Abstract
Using the M13 dideoxy sequencing technique, we have established the DNA sequences of colicins E2 and E3 which encompass the receptor-binding and the catalytic domains of each of the nucleases, and their immunity (imm) genes. The imm gene of plasmid ColE2-P9 is 255 bp long and is separated from the end of the col gene by a dinucleotide. This gene pair is arranged similarly in plasmid ColE3-CA38 except that the intergenic space is 9 bp and the E3 imm gene is one codon shorter than its E2 counterpart. Comparisons of the E2 and E3 imm sequences indicate considerable divergence whereas the receptor-binding domains of both colicins are highly conserved. The two nuclease domains appear to share some sequence homology. A possible evolutionary relationship between colicin E3 and other microbial extracellular ribonucleases is also suggested from the sequence alignment analysis.
Full text
PDF


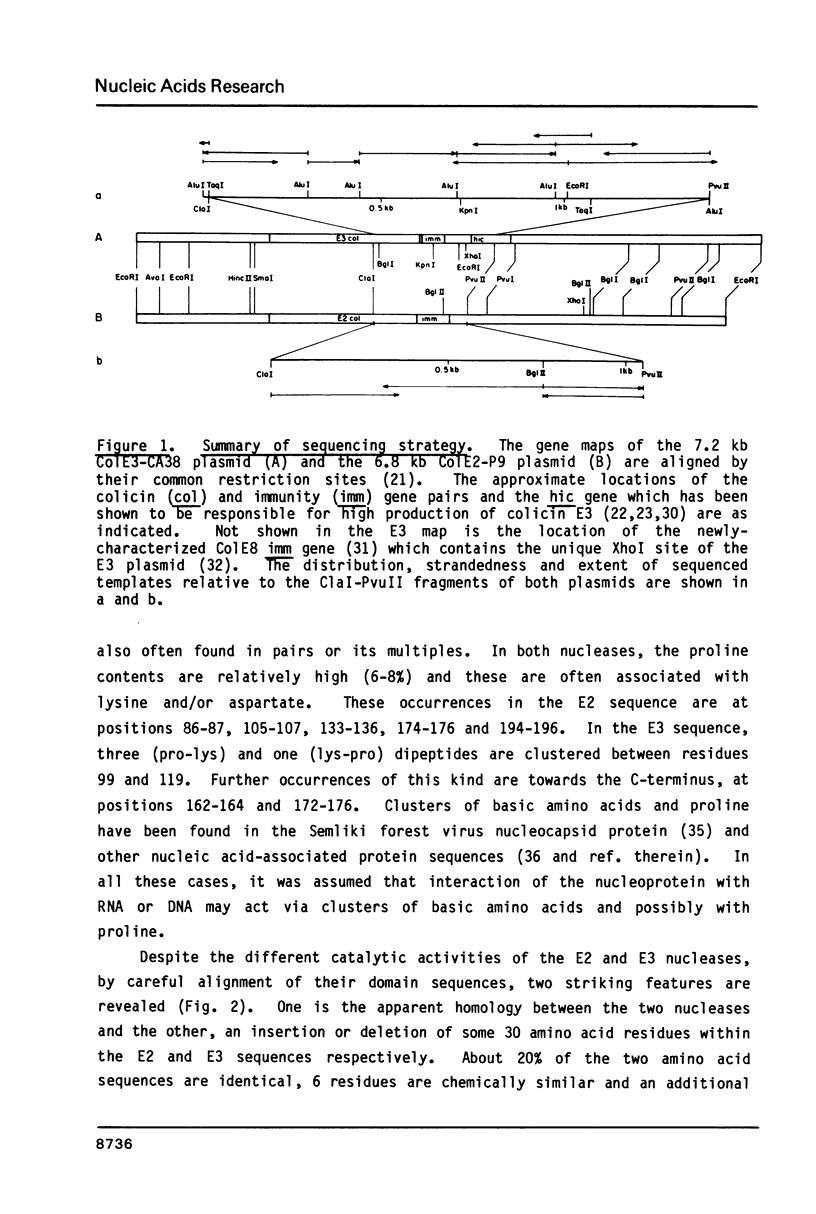

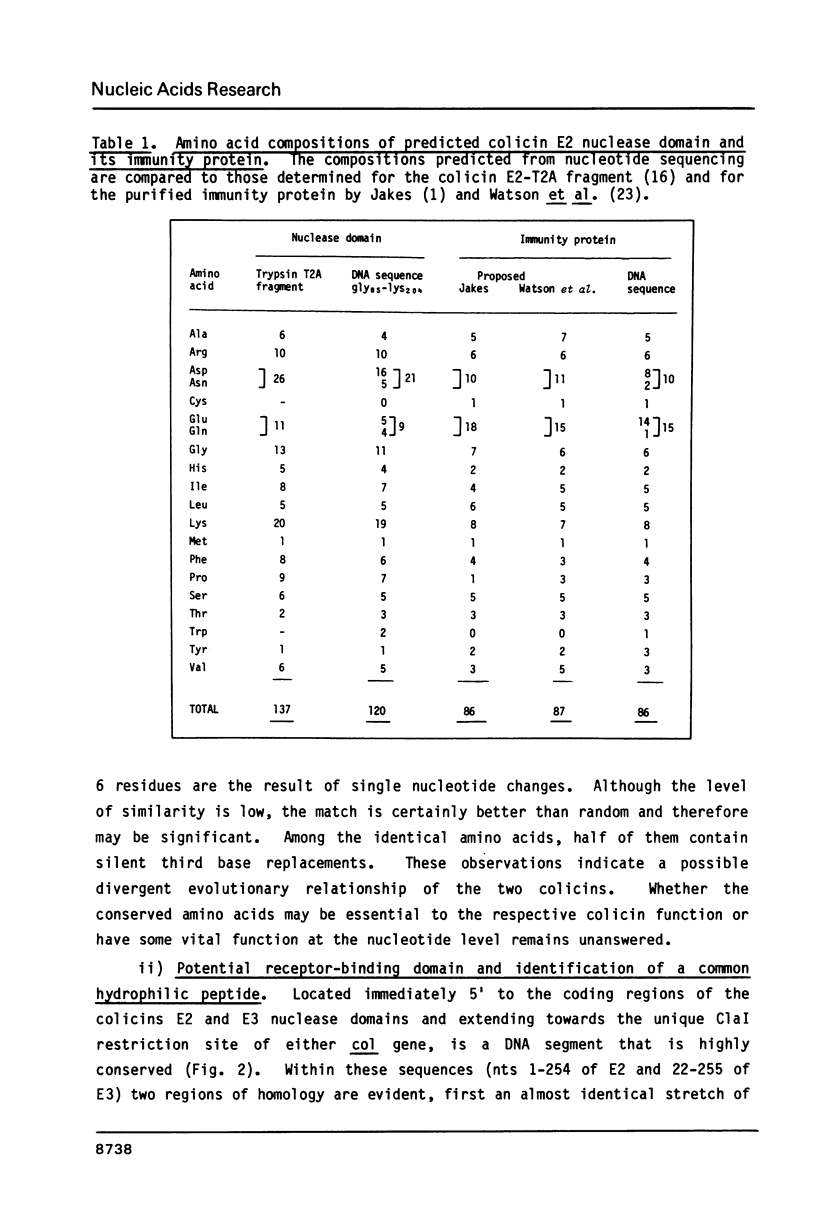
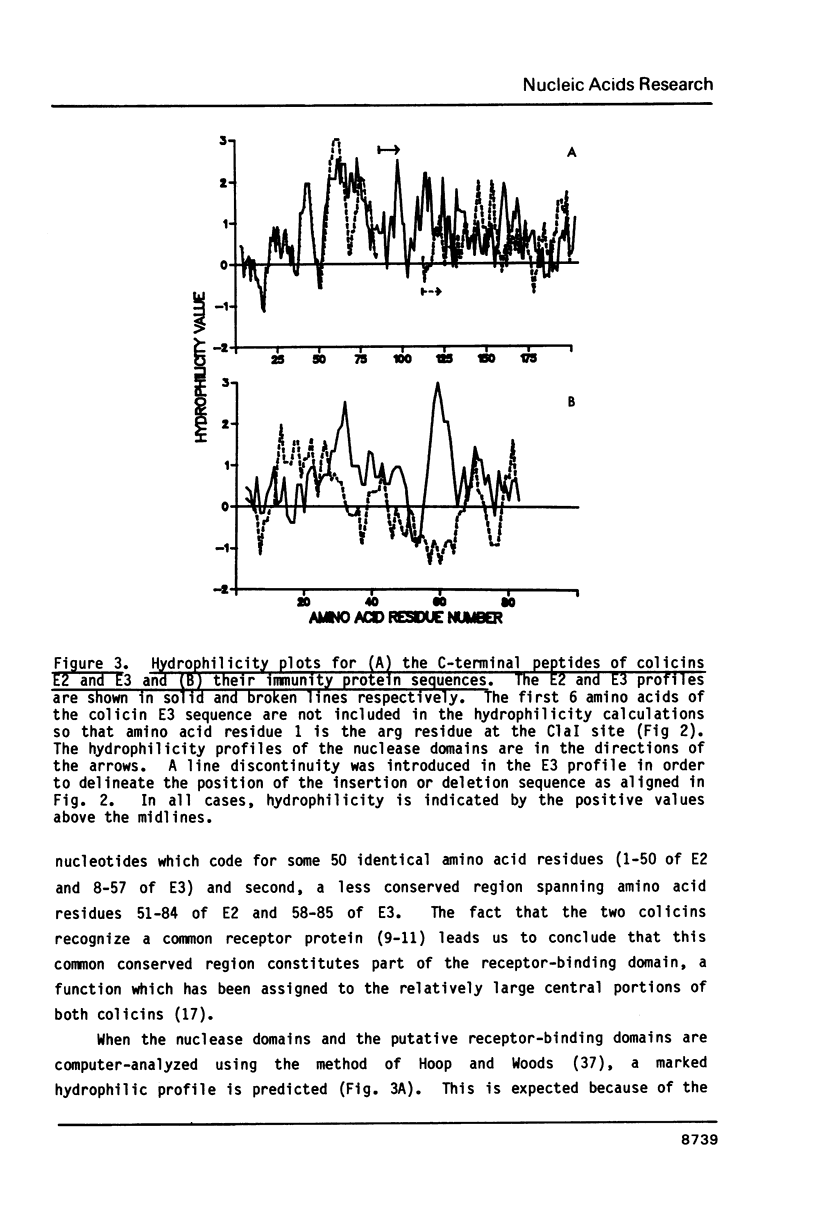


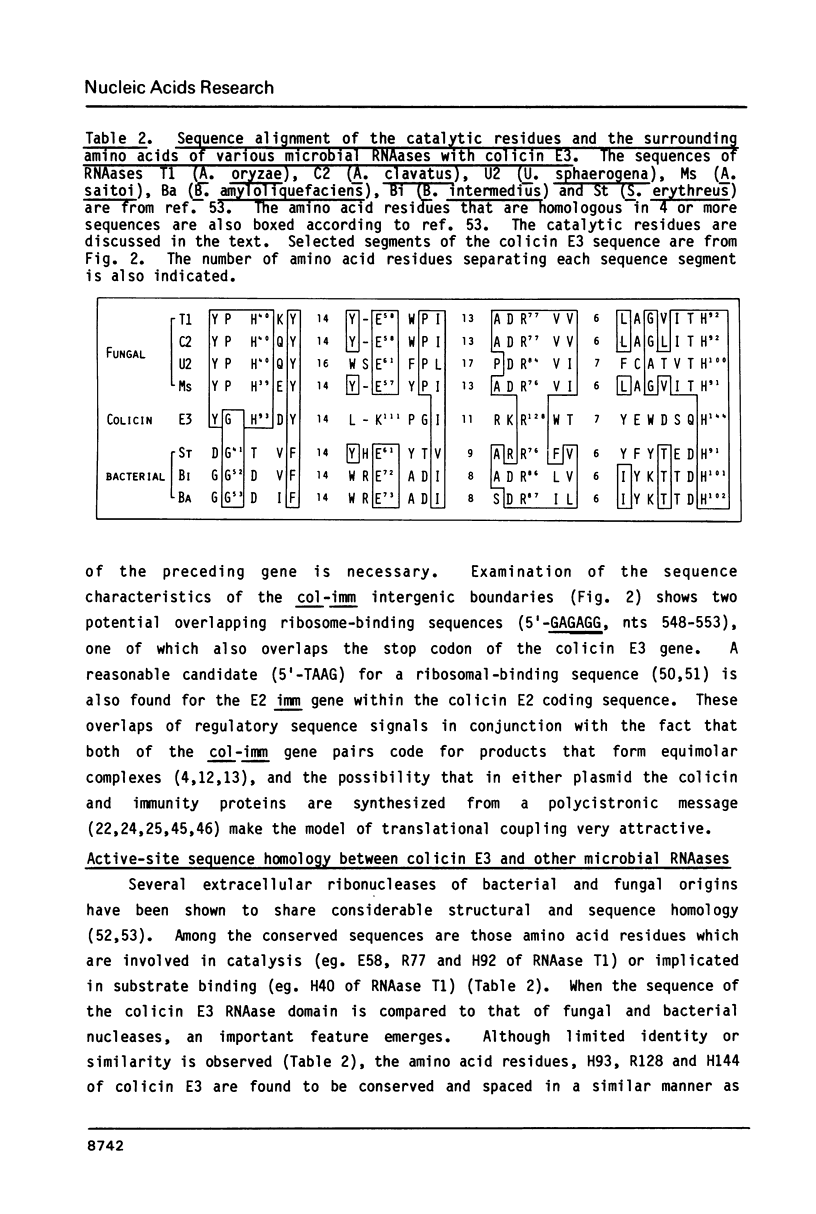



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atkins J. F. Is UAA or UGA part of the recognition signal for ribosomal initiation? Nucleic Acids Res. 1979 Oct 25;7(4):1035–1041. doi: 10.1093/nar/7.4.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajaj M., Blundell T. Evolution and the tertiary structure of proteins. Annu Rev Biophys Bioeng. 1984;13:453–492. doi: 10.1146/annurev.bb.13.060184.002321. [DOI] [PubMed] [Google Scholar]
- Bowman C. M., Dahlberg J. E., Ikemura T., Konisky J., Nomura M. Specific inactivation of 16S ribosomal RNA induced by colicin E3 in vivo. Proc Natl Acad Sci U S A. 1971 May;68(5):964–968. doi: 10.1073/pnas.68.5.964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chak K. F., James R. Localization and characterization of a gene on the ColE3-CA38 plasmid that confers immunity to colicin E8. J Gen Microbiol. 1984 Mar;130(3):701–710. doi: 10.1099/00221287-130-3-701. [DOI] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Prediction of the secondary structure of proteins from their amino acid sequence. Adv Enzymol Relat Areas Mol Biol. 1978;47:45–148. doi: 10.1002/9780470122921.ch2. [DOI] [PubMed] [Google Scholar]
- Cramer W. A., Dankert J. R., Uratani Y. The membrane channel-forming bacteriocidal protein, colicin El. Biochim Biophys Acta. 1983 Mar 21;737(1):173–193. doi: 10.1016/0304-4157(83)90016-3. [DOI] [PubMed] [Google Scholar]
- Di Masi D. R., White J. C., Schnaitman C. A., Bradbeer C. Transport of vitamin B12 in Escherichia coli: common receptor sites for vitamin B12 and the E colicins on the outer membrane of the cell envelope. J Bacteriol. 1973 Aug;115(2):506–513. doi: 10.1128/jb.115.2.506-513.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doolittle R. F. Similar amino acid sequences: chance or common ancestry? Science. 1981 Oct 9;214(4517):149–159. doi: 10.1126/science.7280687. [DOI] [PubMed] [Google Scholar]
- Garoff H., Frischauf A. M., Simons K., Lehrach H., Delius H. The capsid protein of Semliki Forest virus has clusters of basic amino acids and prolines in its amino-terminal region. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6376–6380. doi: 10.1073/pnas.77.11.6376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley R. W. Homology between prokaryotic and eukaryotic ribonucleases. J Mol Evol. 1980 Aug;15(4):355–358. doi: 10.1007/BF01733142. [DOI] [PubMed] [Google Scholar]
- Hopp T. P., Woods K. R. Prediction of protein antigenic determinants from amino acid sequences. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3824–3828. doi: 10.1073/pnas.78.6.3824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imajoh S., Ohno-Iwashita Y., Imahori K. The receptor for colicin E3. Isolation and some properties. J Biol Chem. 1982 Jun 10;257(11):6481–6487. [PubMed] [Google Scholar]
- Inselburg J. Colicin factor DNA: a single non-homologous region in Col E2-E3 heteroduplex molecules. Nat New Biol. 1973 Feb 21;241(112):234–237. doi: 10.1038/newbio241234a0. [DOI] [PubMed] [Google Scholar]
- Jakes K., Zinder N. D., Boon T. Purification and properties of colicin E3 immunity protein. J Biol Chem. 1974 Jan 25;249(2):438–444. [PubMed] [Google Scholar]
- Juillerat M. A., Barkas T., Tzartos S. J. Antigenic sites of the nicotinic acetylcholine receptor cannot be predicted from the hydrophilicity profile. FEBS Lett. 1984 Mar 12;168(1):143–148. doi: 10.1016/0014-5793(84)80224-0. [DOI] [PubMed] [Google Scholar]
- Konisky J. Colicins and other bacteriocins with established modes of action. Annu Rev Microbiol. 1982;36:125–144. doi: 10.1146/annurev.mi.36.100182.001013. [DOI] [PubMed] [Google Scholar]
- Kool A. J., Pols C., Nijkamp H. J. Bacteriocinogenic Clo DF13 minicells of Escherichia coli synthesize a protein that accounts for immunity to bacteriocin Clo DF13: purification and characterization of the immunity protein. Antimicrob Agents Chemother. 1975 Jul;8(1):67–75. doi: 10.1128/aac.8.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. Comparison of initiation of protein synthesis in procaryotes, eucaryotes, and organelles. Microbiol Rev. 1983 Mar;47(1):1–45. doi: 10.1128/mr.47.1.1-45.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau P. C., Rowsome R. W., Watson R. J., Visentin L. P. The immunity genes of colicins E2 and E8 are closely related. Biosci Rep. 1984 Jul;4(7):565–572. doi: 10.1007/BF01121913. [DOI] [PubMed] [Google Scholar]
- Lau P. C., Spencer J. H. An efficient synthetic primer for the M13 cloning dideoxy sequencing system. Biosci Rep. 1982 Sep;2(9):687–696. doi: 10.1007/BF01114830. [DOI] [PubMed] [Google Scholar]
- Levinson B. L., Pickover C. A., Richards F. M. Dimerization by colicin E3 in the absence of immunity protein. J Biol Chem. 1983 Sep 25;258(18):10967–10972. [PubMed] [Google Scholar]
- Masaki H., Ohta T. A plasmid region encoding the active fragment and the inhibitor protein of colicin E3--CA38. FEBS Lett. 1982 Nov 22;149(1):129–132. doi: 10.1016/0014-5793(82)81087-9. [DOI] [PubMed] [Google Scholar]
- Mochitate K., Suzuki K., Imahori K. Amino acid sequence of immunity protein (B subunit) of colicin E3. J Biochem. 1981 May;89(5):1609–1618. doi: 10.1093/oxfordjournals.jbchem.a133356. [DOI] [PubMed] [Google Scholar]
- Mock M., Miyada C. G., Gunsalus R. P. Nucleotide sequence for the catalytic domain of colicin E3 and its immunity protein. Evidence for a third gene overlapping colicin. Nucleic Acids Res. 1983 Jun 11;11(11):3547–3557. doi: 10.1093/nar/11.11.3547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mock M., Pugsley A. P. The BtuB group col plasmids and homology between the colicins they encode. J Bacteriol. 1982 Jun;150(3):1069–1076. doi: 10.1128/jb.150.3.1069-1076.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno-Iwashita Y., Imahori K. Assignment of the functional loci in colicin E2 and E3 molecules by the characterization of their proteolytic fragments. Biochemistry. 1980 Feb 19;19(4):652–659. doi: 10.1021/bi00545a008. [DOI] [PubMed] [Google Scholar]
- Ohno-Iwashita Y., Imahori K. Comparative studies on the structures of colicins E2 and E3. FEBS Lett. 1979 Apr 15;100(2):249–252. doi: 10.1016/0014-5793(79)80344-0. [DOI] [PubMed] [Google Scholar]
- Ohno S., Imahori K. Colicin E3 is an endonuclease. J Biochem. 1978 Dec;84(6):1637–1640. doi: 10.1093/oxfordjournals.jbchem.a132291. [DOI] [PubMed] [Google Scholar]
- Ohno S., Ohno-Iwashita Y., Suzuki K., Imahori K. Purification and characterization of active component and active fragment of colicin E3. J Biochem. 1977 Oct;82(4):1045–1053. doi: 10.1093/oxfordjournals.jbchem.a131775. [DOI] [PubMed] [Google Scholar]
- Oppenheim D. S., Yanofsky C. Translational coupling during expression of the tryptophan operon of Escherichia coli. Genetics. 1980 Aug;95(4):785–795. doi: 10.1093/genetics/95.4.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oudega B., Klaasen-Boor P., De Graaf F. K. Mode of action of the cloacin DF13-immunity protein. Biochim Biophys Acta. 1975 May 5;392(1):184–195. [PubMed] [Google Scholar]
- Pugsley A. P. Autoinduced synthesis of colicin E2. Mol Gen Genet. 1983;190(3):379–383. doi: 10.1007/BF00331062. [DOI] [PubMed] [Google Scholar]
- Pugsley A. P., Schwartz M. A genetic approach to the study of mitomycin-induced lysis of Escherichia coli K-12 strains which produce colicin E2. Mol Gen Genet. 1983;190(3):366–372. doi: 10.1007/BF00331060. [DOI] [PubMed] [Google Scholar]
- Rosenberg M., Court D. Regulatory sequences involved in the promotion and termination of RNA transcription. Annu Rev Genet. 1979;13:319–353. doi: 10.1146/annurev.ge.13.120179.001535. [DOI] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R., Barrell B. G., Smith A. J., Roe B. A. Cloning in single-stranded bacteriophage as an aid to rapid DNA sequencing. J Mol Biol. 1980 Oct 25;143(2):161–178. doi: 10.1016/0022-2836(80)90196-5. [DOI] [PubMed] [Google Scholar]
- Schaller K., Nomura M. Colicin E2 is DNA endonuclease. Proc Natl Acad Sci U S A. 1976 Nov;73(11):3989–3993. doi: 10.1073/pnas.73.11.3989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schümperli D., McKenney K., Sobieski D. A., Rosenberg M. Translational coupling at an intercistronic boundary of the Escherichia coli galactose operon. Cell. 1982 Oct;30(3):865–871. doi: 10.1016/0092-8674(82)90291-4. [DOI] [PubMed] [Google Scholar]
- Suzuki K., Imahori K. Amino acid sequence of an active fragment (T2A) of colicin E3. J Biochem. 1978 Nov;84(5):1031–1039. doi: 10.1093/oxfordjournals.jbchem.a132217. [DOI] [PubMed] [Google Scholar]
- Suzuki K., Imahori K. Preparation and characterization of an active fragment of colicin E3. J Biochem. 1978 Nov;84(5):1021–1029. doi: 10.1093/oxfordjournals.jbchem.a132216. [DOI] [PubMed] [Google Scholar]
- Suzuki K., Ohno S., Imahori K. Studies on the physicochemical structure and stability of an active fragment (T2A) of colicin E3. J Biochem. 1980 Mar;87(3):761–769. doi: 10.1093/oxfordjournals.jbchem.a132805. [DOI] [PubMed] [Google Scholar]
- Tyler J., Sherratt D. J. Synthesis of E colicins in Escherichia coli. Mol Gen Genet. 1975 Oct 22;140(4):349–353. doi: 10.1007/BF00267325. [DOI] [PubMed] [Google Scholar]
- Van Rompuy L., Min Jou W., Huylebroeck D., Devos R., Fiers W. Complete nucleotide sequence of the nucleoprotein gene from the human influenza strain A/PR/8/34 (HON1). Eur J Biochem. 1981 May 15;116(2):347–353. doi: 10.1111/j.1432-1033.1981.tb05341.x. [DOI] [PubMed] [Google Scholar]
- Watson R. J., Lau P. C., Vernet T., Visentin L. P. Characterization and nucleotide sequence of a colicin-release gene in the hic region of plasmid ColE3-CA38. Gene. 1984 Jul-Aug;29(1-2):175–184. doi: 10.1016/0378-1119(84)90178-1. [DOI] [PubMed] [Google Scholar]
- Watson R. J., Visentin L. P. Cloning of the ColE3-CA38 colicin and immunity genes and identification of a plasmid region which enhances colicin production. Gene. 1982 Sep;19(2):191–200. doi: 10.1016/0378-1119(82)90006-3. [DOI] [PubMed] [Google Scholar]
- Watson R., Konarska-Kozlowska M., Iyer V. N., Yaguchi M., Visentin L. P. Comparison of plasmids Co1E2-P9 and Co1E2-CA42 and their immunity proteins. J Bacteriol. 1983 Mar;153(3):1552–1557. doi: 10.1128/jb.153.3.1552-1557.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson R., Rowsome W., Tsao J., Visentin L. P. Identification and characterization of Col plasmids from classical colicin E-producing strains. J Bacteriol. 1981 Aug;147(2):569–577. doi: 10.1128/jb.147.2.569-577.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson R., Visentin L. P. Restriction endonuclease mapping of ColE2-P9 and ColE3-CA38 plasmids. Gene. 1980 Sep;10(4):307–318. doi: 10.1016/0378-1119(80)90151-1. [DOI] [PubMed] [Google Scholar]
- Yamamoto H., Nishida K. I., Beppu T., Arima K. Tryptic digestion of colicin E2 and its active fragment. J Biochem. 1978 Mar;83(3):827–834. doi: 10.1093/oxfordjournals.jbchem.a131979. [DOI] [PubMed] [Google Scholar]
- de Graaf F. K., Niekus H. G., Klootwijk J. Inactivation of bacterial ribosomes in vivo and in vitro by cloacin DF13. FEBS Lett. 1973 Sep 1;35(1):161–165. doi: 10.1016/0014-5793(73)80601-5. [DOI] [PubMed] [Google Scholar]
- van den Elzen P. J., Gaastra W., Spelt C. E., de Graaf F. K., Veltkamp E., Nijkamp H. J. Molecular structure of the immunity gene and immunity protein of the bacteriocinogenic plasmid Clo DF13. Nucleic Acids Res. 1980 Oct 10;8(19):4349–4363. doi: 10.1093/nar/8.19.4349. [DOI] [PMC free article] [PubMed] [Google Scholar]


