Abstract
We present a method to form an optical window in the mouse skull that spans millimeters and is stable for months without inflammation of the brain. This enabled us to repeatedly image blood flow in cortical capillaries of awake animals and determine long-range correlations in speed. We further demonstrate repeated cortical imaging of dendritic spines, microglia, and angioarchitecture, as well as illumination to drive motor output via optogenetics and induce microstrokes via photosensitizers.
Keywords: Microscopy, Imaging, Neuroscience
Chronic observation and manipulation of cells in the cortex is integral to studies on neurons, glia, and microvasculature. An increasingly popular application involves the use of in vivo two-photon laser scanning microscopy (TPLSM) to image deep within mammalian cortex through a window in the skull1. This may be formed by a craniotomy2, in which a section of skull is removed and replaced with glass, or by thinning a section of the skull with a microsurgical blade3. Both methods have been employed for the chronic observation of fluorescently labeled neuronal processes3,4 and microvasculature5, yet have limitations. It has been suggested that cranial windows activate microglia and astrocytes as part of an inflammatory response6 that can alter neuronal physiology7 and pial blood vessels8, although these complications may relate to surgical finesse9. The thinned skull procedure avoids the activation of microglia and astrocytes6, yet access is limited to a very small area, i.e., ~ 0.2 mm2 compared with 1 to 10mm2 for a craniotomy. Further, sequential imaging sessions require re-thinning of the skull to counteract re-growth of the bone, which limits the number of times that imaging can be performed and leaves the area under the thinned portion of skull mechanically vulnerable3. Thus there is a need for a technique that provides readily available optical access across a large area of the brain without the potential to induce inflammation.
We introduce a polished and reinforced thinned-skull (PoRTS) procedure to create a large, chronically stable window in the skull. We attempt to satisfy four goals with this method: (1) Optical clarity, sufficient for imaging with TPLSM as well as optogenetic control, without the need to intervene after the initial surgery; (2) Optical access across many square millimeters; (3) Mechanical stability for imaging in awake animals; and (4) The absence of an inflammatory response.
Our basic idea is to mechanically thin the skull3, polish the cut surface to improve flatness, then use transparent cement and glass to form a clear, rigid window that is fused to the thinned bone. The glass window is designed to stabilize the thinned and thus weakened bone, and both the mechanical stability and the fusion of the window to the bone is reasoned to inhibit regrowth of bone, thus preserving clarity. In detail, the skull is thinned with a high speed burr to a final thickness of 10 to 15 μm, polished without direct pressure on the skull with a slurry of grit in artificial cerebral spinal fluid (ACSF), and then fused to a cover glass with a thin layer of cyanoacrylate cement (Fig. 1A).
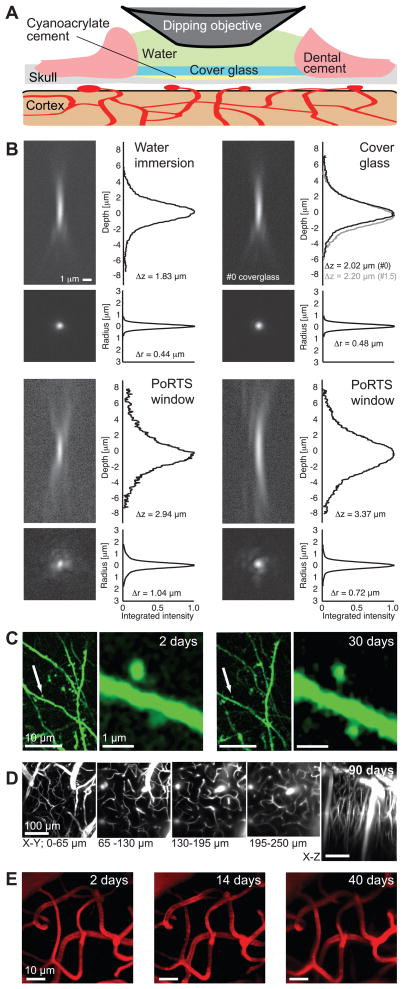
Figure 1. PoRTS window procedure, optical properties, and basic capabilities.
(A) Schematic of the window. The thinned and polished skull is protected with a cover glass attached with cyanoacrylate cement. Dental cement is used to seal the edge of the cover glass and provide support for the meniscus. The thinned area is typically 1 – 2 mm on edge. (B) Point spread functions for TPLSM determined by imaging 0.2 μm fluorescent beads were embedded in 1 % (w/v) agarose and imaged with a 40x, 0.8 NA water dipping objective. The axial depth profile shown the integrated radial intensity along the optical axis, i.e., z-axis, and the radial depth profile shows the integrated axial intensity along the radial axis. The reported Δz and Δr are the full widths that encompass half of the integrated intensity. The top left panel shows a bead imaged through only water along width depth profiles. The top right panel shows a bead imaged under a #0 cover glass (black line) along with depth profiles, along with the profiles for a #1.5 cover glass (grey line). The two bottom panels show separate examples of beads imaged through an excised PoRTS window. (C) Dendrites and spines of thy1-YFP expressing neurons two days (left panel) and 30 days (right panel) after PoRTS window implantation. Images were taken 30 μm below the surface. Reduction in the brightness of the YFP over time necessitated averaging of five frames for images taken one month after implantation; the dwell time was 6 μs per pixel and the average incident power was 35 – 70 mW. Representative image from a set of two mice (D) Maximum z-axis projections across 65 μm of fluorescein-conjugated-dextran filled vasculature through a PoRTS window 90 days after surgery. Each image is the average of six frames, the z-step was 2 μm, the dwell time was 3 μs per pixel and the average power was 25 – 120 mW. Representative images from a pool of 35 mice (E) Subsurface microvessels are stable under the PoRTS window for over a month after implantation. Images show maximum projections across 20 – 70 μm below the surface using an average of 5 frames, a dwell time of 6 μs per pixel, and an average power of 30 – 45 mW. Representative images from a set of two mice.
The optical transfer function for two-photon excited fluorescence measured through the PoRTS window is modestly degraded compared to that found using only a cover glass (Fig. 1B). The resolution varies across the window, presumably as a result of natural inhomogeneities in bone, with a radial width of 0.7 to 1.0 μm (full width of half of the integrated intensity) and an axial resolution of about 3 μm. Further, the TPLSM signal is diminished by a factor of e–1.5 by the thinned bone (Supplementary Fig. 1); this is equivalent to the loss in signal by imaging through 150 μm of brain tissue10. Despite such caveats, we could repeatedly observe the dendritic spines of neurons that express yellow fluorescent protein (YFP) both two days and several weeks after surgery (2 animals) (Fig. 1C). Second, we repeatedly imaged subsurface brain microvessels in animals in which the plasma was stained with fluorophore-conjugated-dextran. Good visibility was obtained up to 250 μm below the pial surface for periods up to three months (35 animals) (Fig. 1D). In a more stringent examination, we found that the pattern of subsurface microvascular architecture was stable when the same group of microvessels was imaged over a period of 40 days through a PoRTS window (3 fields across 2 animals) (Fig. 1E). Consistent with the long-term optical clarity of subsurface brain structures, the PoRTS window appears to impede regrowth of the thinned skull as assayed by measuring the thickness of the skull over time (Supplementary Fig. 2).
A critical biological assessment of the PoRTS window concerns the potential activation of microglia; these cells change their morphology in response to insults to the brain11. We utilized transgenic mice that express enhanced green fluorescent protein (EGFP) in microglia, i.e., line Cx3cr1eGFP+/−. At two days after surgery, which corresponds to the time when microglia are maximally active under a craniotomy6, we observe a diffuse, highly branched morphology of microglia under the PoRTS window that is indicative of non-activation (3 animals) (Supplementary Fig. 3). An essentially identical pattern is seen on the control, contralateral hemisphere. Submicrometer microglial processes, at depths greater than 100 μm below the surface, were readily imaged over a period of two months (Supplementary Fig. 4). Repeated imaging of the same general area showed a continued absence of activated microglia up to at least one month following installation of the PoRTS window (5 animals at 2 days, 3 animals at 14 days, and 3 animals at 30 days) (Supplementary Fig. 5).
A further assessment concerns reporters of brain trauma as evaluated within the area of cortex just below the PoRTS window. First, we assessed the expression of glial fibrillary acidic protein (GFAP), which is up-regulated in reactive astrocytes during brain injury12. The pattern of immunostaining for GFAP was found to be weak and indistinguishable from that seen on the control, contralateral hemisphere (3 animals) (Supplementary Fig. 6). This implies a lack of astrocyte activation. Second, we exploited the past finding that cortical injury can trigger remodeling and outgrowth of surface pial vasculature8. We observe that the pial surface vasculature remains nearly unchanged (2 fields in each of 2 animals) with the PoRTS window (Supplementary Fig. 7). In toto, the astrocyte, microglia and vascular data show that the PoRTS window does not induce a classic inflammatory response in the brain. By contrast, companion experiments reveal an increase in the density of brightly immunostained GFAP-positive reactive astrocytes below a craniotomy (3 animals), as well as an alteration of the topology of the vasculature (2 animals) that occurs primarily through the growth of surface veins following a craniotomy (Supplementary Fig. 7).
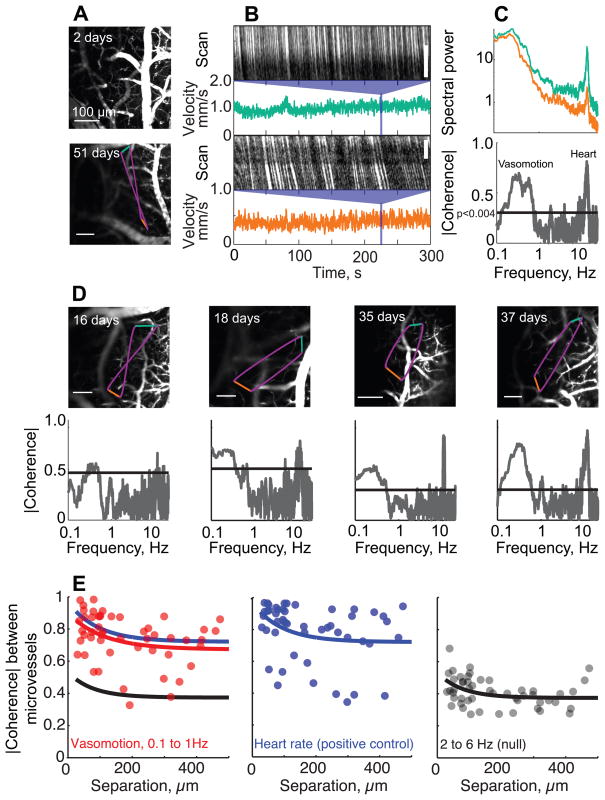
An application that exploits the large area of cortex that is visible through the PoRTS concerns the possibility of long-range correlations in vasomotion10, a 0.1 to 1 Hz oscillation in vascular tone that is hypothesized to be part of the “default” state of brain activity13. We made simultaneous measurements of the flow of red blood cells (RBCs) in pairs of subsurface microvessels, defined as vessels with diameters less than 7.5 μm, in awake animals. The RBCs can be measured in vessels over 100 μm below the pial surface and separated by up to 500 μm (17 animals measured up to 60 days after implantation of the window) (Fig. 2A-D). As a population, the baseline speed was heterogeneous (0.90 ± 0.61mm/sec; mean ± SD; 159 vessels) (Supplementary Fig. 8) and matches the values found for anesthetized rat10. Of critical interest, low-frequency variations in blood flow were strongly and significantly correlated out to 500 μm (Fig. 2E), the longest separation we could probe. Coherence at the heart rate, which serves as a positive control, was strongest between microvessels separated by less than 100 μm and remained significant out to 500 μm, Intermediate frequencies showed considerably less coherence. These data provides direct evidence for high spatial coherence of the 0.1 to 1.0 Hz vasomotion in awake animals.
Figure 2. Long-range coherence of the velocity of RBC flow in capillaries in the cortex of awake head-fixed mice through a PoRTS window.
Example data in panels A to D are taken from the same animal using a 10x,0.3 NA lens and averaging over 5 – 10 frames (A) Top panel is the maximal z projection over 90 μm of images of fluorescein-conjugated-dextran labeled vasculature, based on frames separated by 10 μm, two days after surgery. Bottom panel is an image from a single plane in the same animal, but a different location, 51 days after surgery. The colored lines show the scan path of the laser focus: green and orange are constant velocity segments along capillaries and purple are minimum time segments between capillaries. (B) Space-time plots of one segment of line scan data for each of two capillaries, with the calculated instantaneous velocity for the entire 300 s run shown below. (C) Top panel shows power spectra for the two velocity traces in panel B; 0.083 Hz bandwidth. A rhythmic component at ~ 10 Hz results from the heartbeat. Bottom panel is the magnitude of the spectral coherence between the velocities of the two capillaries as a function of frequency; 0.1 Hz bandwidth. Line denotes a significance of p < 0.004, which corresponds to the inverse of twice the number of degrees of freedom. Note the strong coherence at the heart rate and at the 0.1 – 1 Hz vasomotor frequencies. (D) Examples of spontaneous velocity coherences between capillaries obtained various times after surgery; 0.1 Hz bandwidth. Panels A-D show representative data from a pool of 17 animals monitored up to 60 days after implantation (E) The coherence as a function of distance (50 vessel pairs in 9 mice). Left panel is the mean coherence in the 0.1 – 1.0 Hz vasomotor band. Center panel is the coherence at the heart-rate, which serves as a positive control for the maximum possible coherence. Right panel is the coherence in the 2 to 6Hz band, which serves as the null hypothesis. Colored lines correspond to fits with exponential functions to the corresponding spectral band.
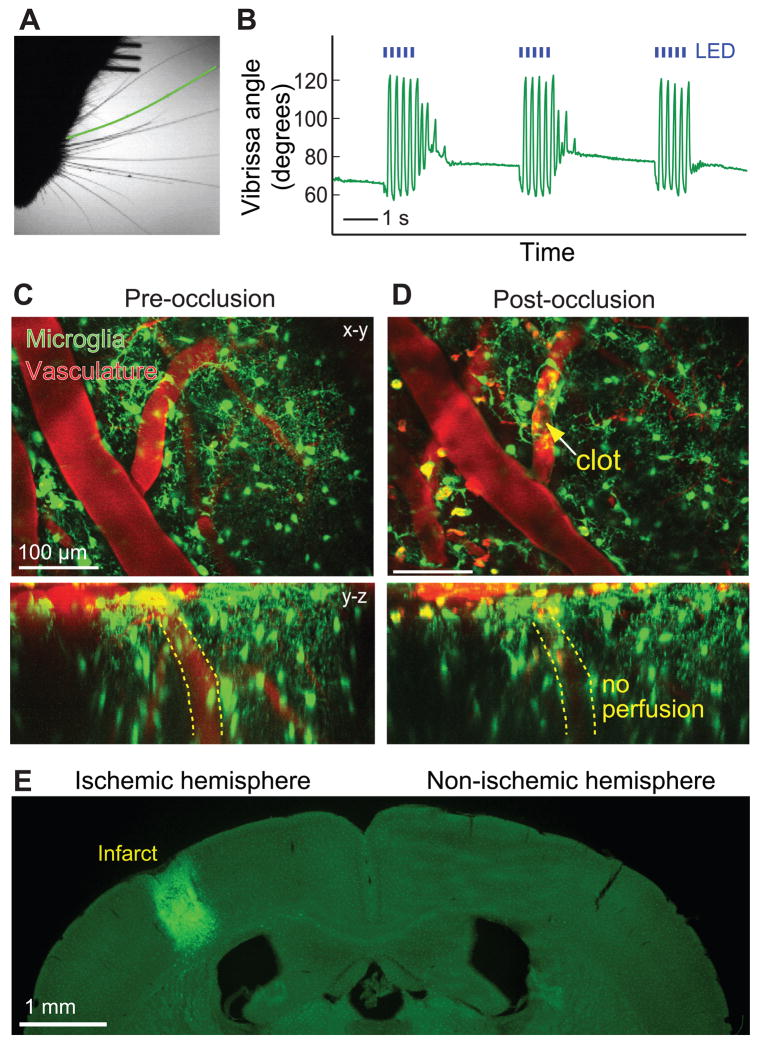
We now turn to the manipulation of brain activity. A first application concerns the excitation of neurons expressing light-sensitive channels through the PoRTS window. We used a strain of mice that express channelrhodopsin-2 (ChR2) in the layer 5 pyramidal neurons, i.e., thy–1–YFP–-ChR2, and found that vibrissa movements were elicited by illumination of motor cortex with blue light (2 awake animals) (Fig. 3A,B). This shows that focal excitation of cells that express ChR2 is compatible with the PoRTS window. As a second application, we show that we can induce microstrokes via photosensitizers through the PoRTS window. An optically-mediated occlusion to a single penetrating arteriole, the vessel that sources blood from the cortical surface to the paranchyma, is induced by focal illumination of the photosensitizer Rose-Bengal, which circulates intravenously, with green laser light. This leads to a localized clot (2 animals) (Fig. 3C,D). Occlusion of this single vessel is sufficient to produce a significant infarct (Fig. 3E), as seen previously with a craniotomy14. Use of the PoRTS window permits the effects of experimental stroke on cortical viability to be characterized while avoiding the possibility of additional damage from the craniotomy procedure. Overall, these examples show the utility of the PoRTS window for physiological manipulation as well as imaging.
Figure 3. Examples of cortical physiology evoked through the PoRTS window.
(A) Sample frame from video of awake, head-fixed ChR2 carrying mouse with a PoRTS window fabricated over vibrissa motor cortex 150 days earlier. Green line marks the position of the caudal vibrissa that is tracked over time. (B) Rostral-caudal motion of a caudal vibrissa in response to pulses of 467 nm light (5 Hz, 100 ms pulse duration; blue lines) from a light emitting diode (LED). Larger angles indicate retraction. A-B representative data from a set of two animals. (C and D) Maximal projection (x-y and y-z) through a 200 μm depth of a Cx3cr1eGFP+/− mouse cortex before (panel C) and 100 minutes after (panel D) occlusion of a single penetrating arteriole using targeted optical activation of Rose Bengal (yellow arrow points to clot). Images are averages of four frames with a dwell time of 3 μs per pixel and 1 μm steps along the z-axis. Note the change in microglia morphology to an amoeboid shape around the occluded arteriole and the interruption of flow as evidenced by the absence of Texas Red in the penetrating vessel highlighted in the y – z projection in the bottom pannels. The occlusion was made one day after implantation of the window. (E) The extent of the infarct, for the same animal as in panels C and D, visualized two days after the optically generated stroke. Note the invasion of eGFP labeled microglia into the cyst. The data in panels C to E are representative images from a set of two mice.
In summary, the PoRTS method enables high resolution chronic imaging and optical manipulation through large windows into the brain for periods of at least three months. The windows do not require regular maintenance, do not induce an apparent inflammatory response nor lead to vascular rearrangement, and may be used with awake animals. The downside of imaging through thinned bone is a loss in resolution, although this may be ameliorated through the use of adaptive optics to correct for distortions of the wave-front of the incident excitation beam. Lastly, the PoRTS window may be combined with miniaturized, head mounted microscopes for chronic imaging in free-ranging animals15.
Supplementary Material
Acknowledgments
We thank Beth Friedman, Carlos Portera-Cailliau and Karel Svoboda for critical comments on an early version of the manuscript, Michael Fuentes and Carl Petersen for advice on head fixation, Daniel N. Hill for discussions on data analysis, Jennifer Lee and Katherine Yang for help with animal husbandry, Jeffrey D. Moore for discussions on optical stimulation, and Steve Tayman for a gift of tin oxide. This work was supported by grants from the NIH (EB003832, MH085499, NS059832 and RR021907 to DK and NS066361 to KA) and the Dana Program in Brain and Immuno-imaging (to KA) and fellowships from the Israeli Science Foundation (to PB), the Canadian Institutes of Health Research and American Heart Association (to AYS), the Human Frontiers Scientific Program (to PMK), and the National Multiple Sclerosis Society (to DD).
Abbreviations
- ACSF
Artificial cerebral spinal fluid
- ChR2
Channelrhodopsin-2
- eGFP
Enhanced green fluorescent protein
- GFAP
Glial fibrillary acidic protein
- PoRTS
Polished and reinforced thinned-skull
- RBC
Red blood cell
- TPLSM
Two photon laser scanning microscopy
- YFP
Yellow fluorescent protein
Footnotes
Author contributions
The PoRTS window was conceived by PJD, AYS and PST, the imaging tools were developed by JDD and DK, the experiments were designed by KA, DD, PJD, DK, AYS and PST and carried out by PJD, PMK, AYS and PST, data analysis was carried out by PB, PJD, DK and PST, and the manuscript was written by PJD, DK and AYS.
Competing interests statement
The authors declare no competing financial interests.
References
- 1.Svoboda K, Denk W, Kleinfeld D, Tank DW. Nature. 1997;385:161. doi: 10.1038/385161a0. [DOI] [PubMed] [Google Scholar]
- 2.Levasseur JE, Wei EP, Raper AJ, Kontos AA, Patterson JL. Stroke. 1975;6:308. doi: 10.1161/01.str.6.3.308. [DOI] [PubMed] [Google Scholar]
- 3.Grutzendler J, Kasthuri N, Gan WB. Nature. 2002;420:812. doi: 10.1038/nature01276. [DOI] [PubMed] [Google Scholar]
- 4.Trachtenberg JT, Chen BE, Knott GW, Feng G, Sanes JR, Welker E, Svoboda K. Nature. 2002;420:788. doi: 10.1038/nature01273. [DOI] [PubMed] [Google Scholar]
- 5.Brown CE, Li P, Boyd JD, Delaney KR, Murphy TH. Journal of Neuroscience. 2007;27:4101. doi: 10.1523/JNEUROSCI.4295-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xu HT, Pan F, Yang G, Gan WB. Nature Neuroscience. 2007;10:549. doi: 10.1038/nn1883. [DOI] [PubMed] [Google Scholar]
- 7.Hauss-Wegrzyniak B, Lynch MA, Vraniak PD, Wenk GL. Experimental Neurology. 2002;176:336. doi: 10.1006/exnr.2002.7966. [DOI] [PubMed] [Google Scholar]
- 8.Sohler TP, Lothrop GN, Forbes HS. Journal of Pharmacology and Experimental Therapeutics. 1941;71:325. [Google Scholar]
- 9.Holtmaat A, Bonhoeffer T, Chow DK, Chuckowree J, De Paola V, Hofer SB, Hubener M, Keck T, Knott G, Lee WC, Mostany R, Mrsic-Flogel TD, Nedivi E, Portera-Cailliau C, Svoboda K, Trachtenberg JT, Wilbrecht L. Nature Protocols. 2009;4:1128. doi: 10.1038/nprot.2009.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kleinfeld D, Mitra PP, Helmchen F, Denk W. Proceedings of the National Academy of Sciences USA. 1998;95:15741. doi: 10.1073/pnas.95.26.15741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stoll G, Jander S. Progress in Neurobiology. 1999;58:233. doi: 10.1016/s0301-0082(98)00083-5. [DOI] [PubMed] [Google Scholar]
- 12.Pekny M, Nilsson M. Glia. 2005;50:427. doi: 10.1002/glia.20207. [DOI] [PubMed] [Google Scholar]
- 13.Fox MD, Raichle ME. Nature Reviews of Neuroscience. 2007;8:700. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- 14.Blinder* P, Shih* AY, Rafie CA, Kleinfeld D. Proceedings of the National Academy of Sciences USA. 2010;107:12670. doi: 10.1073/pnas.1007239107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sawinski J, Wallace DJ, Greenberg DS, Grossmann S, Denk W, Kerr JND. Proceedings of the National Academy of Sciences USA. 2009;106:19557. doi: 10.1073/pnas.0903680106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jung S, Aliberti A, Graemmel P, Sunshine MJ, Kreutzberg GW, Sher A, Littman DR. Molecular and Cellular Biology. 2000;20:4106. doi: 10.1128/mcb.20.11.4106-4114.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arenkiel BR, Peca J, Davison IG, Feliciano C, Deisseroth K, Augustine GJ, Ehlers MD, Feng G. Neuron. 2007;54:205. doi: 10.1016/j.neuron.2007.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Frostig RD, Dory Y, Kwon MC, Masino SA. Proceedings of the National Academy of Sciences USA. 1993;90:9998. doi: 10.1073/pnas.90.21.9998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stosiek C, Garaschuk O, Holthoff K, Konnerth A. Proceedings of the National Academy of Sciences USA. 2003;100:7319. doi: 10.1073/pnas.1232232100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kleinfeld D, Delaney KR. Journal of Comparative Neurology. 1996;375:89. doi: 10.1002/(SICI)1096-9861(19961104)375:1<89::AID-CNE6>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 21.Denk W, Delaney KR, Kleinfeld D, Strowbridge B, Tank DW, Yuste R. Journal of Neuroscience Methods. 1994;54:151. doi: 10.1016/0165-0270(94)90189-9. [DOI] [PubMed] [Google Scholar]
- 22.Tsai PS, Kleinfeld D. In: Methods for In Vivo Optical Imaging, 2nd edition. Frostig RD, editor. CRC Press; Boca Raton: 2009. p. 59. [Google Scholar]
- 23.Tsai PS, Friedman B, Ifarraguerri AI, Thompson BD, Lev-Ram V, Schaffer CB, Xiong Q, Tsien RY, Squier JA, Kleinfeld D. Neuron. 2003;39:27. doi: 10.1016/s0896-6273(03)00370-2. [DOI] [PubMed] [Google Scholar]
- 24.Nguyen Q-T, Dolnick EM, Driscoll J, Kleinfeld D. In: Methods for In Vivo Optical Imaging. 2. Frostig RD, editor. CRC Press; Boca Raton: 2009. p. 117. [Google Scholar]
- 25.Nguyen QT, Tsai PS, Kleinfeld D. Journal of Neuroscience Methods. 2006;156:351. doi: 10.1016/j.jneumeth.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 26.Shih AY, Friedman B, Drew PJ, Tsai PS, Lyden PD, Kleinfeld D. Journal of Cerebral Blood Flow and Metabolism. 2009;29:738. doi: 10.1038/jcbfm.2008.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shih AY, Nishimura N, Nguyen J, Friedman B, Lyden PD, Schaffer CB, Kleinfeld D. In: Imaging in Neuroscience and Development. Yuste R, editor. Vol. 2. Cold Spring Harbor Laboratory Press; New York: 2009. in press. [Google Scholar]
- 28.Valmianski I, Shih AY, Driscoll J, Matthews DM, Freund Y, Kleinfeld D. Journal of Neurophysiology. 2010;104:1803. doi: 10.1152/jn.00484.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Drew PJ, Blinder P, Cauwenberghs G, Shih AY, Kleinfeld D. Journal of Computational Neuroscience. 2010;29:5. doi: 10.1007/s10827-009-0159-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McMenamin PG. Journal of Comparitive Neurology. 1999;405:553. [PubMed] [Google Scholar]
- 31.Ferezou I, Haiss F, Gentet LJ, Aronoff R, Weber B, Petersen CCH. Neuron. 2007;56:907. doi: 10.1016/j.neuron.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 32.Knutsen PM, Derdikman D, Ahissar E. Journal of Neurophysiology. 2005;93:2294. doi: 10.1152/jn.00718.2004. [DOI] [PubMed] [Google Scholar]
- 33.Nishimura N, Schaffer CB, Friedman B, Lyden PD, Kleinfeld D. Proceedings of the National Academy of Sciences USA. 2007;104:365. doi: 10.1073/pnas.0609551104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Born M, Wolf E. Principles of Optics: Electromagnetic Theory of Propagation Interference and Diffraction of Light. 6. Pergamon Press; Oxford: 1980. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.