M.A., Kohno T., Tafuri-Bladt A., Brankow D., Campbell P., Chang D., Chiu L., Dai T., Duncan G., Elliott G.S., Hui A., McCabe S.M., Scully S., Shahinian A., Shaklee C.L., Van G., Mak T.W., Senaldi G. T-cell co-stimulation through B7RP-1 and ICOS. Nature. 1999;402:827–832. doi: 10.1038/45582. [DOI] [PubMed] [Google Scholar]
- 7.Greenwald R.J., Freeman G.J., Sharpe A.H. The B7 family revisited. Annu Rev Immunol. 2005;23:515–548. doi: 10.1146/annurev.immunol.23.021704.115611. [DOI] [PubMed] [Google Scholar]
- 8.Swallow M.M., Wallin J.J., Sha W.C. B7h, a novel costimulatory homolog of B7.1 and B7.2, is induced by TNFalpha. Immunity. 1999;11:423–432. doi: 10.1016/s1074-7613(00)80117-x. [DOI] [PubMed] [Google Scholar]
- 9.McAdam A.J., Chang T.T., Lumelsky A.E., Greenfield E.A., Boussiotis V.A., Duke-Cohan J.S., Chernova T., Malenkovich N., Jabs C., Kuchroo V.K., Ling V., Collins M., Sharpe A.H., Freeman G.J. Mouse inducible costimulatory molecule (ICOS) expression is enhanced by CD28 costimulation and regulates differentiation of CD4+ T cells. J Immunol. 2000;165:5035–5040. doi: 10.4049/jimmunol.165.9.5035. [DOI] [PubMed] [Google Scholar]
- 10.Nurieva R.I., Chung Y., Hwang D., Yang X.O., Kang H.S., Ma L., Wang Y.H., Watowich S.S., Jetten A.M., Tian Q., Dong C. Generation of T follicular helper cells is mediated by interleukin-21 but independent of T helper 1, 2, or 17 cell lineages. Immunity. 2008;29:138–149. doi: 10.1016/j.immuni.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coyle A.J., Gutierrez-Ramos J.C. The role of ICOS and other costimulatory molecules in allergy and asthma. Springer Semin Immunopathol. 2004;25:349–359. doi: 10.1007/s00281-003-0154-y. [DOI] [PubMed] [Google Scholar]
- 12.Lohning M., Hutloff A., Kallinich T., Mages H.W., Bonhagen K., Radbruch A., Hamelmann E., Kroczek R.A. Expression of ICOS in vivo defines CD4+ effector T cells with high inflammatory potential and a strong bias for secretion of interleukin 10. J Exp Med. 2003;197:181–193. doi: 10.1084/jem.20020632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bauquet A.T., Jin H., Paterson A.M., Mitsdoerffer M., Ho I.C., Sharpe A.H., Kuchroo V.K. The costimulatory molecule ICOS regulates the expression of c-Maf and IL-21 in the development of follicular T helper cells and TH-17 cells. Nat Immunol. 2009;10:167–175. doi: 10.1038/ni.1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burmeister Y., Lischke T., Dahler A.C., Mages H.W., Lam K.P., Coyle A.J., Kroczek R.A., Hutloff A. ICOS controls the pool size of effector-memory and regulatory T cells. J Immunol. 2008;180:774–782. doi: 10.4049/jimmunol.180.2.774. [DOI] [PubMed] [Google Scholar]
- 15.Akbari O., Freeman G.J., Meyer E.H., Greenfield E.A., Chang T.T., Sharpe A.H., Berry G., DeKruyff R.H., Umetsu D.T. Antigen-specific regulatory T cells develop via the ICOS-ICOS-ligand pathway and inhibit allergen-induced airway hyperreactivity. Nat Med. 2002;8:1024–1032. doi: 10.1038/nm745. [DOI] [PubMed] [Google Scholar]
- 16.Mak T.W., Shahinian A., Yoshinaga S.K., Wakeham A., Boucher L.M., Pintilie M., Duncan G., Gajewska B.U., Gronski M., Eriksson U., Odermatt B., Ho A., Bouchard D., Whorisky J.S., Jordana M., Ohashi P.S., Pawson T., Bladt F., Tafuri A. Costimulation through the inducible costimulator ligand is essential for both T helper and B cell functions in T cell-dependent B cell responses. Nat Immunol. 2003;4:765–772. doi: 10.1038/ni947. [DOI] [PubMed] [Google Scholar]
- 17.Nurieva R.I., Mai X.M., Forbush K., Bevan M.J., Dong C. B7h is required for T cell activation, differentiation, and effector function. Proc Natl Acad Sci U S A. 2003;100:14163–14168. doi: 10.1073/pnas.2335041100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dong C., Juedes A.E., Temann U.A., Shresta S., Allison J.P., Ruddle N.H., Flavell R.A. ICOS co-stimulatory receptor is essential for T-cell activation and function. Nature. 2001;409:97–101. doi: 10.1038/35051100. [DOI] [PubMed] [Google Scholar]
- 19.Rottman J.B., Smith T., Tonra J.R., Ganley K., Bloom T., Silva R., Pierce B., Gutierrez-Ramos J.C., Ozkaynak E., Coyle A.J. The costimulatory molecule ICOS plays an important role in the immunopathogenesis of EAE. Nat Immunol. 2001;2:605–611. doi: 10.1038/89750. [DOI] [PubMed] [Google Scholar]
- 20.Kopf M., Coyle A.J., Schmitz N., Barner M., Oxenius A., Gallimore A., Gutierrez-Ramos J.C., Bachmann M.F. Inducible costimulator protein (ICOS) controls T helper cell subset polarization after virus and parasite infection. J Exp Med. 2000;192:53–61. doi: 10.1084/jem.192.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ozkaynak E., Gao W., Shemmeri N., Wang C., Gutierrez-Ramos J.C., Amaral J., Qin S., Rottman J.B., Coyle A.J., Hancock W.W. Importance of ICOS-B7RP-1 costimulation in acute and chronic allograft rejection. Nat Immunol. 2001;2:591–596. doi: 10.1038/89731. [DOI] [PubMed] [Google Scholar]
- 22.Tanaka C., Fujimoto M., Hamaguchi Y., Sato S., Takehara K., Hasegawa M. Inducible costimulator ligand regulates bleomycin-induced lung and skin fibrosis in a mouse model independently of the inducible costimulator/inducible costimulator ligand pathway. Arthritis Rheum. 2010;62:1723–1732. doi: 10.1002/art.27428. [DOI] [PubMed] [Google Scholar]
- 23.Dianzani C., Minelli R., Mesturini R., Chiocchetti A., Barrera G., Boscolo S., Sarasso C., Gigliotti C.L., Sblattero D., Yagi J., Rojo J.M., Fantozzi R., Dianzani U. B7h triggering inhibits umbilical vascular endothelial cell adhesiveness to tumor cell lines and polymorphonuclear cells. J Immunol. 2010;185:3970–3979. doi: 10.4049/jimmunol.0903269. [DOI] [PubMed] [Google Scholar]
- 24.Mori R., Kondo T., Nishie T., Ohshima T., Asano M. Impairment of skin wound healing in beta-1,4-galactosyltransferase-deficient mice with reduced leukocyte recruitment. Am J Pathol. 2004;164:1303–1314. doi: 10.1016/s0002-9440(10)63217-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tomita Y., Nomoto K. Prevention of induction of unresponsiveness to class I antigens by veto activity of donor marrow in cylophosphamide-treated mice. Transplantation. 1993;56:1473–1480. doi: 10.1097/00007890-199312000-00037. [DOI] [PubMed] [Google Scholar]
- 26.Salmon-Ehr V., Ramont L., Godeau G., Birembaut P., Guenounou M., Bernard P., Maquart F.X. Implication of interleukin-4 in wound healing. Lab Invest. 2000;80:1337–1343. doi: 10.1038/labinvest.3780141. [DOI] [PubMed] [Google Scholar]
- 27.Gallucci R.M., Simeonova P.P., Matheson J.M., Kommineni C., Guriel J.L., Sugawara T., Luster M.I. Impaired cutaneous wound healing in interleukin-6-deficient and immunosuppressed mice. FASEB J. 2000;14:2525–2531. doi: 10.1096/fj.00-0073com. [DOI] [PubMed] [Google Scholar]
- 28.Lin Z.Q., Kondo T., Ishida Y., Takayasu T., Mukaida N. Essential involvement of IL-6 in the skin wound-healing process as evidenced by delayed wound healing in IL-6-deficient mice. J Leukoc Biol. 2003;73:713–721. doi: 10.1189/jlb.0802397. [DOI] [PubMed] [Google Scholar]
- 29.Barbul A., Shawe T., Rotter S.M., Efron J.E., Wasserkrug H.L., Badawy S.B. Wound healing in nude mice: a study on the regulatory role of lymphocytes in fibroplasia. Surgery. 1989;105:764–769. [PubMed] [Google Scholar]
- 30.Efron J.E., Frankel H.L., Lazarou S.A., Wasserkrug H.L., Barbul A. Wound healing and T-lymphocytes. J Surg Res. 1990;48:460–463. doi: 10.1016/0022-4804(90)90013-r. [DOI] [PubMed] [Google Scholar]
- 31.Barbul A., Breslin R.J., Woodyard J.P., Wasserkrug H.L., Efron G. The effect of in vivo T helper and T suppressor lymphocyte depletion on wound healing. Ann Surg. 1989;209:479–483. doi: 10.1097/00000658-198904000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Davis P.A., Corless D.J., Aspinall R., Wastell C. Effect of CD4(+) and CD8(+) cell depletion on wound healing. Br J Surg. 2001;88:298–304. doi: 10.1046/j.1365-2168.2001.01665.x. [DOI] [PubMed] [Google Scholar]
- 33.Tesciuba A.G., Shilling R.A., Agarwal M.D., Bandukwala H.S., Clay B.S., Moore T.V., Weinstock J.V., Welcher A.A., Sperling A.I. ICOS costimulation expands Th2 immunity by augmenting migration of lymphocytes to draining lymph nodes. J Immunol. 2008;181:1019–1024. doi: 10.4049/jimmunol.181.2.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Beier K.C., Hutloff A., Lohning M., Kallinich T., Kroczek R.A., Hamelmann E. Inducible costimulator-positive T cells are required for allergen-induced local B-cell infiltration and antigen-specific IgE production in lung tissue. J Allergy Clin Immunol. 2004;114:775–782. doi: 10.1016/j.jaci.2004.06.024. [DOI] [PubMed] [Google Scholar]
- 35.Leibovich S.J., Ross R. The role of the macrophage in wound repair: a study with hydrocortisone and antimacrophage serum. Am J Pathol. 1975;78:71–100. [PMC free article] [PubMed] [Google Scholar]
- 36.Danon D., Kowatch M.A., Roth G.S. Promotion of wound repair in old mice by local injection of macrophages. Proc Natl Acad Sci U S A. 1989;86:2018–2020. doi: 10.1073/pnas.86.6.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Peterson J.M., Barbul A., Breslin R.J., Wasserkrug H.L., Efron G. Significance of T-lymphocytes in wound healing. Surgery. 1987;102:300–305. [PubMed] [Google Scholar]
- 38.Gallucci R.M., Sloan D.K., Heck J.M., Murray A.R., O'Dell S.J. Interleukin 6 indirectly induces keratinocyte migration. J Invest Dermatol. 2004;122:764–772. doi: 10.1111/j.0022-202X.2004.22323.x. [DOI] [PubMed] [Google Scholar]
- 39.Sano S., Itami S., Takeda K., Tarutani M., Yamaguchi Y., Miura H., Yoshikawa K., Akira S., Takeda J. Keratinocyte-specific ablation of Stat3 exhibits impaired skin remodeling, but does not affect skin morphogenesis. EMBO J. 1999;18:4657–4668. doi: 10.1093/emboj/18.17.4657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moroguchi A., Ishimura K., Okano K., Wakabayashi H., Maeba T., Maeta H. Interleukin-10 suppresses proliferation and remodeling of extracellular matrix of cultured human skin fibroblasts. Eur Surg Res. 2004;36:39–44. doi: 10.1159/000075073. [DOI] [PubMed] [Google Scholar]
- 41.Sato Y., Ohshima T., Kondo T. Regulatory role of endogenous interleukin-10 in cutaneous inflammatory response of murine wound healing. Biochem Biophys Res Commun. 1999;265:194–199. doi: 10.1006/bbrc.1999.1455. [DOI] [PubMed] [Google Scholar]
- 42.Ishida Y., Kondo T., Takayasu T., Iwakura Y., Mukaida N. The essential involvement of cross-talk between IFN-gamma and TGF-beta in the skin wound-healing process. J Immunol. 2004;172:1848–1855. doi: 10.4049/jimmunol.172.3.1848. [DOI] [PubMed] [Google Scholar]
- 43.Khayyamian S., Hutloff A., Buchner K., Grafe M., Henn V., Kroczek R.A., Mages H.W. ICOS-ligand, expressed on human endothelial cells, costimulates Th1 and Th2 cytokine secretion by memory CD4+ T cells. Proc Natl Acad Sci U S A. 2002;99:6198–6203. doi: 10.1073/pnas.092576699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kajiwara K., Morishima H., Akiyama K., Yanagihara Y. Expression and function of the inducible costimulator ligand B7-H2 in human airway smooth muscle cells. Allergol Int. 2009;58:573–583. doi: 10.2332/allergolint.09-OA-0113. [DOI] [PubMed] [Google Scholar]


