Abstract
In the early stages of reproductive isolation, genomic regions of reduced recombination are expected to show greater levels of differentiation, either because gene flow between species is reduced in these regions or because the effects of selection at linked sites within species is enhanced in these regions. Here we study patterns of DNA sequence variation at 27 autosomal loci among populations of Mus musculus musculus, M. m. domesticus, and M. m. castaneus, three subspecies of house mice with co-linear genomes. We found that some loci exhibit considerable shared variation among subspecies, while others exhibit fixed differences. We used an isolation-with-gene-flow model to estimate divergence times, effective population sizes (Ne) and to disentangle ancestral variation from gene flow. Estimates of divergence time indicate that all three subspecies diverged from one another within a very short period of time roughly 350,000 years ago. Overall, Ne for each subspecies was associated with the degree of genetic differentiation: M. m. musculus had the smallest Ne and the greatest proportion of monophyletic gene genealogies, while M. m. castaneus had the largest Ne and the smallest proportion of monophyletic gene genealogies. M. m. domesticus and M. m. musculus were more differentiated from each other than either was from M. m. castaneus, consistent with greater reproductive isolation between M. m. domesticus and M. m. musculus. FST was significantly greater at loci experiencing low recombination rates compared to loci experiencing high recombination rates in comparisons between M. m. castaneus and M. m. musculus or M. m. domesticus. These results provide evidence that genomic regions with less recombination show greater differentiation, even in the absence of chromosomal rearrangements.
Keywords: divergence, gene flow, polymorphism, recombination, speciation
Introduction
Understanding how new species arise is a central problem in evolutionary genetics. Complex patterns of genetic variation are expected among recently diverged lineages, and these patterns may be governed by both stochastic and deterministic processes (e.g. Maroja et al. 2009). For example, neutral polymorphisms are expected to be shared among daughter populations as a simple consequence of the persistence of polymorphisms present in the ancestral population. Shared polymorphisms may also derive from secondary contact and gene flow between daughter populations. Both processes (persistence of ancestral variation and subsequent gene flow) may occur simultaneously, and while distinguishing between them is difficult, a variety of analytic tools now exist for assessing their relative importance (e.g. Nielsen and Wakeley 2001a; Hey and Nielsen 2004; Becquet and Przeworski 2007; Hey and Nielsen 2007). Selection can also shape the distribution of variation among young lineages, and this can occur in several ways and affect individual regions of the genome differently. Positive directional selection on individual loci may reduce variation within one or both daughter populations, leading to increased differentiation for those loci. In genomic regions with low recombination rates, the effects of selection at linked sites (due to genetic hitchhiking or background selection) can also lead to increased differentiation (Charlesworth 1998). Gene flow between daughter populations may be reduced at some loci if particular alleles reduce fitness in the sister lineage, either as a result of epistasis or selection in a different environment (reviewed in Coyne and Orr 2004).
Studying patterns of genetic variation among newly arising lineages can thus shed light on the history of speciation for a particular group, including the relative importance of demography, gene flow, and selection in shaping observed patterns. By studying many loci throughout the genome, it may be possible to identify particular genomic regions that are important in isolating newly arising species.
Regions of low recombination are of particular interest and are expected to show increased levels of differentiation for two different reasons. First, several speciation models have suggested that gene flow between species may be reduced in such regions (Noor et al. 2001; Rieseberg 2001; Navarro and Barton 2003; Faria and Navarro 2010). Noor’s model (2001) is based on the asymmetric nature of Dobzhansky-Muller incompatibilities and the difficulty of selecting against such mutations when two incompatibilities with opposite asymmetries are locked in a single non-recombining region. The model of Navarro and Barton (2003) emphasizes the accumulation of co-adapted genes within non-recombining regions, while the model of Rieseberg (2001) emphasizes the accumulated effect of multiple genes contributing to isolation when they occur in a single non-recombining region. Second, the effects of selection at linked sites will extend over larger distances in regions of low recombination. Positive selection and associated genetic hitchhiking (Smith and Haigh 1974) as well as background selection (Charlesworth et al. 1993) can reduce levels of genetic variation within species and thereby lead to an inflation of measures like FST between species (Charlesworth 1998). Distinguishing reduced gene flow between species from selection within species as causes of increased differentiation is a difficult problem (Noor and Bennett 2009). Noor and Bennett (2009) suggest that coalescent models in which gene flow is estimated in a non-equilibrium context may help solve this problem, and we utilize this approach here.
The house mouse, Mus musculus, is arguably the best mammalian model for studies of the genetics of speciation. It consists of three subspecies with parapatric distributions: M. m. musculus is found in Eastern Europe and Northern Asia, M. m. castaneus is found in Southeast Asia, and M. m. domesticus is native to the Near East, Northern Africa and Western Europe and has been introduced to the Americas, Africa, and many oceanic islands in association with humans during historical times. These three lineages are young; the available data suggest that they diverged in allopatry within the last 500,000 years (e.g. Boursot et al. 1993; Geraldes et al. 2008a). Regions of secondary contact exist where the subspecies meet in nature. The best studied of these is a narrow hybrid zone between M. m. musculus and M. m. domesticus that stretches from Denmark to the Black Sea in central Europe (e.g. Raufaste et al. 2005; Macholan et al. 2007; Teeter et al. 2008; Teeter et al. 2010). Hybrids have also been extensively studied in the laboratory. M. m. musculus and M. m. domesticus are reproductively isolated primarily by hybrid male sterility (e.g. Forejt 1996; Britton-Davidian et al. 2005). The genetic basis of this sterility is complex and maps in part to the X chromosome (e.g. Storchova et al. 2004; Good et al. 2008). The involvement of the X chromosome in reproductive isolation is also suggested by patterns of reduced gene flow between subspecies on the X chromosome compared to the autosomes (Tucker et al. 1992). Recently the first gene for hybrid male sterility in a vertebrate was identified in crosses between mice that were primarily derived from M. m. musculus and M. m. domesticus (Mihola et al. 2009). Finally, most classical inbred strains of laboratory mice derive from crosses between house mouse subspecies (Frazer et al. 2007; Yang et al. 2007). Thus, the genetic tools and genomic resources for laboratory mice can be applied to the study of mouse speciation, including a genome sequence, expression databases, individual gene knockouts, and many other resources and methods.
Despite our impressive understanding of mouse genetics in general, we still know remarkably little about the distribution of genetic variation in natural populations of the three house mouse subspecies. Our chief goal here is to fill this gap by providing a genome-wide picture of patterns of differentiation among M. m. musculus, M. m. castaneus, and M. m. domesticus in the context of models of speciation. We resequenced 23 autosomal loci and used these data with four loci previously sequenced in the same populations to estimate levels of differentiation and gene flow among mouse subspecies in different regions of the genome.
Materials and Methods
Samples
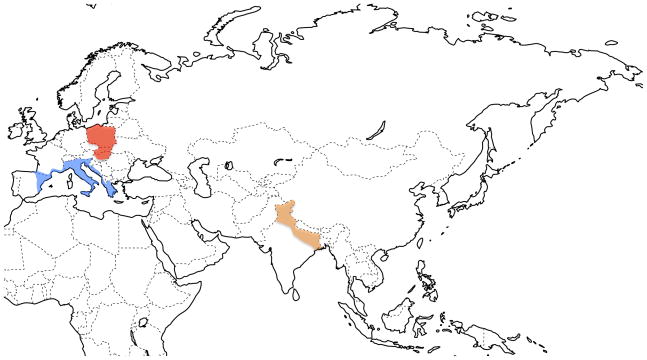
We included 27 Mus musculus domesticus from Western Europe, 26 M. m. musculus from Eastern Europe, and 27 M. m. castaneus from India (Figure 1 and Supplementary Table 1). All mice were collected at least 300 m apart to avoid the sampling of related individuals. Mice from India were kindly provided by B. Harr. One M. caroli and one M. spretus were purchased from the Jackson laboratory and used as outgroups.
Figure 1.
Approximate location of populations sampled in this study. Blue indicates M. m. domesticus, red indicates M. m. musculus and orange indicates M. m. castaneus. Sample sizes, sampling localities names and geographic coordinates are indicated in Supplementary Table 1.
Selection of loci and molecular methods
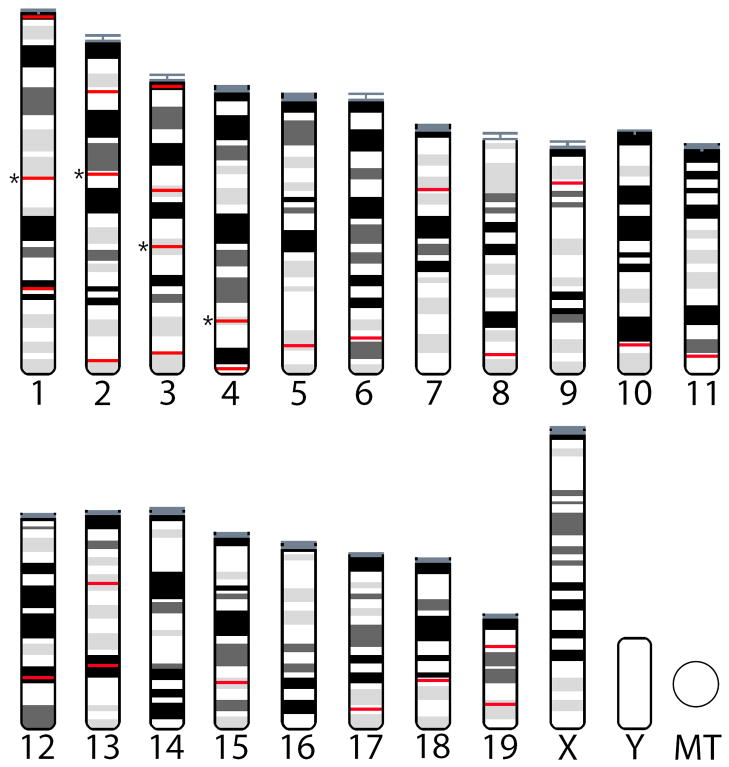
We PCR amplified and sequenced mostly intronic portions of 23 autosomal loci (Table 1 and Figure 2). Loci were chosen to cover a wide range of recombination rates. Recombination rates were calculated by regressing marker positions on the genetic map (Shifman et al. 2006; Cox et al. 2009) against physical positions on the mouse genome sequence (NCBI build 37) for a 10 Mb window centered on each locus. These recombination rates are derived from crosses involving inbred strains that are largely of M. m. domesticus origin. We assume that recombination rates are broadly similar across the three subspecies (Dumont et al. 2011). We also assume that recombination rates estimated over 10 Mb distances are a reasonable proxy for local recombination rates. To the extent that this is not true, we will have less power to detect true differences among regions differing in recombination rate. The genomes of these three subspecies are co-linear at the level of cytogenetic resolution. We cannot exclude the possibility that small inversion differences exist between subspecies. For each locus we amplified two overlapping fragments and sequenced both. This strategy provided at least two-fold coverage for every base, typically with one sequence from each strand. It also enabled us to detect rare instances of allele-specific PCR. In many cases, PCR primers were placed in exons to amplify intervening introns. PCR and sequencing primers are listed in Supplementary Table 2. Sanger sequencing was performed on an ABI 3700 automated sequencer by the University of Arizona’s Genomic Analysis and Technology Core facility. An additional four autosomal loci previously sequenced in the same populations were included (Geraldes et al. 2008a).
Table 1.
Loci surveyed, GC content, gene density and recombination rate
| Locus | Chromosome | Start position (bp)1 | GC content (%)2 | Gene density/Mb3 | Recombination rate (cM/Mb)4 |
|---|---|---|---|---|---|
| Atp6v1h | 1 | 5,090,103 | 0.394 | 2.9 | 0.082 |
| Chrng* | 1 | 89,102,229 | 0.455 | 9.7 | 0.364 |
| Fbxo28 | 1 | 184,268,340 | 0.449 | 8.0 | 0.898 |
| Ndufa8 | 2 | 35,893,440 | 0.438 | 13.9 | 0.321 |
| Med19* | 2 | 84,522,848 | 0.388 | 25.3 | 0.174 |
| Atp5e | 2 | 174,287,027 | 0.448 | 9.9 | 1.232 |
| Zfhx4 | 3 | 5,223,313 | 0.381 | 2.1 | 0.072 |
| Ssr3 | 3 | 65,187,464 | 0.403 | 5.1 | 0.280 |
| Prpf3* | 3 | 95,653,041 | 0.443 | 19.9 | 0.431 |
| Fpgt | 3 | 154,752,158 | 0.415 | 4.2 | 0.735 |
| Clcn6* | 4 | 147,391,868 | 0.400 | 15.2 | 0.600 |
| Acot7 | 4 | 151,573,815 | 0.496 | 17.1 | 1.112 |
| Sfrs8 | 5 | 130,008,014 | 0.458 | 7.3 | 1.203 |
| Cmas | 6 | 142,713,639 | 0.425 | 6.5 | 1.153 |
| Nomo1 | 7 | 53,333,140 | 0.466 | 22.1 | 0.479 |
| Usp10 | 8 | 122,476,548 | 0.496 | 13.3 | 1.203 |
| Ncapd3 | 9 | 26,842,239 | 0.416 | 5.4 | 0.948 |
| Rab21 | 10 | 114,735,869 | 0.404 | 6.3 | 1.107 |
| Slc39a11 | 11 | 113,294,508 | 0.459 | 15.8 | 1.283 |
| Golga5 | 12 | 103,717,894 | 0.451 | 10.0 | 0.915 |
| Iars | 13 | 49,816,700 | 0.448 | 8.0 | 0.644 |
| Rgs7bp | 13 | 105,749,379 | 0.407 | 6.4 | 0.835 |
| Atxn10 | 15 | 85,226,251 | 0.486 | 14.8 | 0.741 |
| Lrpprc | 17 | 85,144,001 | 0.445 | 6.6 | 0.987 |
| Fbxo38 | 18 | 62,704,865 | 0.441 | 8.6 | 0.780 |
| Mamdc2 | 19 | 23,518,001 | 0.426 | 5.5 | 1.162 |
| Shoc2 | 19 | 54,090,657 | 0.431 | 5.2 | 1.178 |
Position in NCBI mouse build 37 of the sequenced region.
In a 10 Mb window centered around the start position of the sequenced region.
In a 10 Mb window centered around the start position of the sequenced region. Only protein coding genes were considered.
Recombination rates were calculated for 10 Mb windows centered on the sequenced region by regressing the genetic position of the markers (Shifman et al. 2006; Cox et al. 2009) in the genetic map against their physical position on mouse NCBI build 37.
Data from Geraldes et al. 2008a
Figure 2.
Location of the loci surveyed in this study. Red lines indicate the approximate location of each locus on the ideogram of the house mouse karyotype. Exact location and locus details are provided in Table 1. Asterisks mark 4 loci from Geraldes et al (2008a) reanalyzed in this study.
Sequence assembly and editing
Sequences were trimmed to exclude all exonic regions; all data analyzed are from introns. Assembly and editing of contigs was performed with phred/phrap/consed/polyphred (Nickerson et al. 1997; Ewing and Green 1998; Ewing et al. 1998; Gordon et al. 1998). Alignments for each locus were generated with Clustal X (Thompson et al. 1994) and checked manually with BioEdit (Hall 1999). All indel polymorphisms were excluded from further analyses. For each locus, individuals and sites with more than 10% missing data were excluded from further analyses. All resulting alignments were deposited in Genbank under accession numbers JF336572-JF338137. Haplotypes were inferred with Phase 2.1.1 (Stephens et al. 2001; Stephens and Donnelly 2003) after checking for convergence of three independent runs.
Population genetic analyses
For each locus and each subspecies we estimated π, the average number of pairwise differences between sequences (Nei and Li 1979), and θ, the proportion of segregating sites (Watterson 1975). Divergence between each subspecies and M. caroli was estimated with DXY (Nei 1987), the average pairwise divergence. Deviations from the expected frequency spectrum of polymorphisms under a neutral model were assessed with Tajima’s D (Tajima 1989) and Fu and Li’s D (Fu and Li 1993). These analyses were performed using the program SITES (Wakeley and Hey 1997). Statistical significance for Tajima’s D and Fu and Li’s D was assessed after 1000 coalescent simulations with parameters estimated from the data for each locus using the program HKA (http://genfaculty.rutgers.edu/hey/software). Evolutionary relationships among haplotypes were inferred using the neighbor-joining method (Saitou and Nei 1987) in MEGA4 (Kumar et al. 2008). This was done using all of the data as well as the largest non-recombining segment for each locus. Trees were rooted with M. caroli, and bootstrap values were calculated after 1000 replicates.
Differentiation among subspecies
Levels of differentiation between pairs of subspecies were assessed in several ways. First, we used the program SITES (Wakeley and Hey 1997) to estimate FST (Wright 1951) using equation 3 of Hudson et al. (1992). We conducted computer simulations to ask whether the mean and range of observed FST values were greater than expected under a simple model of divergence without gene flow, using the program Make Sample (Hudson 2002). We simulated gene genealogies for three populations, with current population sizes of 36,600, 82,600 and 366,000, that diverged instantaneously 326,000 years ago from an ancestral population with a size of 277,000. The parameters used for these simulations were based on the IMa results (see below). The geometric mean mutation rate across all loci was also taken from IMa analyses. We simulated 1,000 datasets with 27 gene genealogies each. We calculated the mean and range of FST values in these simulated datasets and then compared these simulated values to the observed values.
Nei (1973) noted that measures of differentiation such as FST are heavily influenced by levels of genetic variation within sub-populations, and Charlesworth (1998) pointed out that forces which reduce variation within sub-populations, such as background selection or genetic hitchhiking, will increase FST. Thus, we calculated DXY between each pair of subspecies, a measure which is independent of within-subspecies diversity. We also calculated DA, or net nucleotide divergence corrected for within-subspecies diversity (Nei 1987). Comparison between DA and DXY may help to distinguish between competing hypotheses (i.e. reduced gene flow or diversity-reducing selection) for high levels of differentiation in some genomic regions (Noor and Bennett 2009).
The measures of genetic differentiation described above provide a summary of the data, but cannot be used to directly make inferences about processes such as gene flow, except under a number of simplifying assumptions (Whitlock and McCauley 1999). For example, FST=1/(4Nm +1), where Nm is the number of migrants per generation, assumes that populations are at equilibrium with respect to migration and drift, a condition that is almost certainly not met between subspecies of house mice. One solution to this problem is to use more complex models that relax some of these assumptions (Noor and Bennett 2009). In particular, coalescent-based methods have been developed to distinguish on-going gene flow from ancestral shared variation in the context of recently diverging populations (e.g. Hey and Nielsen 2004; Becquet and Przeworski 2007; Hey and Nielsen 2007). Here, we used IM and IMa, which implement a Markov Chain Monte Carlo method for analysis of genetic data under an isolation-with-migration model (Nielsen and Wakeley 2001b; Hey and Nielsen 2004; Hey and Nielsen 2007), to obtain maximum likelihood estimates of population sizes, divergence times, and migration rates under non-equilibrium conditions. This model assumes that there is no recombination within loci and free recombination between loci. We used IMgc (Woerner et al. 2007) to obtain the longest region within each locus without four gametic types. Estimates from IMa have been shown to be robust when data sets are trimmed to apparently non-recombining blocks (Strasburg and Rieseberg 2010). Using this non-recombining dataset (Supplementary Table 3) we used IMa to compare different models of population divergence. The full isolation-with-migration model has six parameters: the effective population size of each contemporary population (Ne1 and Ne2), the ancestral population size (Nea), the time since divergence (t) and migration rates between populations in each direction (2Nm1 and 2Nm2). These parameters are estimated jointly in the model. Thus, estimates of gene flow take into account past and current Ne, and the model does not require that populations be at migration-drift equilibrium. We first estimated parameters for each of the three subspecies in pairwise comparisons. Then using a likelihood ratio test, we compared the log likelihood of our data under this fully parameterized model to the log likelihood of our data under nested models in which migration was set to zero in both directions (no gene flow), or in each direction separately (fully asymmetric gene flow). For each analysis, we ran the program under Metropolis Coupled Monte Carlo Markov Chains using at least 28 chains with a two-step heating scheme and parameters that allowed for proper chain swapping. We ran the program for at least two million steps. For each analysis we checked for convergence between two replicates, and we present results from just one replicate of each analysis. The geometric mean of the mutation rate of all loci was used to convert the estimated parameters (θ and t, which are scaled to the mutation rate) to demographic parameters (Ne and t). We estimated mutation rates per year for each locus assuming that the divergence to M. caroli represents 4.3 MY (Suzuki et al. 2004). Estimating the mutation rate per generation further requires knowledge of the number of generations per year. Wild mice are expected to have between one and two generations per year (Bronson 1979; Geraldes et al. 2008a). Since the precise generation time is unknown, we give estimates of Ne assuming both one and two generations per year. Finally we used IM to estimate per locus migration rates into each subspecies (except for M. m. domesticus, since a model with no gene flow into this subspecies was not significantly different from the fully parameterized model, see below). Migration rates per locus are given in terms of the joint parameter 2Nm.
Results
Overall levels of genetic differentiation among subspecies
Gene genealogies for 27 autosomal loci sampled among 26 M. m. musculus, 27 M. m. castaneus, and 27 M. m. domesticus are shown in Supplementary Figure 1 and are summarized in Figure 3. We observed different genealogical patterns among different loci, as expected for taxa at an early stage of divergence. For some loci such as Prpf3, alleles corresponding to the three subspecies were fully sorted (i.e. each was monophyletic, with alleles within each subspecies more closely related to other alleles within that subspecies than to any alleles in other subspecies). For other loci, subspecies were intermingled on the gene genealogy, and were either paraphyletic such as Rab21 (i.e. with one subspecies nested within another) or polyphyletic such as Golga5 (with multiple unrelated lineages for each subspecies). We compared each subspecies to every other subspecies, and calculated the proportion of gene trees that were reciprocally monophyletic, paraphyletic, or polyphyletic (Figure 3a). In comparisons between M. m. domesticus and M. m. musculus (DM), a greater proportion of loci were fully sorted than in comparisons between M. m. domesticus and M. m. castaneus (DC) or between M. m. musculus and M. m. castaneus (MC) (Figure 3a), indicating that DM are more differentiated than DC or MC. The same pattern was observed when only the largest non-recombining blocks for each locus were used to generate genealogies (Supplementary Figure 2). Similar patterns were seen using only nodes with high bootstrap support (>80%; Supplementary Figure 1).
Figure 3.
Summary of genealogical relationships among subspecies of house mouse for the 27 loci surveyed in this study. A) Proportion of loci that are reciprocally monophyletic (black), paraphyletic (gray) or polyphyletic (white), in pairwise comparisons among subspecies. B) proportion of loci where each subspecies is either monophyletic (black) or not (white), in relation to the other two subspecies.
Levels of population differentiation between pairs of subspecies were assessed using FST for sequence data (Hudson et al. 1992). FST was significantly higher for DM (average FST=0.72) than for DC (average FST=0.43; Wilcoxon signed-rank test P<0.0001) or MC (average FST=0.49; Wilcoxon signed-rank test P<0.0001) (Table 2), consistent with the observed genealogical patterns (Figure 3). Higher overall differentiation for DM could be due to lower levels of gene flow, smaller effective population sizes, or some combination of these factors.
Table 2.
Estimates of population differentiation and gene flow among house mouse subspecies.
| Locus | Rec. Rate(1) | FST |
Fixed/Total(2) |
2Nm(3) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DM | DC | MC | DM | DC | MC | into M. m. musculus |
into M. m. castaneus |
||||||
| from M. m. domesticus | from M. m castaneus | total | from M. m. domesticus | from M. m. musculus | total | ||||||||
| Zfhx4 | 0.072 | 0.75 | 0.62 | 0.80 | 0.44 | 0.14 | 0.36 | 0.00 | 0.00 | 0.00 | 0.01 | 0.02 | 0.03 |
| Atp6v1h | 0.082 | 0.50 | 0.40 | 0.27 | 0.00 | 0.00 | 0.00 | 0.34 | 0.00 | 0.34 | 0.95 | 1.39 | 2.34 |
| Med19 | 0.174 | 0.80 | 0.73 | 0.70 | 0.17 | 0.00 | 0.04 | 0.00 | 0.00 | 0.00 | 0.27 | 0.02 | 0.29 |
| Ssr3 | 0.280 | 0.81 | 0.39 | 0.55 | 0.33 | 0.02 | 0.00 | 0.01 | 0.00 | 0.01 | 0.36 | 0.61 | 0.97 |
| Ndufa8 | 0.321 | 0.83 | 0.83 | 0.80 | 0.50 | 0.07 | 0.02 | 0.01 | 0.00 | 0.01 | 0.01 | 0.02 | 0.03 |
| Chrng | 0.364 | 0.69 | 0.32 | 0.45 | 0.13 | 0.00 | 0.03 | 0.00 | 0.00 | 0.00 | 0.01 | 0.02 | 0.03 |
| Prpf3 | 0.431 | 0.94 | 0.75 | 0.90 | 0.74 | 0.08 | 0.46 | 0.00 | 0.01 | 0.01 | 0.01 | 0.02 | 0.03 |
| Nomo1 | 0.479 | 0.89 | 0.54 | 0.46 | 0.36 | 0.00 | 0.00 | 0.00 | 0.20 | 0.20 | 0.56 | 0.02 | 0.58 |
| Clcn6 | 0.600 | 0.50 | 0.38 | 0.18 | 0.00 | 0.01 | 0.00 | 0.15 | 0.54 | 0.69 | 0.01 | 0.05 | 0.06 |
| Iars | 0.644 | 0.41 | 0.26 | 0.37 | 0.00 | 0.00 | 0.00 | 0.00 | 0.01 | 0.01 | 0.30 | 0.64 | 0.94 |
| Fpgt | 0.735 | 0.86 | 0.45 | 0.30 | 0.11 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.53 | 2.90 | 3.43 |
| Atxn10 | 0.741 | 0.93 | 0.39 | 0.40 | 1.08 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.16 | 1.39 | 1.55 |
| Fbxo38 | 0.780 | 0.92 | 0.58 | 0.36 | 1.14 | 0.01 | 0.00 | 0.00 | 0.10 | 0.10 | 0.04 | 0.95 | 0.99 |
| Rgs7bp | 0.835 | 0.88 | 0.38 | 0.55 | 0.44 | 0.00 | 0.03 | 0.00 | 0.00 | 0.00 | 0.67 | 0.20 | 0.87 |
| Fbxo28 | 0.898 | 0.63 | 0.28 | 0.63 | 0.08 | 0.00 | 0.00 | 0.00 | 0.01 | 0.01 | 0.13 | 0.52 | 0.65 |
| Golga5 | 0.915 | 0.48 | 0.20 | 0.32 | 0.00 | 0.00 | 0.00 | 0.29 | 0.35 | 0.64 | 1.47 | 0.02 | 1.49 |
| Ncapd3 | 0.948 | 0.62 | 0.41 | 0.42 | 0.08 | 0.00 | 0.00 | 0.00 | 0.02 | 0.02 | 0.98 | 0.02 | 1.00 |
| Lrpprc | 0.987 | 0.94 | 0.36 | 0.58 | 0.54 | 0.00 | 0.03 | 0.00 | 0.00 | 0.00 | 1.15 | 0.02 | 1.17 |
| Rab21 | 1.107 | 0.55 | 0.27 | 0.48 | 0.09 | 0.00 | 0.04 | 0.00 | 0.00 | 0.00 | 0.01 | 0.02 | 0.03 |
| Acot7 | 1.112 | 0.84 | 0.46 | 0.62 | 0.31 | 0.00 | 0.07 | 0.00 | 0.01 | 0.01 | 0.10 | 0.49 | 0.59 |
| Cmas | 1.153 | 0.67 | 0.32 | 0.27 | 0.00 | 0.00 | 0.00 | 0.21 | 0.16 | 0.37 | 0.58 | 0.64 | 1.22 |
| Mamdc2 | 1.162 | 0.87 | 0.60 | 0.67 | 0.35 | 0.05 | 0.06 | 0.00 | 0.00 | 0.00 | 0.01 | 0.02 | 0.03 |
| Shoc2 | 1.178 | 0.77 | 0.64 | 0.39 | 0.00 | 0.00 | 0.00 | 0.27 | 0.11 | 0.38 | 0.36 | 0.02 | 0.38 |
| Usp10 | 1.203 | 0.77 | 0.52 | 0.61 | 0.00 | 0.01 | 0.00 | 0.21 | 0.24 | 0.45 | 0.07 | 0.17 | 0.24 |
| Sfrs8 | 1.203 | 0.57 | 0.06 | 0.47 | 0.00 | 0.00 | 0.00 | 0.35 | 0.08 | 0.43 | 6.68 | 0.02 | 6.70 |
| Atp5e | 1.232 | 0.47 | 0.19 | 0.32 | 0.00 | 0.00 | 0.00 | 0.01 | 0.10 | 0.11 | 1.98 | 0.02 | 2.00 |
| Slc39a11 | 1.283 | 0.49 | 0.32 | 0.30 | 0.00 | 0.00 | 0.00 | 0.37 | 0.00 | 0.37 | 0.01 | 1.24 | 1.25 |
| Average Low (8) | 0.275 | 0.78 | 0.57 | 0.62 | 0.32 | 0.03 | 0.07 | 0.05 | 0.03 | 0.07 | 0.27 | 0.26 | 0.54 |
| Average High (19) | 0.985 | 0.69 | 0.37 | 0.43 | 0.15 | 0.00 | 0.01 | 0.10 | 0.09 | 0.19 | 0.80 | 0.49 | 1.29 |
| Average all (27) | 0.775 | 0.72 | 0.43 | 0.49 | 0.19 | 0.01 | 0.02 | 0.08 | 0.07 | 0.15 | 0.65 | 0.42 | 1.07 |
Recombination rates were calculated for 10 Mb windows centered on the sequenced region by regressing the genetic position of the markers (Shifman et al. 2006; Cox et al. 2009) in the genetic map against their physical position on mouse NCBI build 37.
Ratio of fixed differences between subspecies to all other polymorphisms (shared, among subspecies and exclusive to either one)
IM locus specific estimates of the effective rate at which genes come into each subspecies from the gene pool of the other.
To explore these issues, we fitted the polymorphism data to a model of divergence-with-gene-flow (IMa) (Nielsen and Wakeley 2001b; Hey and Nielsen 2004; Hey and Nielsen 2007) and obtained maximum-likelihood estimates (MLE) of demographic parameters. These analyses indicate that DM started to diverge about 321,000 years ago, DC about 314,000 years ago and MC about 346,000 years ago (Table 3). Although the uncertainty in these estimates is fairly high [e.g., 90% of the posterior probability density (HPD90) for the estimate of divergence time between DC falls between 247,000 and 373,000 years ago], these analyses indicate that all three pairs of subspecies started to diverge at approximately the same time.
Table 3.
Maximum likelihood estimates (MLE) and 90% posterior density intervals (in parentheses) of demographic parameters obtained with IMa for subspecies of house mice. Estimates of effective population size (Ne) assuming a generation length of 1 and 0.5 years are given.
| Generation Length | Sub-species 1 | Sub-species 2 | Ne species 1 | Ne species 2 | Ne Ancestral | t1 | 2Nm(species 1)2 | 2Nm(species 2)3 | Average 2Nm |
|---|---|---|---|---|---|---|---|---|---|
| 1 year | domesticus | musculus | 82,552 (67,951–97,153) | 38,096 (28,679–49,226) | 272,365 (133,094–408,266) | 320,764 | 0.003 (0.000–0.023) | 0.057 (0.031–0.093) | 0.030 |
| domesticus | castaneus | 82,653 (67,552–97,575) | 370,264 (322,590–427,472) | 220,887 (166,857–284,451) | 313,822 (247,268–372,981) | 0.000 (0.000–0.025) | 0.193 (0.088–0.336) | 0.097 | |
| musculus | castaneus | 35,060 (24,672–46,314) | 363,101 (312,802–419,689) | 190,196 (111,603–278,221) | 345,752 | 0.058 (0.027–0.106) | 0.190 (0.078–0.346) | 0.124 | |
| 0.5 years | domesticus | musculus | 165,104 (135,902–194,306) | 76,193 (57,359–98,451) | 544,730 (266,187–816,533) | ||||
| domesticus | castaneus | 165,126 (135,103–195,149) | 740,527 (645,180–854,943) | 441,774 (333,714–568,903) | |||||
| musculus | castaneus | 70,120 (49,344–92,628) | 726,202 (625,603–839,377) | 380,391 (223,205–556,441) |
In cases where the posterior density distribution failed to return to zero, confidence intervals cannot be reliably estimated and were left blank.
Number of years since species begun to diverge.
The effective rate at which genes come into the subspecies one gene pool from subspecies two.
The effective rate at which genes come into the subspecies two gene pool from subspecies one. M. caroli, for the eight low and 19 high recombination loci and
Higher differentiation for DM compared to DC or MC could arise if M. m. domesticus and M. m. musculus have lower Ne than M. m. castaneus. Genetic drift will be greater in smaller populations, leading to greater changes in allele frequencies from the ancestral population. Levels of nucleotide polymorphism (π) were significantly lower in M. m. domesticus (average π=0.213%) and in M. m. musculus (average π=0.166%) compared to M. m. castaneus (average π=0.585%; Wilcoxon signed-rank tests P<0.0001 for both), suggesting that Ne is higher in castaneus (Table 4). Maximum likelihood estimates of Ne were obtained with IMa (Table 3). Ne was ~82,500 for M. m. domesticus, ~36,500 for M. m. musculus, and ~366,500 for M. m. castaneus, assuming a generation time of one year. If a generation time of 0.5 years is assumed, all values are twice as large (Table 3). These estimates of Ne are in good agreement with the proportion of monophyletic gene genealogies for each subspecies (Figure 3b). M. m. musculus has the greatest proportion of monophyletic loci and the smallest Ne, while M. m. castaneus has the smallest proportion of monophyletic loci and the largest Ne. Thus, it seems likely that genetic drift plays an important role in shaping genome-wide differentiation between house mouse subspecies.
Table 4.
Average levels of polymorphism within subspecies of house mice and divergence (DXY) between these and the entire data set.
| Locus | Subspecies | n(1) | L(2) | S(3) | π (%) | θ (%) | Tajima’s D(4) | Fu and Li’s D(5) | DXY (%) | θ/DXY(5) |
|---|---|---|---|---|---|---|---|---|---|---|
| Average Low (8) | M. m. domesticus | 58 | 1867 | 15 | 0.139 | 0.179 | −0.5667 | −0.4564 | 3.408 | 0.052 |
| M. m. musculus | 34 | 1865 | 7 | 0.087 | 0.100 | −0.2222 | −0.4227 | 3.470 | 0.029 | |
| M. m. castaneus | 43 | 1833 | 41 | 0.470 | 0.540 | −0.7017* | −0.4791 | 3.393 | 0.159 | |
| Average High (19) | M. m. domesticus | 53 | 1653 | 16 | 0.244 | 0.227 | −0.2493 | −0.1305 | 3.886 | 0.058 |
| M. m. musculus | 48 | 1652 | 12 | 0.199 | 0.168 | 0.3950* | 0.5877** | 3.918 | 0.043 | |
| M. m. castaneus | 42 | 1634 | 48 | 0.634 | 0.716 | −0.4240 | −0.0968 | 3.863 | 0.185 | |
| Average All (27) | M. m. domesticus | 55 | 1717 | 16 | 0.213 | 0.213 | −0.3370 | −0.2271 | 3.744 | 0.059 |
| M. m. musculus | 44 | 1716 | 11 | 0.166 | 0.148 | 0.2121 | 0.2883* | 3.785 | 0.041 | |
| M. m castaneus | 42 | 1693 | 46 | 0.585 | 0.664 | −0.5063** | −0.2101 | 3.724 | 0.183 |
0.05>P>0.01;
0.01>P>0.001;
P<0.001
Number of chromosomes analyzed.
Number of bp analyzed.
Number of segregating sites.
Statistical significance for Tajima’s D and Fu and Li’s D was assessed after 10000 coalescent simulations under the program HKA.
Differences in migration rate among subspecies could also lead to differences in the levels of overall differentiation. Maximum likelihood estimates of gene flow between each pair of subspecies were generally low (Table 3). Nonetheless, nested models in which gene flow was constrained to be 0 in one or both directions were rejected as significantly worse than models with gene flow in both directions using likelihood ratio tests except for DM, where a model without gene flow could not be rejected (Table 5). The average migration rate into each population was lower for DM (2Nm=0.030), than for DC (2Nm= 0.097) or MC (2Nm= 0.124). Gene flow into M. m. castaneus was higher from both M. m. domesticus (2Nm=0.193) and from M. m. musculus (2Nm=0.190) than gene flow into M. m. domesticus (2Nm=0.003 from M. m. musculus and 2Nm=0.000 from M. m. castaneus) or into M. m. musculus (2Nm=0.057 from M. m. domesticus and 2Nm=0.058 from M. m. castaneus). Thus, low migration into M. m. domesticus and M. m. musculus may also contribute to the high overall differentiation observed for DM.
Table 5.
Likelihood ratio tests (2LLR) of nested IMa models against the fully parameterized (5 parameters) isolation with migration model. Presented are the results for symmetric migration rates (4 parameters), unidirectional gene flow (4 parameters) and no gene flow (3 parameters).
| Subspecies comparison | Model | log (P) | 2LLR | p-value |
|---|---|---|---|---|
| M. m. domesticus-M. m. musculus | mD1=mM2 | 1.4328 | 11.2443 | 0.0008 |
| mD mM=0 | −28.9782 | 72.0654 | <0.0001 | |
| mD=0 mM | 6.8006 | 0.5079 | 0.4760 | |
| mD=mM=0 | −28.9777 | 72.0644 | <0.0001 | |
| M. m. domesticus-M. m. castaneus | mD=mC3 | 5.0164 | 4.6939 | 0.0303 |
| mD mC=0 | −18.4373 | 51.6011 | <0.0001 | |
| mD=0 mC | 7.3601 | 0.0065 | 0.9357 | |
| mD=mC=0 | −26.3559 | 67.4384 | <0.0001 | |
| M. m. musculus-M. m. castaneus | mM=mC | 3.8046 | 2.8861 | 0.0894 |
| mM mC=0 | −4.2169 | 18.9291 | <0.0001 | |
| mM=0 mC | −12.1585 | 34.8123 | <0.0001 | |
| mM=mC=0 | −55.7118 | 121.9188 | <0.0001 |
mD= migration into M. m. domesticus,
mM= migration into M. m. musculus and
mC= migration into M. m. castaneus.
Heterogeneity among loci in levels of differentiation and variation in recombination rate
FST showed considerable variation among loci. FST ranged from 0.06 to 0.83 for DC, from 0.18 to 0.90 for MC and from 0.41 to 0.94 for DM (Table 2 and Figure 4). To determine whether the mean and range of FST values were higher than expected under a simple model without gene flow, we performed coalescent simulations of 1000 sets of 27 gene genealogies and compared observed values with the simulated distributions. Despite the inference of gene flow from IMa (Table 5), we found that the observed range of FST falls within 95% of the simulated ranges for both DM and MC (DM, P=0.717, MC, P=0.191). For DC, the result was marginally significant, with 5.7% of the simulations showing FST ranges greater than the observed range (i.e. P=0.057). These simulations highlight that a wide range of FST is expected even in the absence of gene flow.
Figure 4.
Scatter plots showing the distribution of FST values between pairs of house mouse subspecies as a function of the recombination rate for the 27 loci included in this study. From left to right, DM (M. m. domesticus and M. m. musculus; r=−0.209, P=0.150), DC (M. m. domesticus and M. m. castaneus; r=−0.398, P=0.020) and MC (M. m. musculus and M. m. castaneus; r=−0.210, P=0.147).
Recombination rates for the 27 loci varied between 0.072 cM/Mb and 1.283 cM/Mb (Table 2). We compared recombination rate and FST in each of the three pairwise comparisons (Figure 4). There was a significant negative correlation between FST and recombination rate for DC, and a non-significant trend in the same direction for the other two comparisons (DC r=−0.398, P=0.020; MC r=−0.210, P=0.147; DM r=−0.209, P=0.150). We also divided all loci into two groups, those with recombination rates above the genomic average and those with recombination rates below the genomic average, and we obtained similar results. FST for low recombination loci was significantly greater than FST for high recombination loci for DC (average FST=0.57 for low and FST=0.37 for high, Mann-Whitney U test P=0.008) and for MC (average FST=0.62 for low and FST=0.43 for high, Mann-Whitney U test P=0.026) (Table 2). For DM the difference was not significant but tended in the same direction (average FST=0.78 for low and FST=0.69 for high, Mann-Whitney U test P=0.164). We also compared the ratio of fixed differences to total polymorphism between each pair of subspecies for low and high recombination loci in a 2×2 contingency table and found that in each case the ratio of fixed differences to polymorphism was significantly higher for low recombination loci (P<0.001) (Table 6). These results show that regions of low recombination are more differentiated, on average, than regions of high recombination, but they do not speak directly to the cause of this greater differentiation.
Table 6.
Fisher’s exact tests comparing polymorphisms and fixed differences for regions of low and high recombination rate.
| Comparison | Recombination rate | Polymorphisms | Fixed differences | p-value |
|---|---|---|---|---|
| M. m. domesticus-M. m. musculus | Low | 169 | 54 | |
| High | 502 | 73 | 8.2×10−05 | |
| M. m. domesticus-M. m. castaneus | Low | 410 | 11 | |
| High | 1094 | 5 | 8.2×10−04 | |
| M. m. musculus-M. m. castaneus | Low | 360 | 24 | |
| High | 1036 | 10 | 9.0×10−08 |
To explore whether selection at linked sites (i.e. background selection or genetic hitchhiking) is reducing variation within subspecies and thereby increasing differentiation in regions of low recombination, we compared levels of nucleotide diversity () within each subspecies to recombination rate among loci. There was a significant positive correlation for M. m. musculus (Spearman rank order correlation r=0.383; P=0.02), but not for the other two subspecies. Thus, the effects of selection at linked sites within subspecies appears to be modest. Similarly, standard tests of a neutral model based on the distribution of allele frequencies were generally not-significant. We calculated Tajima’s D (Tajima 1989) and Fu and Li’s D (Fu and Li 1993) for each of the three subspecies (Supplementary Table 4) and found that only two loci in M. m. domesticus showed deviations from neutral expectations after a Bonferroni correction for multiple tests (Iars: FLD=1.9284, P=0.0014 and Lrpprc: TD=−2.2657, p=0.0011 and FLD=−4.0980, P=0.0005).
To explore whether reduced gene flow is contributing to increased differentiation in regions of low recombination, we calculated DXY and DA between pairs of subspecies. Reduced gene flow should increase both DXY and DA, while genetic hitchhiking or background selection should primarily increase DA and may reduce DXY (Noor and Bennett 2009). We found that both Dxy and Da are higher in regions of low recombination for DC and MC (but not for DM; Supplementary Table 5), though these differences were not significant (Mann-Whitney U tests P>0.05 for all).
Finally, we compared estimates of Nm from IM with estimates of recombination rate for all loci. We found a significant positive correlation between recombination rate and estimates of 2Nm into M. m. musculus (Spearman rank order correlation r=0.359 P=0.033) and a marginally significant correlation between recombination rate and estimates of 2Nm into M. m. castaneus (Spearman rank order correlation r=0.290 P=0.071). We also found that for each of the unidirectional estimates of gene flow (i.e. from a particular subspecies to a particular subspecies), the average Nm was roughly two-fold higher for loci in regions of high recombination compared to loci in regions of low recombination (Table 2).
Discussion
This study represents the largest survey of DNA sequence variation in wild populations of the three Mus musculus subspecies. Our main observations are as follows. (i) All three subspecies started to diverge at roughly the same time ~350,000 years ago, yet M. m. musculus and M. m. domesticus are more differentiated from each other than either is from M. m. castaneus. (ii) There is heterogeneity among loci in levels of population differentiation between pairs of subspecies, with FST ranging from almost zero to one. Genomic regions with lower rates of recombination are more differentiated, on average, than regions of higher recombination.
History of speciation in house mice
The data and analyses presented here provide insight into the temporal and demographic history of speciation in house mice. Models of isolation-with-gene-flow enabled us to estimate parameters while allowing for gene flow following divergence from an ancestral population. Previous studies suggested that house mouse subspecies started to diverge within the last 500,000 years (She et al. 1990; Geraldes et al. 2008a). Our analyses, based on considerably more data, suggest that the divergence may be even younger and closer to 350,000 years ago. Interestingly, the three estimates of t in pairwise comparisons between subspecies were very similar, suggesting that all three subspecies diverged at roughly the same time. This is consistent with a recent study in which the genome sequences of one M. m. domesticus, one M. m. musculus, and one M. m. castaneus were analyzed to estimate the species phylogeny (White et al. 2009). White et al. (2009) found highly discordant patterns among loci, with only 39% supporting the best-supported topology (M. m. domesticus as the sister group to a clade containing M. m. musculus and M. m. castaneus), which is close to the 33% expected if all three lineages diverged at approximately the same time as suggested by our results.
Maximum likelihood estimates of Ne were different for the three subspecies. Ne for M. m. castaneus (~366,500) was substantially larger than Ne for M. m domesticus (~82,500) which in turn was larger than Ne for M. m. musculus (~36,500). These values are based on a generation time of one year, and are twice as large if a generation time of 0.5 years is assumed. Probably the biggest source of error in estimates of Ne is the uncertainty in estimates of generation time. While laboratory mice can have four or more generations per year, wild mice typically breed seasonally (Bronson 1979). Moreover, abundant food for commensal mice, which is associated with more continuous breeding, occurred only after the development of agriculture about 10,000 years ago. This represents a small fraction of the ~350,000 year history of these lineages. It thus seems likely that wild M. musculus have had a generation time of closer to one year over much of their history. However, uncertainty in the exact value precludes more precise estimates of Ne. The estimates of Ne here are slightly different from previous estimates which were based on fewer loci and more individuals (Geraldes et al. 2008a). Geraldes et al. (2008a) sampled eight loci (of which only four were autosomal) and two or three major geographic regions for each subspecies, while the present study sampled 27 loci and one major geographic region for each subspecies. These factors are likely to account for the modest differences in estimates of Ne. Nonetheless, the two studies are in good agreement about the relative values of Ne for the three subspecies.
Despite the fact that all three subspecies diverged from each other within a very short period of time, differentiation was greater for DM than for DC or MC. This was seen in the higher average FST for DM (0.72) compared to either DC or MC (FST=0.43 and 0.49, respectively) and in the greater proportion of monophyletic gene genealogies in DM compared to DC or MC (Figure 3). Similarly, the ratio of fixed differences to intra-subspecific polymorphisms was 19% for DM but only 1% for DC and 2% for MC. The proportion of monophyletic gene trees within each subspecies (Figure 3b) accords well with the relative Ne for each subspecies, suggesting that genetic drift plays a major role in determining the amount of differentiation between subspecies. It is also likely that gene flow following divergence accounts for some of this overall pattern, since gene flow between DM is less than between DC or MC (Table 3).
Interestingly, the greater differentiation observed for DM compared to DC or MC is consistent with the known greater degree of reproductive isolation for DM than for DC or MC. M. m. musculus and M. m. domesticus form a narrow hybrid zone in central Europe (Teeter et al. 2010), and lab crosses between these taxa produce sterile hybrid males (Forejt 1996). In contrast, M. m. domesticus and M. m. castaneus appear to have hybridized freely in Califiornia (Orth et al. 1998), and M. m. musculus and M. m. castaneus hybridized to form M. m. molossinus in Japan (Boursot et al. 1993).
Recombination rate variation and patterns of differentiation
Recent speciation models have invoked the importance of suppressed recombination in rearranged regions of the genome as a mechanism to facilitate species divergence (Noor et al. 2001; Rieseberg 2001; Navarro and Barton 2003). House mice provide a useful system for testing the generality of these models, since there is considerable genomic variation in recombination rate (Shifman et al. 2006) but the three subspecies are not distinguished by chromosomal rearrangements. FST was significantly greater at loci experiencing low recombination rates compared to loci experiencing high recombination rates between M. m. musculus and M. m. castaneus and between M. m. domesticus and M. m. castaneus. In the comparison between M. m. musculus and M. m. domesticus, we observed a trend in the same direction. We found a significant negative correlation between FST and recombination rate for DC and a non-significant trend in the same direction for DM and MC. For all three comparisons, the average FST was higher for loci in regions of low recombination.
These differences in FST could be due to differences in gene flow or due to diversity-reducing selection, or some combination of both processes. Teasing apart these processes is exceptionally difficult (Noor and Bennet 2009), and many previous studies that have documented a correlation between recombination rate and FST have made no attempt to consider these alternatives (e.g. Takahashi et al. 2004). The IM analyses performed here show that gene flow is occuring between DC and MC. Moreover, genes with high recombination rates (and low FST) had the highest IM-estimates of gene flow. However, caution is warranted in interpreting this pattern; FST and IM-estimates of gene flow are not independent. Thus, while this pattern is consistent with reduced gene flow in regions of low recombination, we cannot rule out the alternative that selection at linked sites is also a contributing factor. One approach to disentangling these processes will come from mapping genes underlying reproductive isolation. The speciation models of Noor et al. (2001), Rieseberg (2001), and Navarro and Barton (2003) predict that such genes will be found in regions of low recombination.
Several studies have now documented correlated variation in recombination rate and levels of differentiation among populations or closely related species. In Drosophila melanogaster, FST between Africa and North America is greatest in regions of low recombination (Begun and Aquadro 1993). In humans, FST among African, Asian and European populations is negatively correlated with local rates of recombination (Keinan and Reich 2010). Similarly, a study based on two inbred strains from each subspecies of house mice revealed that GST was negatively correlated with recombination rate (Takahashi et al. 2004). In all of these cases, patterns were attributed to selection at linked sites reducing variation in regions of the genome with low recombination. Other studies have documented variation in levels of differentiation across the genome but placed the emphasis on the interplay between recombination rate and gene flow (e.g. Rieseberg et al. 1999; Machado et al. 2002; Geraldes et a. 2006). These studies include some taxa in which rearranged chromosomes are believed to suppress recombination (e.g. sunflowers, Rieseberg et al. 1999; Drosophila, Machado et al. 2002) and some studies in which the suppression of recombination is associated with proximity to a centromere (e.g. rabbits, Geraldes et al. 2006; Geraldes et al. 2008b; Carneiro et al. 2009). The genomes of the three subspecies of house mice are collinear, and the loci studied here do not lie near centromeres. Therefore the observation of variation in levels of differentiation cannot be attributed to chromosomal rearrangements or to proximity to centromeres. Greater differentiation in regions of low recombination therefore appears to be a fairly general phenomenon.
Supplementary Material
Supplementary Figure 1- Gene genealogies for each locus surveyed in this study. These were generated with the entire data set (top) and with the largest non-recombining blocks within Mus musculus (bottom; generated with IMgc). Bootstrap values above 80% are shown next to the corresponding nodes and the species in which each haplotype was observed are indicated by boxes at the tips of the tree branches. White boxes represent M. musculus domesticus, gray boxes, M. m. castaneus and black boxes M. m. musculus. The length of each box is proportional to the number of times each haplotype was observed. For genealogies with the largest non-recombining block, one allele of M. caroli was added to the data set after IMgc analyses. For details on each locus refer to Table 1 and Figure 2.
Supplementary Figure 2. Summary of genealogical relationships among subspecies of house mouse for the 27 loci surveyed in this study. A) Proportion of loci that are reciprocally monophyletic (black), paraphyletic (gray) or polyphyletic (white), in pairwise comparisons among subspecies. B) same as A but using only the largest non-recombining block of data for each locus. C) proportion of loci where each subspecies is either monophyletic (black) or not (white), in relation to the other two subspecies. D) same as C but using only the largest non-recombining block of data for each locus.
Acknowledgments
We thank B. Harr for kindly providing samples from India. We thank M. Carneiro, M. Dean, J. Good, T. Salcedo, M. Sans Fuentes, R. Storchova, M. Whitlock and G. Wlasiuk for useful discussions. This work was supported by an NIH grant (RO1 GM074245) to MWN, a fellowship from the Fundação para a Ciência e Tecnologia (SFRH/BPD/24743/2005) to AG and a fellowship from the Swiss National Science Foundation for a Post-Doctoral fellowship (PBLAA- 111572) to PB.
Footnotes
Literature Cited
- Becquet C, Przeworski M. A new approach to estimate parameters of speciation models with application to apes. Genome Res. 2007;17:1505–1519. doi: 10.1101/gr.6409707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begun DJ, Aquadro CF. African and North-American populations of Drosophila melanogaster are very different at the DNA level. Nature. 1993;365:548–550. doi: 10.1038/365548a0. [DOI] [PubMed] [Google Scholar]
- Boursot P, Auffray JC, Brittondavidian J, Bonhomme F. The Evolution of House Mice. Annual Review of Ecology and Systematics. 1993;24:119–152. [Google Scholar]
- Britton-Davidian J, Fel-Clair F, Lopez J, Alibert P, Boursot P. Postzygotic isolation between the two European subspecies of the house mouse: estimates from fertility patterns in wild and laboratory-bred hybrids. Biological Journal of the Linnean Society. 2005;84:379–393. [Google Scholar]
- Bronson FH. The reproductive ecology of the house mouse. Q Rev Biol. 1979;54:265–299. doi: 10.1086/411295. [DOI] [PubMed] [Google Scholar]
- Carneiro M, Ferrand N, Nachman MW. Recombination and speciation: loci near centromeres are more differentiated than loci near telomeres between subspecies of the European rabbit (Oryctolagus cuniculus) Genetics. 2009;181:593–606. doi: 10.1534/genetics.108.096826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlesworth B. Measures of divergence between populations and the effect of forces that reduce variability. Mol Biol Evol. 1998;15:538–543. doi: 10.1093/oxfordjournals.molbev.a025953. [DOI] [PubMed] [Google Scholar]
- Charlesworth B, Morgan MT, Charlesworth D. The effect of deleterious mutations on neutral molecular variation. Genetics. 1993;134:1289–1303. doi: 10.1093/genetics/134.4.1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox A, Ackert-Bicknell CL, Dumont BL, Ding Y, Bell JT, et al. A new standard genetic map for the laboratory mouse. Genetics. 2009;182:1335–1344. doi: 10.1534/genetics.109.105486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyne JA, Orr HA. Speciation. Sinauer Associates, Inc; Sunderland: 2004. [Google Scholar]
- Dumont BL, White MA, Steffy B, Wiltshire T, Payseur BA. Extensive recombination rate variation in the house mouse species complex inferred from genetic linkage maps. Genome Research. 2011;21:114–125. doi: 10.1101/gr.111252.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewing B, Green P. Base-calling of automated sequencer traces using phred. II. Error probabilities. Genome Res. 1998;8:186–194. [PubMed] [Google Scholar]
- Ewing B, Hillier L, Wendl MC, Green P. Base-calling of automated sequencer traces using phred. I. Accuracy assessment. Genome Res. 1998;8:175–185. doi: 10.1101/gr.8.3.175. [DOI] [PubMed] [Google Scholar]
- Faria R, Navarro A. Chromosomal speciation revisited: rearranging theory with pieces of evidence. Trends in Ecology and Evolution. 2010;25:660–669. doi: 10.1016/j.tree.2010.07.008. [DOI] [PubMed] [Google Scholar]
- Forejt J. Hybrid sterility in the house mouse. Trends in Genetics. 1996;12:412–417. doi: 10.1016/0168-9525(96)10040-8. [DOI] [PubMed] [Google Scholar]
- Frazer KA, Eskin E, Kang HM, Bogue MA, Hinds DA, Beilharz EJ, Gupta RV, Montgomery J, Morenzoni MM, Nilsen GB, Pethiyagoda CL, Stuve LL, Johnson FM, Daly MJ, Wade CM, Cox DR. A sequence-based variation map of 8.27 million SNPs in inbred mouse strains. Nature. 2007;448:1050–1053. doi: 10.1038/nature06067. [DOI] [PubMed] [Google Scholar]
- Fu YX, Li WH. Statistical tests of neutrality of mutations. Genetics. 1993;133:693–709. doi: 10.1093/genetics/133.3.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geraldes A, Ferrand N, Nachman MW. Contrasting patterns of introgression at X-linked loci across the hybrid zone between subspecies of the European rabbit (Oryctolagus cuniculus) Genetics. 2006;173:919–933. doi: 10.1534/genetics.105.054106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geraldes A, Basset P, Gibson B, Smith KL, Harr B, et al. Inferring the history of speciation in house mice from autosomal, X-linked, Y-linked and mitochondrial genes. Mol Ecol. 2008a;17:5349–5363. doi: 10.1111/j.1365-294X.2008.04005.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geraldes A, Carneiro M, Delibes-Mateos M, Villafuerte R, Nachman MW, et al. Reduced introgression of the Y chromosome between subspecies of the European rabbit (Oryctolagus cuniculus) in the Iberian Peninsula. Mol Ecol. 2008b;17:4489–4499. doi: 10.1111/j.1365-294X.2008.03943.x. [DOI] [PubMed] [Google Scholar]
- Good JM, Handel MA, Nachman MW. Asymmetry and polymorphism of hybrid male sterility during the early stages of speciation in house mice. Evolution. 2008;62:50–65. doi: 10.1111/j.1558-5646.2007.00257.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon D, Abajian C, Green P. Consed: A graphical tool for sequence finishing. Genome Research. 1998;8:195–202. doi: 10.1101/gr.8.3.195. [DOI] [PubMed] [Google Scholar]
- Hall TA. BioEdit: a user friendly biological sequence alignment editor and analyses program for Windows 95/98/NT. Nucleic Acids Symposium Series; 1999. pp. 95–98. [Google Scholar]
- Hey J, Nielsen R. Multilocus methods for estimating population sizes, migration rates and divergence time, with applications to the divergence of Drosophila pseudoobscura and D. persimilis. Genetics. 2004;167:747–760. doi: 10.1534/genetics.103.024182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hey J, Nielsen R. Integration within the Felsenstein equation for improved Markov chain Monte Carlo methods in population genetics. Proc Natl Acad Sci U S A. 2007;104:2785–2790. doi: 10.1073/pnas.0611164104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson RR. Generating samples under a Wright-Fisher neutral model of genetic variation. Bioinformatics. 2002;18:337–338. doi: 10.1093/bioinformatics/18.2.337. [DOI] [PubMed] [Google Scholar]
- Hudson RR, Kreitman M, Aguade M. A test of neutral molecular evolution based on nucleotide data. Genetics. 1987;116:153–159. doi: 10.1093/genetics/116.1.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson RR, Slatkin M, Maddison WP. Estimation of levels of gene flow from sequence data. Genetics. 1992;132:583–589. doi: 10.1093/genetics/132.2.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keinan A, Reich D. Human population differentiation is strongly correlated with local recombination rate. PLoS Genet. 2010;6:e1000886. doi: 10.1371/journal.pgen.1000886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Nei M, Dudley J, Tamura K. MEGA: a biologist-centric software for evolutionary analysis of DNA and protein sequences. Brief Bioinform. 2008;9:299–306. doi: 10.1093/bib/bbn017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado CA, Kliman RM, Markert JA, Hey J. Inferring the history of speciation from multilocus DNA sequence data: the case of Drosophila pseudoobscura and close relatives. Mol Biol Evol. 2002;19:472–488. doi: 10.1093/oxfordjournals.molbev.a004103. [DOI] [PubMed] [Google Scholar]
- Macholan M, Munclinger P, Sugerkova M, Dufkova P, Bimova B, et al. Genetic analysis of autosomal and X-linked markers across a mouse hybrid zone. Evolution. 2007;61:746–771. doi: 10.1111/j.1558-5646.2007.00065.x. [DOI] [PubMed] [Google Scholar]
- Maroja LS, Andres JA, Harrison RG. Genealogical discordance and patterns of introgression and selection across a cricket hybrid zone. Evolution. 2009;63:2999–3015. doi: 10.1111/j.1558-5646.2009.00767.x. [DOI] [PubMed] [Google Scholar]
- Mihola O, Trachtulec Z, Vlcek C, Schimenti JC, Forejt J. A mouse speciation gene encodes a meiotic histone H3 methyltransferase. Science. 2009;323:373–375. doi: 10.1126/science.1163601. [DOI] [PubMed] [Google Scholar]
- Navarro A, Barton NH. Accumulating postzygotic isolation genes in parapatry: A new twist on chromosomal speciation. Evolution. 2003;57:447–459. doi: 10.1111/j.0014-3820.2003.tb01537.x. [DOI] [PubMed] [Google Scholar]
- Nei M. Analysis of gene diversity in subdivided populations. Proc Natl Acad Sci U S A. 1973;70:3321–3323. doi: 10.1073/pnas.70.12.3321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nei M. Molecular Evolutionary Genetics. Columbia University Press; New York: 1987. [Google Scholar]
- Nei M, Li WH. Mathematical model for studying genetic variation in terms of restriction endonucleases. Proc Natl Acad Sci U S A. 1979;76:5269–5273. doi: 10.1073/pnas.76.10.5269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickerson DA, V, Tobe O, Taylor SL. PolyPhred: automating the detection and genotyping of single nucleotide substitutions using fluorescence-based resequencing. Nucleic Acids Res. 1997;25:2745–2751. doi: 10.1093/nar/25.14.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen R, Wakeley J. Distinguishing migration from isolation: a Markov chain Monte Carlo approach. Genetics. 2001a;158:885–896. doi: 10.1093/genetics/158.2.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen R, Wakeley J. Distinguishing migration from isolation: A Markov chain Monte Carlo approach. Genetics. 2001b;158:885–896. doi: 10.1093/genetics/158.2.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noor MA, Bennet SM. Islands of speciation or mirages in the desert? Examining the role of restricted recombination in maintaining species. Heredity. 2009;103:439–444. doi: 10.1038/hdy.2009.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noor MA, Grams KL, Bertucci LA, Reiland J. Chromosomal inversions and the reproductive isolation of species. Proc Natl Acad Sci U S A. 2001;98:12084–12088. doi: 10.1073/pnas.221274498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orth A, Adama T, Din W, Bonhomme F. Natural hybridization of two subspecies of house mice, Musculus domesticus and Mus musculus castaneus, near Lake Casitas (California) Genome. 1998;41:104–110. doi: 10.1139/g97-109. [DOI] [PubMed] [Google Scholar]
- Raufaste N, Orth A, Belkhir K, Senet D, Smadja C, et al. Inferences of selection and migration in the Danish house mouse hybrid zone. Biological Journal of the Linnean Society. 2005;84:593–616. [Google Scholar]
- Rieseberg LH. Chromosomal rearrangements and speciation. Trends Ecol Evol. 2001;16:351–358. doi: 10.1016/s0169-5347(01)02187-5. [DOI] [PubMed] [Google Scholar]
- Rieseberg LH, Whitton J, Gardner K. Hybrid zones and the genetic architecture of a barrier to gene flow between two sunflower species. Genetics. 1999;152:713–727. doi: 10.1093/genetics/152.2.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitou N, Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987;4:406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- She JX, Bonhomme F, Boursot P, Thaler L, Catzeflis F. Molecular phylogenies in the genus Mus – comparative analysis of electrophoretic, scnDNA hybridization, and mtDNA RFLP data. Biological Journal of the Linnean Society. 1990;41:83–103. [Google Scholar]
- Shifman S, Bell JT, Copley RR, Taylor MS, Williams RW, et al. A high-resolution single nucleotide polymorphism genetic map of the mouse genome. Plos Biology. 2006;4:2227–2237. doi: 10.1371/journal.pbio.0040395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JM, Haigh J. The hitch-hiking effect of a favourable gene. Genet Res. 1974;23:23–35. [PubMed] [Google Scholar]
- Stephens M, Donnelly P. A comparison of bayesian methods for haplotype reconstruction from population genotype data. Am J Hum Genet. 2003;73:1162–1169. doi: 10.1086/379378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens M, Smith NJ, Donnelly P. A new statistical method for haplotype reconstruction from population data. Am J Hum Genet. 2001;68:978–989. doi: 10.1086/319501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storchova R, Gregorova S, Buckiova D, Kyselova V, Divina P, Forejt J. Genetic analysis of X-linked hybrid sterility in the house mouse. Mammal Genome. 2004;15:515–524. doi: 10.1007/s00335-004-2386-0. [DOI] [PubMed] [Google Scholar]
- Strasburg JL, Rieseberg LH. How robust are “Isolation with migration” analyses to violations of the IM model? A simulation study. Mol Bio Evol. 2010;27(2):297–310. doi: 10.1093/molbev/msp233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki H, Shimada T, Terashima M, Tsuchiya K, Aplin K. Temporal, spatial, and ecological modes of evolution of Eurasian Mus based on mitochondrial and nuclear gene sequences. Mol Phylogenet Evol. 2004;33:626–646. doi: 10.1016/j.ympev.2004.08.003. [DOI] [PubMed] [Google Scholar]
- Tajima F. Statistical method for testing the neutral mutation hypothesis by DNA polymorphism. Genetics. 1989;123:585–595. doi: 10.1093/genetics/123.3.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi A, Liu YH, Saitou N. Genetic variation versus recombination rate in a structured population of mice. Mol Biol Evol. 2004;21:404–409. doi: 10.1093/molbev/msh030. [DOI] [PubMed] [Google Scholar]
- Teeter KC, Payseur BA, Harris LW, Bakewell MA, Thibodeau LM, et al. Genome-wide patterns of gene flow across a house mouse hybrid zone. Genome Res. 2008;18:67–76. doi: 10.1101/gr.6757907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teeter KC, Thibodeau LM, Gompert Z, Buerkle CA, Nachman MW, et al. The variable genomic architecture of isolation between hybridizing species of house mice. Evolution. 2010;64:472–485. doi: 10.1111/j.1558-5646.2009.00846.x. [DOI] [PubMed] [Google Scholar]
- Thompson JD, Higgins DG, Gibson TJ. Clustal-W - Improving the Sensitivity of Progressive Multiple Sequence Alignment through Sequence Weighting, Position-Specific Gap Penalties and Weight Matrix Choice. Nucleic Acids Research. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker PK, Sage RD, Warner J, Wilson AC, Eicher EM. Abrupt Cline for Sex-Chromosomes in a Hybrid Zone between 2 Species of Mice. Evolution. 1992;46:1146–1163. doi: 10.1111/j.1558-5646.1992.tb00625.x. [DOI] [PubMed] [Google Scholar]
- Wakeley J, Hey J. Estimating ancestral population parameters. Genetics. 1997;145:847–855. doi: 10.1093/genetics/145.3.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watterson GA. On the number of segregating sites in genetical models without recombination. Theor Popul Biol. 1975;7:256–276. doi: 10.1016/0040-5809(75)90020-9. [DOI] [PubMed] [Google Scholar]
- White MA, Ane C, Dewey CN, Larget BR, Payseur BA. Fine-scale phylogenetic discordance across the house mouse genome. PLoS Genet. 2009;5:e1000729. doi: 10.1371/journal.pgen.1000729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitlock MC, McCauley DE. Indirect measures of gene flow and migration: Fst • 1/(4Nm +1) Heredity. 1999;82:117–125. doi: 10.1038/sj.hdy.6884960. [DOI] [PubMed] [Google Scholar]
- Woerner AE, Cox MP, Hammer MF. Recombination-filtered genomic datasets by information maximization. Bioinformatics. 2007;23:1851–1853. doi: 10.1093/bioinformatics/btm253. [DOI] [PubMed] [Google Scholar]
- Wright S. The genetical structure of populations. Annals of Eugenics. 1951;15:323–354. doi: 10.1111/j.1469-1809.1949.tb02451.x. [DOI] [PubMed] [Google Scholar]
- Yang H, Bell TA, Churchill GA, Pardo-Manuel de Villena F. On the subspecific origin of the laboratory mouse. Nature Genetics. 2007;39:1100–1107. doi: 10.1038/ng2087. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1- Gene genealogies for each locus surveyed in this study. These were generated with the entire data set (top) and with the largest non-recombining blocks within Mus musculus (bottom; generated with IMgc). Bootstrap values above 80% are shown next to the corresponding nodes and the species in which each haplotype was observed are indicated by boxes at the tips of the tree branches. White boxes represent M. musculus domesticus, gray boxes, M. m. castaneus and black boxes M. m. musculus. The length of each box is proportional to the number of times each haplotype was observed. For genealogies with the largest non-recombining block, one allele of M. caroli was added to the data set after IMgc analyses. For details on each locus refer to Table 1 and Figure 2.
Supplementary Figure 2. Summary of genealogical relationships among subspecies of house mouse for the 27 loci surveyed in this study. A) Proportion of loci that are reciprocally monophyletic (black), paraphyletic (gray) or polyphyletic (white), in pairwise comparisons among subspecies. B) same as A but using only the largest non-recombining block of data for each locus. C) proportion of loci where each subspecies is either monophyletic (black) or not (white), in relation to the other two subspecies. D) same as C but using only the largest non-recombining block of data for each locus.