Abstract
Transforming growth factor (TGF)-β antagonizes mitogenic Ras signaling during epithelial regeneration, but TGF-β and Ras act synergistically in driving tumor progression. Insights into these apparently contradictory effects have come from recent detailed analyses of the TGF-β signaling process. Here, we summarize the different modes of TGF-β/Ras signaling in normal epithelium and neoplasms and show how perturbation of TGF-β signaling by Ras may contribute to a shift from tumor-suppressive to protumorigenic TGF-β activity during tumor progression. Smad proteins, which convey signals from TGF-β receptors to the nucleus, have intermediate linker regions between conserved Mad homology (MH) 1 and MH2 domains. TGF-β Type I receptor and Ras-associated kinases differentially phosphorylate Smad2 and Smad3 to create C-terminally (C), linker (L) or dually (L/C) phosphorylated (p) isoforms. In epithelial homeostasis, TGF-β-mediated pSmad3C signaling opposes proliferative responses induced by mitogenic signals. During carcinogenesis, activation of cytoplasmic Ras-associated kinases including mitogen-activated protein kinase confers a selective advantage on benign tumors by shifting Smad3 signaling from a tumor-suppressive pSmad3C to an oncogenic pSmad3L pathway, leading to carcinoma in situ. Finally, at the edges of advanced carcinomas invading adjacent tissues, nuclear Ras-associated kinases such as cyclin-dependent kinases, together with cytoplasmic kinases, alter TGF-β signals to more invasive and proliferative pSmad2L/C and pSmad3L/C signaling. Taken together, TGF-β signaling specificity arises from spatiotemporal dynamics of Smad phosphoisoforms. Based on these findings, we have reason to hope that pharmacologic inhibition of linker phosphorylation might suppress progression to human advanced carcinomas by switching from protumorigenic to tumor-suppressive TGF-β signaling.
Introduction
Transforming growth factor (TGF)-β inhibits proliferation of normal epithelial, endothelial and hematopoietic cells, thus being crucial for homeostasis of various tissues (1,2). During carcinogenesis, the physiological balance between proliferation and differentiation in normal epithelial cell homeostasis is lost. TGF-β signaling appears to be important for prevention of early-stage carcinogenesis, acting to maintain normal tissue architecture (3). The cytostatic function of TGF-β is inhibited in cancer as a result of mutations that directly inactivate components of the TGF-β signaling pathway (4,5). However, genetic inactivation of the TGF-β signaling molecules occurs in only ∼10% of all cancers (5). Many tumor cells without known mutations in these components are refractory to growth inhibition by TGF-β.
What are the mutations that interrupt growth inhibition by TGF-β and confer growth advantages at an early stage of tumor development? Among genes found so far to be involved in human colorectal carcinogenesis as an example, the proto-oncogene K-Ras (a member of the Ras gene family) stands out as most frequently mutated (6). During carcinogenesis, genetic mutations involving Ras and other oncogenic pathways gradually accumulate in a benign tumor, as it becomes carcinoma in situ (7–9). Finally, in advanced carcinomas invading adjacent tissues, TGF-β signaling acts in concert with the Ras and other oncogenic pathways to induce a proliferative and invasive phenotype (10,11). Both major effects of TGF-β, one antagonizing and the other favoring tumor progression, are clearly in direct conflict with each other. Cancer cells resolve this dilemma by evolving to evade the cytostatic effects of TGF-β, while promoting other responses favoring tumor cell invasiveness (12–14).
Progress over the past 10 years has disclosed important details of how TGF-β elicits its responses. Smads, central mediators conveying signals from receptors for TGF-β superfamily members to the nucleus (15–19), are modular proteins with conserved Mad homology (MH)1, intermediate linker and MH2 domains (16). In cell signaling pathways, various transcription factors (TFs) are phosphorylated at multiple sites by upstream kinases. Catalytically, active TGF-β Type I receptor (TβRI) phosphorylates COOH-tail serine residues of receptor-activated Smad (R-Smad) (17), which include Smad2 and the highly similar protein Smad3. Mitogenic signals acting via the Ras pathway alternatively cause phosphorylation of R-Smad at specific sites in their middle linker regions (20–25).
Monitoring phosphorylation status of signaling molecules is a key step in dissecting their pathways. In Smad signaling, phosphorylation of not only the COOH-tail but also the linker regions of R-Smads is important in regulating Smad activity under physiologic and pathologic conditions (26). In this review, we first examine Smad signaling specificity derived from the target gene profile in response to changes in spatial and temporal dynamics of domain-specific R-Smad phosphorylation. We then consider how these phosphorylated R-Smad signals determine specific cellular responses to TGF-β in normal epithelial cells, benign tumors, carcinomas in situ and invasive carcinomas. Finally, we discuss how enhanced understanding of phosphorylated R-Smad signaling could lead to improved methods for cancer prevention and treatment.
The canonical TGF-β and Ras-activated mitogen-activated protein kinase pathways
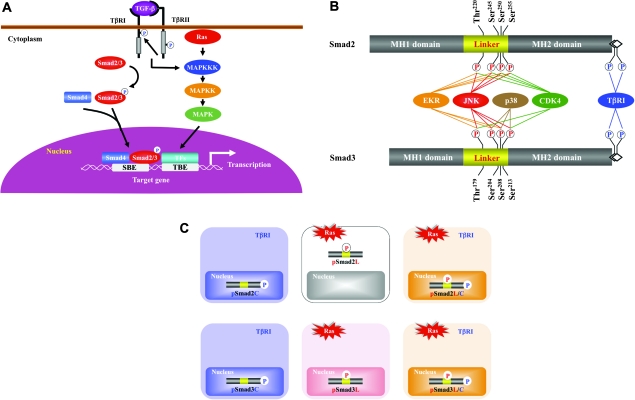
The canonical TGF-β pathway involves R-Smad signaling through direct serine phosphorylation of C-termini by TβRI upon TGF-β binding [Figure 1A, left (16,19)]. TβRI-mediated phosphorylation of R-Smads induces their association with the shared partner Smad4. The complexes accumulate in the nucleus, where they interact with other TFs, coactivators and corepressors to regulate the transcription of specific genes (15–19,27). Although Smad3 makes direct contact with DNA at a 5′-AGAC-3′ sequence known as a Smad-binding element, Smad2 cannot bind directly to DNA (28,29).
Fig. 1.
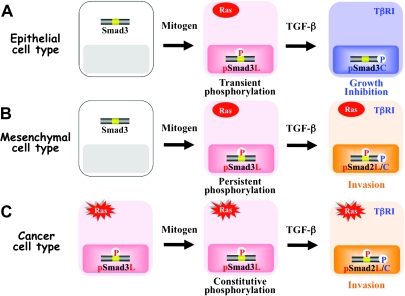
Multiple Smad phosphoisoforms exist. (A) The canonical TGF-β and Ras-activated MAPK pathways. Following phosphorylation (p) of their C-termini by TβRI, Smad2 and Smad3 partner with the common mediator Smad4 and translocate to the nucleus. TGF-β also activates the Ras/MAPK pathway; the MAPK pathway induces the phosphorylation of a variety of TFs that co-operate with nuclear Smads in mediating TGF-β-induced transcriptional responses. SBE, Smad-binding element; TBE, transcription factor-binding element. (B) Schematic representation of phosphorylation sites in Smad2 and Smad3. Catalytically, active TβRI phosphorylates COOH-tail serine residues. ERK, JNK, p38 MAPK and CDK4 alternatively phosphorylate Smad2/3 at specific sites in their middle linker regions as lines indicate. Several other kinases such as glycogen synthase kinase-3β also phosphorylate Smad2/3 at the specific sites in the linker segments. (C) Three Smad phosphoisoform types: pSmad2C and pSmad3C; pSmad2L and pSmad3L and pSmad2L/C and pSmad3L/C. TβRI and Ras-associated kinases differentially phosphorylate Smad2/3 to create three phosphorylated forms (phosphoisoforms): C-terminally phosphorylated Smad2/3 (pSmad2C and pSmad3C); linker phosphorylated Smad2/3 (pSmad2L and pSmad3L) and dually phosphorylated Smad2/3 (pSmad2L/C and pSmad3L/C). Except for cytoplasmic localization of pSmad2L, the other phosphoisoforms localize to cell nuclei.
Mitogen-activated protein kinase (MAPK), including extracellular signal-regulated kinase (ERK), c-Jun N-terminal kinase (JNK) and p38 MAPK, is an evolutionarily conserved regulator essential for a variety of cellular events (30). Multiple extracellular stimuli can initiate a cascade of serial phosphorylation activation from MAP kinase kinase kinase (MAPKKK) to MAP kinase kinase (MAPKK) and finally MAPK (Figure 1A, right). One of the best-characterized triggers for the MAPK pathway is Ras activation, which propagates mitogenic signals from a number of ligand- or self-activated receptor tyrosine kinase (RTK). In addition, MAPK can be regulated by TGF-β stimulation, which represents an important mechanism for non-Smad TGF-β signaling (31). MAPK phosphorylates nuclear TFs, such as c-Jun, Fos, Maf and ATF subfamilies (32), which can physically interact with Smads and regulate TGF-β responses (5). Other non-Smad pathways include phosphoinositol-3 kinase, RhoA, Rac1 and Cdc42 guanosine triphosphatases (33). Imbalance may occur between signaling through the non-Smad and Smad pathways during carcinogenesis, and some co-operation between these pathways mediates protumorigenic effects of TGF-β (5,33).
Multiple Smad phosphoisoforms exist
Although COOH-tail phosphorylation by TβRI is a key event in R-Smad activation, additional phosphorylation by intracellular protein kinases can positively and negatively regulate R-Smads. R-Smads contain two conserved polypeptide segments, the MH1 and MH2 domains, joined by a less conserved linker region (16). The linker domain undergoes regulatory phosphorylation by MAPK, cyclin-dependent kinase (CDK), glycogen synthase kinase 3-β, Ca (2+)-calmodulin-dependent protein kinase II and G protein-coupled receptor kinase-2 [Figure 1B (20–26,34–38)]. Among these kinases, MAPK and CDK are major groups of protein kinases that exhibit preference for specific serine/threonine residues in the linker regions (26,38,39). Phosphorylation in the linker regions serves an important function in regulating stability, activity and transport of R-Smads. Smurfs have been found to interact with R-Smads (40–42), thereby directly targeting R-Smads for ubiquitin-mediated degradation via the proteasome pathway. Whereas Smurf 1 preferentially interacts with bone morphogenetic protein R-Smads, Smurf2 can associate with TGF-β/activin R-Smads as well as bone morphogenetic protein R-Smads (40–42). Smurf-mediated degradation of R-Smads induces a decrease in cellular competence for TGF-β family-induced responses (40,42). Linker phosphorylation of R-Smads by TGF-β facilitates the binding of the E3 ubiquitin ligase NEDD4L to the R-Smads and consequently results in R-Smad polyubiquitination and degradation (43).
Ras signaling simultaneously activates linker-phosphorylated R-Smads and non-Smad pathways, with both usually operating in parallel. Biologic significance of linker-phosphorylated R-Smad pathways is therefore difficult to assess in isolation. Here, we will review recent work in this area, with a particular focus on how the Ras pathway modulates TGF-β signaling through Smad linker phosphorylation, using colon and liver cancer as examples. Antibodies (Abs) reactive with structurally related phosphorylated peptides are emerging as valuable tools for determining phosphorylation sites in vivo and for investigating their distinct signals via phosphorylated domains. Domain-specific phospho-R-Smad Abs have allowed us to reveal that TβRI and Ras-associated kinases differentially phosphorylate R-Smads to create three phosphorylated forms (phosphoisoforms): C-terminally phosphorylated R-Smad (pSmad2C and pSmad3C), linker-phosphorylated R-Smad (pSmad2L and pSmad3L) and dually phosphorylated R-Smad (pSmad2L/C and pSmad3L/C) (39,44,45). Except for pSmad2L with cytoplasmic localization (20,21,36), the other phosphoisoforms are localized to cell nuclei [Figure 1C (21,24,25,35,38,46–51)]. Linker phosphorylation can modify C-terminally phosphorylated R-Smad signaling (20–22,24–26,34–36). Differential localization of kinases and phosphatases in the cytoplasm or nucleus raises the intriguing possibility of different temporal dynamics for cytoplasmic or nuclear R-Smad phosphoisoforms and adds to the repertoire of signaling responses that determine cell-fate decisions. Immunohistochemical and immunofluorescence analyses using specific Abs in human tissues can examine clinical significance of context-dependent and cell type-specific signaling mediated by R-Smad phosphoisoforms by comparing tissue/cellular localization of these phosphoisoforms in various pathologic specimens.
Cytostatic TGF-β signaling: involvement of the pSmad3C pathway
CDK, cyclins and CDK inhibitors are important molecules for understanding both TGF-β and Ras signaling. Growth arrest by TGF-β occurs via interference with cell cycle progression. Depending on the cell type, TβRI/pSmad3C signal inhibits proliferation by suppressing the expression of c-Myc (52) and by inducing the CDK inhibitors p15INK4B and p21WAF1 (53,54), shutting down cell cycle progression in the early/mid G1 phase of the cell cycle (Figure 2A, right). Another CDK inhibitor p27KIP1 functions as a tumor suppressor via TGF-β signal (55,56). Organisms attempt to block development of cancer through actions of the pSmad3C pathway, which can cause normal epithelial cells to cease growth and enter apoptosis after cell proliferation, in part through the ability of pSmad3C to induce or repress expression of a number of apoptosis-associated proteins such as Bcl2 (57).
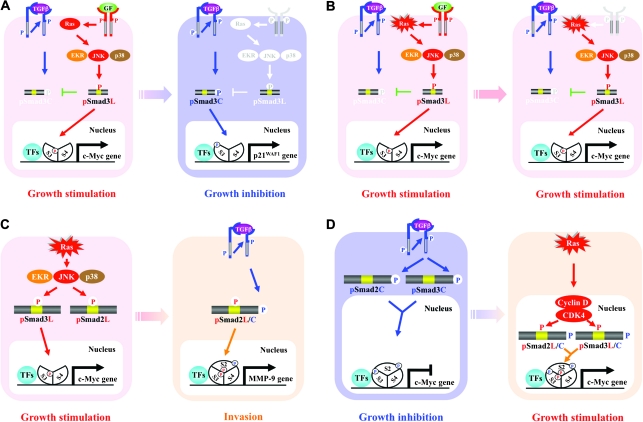
Fig. 2.
Different modes of Smad phosphoisoform signaling in normal epithelium and neoplasms. Ligand-dependent pSmad3L signaling in normal epithelial cells (A) gives way to constitutive pSmad3L signaling in tumor cells (B). (A) In normal epithelial cells, the RTK activated by growth factor (GF) results in activation of the Ras/MAPK pathway, further leading to mitogenic pSmad3L signaling. pSmad3L forms a heterodimeric complex with Smad4, and moves to the nucleus (supplementary Figure S1 is available at Carcinogenesis Online). The nuclear Smad oligomer binds to DNA and associates with other TFs to transmit a mitogenic signal by upregulating transcription of c-Myc gene (supplementary Figures S2 and S3 are available at Carcinogenesis Online). In normal epithelial cells, pSmad3L appears only transiently in response to GFs such as epidermal growth factor (left) and is rapidly reversed to cytostatic pSmad3C signaling once GFs are absent (right). (B) In tumor cells, somatic mutants such as a hyperactive Ras mutant constitutively induce the phosphorylation of Smad3 at its linker region; this pSmad3L loses sensitivity to growth inhibition by pSmad3C (left). In this case, pSmad3L continues to transmit its mitogenic signal even without extracellular GFs (right; supplementary Figure S2 is available at Carcinogenesis Online). Protumorigenic TGF-β signaling: involvement of the pSmad2L/C (C) and pSmad3L/C (D) pathways. (C) Ras activates MAPK, which phosphorylates Smad2L and Smad3L (left). After the COOH-tail phosphorylation of cytoplasmic pSmad2L by TβRI, pSmad2L/C undergoes translocation to the nucleus, where it interacts with pSmad3L and Smad4. Together with TFs, the heterotrimeric complex of pSmad2L/C and pSmad3L with Smad4 stimulates MMP-9 transcription and cellular invasion (right). (D) TGF-β inhibits cell growth by downregulating c-Myc oncoprotein via the pSmad2C and pSmad3C pathways (left); TGF-β signaling in turn enhances cell growth by upregulating c-Myc via the CDK4-dependent pSmad2L/C and pSmad3L/C pathways (right).
Mitogenic Ras signaling: involvement of the pSmad3L pathway
RTK ligands strongly activate the Ras/MAPK pathway, as TGF-β does more weakly (31). Ras/MAPK signaling was shown previously to induce phosphorylation of Smad2 and Smad3 at their linker regions (20). Smad2 phosphorylation at the linker region inhibits nuclear accumulation of Smad2 without interfering with TGF-β-induced phosphorylation of its COOH-tail (25,58–66). In contrast, linker phosphorylation does not retain Smad3 in the cytoplasm, permitting further consequences of the Ras/MAPK signaling. The mechanisms underlying this difference between the two R-Smads are not known, but phosphorylation sites of Smad3 at clusters of three serine residues in its linker region (Ser204, Ser208 and Ser213) are somewhat different in sequence from the corresponding linker phosphorylation sites (Ser245, Ser250 and Ser255) of Smad2 (Figure 1B).
Several lines of evidence indicate that Ras/MAPK transmits mitogenic signals via the pSmad3L pathway (Figure 2A, left). Firstly, RTK ligands such as epidermal growth factor and hepatocyte growth factor transiently induce Smad3 phosphorylation at the linker region and active Ras and ERK mutants constitutively phosphorylate the linker site (20,21,24). Secondly, RTK ligands or Ras-activated MAPKs can directly phosphorylate the linker site in vitro, and various MAPK inhibitors including ERK, JNK and p38 MAPK block linker phosphorylation in vivo (20,21,23,24,35). Thirdly, RTK ligands and constitutively active Ras translocate pSmad3L into the nucleus (21,24,25). Fourthly, nuclear pSmad3L forms a heterocomplex with Smad4 [(21,35), supplementary Figure S1 is available at Carcinogenesis Online]. Fifthly, nuclear pSmad3L binds to Smad-binding element in the promoter with high affinity and specificity (35,67–69). Finally, RTK ligands and Ras induce growth of normal epithelial cells, and such mitogenic effects are blocked by Smad3 mutants lacking linker phosphorylation sites [(24), supplementary Figures S2 and S3 are available at Carcinogenesis Online] and by various MAPK inhibitors (24). These results strongly support the notion that Ras/MAPK specifically signals through Smad3 (13,70).
Reversibility of phospho-Smad3 signaling between cell growth and inhibition
pSmad3L acts as not only a functional molecule actually contributing to the mitogenic effect of Ras but also an antagonist for the cytostatic pSmad3C signaling. Ras-mediated pSmad3L and TβRI-mediated pSmad3C signals oppose each other; most importantly, the balance can shift between cell growth and inhibition (Figure 2A). Linker phosphorylation of Smad3 blocks COOH-tail phosphorylation by TβRI [Figure 2A, left (21,24,36,49,50)]. Mitogenic signaling accelerates nuclear transport of pSmad3L from the cytoplasm, while preventing Smad3C phosphorylation, pSmad3C-mediated transcription and antiproliferative effects of TGF-β [Figure 2A, left (24)]. Smad3 mutants lacking linker phosphorylation sites, as well as various MAPK inhibitors, can restore growth inhibitory and transcriptional responses to TGF-β in Ras-transformed cells and rat carcinomas, both in vitro and in vivo (24,49,50). Our model implies that the Ras/MAPK pathway directly or indirectly modulates pSmad3C and pSmad3L-mediated signaling to regulate target genes, resulting in an antagonistic relationship between cell growth and inhibition. Thus, effectiveness of cytostatic TGF-β signaling can depend on the extent of Smad3 phosphorylation at the linker region.
The Ras/MAPK pathway enhances c-Myc expression, which advances cell cycle and/or promotes cell survival (71). The c-Myc oncoprotein can antagonize the two CDK inhibitors p21WAF1 and p15INK4B (72,73). In the initial 1–2 h of TGF-β treatment, c-Myc inhibits the two CDK inhibitors through binding to Smad2 and Smad3 and suppression of their function (73). After c-Myc decreases, Smads act together with cellular factors to activate transcription of CDK inhibitors (74–78). By repressing expression of the genes encoding these CDK inhibitors, c-Myc eliminates two major obstacles to cycle progression. Stated differently, the MAPK/pSmad3L/c-Myc pathway strongly suppresses the TβRI/pSmad3C/the CDK inhibitor pathway, thereby paving the way for vigorous cell cycle advancement.
In normal epithelial cell homeostasis, the pSmad3L/c-Myc signaling is dependent on mitogens, rapidly disappearing when extracellular mitogenic stimuli has been withdrawn [Figure 2A (21,24)]. Most tumor cells, however, produce constitutively active forms of one or more intracellular signal transduction proteins that cause growth-promoting signaling in the absence of mitogenic stimuli (79). Importantly, mutations in the key pathway components lead to sustained linker phosphorylation. For example, a constitutively active Ras persistently induces the phosphorylation of Smad3 at its linker region [Figure 2B (20,24), supplementary Figure S2 is available at Carcinogenesis Online]. Highly phosphorylated Smad3L is likely to impair sensitivity to growth inhibition by pSmad3C in tumor cells (48–51). Furthermore, a frequent hallmark of tumor cells is overexpression and/or amplification of cell surface growth factor receptors (80), resulting in aberrant constitutive linker phosphorylation of Smad3 in the absence of extracellular ligand.
Overactivation of Ras pathways contributes to carcinogenesis in several ways, including interference with cell cycle regulation via another CDK inhibitor p27KIP1 and disruption of TGF-β antiproliferative activity. p27KIP1 acts as a tumor suppressor by inhibiting CDK activity in the nucleus (81), whereas proteolytic degradation and cytoplasmic mislocalization of p27KIP1 may play a carcinogenic role (82–84). Importantly, exclusion of p27KIP1 from the nucleus by Ras is sufficient to impair TGF-β-mediated growth inhibition (85). Overall, the net loss of nuclear p27KIP1 is correlated with tumor aggressiveness and poor clinical outcome (86).
Protumorigenic TGF-β signaling: involvement of the pSmad2L/C and pSmad3L/C pathways
In later stages of cancer, TGF-β co-operates with the mitogenic Ras pathway to induce an invasive and proliferative tumor phenotype (10,11,87,88). Important effectors of cellular invasion include the matrix metalloproteinase (MMP). Many carcinomas release MMPs, notably MMP-2 and MMP-9, which are expressed at the invasive fronts of various advanced carcinomas. MMP-2/9 can act at several stages of the invasion–metastasis cascade, including local invasion by the primary tumors, intravasation and extravasation (89). In a colorectal model, we reported that promotion of cellular invasion and MMP-9 expression requires both complete linker and COOH-tail phosphorylation of Smad2, whereas an MMP-9 inhibitor blocks platelet-derived growth factor (PDGF)- and TGF-β-driven cellular invasion, indicating that TGF-β together with PDGF induces MMP-9-mediated cellular invasion via the pSmad2L/C pathway [Figure 2C, right (25)]. After COOH-tail phosphorylation of cytoplasmic pSmad2L by TβRI, pSmad2L/C undergoes translocation to the nucleus where it binds to the pSmad3L and Smad4 complex (25). Consequently, the Smad complex stimulates MMP-9 transcription and cellular invasion (25,90). Smad2 accumulates in the nucleus only if its C-terminus is phosphorylated under conditions of sustained linker phosphorylation by MAPK. In this case, MAPK-mediated Smad2 phosphorylation at the linker region serves to ‘prime’ for TβRI docking and further phosphorylation at the C-terminus (25).
Recent understandings of molecular aspects of Smad phosphoisoform signaling further offer potential for understanding the molecular mechanisms regulating the two opposing effects of TGF-β, namely growth inhibition and stimulation. Only Rb family members were known to be substrates of CDK4 until the Liu group reported that Smad3 is phosphorylated by both CDK4 and CDK2 in vivo and in vitro (22). CDK4 phosphorylation of Smad3 at its linker region inhibits its transcriptional activity and the antiproliferative activity of TGF-β (22,26). COOH-tail phosphorylation of Smad3 is necessary for TGF-β-induced phosphorylation of Smad3 at its linker region (91,92). Consistent with these observations about Smad3, we confirmed that nuclear cyclin D1·CDK4 complex activated by TGF-β signaling directly phosphorylates the linker segment of pSmad2C to produce pSmad2L/C (25). Other nuclear CDK members including CDK8 and CDK9 phosphorylate the linker portions of pSmad1C, pSmad2C and pSmad3C to create pR-SmadL/C (38). Expression of c-Myc in fibroblasts is initially repressed by TGF-β, but subsequent TGF-β signaling undergoes a complete change to stimulate c-Myc (25). In contrast, TGF-β persistently inhibits c-Myc expression and growth in fibroblasts carrying Smad2/3 mutants lacking CDK phosphorylation sites in their linker regions (Figure 1B). Collectively, TGF-β inhibits cell growth by downregulating the c-Myc oncoprotein via the pSmad2C and pSmad3C pathway (Figure 2D, left). However, TGF-β can enhance cell growth by upregulating c-Myc via the CDK4-dependent pSmad2L/C and pSmad3L/C pathways in the nuclei of fibroblasts (Figure 2D, right).
Pin1 is a peptidyl-prolyl cis/trans isomerase that recognizes phosphorylated serine–proline motifs in certain proteins, catalyzing proly cis/trans isomerization (93). Nakao et al. (94) reported that Pin1 can associate with Smad2 and Smad3 to enhance their interaction with Smurf2 and a homologous to E6-associated protein C-terminus domain E3 ubiquitin ligase, resulting in enhanced Smad ubiquitination and reduction in Smad2/3. Interestingly, a constitutively activated Ras, which leads to Smad2/3 phosphorylation at their linker regions, can induce Smad2/3 binding to Pin1 in response to TGF-β. Further analyses by Matsuura et al. showed that Smad3 phosphorylation at both the COOH-tail and at Thr179 in the linker segment (Figure 1B) is necessary for Pin1 binding and that knockdown of Pin1 results in inhibition of TGF-β-mediated migration and invasion (92).
To acquire motility and invasiveness, carcinomas must shed much of their epithelial phenotypes, detach from epithelial sheets and undergo a drastic epithelial–mesenchymal transition (EMT), which normally occurs early in embryogenesis (79). EMT involves loss of an epithelial cell gene expression program and acquisition of mesenchymal gene expression, which allows tumor cells to acquire motility and invasiveness. In one set of influential experiments, exposure of Ras transformed, but not normal, epithelial cells to TGF-β result in progressive reduction in epithelial morphology and in epithelial markers including cytokeratins and E-cadherin (87). At the same time, these transformed cells acquire mesenchymal protein markers such as vimentin and assume a morphology resembling that of fibroblasts.
TGF-β has emerged as a major inducer of EMT through activation of downstream signaling pathways, including non-Smad signaling pathways [Figure 1A (31,33)]. On the other hand, investigation concerning certain fibrotic diseases using Smad3-null mice indicates an essential role of Smad3 in EMT (95). Several TFs, including the zinc-finger factors Snail and Slug, play critical roles in induction of EMT (19). Horiguchi et al. (96) recently reported co-operation between Ras and TGF-β-Smad signaling in induction of Snail. Snail induction occurs independently of R-Smad phosphorylation at the linker regions. In support of this notion, selective prevention of linker phosphorylation using a Smad3 mutant lacking phosphorylation sites in this region cannot completely suppress TGF-β-mediated EMT in our Ras-transformed cells (Matsozaki K, unpublished data). However, absence of R-Smad linker phosphorylation results in moderate reduction of plasminogen activator inhibitor Type I expression and strong reduction of c-Myc and MMPs expression (24). Thus, pSmad2L/C and pSmad3L/C have specific roles in promoting invasion and proliferation in response to TGF-β, apparently depending on the promoter context (92).
Physiologic phospho-Smad3 signaling: mitogenic pSmad3L signaling followed by cytostatic pSmad3C signaling
Cell proliferation occurs continuously as a constant tissue-renewal strategy (97). In the colon, enterocytes are constantly renewed by immature cells, which proliferate at the base of mucosal crypts and then migrate upward to the luminal surface. This process is tightly controlled by a delicate balance between proliferation and differentiation of enterocytes (98). The phosphorylation pattern of Smad3 in normal colonic mucosa suggests important participation of Smad3 in maintaining this balance (51). In immature enterocytes near the bottom of normal colonic crypts, intracellular phosphorylation at Smad3L is high (Figure 3A). Translocated to the nucleus, pSmad3L stimulates c-Myc transcription; this increases proliferation of enterocytes and opposes the cytostatic action of the pSmad3C/p21WAF1 pathway (Figure 2A, left). Accordingly, pSmad3C/p21WAF1 is undetectable at the bottom of normal crypts: escape from TGF-β-induced cytostasis is crucial in a subset of progenitor cells devoted to ensuring epithelial renewal. As enterocytes migrate upward from the crypt base to the lumen, the growth-stimulatory signal via pSmad3L ceases in the differentiated enterocytes. Decreased pSmad3L can lead to increased sensitivity to phosphorylation at Smad3C by TβRI (24) and TGF-β can then activate the promoter of p21WAF1 via pSmad3C to block cell cycle advance.
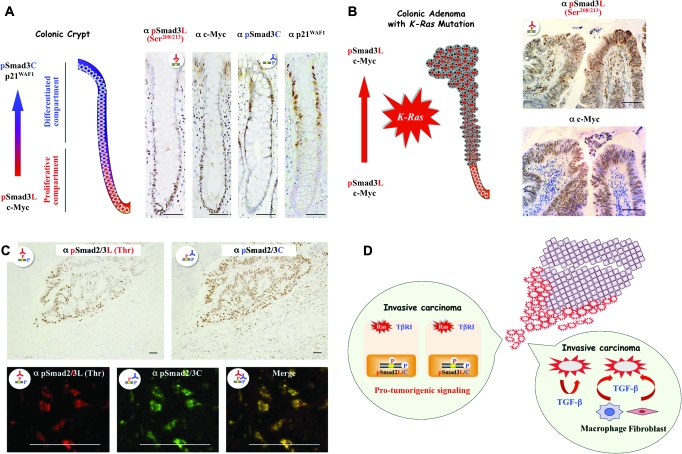
Fig. 3.
Smad phosphoisoform signaling can determine specific cellular responses to TGF-β in normal enterocytes, a colonic adenoma and an invasive carcinoma metastasized from colon. Phospho-Smad3 signaling and the biology of colonic crypt (A) and adenoma (B). Formalin-fixed paraffin-embedded sections of normal colonic crypt (A) and adenoma (B) were stained with anti-pSmad3L (Ser208/213) Ab [α pSmad3L (Ser208/213) column], anti-c-Myc Ab (α c-Myc column), anti-pSmad3C Ab (α pSmad3C column) or anti-p21WAF1 Ab (α p21WAF1 column). Sections stained for pSmad3L (Ser208/213) or pSmad3C were paired with adjacent sections stained with anti-c-Myc Ab or anti-p21WAF1 Ab, respectively. All sections were counterstained with hemotoxylin (blue). Brown product indicates specific Ab reactivity. Scale bars = 100 μm. (A) Physiologic roles of pSmad3L and pSmad3C in normal colonic crypts. Immunoreactivity for pSmad3L shows a striking distribution, localized to the nuclei of c-Myc-immunoreactive progenitor cells at the bottom of mucosal crypts [proliferative compartments: lower portions of crypts in α pSmad3L (Ser208/213) and α c-Myc columns]. In the colonic progenitor cells, linker phosphorylation of Smad3 prevents the cytostatic p21WAF1 effect by shutting down phosphorylation at Smad3C. As enterocytes migrate upward from the crypt base to the lumen, the growth-stimulatory signal via pSmad3L ceases in the differentiated enterocytes (differentiated compartments). TGF-β can then activate the promoter of p21WAF1 via pSmad3C to arrest the growth of enterocytes (upper portions in α pSmad3C and α p21WAF1 columns). (B) pSmad3L/c-Myc-immunoreactive adenomas accumulate within entire crypts and ultimately form an adenomatous polyp. pSmad3L/c-Myc-immunoreactive colonic adenoma carrying K-Ras mutation [α pSmad3L (Ser208/213) and α c-Myc columns] becomes able to divide indefinitely (red arrow), instead of undergoing physiologic pSmad3C/p21WAF1-mediated differentiation and death. At the edges of advanced carcinomas invading adjacent tissues, pSmad2L/C and pSmad3L/C transmit pro-tumorigenic TGF-β signal (C and D). (C) The upper panels show formalin-fixed, paraffin-embedded sections of invasive carcinomas carrying K-Ras mutation, which had metastasized from the colon, as well as uninvolved liver. Tissues were stained with anti-pSmad2/3L (Thr) Ab [α pSmad2/3L (Thr) column] or anti-pSmad2/3C Ab (α pSmad2/3C column). Sections stained for pSmad2/3L (Thr) were paired with adjacent sections stained with pSmad2/3C. All sections were counterstained with hemotoxylin (blue). Brown product indicates specific Ab reactivity. Scale bars = 100 μm. Fibroblasts, macrophages and hepatocytes in the uninvolved liver tissue (lower portions) show little phosphorylation of Smad2/3 at the linker regions [α pSmad2/3L (Thr) column], but moderate phosphorylation at their C-tail regions (α pSmad2/3C column). In the carcinomas (upper portions) invading adjacent liver tissue, nuclear Smad2 and Smad3 are highly phosphorylated at both their linker and C-tail regions [α pSmad2/3L (Thr) and α pSmad2/3C columns]. The lower panels show sections of invasive tumor tissue stained for immunofluorescence to simultaneously detect pSmad2/3L (Thr) (red) and pSmad2/3C (green). Yellow color indicates the presence of both pSmad2L/C and pSmad3L/C. Scale bars = 100 μm. At invasion fronts of the carcinomas, nuclear pSmad2/3L (Thr) co-localizes with pSmad2/3C. (D) upper right: Tumor cells (pink) take on an invasive phenotype at the edge of carcinomas (red) invading adjacent liver tissue. Balloon, left: pSmad2L/C and pSmad3L/C transmit the protumorigenic TGF-β signal in combination with oncogenic Ras. Balloon, lower right: Invasive carcinomas carrying K-Ras mutation gain access to abundant TGF-β from either autocrine or microenvironmental sources. Macrophages and fibroblasts release TGF-β in the reactive stroma.
The pSmad3L/c-Myc signaling in colonic progenitor cells works to inhibit enterocytic differentiation. Conversely, the pSmad3C/p21WAF1 pathway is needed to halt proliferation of enterocytes and to facilitate their differentiation. The mature enterocytes ultimately begin the process of apoptosis via the cytostatic pSmad3C signaling. In normal enterocytes, pSmad3L appears only transiently in response to extracellular mitogenic signals (Figure 2A). This entire process of outward migration and cell death takes only 3–4 days.
Alteration of phospho-Smad3 signaling during carcinogenesis: reciprocal change in oncogenic pSmad3L and tumor-suppressive pSmad3C pathways
The above regulatory mechanism, which avoids accumulation of deleterious mutations in genes that promote cell growth and division, must be disrupted before enterocytes can throw off their normal restraints and behave as a social cancer cells. Somatic mutations in colorectal adenoma include changes in K-Ras gene that favor adenoma progression (6). In adenoma cell nuclei, pSmad3L/c-Myc can accumulate when the Ras mutation constitutively activates the MAPK pathway to phosphorylate Smad3 at the linker region (Figure 3B). More specifically, the proliferative effect mediated via the pSmad3L/c-Myc pathway keeps on suppressing the growth-inhibitory pSmad3C/p21WAF1 pathway in the nuclei of the benign tumor cells (Figure 2B). Adenoma cells are relatively resistant to apoptosis by the pSmad3C pathway (99), which allows them to increase in number and survive where they should not. As a result, proliferative tumor cells accumulate within entire crypts, ultimately forming an adenomatous polyp [Figure 3B (46,51)].
Adenomatous polyps are believed to be precursors of a large proportion of colorectal cancers (6). In microscopic sections of polyps <1 cm in diameter, the cells and their arrangement in the epithelium typically appear nearly normal. The larger the polyp, the more likely it contains cells with abnormally undifferentiated cytologic features and a tendency to form abnormally organized glandular structures. Sometimes, two or more distinct areas can be distinguished within a single polyp, with cells in one area appearing relatively normal and those in the other appearing clearly cancerous, as though they have arisen as a mutant subclone within the original adenoma clone. Mutational activation of the K-Ras oncogene seems dependent on size and shape of colorectal adenomas (100). K-Ras mutations are present in 10% of colorectal adenomas with diameters of <1 cm, but in 30% of adenomas >2 cm. Furthermore, K-Ras mutations are detected in up to 60% of protruding adenomas but are rare in superficial flat adenomas. Reflecting these patterns of K-Ras mutation frequency, immunohistochemical studies have shown that Smad3-mediated signaling shifts from the tumor-suppressive pSmad3C/p21WAF1 to the oncogenic pSmad3L/c-Myc pathway as human colorectal adenomas progress to colorectal cancers (46,51).
A constitutively active Ras persistently stimulates enterocytes to proliferate in an organ that normally does not experience the continuous proliferation: proliferation by progenitor cells in normal crypts is tightly regulated by the cytostatic pSmad3C signaling (Figure 3A). Such signaling represents a highly effective defense mechanism against development of colorectal cancer since normal epithelial cells containing pSmad3C that sustain any mutations are destined to die (57). On the other hand, escaping the cytostatic action of pSmad3C is a critical step for progression to full malignancy in cancers. The TGF-β/pSmad3C pathway is also required for maintenance of genomic stability, induction of replicative senescence and suppression of telomerase (101–103). It is probably that these activities of TGF-β contribute to tumor suppression along with its cytostatic effect.
At the edges of advanced carcinomas invading adjacent tissues, pSmad2L/C and pSmad3L/C transmit protumorigenic TGF-β signals
Profound shifts in cell phenotype are often initiated by collaboration between specific mutant alleles harbored in cancer cell genomes (e.g. Ras oncogene) and TGF-β signal that carcinomas receive in some tissue microenvironments, specifically at the boundary between tumor epithelium and reactive stroma (10). Does each step in the cascade of malignancy require actions of specific TGF-β signaling pathways that become altered during tumor progression? To answer this question, we have focused on the Smad pathway, investigating the localization of pSmad2L/C and pSmad3L/C in human advanced colorectal carcinomas carrying K-Ras mutation since these phosphoisoforms transmit invasive and proliferative TGF-β signals (Figure 2C and D). The results indicate nuclear localization of pSmad2L/C and pSmad3L/C at the boundary between tumor epithelium and reactive stroma in advanced carcinomas invading adjacent tissue [Figure 3C and D (25)]. In particular, strong Smad2/3 phosphorylation is observed at threonine residues in the linker regions (Figure 1B). In vitro kinase assay confirms that nuclear CDK4 and cytoplasmic JNK obtained from the cancerous tissues can phosphorylate Smad2 or Smad3 at the linker regions (25). These results point to an additive requirement of JNK and CDK4 activities for pSmad2L/C and pSmad3L/C. We conclude that perturbation of TGF-β signaling by aberrant activation of nuclear CDK4 and cytoplasmic JNK underlies the critical role of TGF-β in protumorigenic behavior at the edges of human advanced carcinomas. Because Ras-associated kinases such as CDK4 and JNK are commonly activated in various types of human cancers (104,105) and invasive carcinomas gain access to abundant TGF-β from either autocrine or microenvironmental sources (Figure 3D), these Ras-associated kinases in cancerous tissues could confer protumorigenic activity on otherwise tumor-suppressive TGF-β signals at the invasion fronts of advanced carcinomas.
Invasive pSmad2L/C together with oncogenic pSmad3L pathways characterize TGF-β signaling shared between Ras-transformed cells and human advanced carcinomas
Human advanced carcinomas usually retain the protumorigenic TGF-β signaling component but have lost the capacity to respond to TGF-β with growth arrest (13,14,75). Such a state of altered TGF-β responsiveness is observed in Ras-transformed cells. These cells typically exhibit a limited growth-inhibitory response to TGF-β instead responding to TGF-β with invasive (87) and metastatic behavior (61). A clue to understanding the molecular mechanisms is suggested by differential cellular localization of pSmad2L and pSmad3L in Ras-transformed cells and carcinoma in situ (Figures 2 and 4A). Linker phosphorylation of Smad2 at Ser250/255 is associated with its cytoplasmic retention [Figure 1C (46)], whereas pSmad3L (Ser208/213) is predominantly localized in cell nuclei of actively growing colorectal cancer and hepatocellular carcinoma [HCC; Figure 1C (46–51)]. Likewise, hyperactive Ras retains most Smad2 protein in the cytoplasm (20,24) but facilitates nuclear accumulation of pSmad3L (24,25).
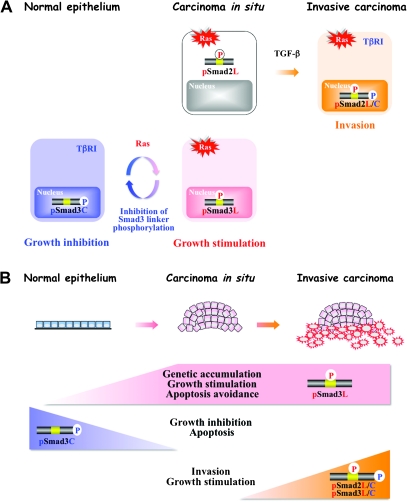
Fig. 4.
Invasive pSmad2L/C together with oncogenic pSmad3L pathways characterize TGF-β signaling shared between Ras-transformed cells and human advanced carcinomas. (A) Tumor cells must evade pSmad3C-imposed growth inhibition if they are to thrive. More specifically, such tumor cells depend on highly activated Ras/pSmad3L signaling to drive their proliferation. Selective blockade of linker phosphorylation can suppress the Ras/pSmad3L pathway, while restoring the lost cytostatic pSmad3C signaling as present in mature epithelial cells. Later, in tumor development, TGF-β transmits an invasive signal via the pSmad2L/C pathway together with oncogenic pSmad3L signaling. (B) During human carcinogenic process, benign tumors affected by somatic mutations including Ras undergo transition from the tumor-suppressive pSmad3C pathway to the oncogenic pSmad3L pathway, becoming carcinoma in situ. Advanced carcinomas acquire more invasive and proliferative properties via the pSmad2L/C and pSmad3L pathways when carcinoma in situ receives TGF-β signals from the reactive stroma.
Phenotypes of carcinoma in situ are dictated by genotype and tumorigenic growth is essentially a cell-autonomous phenomenon that involves a shift from the tumor-suppressive pSmad3C pathway to the oncogenic pSmad3L pathway induced by alterations in the cancer cell genome such as Ras oncogene (Figure 4A). These results suggest an intriguing mechanistic clue as to why Smad3 is seldom mutated in human cancer, but rather is posttranscriptionally regulated (13). Later in tumor progression, TGF-β utilizes different phospho-Smad pathways to mediate protumorigenic effects. Invasive behavior is strongly influenced by stromally produced TGF-β signal, which is processed by the pSmad2L/C pathway to modulate cell shape, adhesion and localized proteolysis in the nearby extracellular matrix (Figures 3C and 4A). Our current data support a multistep model of tumor growth and invasion that involves progressive increases of pSmad2L/C and pSmad3L with concomitant suppression of the cytostatic pSmad3C signaling (Figure 4A and B). Thus, invasive pSmad2L/C together with oncogenic pSmad3L can mediate the protumorigenic TGF-β signaling that allows carcinomas to acquire invasive and proliferative properties needed for progression. As a result, human advanced carcinomas lose responsiveness to TGF-β in terms of growth inhibition, whereas TGF-β can still induce invasiveness.
Cell type-specific spatial and temporal dynamics of R-Smad phosphoisoforms
In contrast to the presence of COOH-tail phosphorylation of R-Smads in almost all cell types and tissues, timing, duration, extent and functional implications of linker phosphorylation depend on cell type and context. Therefore, the influence of linker phosphorylation on COOH-tail phosphorylation has been an unsettled subject with various data suggesting that Ras-mediated linker phosphorylation either inhibits (20,21,24,34,36,37,106–112) or enhances (22,23,25,35,47,58,61,62,65,66,91,92,113–119) events downstream of TβRI. Several possible explanations exist for these different outcomes.
Firstly, involvement of different Ras-associated kinases may explain outcomes differing among various cell types and contexts. Normal epithelial cells generally show rapid phosphorylation at the linker regions in response to various mitogens, and the responsible kinases appear to act before R-Smads reaches the nucleus (Figure 2A). Both JNK and ERK are localized in the cytoplasm and directly phosphorylate the linker regions, creating pSmad2L and pSmad3L (20,24). In contrast, mesenchymal cells show slow phosphorylation of R-Smads at their linker regions, and their kinases act after nuclear translocation of pSmad2C and pSmad3C in response to TGF-β [Figure 2D (25,38,92)]. CDKs are localized in the nucleus and directly phosphorylate the linker regions of pSmad2C and pSmad3C, producing pSmad2L/C and pSmad3L/C (22,25,38). As epithelial cells are transformed into carcinomas, they come to exhibit strong constitutive linker phsophorylation [Figure 2B (20,24)]. Nuclear CDKs together with cytoplasmic MAPKs convert the tumor-suppressive pSmad2/3C signal into pSmad2L/C and/or pSmad3L/C-mediated protumorigenic character.
TGF-β and mitogens exert mutually antagonistic effects on cell cycle control and apoptosis in normal epithelial cells (Figures 2A and 3A). Mitogens drastically alter Smad3 signaling via the MAPK pathway, increasing basal nuclear pSmad3L activity while shutting down TGF-β-dependent pSmad3C that otherwise would be available to act in the nuclei of normal epithelial cells (Figure 2A). Because mitogenic pSmad3L signaling is followed by cytostatic pSmad3C signaling during normal epithelial regeneration, pSmad2L/C and pSmad3L/C rarely exist in normal epithelial cells either in vitro or in vivo (Figures 3A and 5A). In contrast, TGF-β and mitogen/Ras signaling synergistically promote growth and invasion in mesenchymal cells (25,92). Blocking either linker or COOH-tail phosphorylation of Smad2 abrogates the synergistic responses of fibroblasts to TGF-β and PDGF (25), indicating involvement of pSmad2L/C in this synergistic mesenchymal cell response (Figure 5B). As in mesenchymal cells, an invasive phenotype is favored by the pSmad2L/C pathway in response to a mixture of signals converging on carcinoma in situ, which receives TGF-β signal from the stroma together with intracellular signal released by Ras oncogene (Figures 3C and 5C). Thus, TGF-β signaling confers a selective advantage upon carcinomas by shifting from the pSmad2C and pSmad3C pathways characteristic of mature epithelial cells (Figures 3A and 5A) to the pSmad2L/C and pSmad3L/C pathways (Figures 3C and 5C), which is more characteristic of the state of flux shown by activated mesenchymal cells. Loss of epithelial homeostasis and acquisition of a migratory, mesenchymal phenotype are essential for invasion in later stages of human cancer (79).
Fig. 5.
Cell type-specific temporal dynamics of R-Smad phosphoisoforms. Although linker phosphorylation is transient after mitogen treatment of normal epithelial cells (A), mitogen-inducible phosphorylation generally persists in various mesenchymal cells (B). Moreover, constitutive linker phosphorylation is found in almost all types of carcinomas and Ras-transformed cells (C). Because mitogenic pSmad3L signaling is followed by the cytostatic pSmad3C signaling in normal epithelial homeostasis, pSmad2L/C and pSmad3L/C rarely exist in normal epithelial cells (A). Resembling observations in mesenchymal cells (B), carcinomas acquire an invasive phenotype via the pSmad2L/C pathway created by a combination of TGF-β signal with intracellular Ras signal (C).
Kinases play prominent roles in directing R-Smad phosphoisoforms, depending on where the kinases phosphorylate R-Smads: plasma membrane, cytoplasm or nucleus. The first level TβRI kinase is activated on the plasma membrane in response to TGF-β. Secondly, MAPK activation can occur in the cytoplasm; at the third level, CDKs are confined to the cell nucleus. For example, JNK activated in the cytoplasm phosphorylates Smad2, converting it to pSmad2L (Figure 2C). TβRI activated at the plasma membrane phosphorylates cytoplasmic pSmad2L to form pSmad2L/C, which enters the nucleus. Alternatively, TβRI phosphorylates Smad2 to convert it to pSmad2C, which moves to the nucleus (Figure 2D). Then, CDKs phosphorylate nuclear pSmad2C to form pSmad2L/C. Therefore, depending on the input, differential subcellular R-Smad phosphoisoforms can allow kinase modules to signal to different phosphoisoforms.
A second explanation for varied linker phosphorylation effects involves differential duration of linker phosphorylation among various cell types. Although linker phosphorylation is transient after mitogen treatment of normal epithelial cells [Figure 5A (21,24)], mitogen-inducible phosphorylation is generally persistent in various mesenchymal cells [Figure 5B (25,47)]. Moreover, constitutive linker phosphorylation is found in almost all types of cancer cells including Ras-transformed cells and human carcinomas [Figure 5C (24,25,46,49,51)].
Activities of any proteins regulated by phosphorylation depend on the balance at any time point between activities of kinases that phosphorylate them and those of phosphatases that dephosphorylate them. Several lines of evidence identify small C-terminal domain phosphatases (SCP1–3) as R-Smad linker-specific phosphatases (44,120), which dephosphorylate Ser245, Ser250 and Ser255 sites in Smad2L or Ser204, Ser208 and Ser213 sites in Smad3L [Figure 1B (120)]. According to cell type-specific duration of linker phosphorylation, mesenchymal and cancer cells may not be able to induce or activate the relevant phosphatases. It should be noted that phosphorylation at Thr220 in Smad2L and at Thr179 in Smad3L is not dephosphorylated by SCP1–3 in vivo or in vitro, although several kinases (MAPKs and CDKs) are able to phosphorylate these threonine residues [Figure 1B (20,22,24,26,38,120)], through which advanced carcinomas continually transmit protumorigenic signals [Figure 3C (25)]. In sum, spatial distribution and temporal qualities of kinases and phosphatases can markedly influence qualitative and quantitative features of downstream R-Smad phosphoisoform signaling.
Risk categorization for cancer occurrence based on pSmad3L and pSmad3C
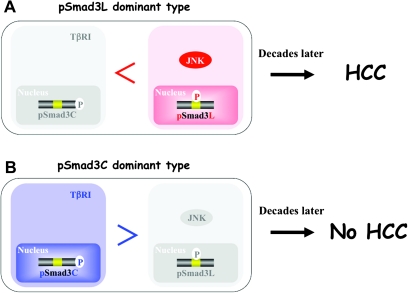
Clinical analyses of pSmad3L and pSmad3C in human tumor formation have provided substantial mechanistic insight. For example, human livers infected by hepatitis B virus progress from chronic hepatitis B to HCC several decades later (121). Specimens from patients with chronic hepatitis B who develop HCC show abundant Smad3L but limited Smad3C phosphorylation in hepatocytic nuclei [Figure 6A (49)], whereas other patients with abundant hepatocytic pSmad3C but limited pSmad3L do not develop HCC (Figure 6B). The same relationships are observed in human hepatitis C virus-related hepatocarcinogenesis (48). These clinical observations support roles for pSmad3C as a tumor suppressor and pSmad3L as a promoter during carcinogenesis.
Fig. 6.
Risk categorization for cancer occurrence based on pSmad3L and pSmad3C. Preneoplastic epithelial cells are in danger of eventually becoming carcinomas. Early chronic hepatitis B has presented a quandary to oncologists with respect to highly variable patient outcomes, with some patients developing HCC and others being cured. Although liver specimens in early chronic hepatitis B have a very similar appearance under the microscope, use of Smad3 phosphorylation profiles allows patients with chronic hepatitis B to be divided into two distinct categories with different clinical outcomes: patients with high or low risk for HCC. (A) HCC subsequently develops in patients whose chronic hepatitis B specimens show strong hepatocytic nuclear positivity for pSmad3L. (B) HCC does not occur in patients whose chronic hepatitis B specimens show strong hepatocytic positivity for pSmad3C.
Smad phosphorylation profiles show great promise by allowing clinicians to stratify preneoplastic epithelia into subgroups with distinct biologic properties including oncogenic potential. The long-term goal of this analytic technique is to accurately assess cancer risk and to supplement the existing clinical criteria for judging whether drugs should be given to patients with high cancer risk (122). Such an approach offers high selectivity for narrowly defined patient populations.
The JNK/pSmad3L pathway is an important target for therapy devised to reduce emergence of cancer
Acquisition of a preneoplastic phenotype is usually accompanied by defective apoptosis or differentiation, associated with entrance into a mitotic state (123). JNK acts as an important regulator of Smad3 signaling by increasing the basal amount of hepatocytic pSmad3L available for cell growth, while inactivating the TGF-β-dependent cytostatic actions of pSmad3C [Figure 2B (24,48,49)]. These behaviors suggest an attractive strategy for preventing HCC. A key therapeutic aim in chronic liver disorders is restoration of lost tumor-suppressive function as observed in normal hepatocytes, at the expense of effects promoting hepatic carcinogenesis (124). To accomplish this difficult aim, Nagata et al. (50) administered a JNK inhibitor SP600125 to rats and were able to suppress chemical hepatocarcinogenesis by shifting hepatocytic Smad3 signaling from the oncogenic pSmad3L pathway to the tumor-suppressive pSmad3C pathway [Figure 4A (50)]. This study provides proof-of-principle that the JNK/pSmad3L pathway is an important target for therapy devised to reduce emergence of HCC in the context of chronic liver injury.
Conclusions and future perspectives
We have changed our perception of R-Smad signaling pathways from a simple pipeline to sophisticated networks. We also have begun to understand how kinases and phosphatases can determine the kinetics of distinct biochemical processes to predictably translate them into specific biologic responses. Although Smad2 and Smad3 are grouped together as TGF-β-specific R-Smads (15–19), the differential function of Smad2 and Smad3 is of major importance for understanding not only Smad signaling during carcinogenesis but also protumorigenic role of TGF-β in cancer cells (125). Particularly, fibroblasts from Smad3 knockout mice still produce matrix material in response to TGF-β, leading to suggestion that Smad2 is the specific mediator of many extracellular matrix responses involving cell invasion (126). In contrast, Smad3 plays a critical role in both regulation of epithelial cell growth and protumorigenic TGF-β signaling (13). Taken together, we are realizing that TGF-β signaling specificity at the different stages of tumors arises from combined assemblies and spatiotemporal dynamics of R-Smad phosphoisoforms.
Most of our main information has been obtained by studying cells in culture and by examining samples from human patients. Ultimately, we need to examine whether or not domain-specific phosphorylation of R-Smads is essential for carcinogenesis and cancer progression. Conditional knockout mice selectively altered with respect to domain-specific phosphorylation will provide definitive knowledge about R-Smad phosphoisoform pathways and gene targets that shift from tumor-suppressive to protumorigenic through oncogenic influences (research currently underway). This information is also critical for design of new approaches to inhibit invasion and metastasis as well as for planning use of domain-specific phosphorylation inhibitors to treat other chronic conditions including fibrotic diseases.
Supplementary material
Supplementary Figures S1–S3can be found at http://carcin.oxfordjournals.org/
Funding
Ministry of Education, Science and Culture of Japan (22390153 to K.M).
Supplementary Material
Acknowledgments
We are grateful to Drs Wakefield (National Institutes of Health) and Popov (Harvard University) for critical reading of the manuscript. We recognize many outstanding published contributions of investigators in the fields of TGF-β signaling and cancer. We apologize to the researchers whose study is not included in this review owing to space limitations.
Conflict of Interest Statement: None declared.
Glossary
Abbreviations
- CDK
cyclin-dependent kinase
- EMT
epithelial-to-mesenchymal transition
- ERK
extracellular signal-regulated kinase
- HCC
hepatocellular carcinoma
- JNK
c-Jun N-terminal kinase
- MAPK
mitogen-activated protein kinase
- MH
Mad homology
- MMP
matrix metalloproteinase
- PDGF
platelet-derived growth factor
- R-Smad
receptor-activated Smad
- RTK
receptor tyrosine kinase
- TGF
transforming growth factor
- TF
transcription factor
- TβRI
TGF-β Type I receptor
References
- 1.Moses HL, et al. TGF-β stimulation and inhibition of cell proliferation: new mechanistic insights. Cell. 1990;63:245–247. doi: 10.1016/0092-8674(90)90155-8. [DOI] [PubMed] [Google Scholar]
- 2.Roberts AB, et al. The transforming growth factor-βs. In: Sporn MB, Roberts AB, editors. Peptide Growth Factors and Their Receptors. Berlin, Germany: Springer; 1990. pp. 419–472. [Google Scholar]
- 3.Bellam N, et al. TGF-β signaling alterations and colon cancer. Cancer Treat. Res. 2010;155:85–103. doi: 10.1007/978-1-4419-6033-7_5. [DOI] [PubMed] [Google Scholar]
- 4.Markowitz S, et al. Inactivation of the type II TGF-β receptor in colon cancer cells with microsatellite instability. Science. 1995;268:1336–1338. doi: 10.1126/science.7761852. [DOI] [PubMed] [Google Scholar]
- 5.Moustakas A, et al. Non-Smad TGF-β signals. J. Cell Sci. 2005;118:3573–3584. doi: 10.1242/jcs.02554. [DOI] [PubMed] [Google Scholar]
- 6.Fearon ER, et al. A genetic model for colorectal tumorigenesis. Cell. 1990;61:759–767. doi: 10.1016/0092-8674(90)90186-i. [DOI] [PubMed] [Google Scholar]
- 7.Hahn WC, et al. Rules for making human tumor cells. N. Engl. J. Med. 2002;347:1593–1603. doi: 10.1056/NEJMra021902. [DOI] [PubMed] [Google Scholar]
- 8.Loeb LA, et al. Multiple mutations and cancer. Proc. Natl Acad. Sci. USA. 2003;100:776–781. doi: 10.1073/pnas.0334858100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Futreal PA, et al. A census of human cancer genes. Nat. Rev. Cancer. 2004;4:177–183. doi: 10.1038/nrc1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kalluri R, et al. The basics of epithelial-mesenchymal transition. J. Clin. Invest. 2009;119:1420–1428. doi: 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thiery JP, et al. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139:871–890. doi: 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 12.de Caestecker MP, et al. Role of transforming growth factor-β signaling in cancer. J. Natl Cancer Inst. 2000;92:1388–1402. doi: 10.1093/jnci/92.17.1388. [DOI] [PubMed] [Google Scholar]
- 13.Roberts AB, et al. Smad3 is key to TGF-β-mediated epithelial-to-mesenchymal transition, fibrosis, tumor suppression and metastasis. Cytokine Growth Factor Rev. 2006;17:19–27. doi: 10.1016/j.cytogfr.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 14.Saunier EF, et al. TGF-β inhibition for cancer therapy. Curr. Cancer Drug Targets. 2006;6:565–578. doi: 10.2174/156800906778742460. [DOI] [PubMed] [Google Scholar]
- 15.Heldin CH, et al. TGF-β signaling from cell membrane to nucleus through SMAD proteins. Nature. 1997;390:465–471. doi: 10.1038/37284. [DOI] [PubMed] [Google Scholar]
- 16.Massagué J. TGF-β signal transduction. Annu. Rev. Biochem. 1998;67:753–791. doi: 10.1146/annurev.biochem.67.1.753. [DOI] [PubMed] [Google Scholar]
- 17.Wrana JL. Regulation of Smad activity. Cell. 2000;100:189–192. doi: 10.1016/s0092-8674(00)81556-1. [DOI] [PubMed] [Google Scholar]
- 18.Datto M, et al. The Smads: transcriptional regulation and mouse models. Cytokine Growth Factor Rev. 2000;11:37–48. doi: 10.1016/s1359-6101(99)00027-1. [DOI] [PubMed] [Google Scholar]
- 19.Derynck R, et al. The TGF-β family. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 2008. [Google Scholar]
- 20.Kretzschmar M, et al. A mechanism of repression of TGF-β/Smad signaling by oncogenic Ras. Genes Dev. 1999;13:804–816. doi: 10.1101/gad.13.7.804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mori S, et al. TGF-β and HGF transmit the signals through JNK-dependent Smad2/3 phosphorylation at the linker regions. Oncogene. 2004;23:7416–7429. doi: 10.1038/sj.onc.1207981. [DOI] [PubMed] [Google Scholar]
- 22.Matsuura I, et al. Cyclin-dependent kinases regulate the antiproliferative function of Smads. Nature. 2004;430:226–231. doi: 10.1038/nature02650. [DOI] [PubMed] [Google Scholar]
- 23.Kamaraju AK, et al. Role of Rho/ROCK and p38 MAP kinase pathways in transforming growth factor-β-mediated Smad-dependent growth inhibition of human breast carcinoma cells in vivo. J. Biol. Chem. 2005;280:1024–1036. doi: 10.1074/jbc.M403960200. [DOI] [PubMed] [Google Scholar]
- 24.Sekimoto G, et al. Reversible Smad-dependent signaling between tumor suppression and oncogenesis. Cancer Res. 2007;67:5090–5096. doi: 10.1158/0008-5472.CAN-06-4629. [DOI] [PubMed] [Google Scholar]
- 25.Matsuzaki K, et al. Smad2 and Smad3 phosphorylated at both linker and COOH-terminal regions transmit malignant TGF-β signal in later stages of human colorectal cancer. Cancer Res. 2009;69:5321–5330. doi: 10.1158/0008-5472.CAN-08-4203. [DOI] [PubMed] [Google Scholar]
- 26.Liu F. Smad3 phosphorylation by cyclin-dependent kinases. Cytokine Growth Factor Rev. 2006;17:9–17. doi: 10.1016/j.cytogfr.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 27.Ikushima H, et al. TGF-βsignal transduction spreading to a wider field: a broad variety of mechanisms for context-dependent effects of TGF-β. Cell Tissue Res. 2011 doi: 10.1007/s00441-011-1179-5. , in press. [DOI] [PubMed] [Google Scholar]
- 28.ten Dijke P, et al. New insights into TGF-β-Smad signaling. Trends Bio. Sci. 2004;29:265–273. doi: 10.1016/j.tibs.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 29.Yagi K, et al. Alternatively spliced variant of Smad2 lacking exon 3. Comparison with wild-type Smad2 and Smad3. J. Biol. Chem. 1999;274:703–709. doi: 10.1074/jbc.274.2.703. [DOI] [PubMed] [Google Scholar]
- 30.Chang L, et al. Mammalian MAP kinase signalling cascades. Nature. 2001;410:37–40. doi: 10.1038/35065000. [DOI] [PubMed] [Google Scholar]
- 31.Derynck R, et al. Smad-dependent and Smad-independent pathways in TGF-β family signaling. Nature. 2003;425:577–584. doi: 10.1038/nature02006. [DOI] [PubMed] [Google Scholar]
- 32.Shaulian E, et al. AP-1 as a regulator of cell life and death. Nat. Cell Biol. 2002;4:E131–E136. doi: 10.1038/ncb0502-e131. [DOI] [PubMed] [Google Scholar]
- 33.Zhang YE. Non-Smad pathways in TGF-β signaling. Cell Res. 2009;19:128–139. doi: 10.1038/cr.2008.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wicks SJ, et al. Inactivation of Smad-transforming growth factor β signaling by Ca(2+)-calmodulin-dependent protein kinase II. Mol. Cell. Biol. 2000;20:8103–8111. doi: 10.1128/mcb.20.21.8103-8111.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Furukawa F, et al. p38 MAPK mediates fibrogenic signal through Smad3 phosphorylation in rat myofibroblasts. Hepatology. 2003;38:879–889. doi: 10.1053/jhep.2003.50384. [DOI] [PubMed] [Google Scholar]
- 36.Ho J, et al. The G protein-coupled receptor kinase-2 is a TGF-β-inducible antagonist of TGF-β signal transduction. EMBO J. 2005;24:3247–3258. doi: 10.1038/sj.emboj.7600794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Millet C, et al. A negative feedback control of transforming growth factor-β signaling by glycogen synthase kinase 3-mediated Smad3 linker phosphorylation at Ser-204. J. Biol. Chem. 2009;284:19808–19816. doi: 10.1074/jbc.M109.016667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Alarcón C, et al. Nuclear CDKs drive Smad transcriptional activation and turnover in BMP and TGF-β pathways. Cell. 2009;139:757–769. doi: 10.1016/j.cell.2009.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Matsuzaki K. Smad3 phosphoisoform-mediated signaling during sporadic human colorectal carcinogenesis. Histol. Histopathol. 2006;21:645–662. doi: 10.14670/HH-21.645. [DOI] [PubMed] [Google Scholar]
- 40.Zhu H, et al. A SMAD ubiquitin ligase targets the BMP pathway and affects embryonic pattern formation. Nature. 1999;400:687–693. doi: 10.1038/23293. [DOI] [PubMed] [Google Scholar]
- 41.Lin X, et al. Smurf2 is a ubiquitin E3 ligase mediating proteasome-dependent degradation of Smad2 in transforming growth factor-β signaling. J. Biol. Chem. 2000;275:36818–36822. doi: 10.1074/jbc.C000580200. [DOI] [PubMed] [Google Scholar]
- 42.Zhang Y, et al. Regulation of Smad degradation and activity by Smurf2, an E3 ubiquitin ligase. Proc. Natl Acad. Sci. USA. 2001;98:974–979. doi: 10.1073/pnas.98.3.974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gao S, et al. Ubiquitin ligase Nedd4L targets activated Smad2/3 to limit TGF-β signaling. Mol. Cell. 2009;36:457–468. doi: 10.1016/j.molcel.2009.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sapkota G, et al. Dephosphorylation of the linker regions of Smad1 and Smad2/3 by small COOH-terminal domain phosphatases has distinct outcomes for bone morphogenetic protein and transforming growth factor-β pathways. J. Biol. Chem. 2006;281:40412–40419. doi: 10.1074/jbc.M610172200. [DOI] [PubMed] [Google Scholar]
- 45.Wrighton KH, et al. Phospho-control of TGF-β superfamily signaling. Cell Res. 2009;19:8–20. doi: 10.1038/cr.2008.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yamagata H, et al. Acceleration of Smad2 and Smad3 phosphorylation via c-Jun NH(2)-terminal kinase during human colorectal carcinogenesis. Cancer Res. 2005;65:157–165. [PubMed] [Google Scholar]
- 47.Yoshida K, et al. Transforming growth factor-β and platelet-derived growth factor signal via c-Jun N-terminal kinase-dependent Smad2/3 phosphorylation in rat hepatic stellate cells after acute liver injury. Am. J. Pathol. 2005;166:1029–1039. doi: 10.1016/s0002-9440(10)62324-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Matsuzaki K, et al. Chronic inflammation associated with hepatitis C virus infection perturbs hepatic transforming growth factor β signaling, promoting cirrhosis and hepatocellular carcinoma. Hepatology. 2007;46:48–57. doi: 10.1002/hep.21672. [DOI] [PubMed] [Google Scholar]
- 49.Murata M, et al. Hepatitis B virus X protein shifts human hepatic TGF-β signaling from tumor-suppression to oncogenesis in early chronic hepatitis B. Hepatology. 2009;49:1203–1217. doi: 10.1002/hep.22765. [DOI] [PubMed] [Google Scholar]
- 50.Nagata H, et al. Inhibition of c-Jun NH2-terminal kinase switches Smad3 signaling from oncogenesis to tumor-suppression in rat hepatocellular carcinoma. Hepatology. 2009;49:1944–1953. doi: 10.1002/hep.22860. [DOI] [PubMed] [Google Scholar]
- 51.Kawamata S, et al. Oncogenic Smad3 signaling induced by chronic inflammation is an early event in ulcerative colitis-associated carcinogenesis. Inflamm. Bowel Dis. 2011;17:683–695. doi: 10.1002/ibd.21395. [DOI] [PubMed] [Google Scholar]
- 52.Frederick JP, et al. Transforming growth factor β-mediated transcriptional repression of c-myc is dependent on direct binding of Smad3 to a novel repressive Smad binding element. Mol. Cell. Biol. 2004;24:2546–2559. doi: 10.1128/MCB.24.6.2546-2559.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Datto MB, et al. Transforming growth factor β induces the cyclin-dependent kinase inhibitor p21 through a p53-independent mechanism. Proc. Natl Acad. Sci. USA. 1995;92:5545–5549. doi: 10.1073/pnas.92.12.5545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hannon GJ, et al. p15INK4B is a potential effector of TGF-β-induced cell cycle arrest. Nature. 1994;371:257–261. doi: 10.1038/371257a0. [DOI] [PubMed] [Google Scholar]
- 55.Polyak K, et al. p27Kip1, a cyclin-Cdk inhibitor, links transforming growth factor-β and contact inhibition to cell cycle arrest. Genes Dev. 1994;8:9–22. doi: 10.1101/gad.8.1.9. [DOI] [PubMed] [Google Scholar]
- 56.Sherr CJ, et al. CDK inhibitors: positive and negative regulators of G1-phase progression. Genes Dev. 1999;13:1501–1512. doi: 10.1101/gad.13.12.1501. [DOI] [PubMed] [Google Scholar]
- 57.Yang YA, et al. Smad3 reduces susceptibility to hepatocarcinoma by sensitizing hepatocytes to apoptosis through downregulation of Bcl-2. Cancer Cell. 2006;9:445–457. doi: 10.1016/j.ccr.2006.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.de Caestecker MP, et al. Smad2 transduces common signals from receptor serine-threonine and tyrosine kinases. Genes Dev. 1998;12:1587–1592. doi: 10.1101/gad.12.11.1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lehmann K, et al. Raf induces TGF-β production while blocking its apoptotic but not invasive responses: a mechanism leading to increased malignancy in epithelial cells. Genes Dev. 2000;14:2610–2622. doi: 10.1101/gad.181700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yue J, et al. Requirement of Ras/MAPK pathway activation by transforming growth factor β for transforming growth factor β1 production in a Smad-dependent pathway. J. Biol. Chem. 2000;275:30765–30773. doi: 10.1074/jbc.M000039200. [DOI] [PubMed] [Google Scholar]
- 61.Oft M, et al. Metastasis is driven by sequential elevation of H-ras and Smad2 levels. Nat. Cell Biol. 2002;4:487–494. doi: 10.1038/ncb807. [DOI] [PubMed] [Google Scholar]
- 62.Funaba M, et al. Modulation of Smad2-mediated signaling by extracellular signal-regulated kinase. J. Biol. Chem. 2002;277:41361–41368. doi: 10.1074/jbc.M204597200. [DOI] [PubMed] [Google Scholar]
- 63.Janda E, et al. Ras and TGF-β cooperatively regulate epithelial cell plasticity and metastasis: dissection of Ras signaling pathways. J. Cell Biol. 2002;156:299–313. doi: 10.1083/jcb.200109037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Suzuki K, et al. Transforming growth factor β signaling via Ras in mesenchymal cells requires p21-activated kinase 2 for extracellular signal-regulated kinase-dependent transcriptional responses. Cancer Res. 2007;67:3673–3682. doi: 10.1158/0008-5472.CAN-06-3211. [DOI] [PubMed] [Google Scholar]
- 65.Burch ML, et al. TGF-β stimulates biglycan synthesis via p38 and ERK phosphorylation of the linker region of Smad2. Cell. Mol. Life Sci. 2010;67:2077–20790. doi: 10.1007/s00018-010-0315-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jiang W, et al. Role of cross-talk between the Smad2 and MAPK pathways in TGF-β1-induced collagen IV expression in mesangial cells. Int. J. Mol. Med. 2010;26:571–576. doi: 10.3892/ijmm_00000501. [DOI] [PubMed] [Google Scholar]
- 67.Wang G, et al. The Smad3 linker region contains a transcriptional activation domain. Biochem. J. 2005;386:29–34. doi: 10.1042/BJ20041820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Prokova V, et al. Characterization of a novel transcriptionally active domain in the transforming growth factor β-regulated Smad3 protein. Nucleic Acids Res. 2005;33:3708–3721. doi: 10.1093/nar/gki679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Vasilaki E, et al. Novel regulation of Smad3 oligomerization and DNA binding by its linker domain. Biochemistry. 2009;48:8366–8378. doi: 10.1021/bi9005489. [DOI] [PubMed] [Google Scholar]
- 70.Wakefield LM, et al. TGF-β signaling: positive and negative effects on tumorigenesis. Curr. Opin. Genet. Dev. 2002;12:22–29. doi: 10.1016/s0959-437x(01)00259-3. [DOI] [PubMed] [Google Scholar]
- 71.Soucek L, et al. The ups and downs of Myc biology. Curr. Opin. Genet. Dev. 2010;20:91–95. doi: 10.1016/j.gde.2009.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Claassen GF, et al. A role for transcriptional repression of p21CIP1 by c-Myc in overcoming transforming growth factor β-induced cell-cycle arrest. Proc. Natl Acad. Sci. USA. 2000;97:9498–9503. doi: 10.1073/pnas.150006697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Feng X-H, et al. Direct interaction of c-Myc with Smad2 and Smad3 to inhibit TGF-β-mediated induction of the CDK inhibitor p15Ink4B. Mol. Cell. 2002;9:133–143. doi: 10.1016/s1097-2765(01)00430-0. [DOI] [PubMed] [Google Scholar]
- 74.Moustakas A, et al. Regulation of the human p21/WAF1/Cip1 promoter in hepatic cells by functional interactions between Sp1 and Smad family members. Proc. Natl Acad. Sci. USA. 1998;95:6733–6738. doi: 10.1073/pnas.95.12.6733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pardali K, et al. Actions of TGF-β as tumor suppressor and pro-metastatic factor in human cancer. Biochim. Biophys. Acta. 2007;1775:21–62. doi: 10.1016/j.bbcan.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 76.Feng X-H, et al. Smad2, Smad3 and Smad4 cooperate with Sp1 to induce p15Ink4B transcription in response to TGF-β. EMBO J. 2000;19:5178–5193. doi: 10.1093/emboj/19.19.5178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Seoane J, et al. TGF-β influences Myc, Miz-1 and Smad to control the CDK inhibitor p15INK4b. Nat. Cell Biol. 2001;3:400–408. doi: 10.1038/35070086. [DOI] [PubMed] [Google Scholar]
- 78.Seoane J, et al. Myc suppression of the p21Cip1 Cdk inhibitor influences the outcome of the p53 response to DNA damage. Nature. 2002;419:729–734. doi: 10.1038/nature01119. [DOI] [PubMed] [Google Scholar]
- 79.Weinberg RA. The Biology of Cancer. New York, NY: Garlang Science; 2007. [Google Scholar]
- 80.Jones PA, et al. The epigenomics of cancer. Cell. 2007;128:683–692. doi: 10.1016/j.cell.2007.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hengst L, et al. Translational control of p27Kip1 accumulation during the cell cycle. Science. 1996;271:1861–1864. doi: 10.1126/science.271.5257.1861. [DOI] [PubMed] [Google Scholar]
- 82.Reynisdottir I, et al. The subcellular locations of p15Ink4b and p27Kip1 coordinate their inhibitory interactions with cdk4 and cdk2. Genes Dev. 1997;11:492–503. doi: 10.1101/gad.11.4.492. [DOI] [PubMed] [Google Scholar]
- 83.Chu IM, et al. p27 phosphorylation by Src regulates inhibition of cyclin E-Cdk2. Cell. 2007;128:281–294. doi: 10.1016/j.cell.2006.11.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Grimmler M, et al. Cdk-inhibitory activity and stability of p27Kip1 are directly regulated by oncogenic tyrosine kinases. Cell. 2007;128:269–280. doi: 10.1016/j.cell.2006.11.047. [DOI] [PubMed] [Google Scholar]
- 85.Serres MP, et al. Cytoplasmic p.27 is oncogenic and cooperates with Ras both in vivo and in vitro. Oncogene. 2011;30:2846–2858. doi: 10.1038/onc.2011.9. [DOI] [PubMed] [Google Scholar]
- 86.Chu IM, et al. The Cdk inhibitor p27 in human cancer: prognostic potential and relevance to anticancer therapy. Nat. Rev. Cancer. 2008;8:253–267. doi: 10.1038/nrc2347. [DOI] [PubMed] [Google Scholar]
- 87.Oft M, et al. TGF-β1 and Ha-Ras collaborate in modulating the phenotypic plasticity and invasiveness of epithelial tumor cells. Genes Dev. 1996;10:2462–2477. doi: 10.1101/gad.10.19.2462. [DOI] [PubMed] [Google Scholar]
- 88.Guo X, et al. Signaling cross-talk between TGF-β/BMP and other pathways. Cell Res. 2009;19:71–88. doi: 10.1038/cr.2008.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kessenbrock K, et al. Matrix metalloproteinases: regulators of the tumor microenvironment. Cell. 2010;141:52–67. doi: 10.1016/j.cell.2010.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Stuelten CH, et al. Breast cancer cells induce stromal fibroblasts to express MMP-9 via secretion of TNF-α and TGF-β. J. Cell Sci. 2005;118:2143–2153. doi: 10.1242/jcs.02334. [DOI] [PubMed] [Google Scholar]
- 91.Wang G, et al. Transforming growth factor-β-inducible phosphorylation of Smad3. J. Biol. Chem. 2009;284:9663–9673. doi: 10.1074/jbc.M809281200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Matsuura I, et al. Pin1 promotes transforming growth factor-β-induced migration and invasion. J. Biol. Chem. 2010;285:1754–1764. doi: 10.1074/jbc.M109.063826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yeh ES, et al. PIN1, the cell cycle and cancer. Nat. Rev. Cancer. 2007;7:381–388. doi: 10.1038/nrc2107. [DOI] [PubMed] [Google Scholar]
- 94.Nakano A, et al. Pin1 down-regulates transforming growth factor-β (TGF-β) signaling by inducing degradation of Smad proteins. J. Biol. Chem. 2009;284:6109–6115. doi: 10.1074/jbc.M804659200. [DOI] [PubMed] [Google Scholar]
- 95.Saika S, et al. Smad3 signaling is required for epithelial-mesenchymal transition of lens epithelium after injury. Am. J. Pathol. 2004;164:651–663. doi: 10.1016/S0002-9440(10)63153-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Horiguchi K, et al. Role of Ras signaling in the induction of snail by transforming growth factor-β. J. Biol. Chem. 2009;284:245–253. doi: 10.1074/jbc.M804777200. [DOI] [PubMed] [Google Scholar]
- 97.Green DR, et al. A matter of life and death. Cancer Cell. 2002;1:19–30. doi: 10.1016/s1535-6108(02)00024-7. [DOI] [PubMed] [Google Scholar]
- 98.Zhu L, et al. Coordinating cell proliferation and differentiation. Curr. Opin. Genet. Dev. 2001;11:91–97. doi: 10.1016/s0959-437x(00)00162-3. [DOI] [PubMed] [Google Scholar]
- 99.Hague A, et al. Sodium butyrate induces apoptosis in human colonic tumour cell lines in a p53-independent pathway: implications for the possible role of dietary fibre in the prevention of large-bowel cancer. Int. J. Cancer. 1993;55:498–505. doi: 10.1002/ijc.2910550329. [DOI] [PubMed] [Google Scholar]
- 100.Maltzman T, et al. K-ras proto-oncogene mutations in sporadic colorectal adenomas: relationship to histologic and clinical characteristics. Gastroenterology. 2001;121:302–309. doi: 10.1053/gast.2001.26278. [DOI] [PubMed] [Google Scholar]
- 101.Glick AB, et al. Transforming growth factor β1 suppresses genomic instability independent of a G1 arrest, p53, and Rb. Cancer Res. 1996;56:3645–3650. [PubMed] [Google Scholar]
- 102.Tremain R, et al. Defects in TGF-β signaling overcome senescence of mouse keratinocytes expressing v-Ha-ras. Oncogene. 2000;19:1698–1709. doi: 10.1038/sj.onc.1203471. [DOI] [PubMed] [Google Scholar]
- 103.Yang H, et al. Autocrine transforming growth factor β suppresses telomerase activity and transcription of human telomerase reverse transcriptase in human cancer cells. Cell Growth Differ. 2001;12:119–127. [PubMed] [Google Scholar]
- 104.Derijard B, et al. JNK1: a protein kinase stimulated by UV light and Ha-Ras that binds and phosphorylates the c-Jun activation domain. Cell. 1994;76:1025–1037. doi: 10.1016/0092-8674(94)90380-8. [DOI] [PubMed] [Google Scholar]
- 105.Malumbres M, et al. Cell cycle, CDKs and cancer: a changing paradigm. Nat. Rev. Cancer. 2009;9:153–166. doi: 10.1038/nrc2602. [DOI] [PubMed] [Google Scholar]
- 106.Engel ME, et al. Interdependent SMAD and JNK signaling in transforming growth factor-β-mediated transcription. J. Biol. Chem. 1999;274:37413–37420. doi: 10.1074/jbc.274.52.37413. [DOI] [PubMed] [Google Scholar]
- 107.Grimm OH, et al. Nuclear exclusion of Smad2 is a mechanism leading to loss of compete. Nat. Cell Biol. 2002;4:519–522. doi: 10.1038/ncb812. [DOI] [PubMed] [Google Scholar]
- 108.Javelaud D, et al. Crosstalk mechanisms between the mitogen-activated protein kinase pathways and Smad signaling downstream of TGF-β: implications for carcinogenesis. Oncogene. 2005;24:5742–5750. doi: 10.1038/sj.onc.1208928. [DOI] [PubMed] [Google Scholar]
- 109.Arany PR, et al. Smad3 deficiency inhibits v-ras-induced transformation by suppression of JNK MAPK signaling and increased farnesyl transferase inhibition. Oncogene. 2008;27:2507–2512. doi: 10.1038/sj.onc.1210889. [DOI] [PubMed] [Google Scholar]
- 110.Hamajima H, et al. Modulation of the transforming growth factor-β1-induced Smad phosphorylation by the extracellular matrix receptor beta1-integrin. Int. J. Oncol. 2009;35:1441–1447. doi: 10.3892/ijo_00000463. [DOI] [PubMed] [Google Scholar]
- 111.Lin Q, et al. SKI promotes Smad3 linker phosphorylations associated with the tumor-promoting trait of TGF-β. Cell Cycle. 2010;9:1684–1689. doi: 10.4161/cc.9.9.11292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hong HY, et al. 14-3-3 sigma and 14-3-3 zeta plays an opposite role in cell growth inhibition mediated by transforming growth factor-β 1. Mol. Cells. 2010;29:305–309. doi: 10.1007/s10059-010-0037-8. [DOI] [PubMed] [Google Scholar]
- 113.Brown JD, et al. MEKK-1, a component of the stress (stress-activated protein kinase/c-Jun N-terminal kinase) pathway, can selectively activate Smad2-mediated transcriptional activation in endothelial cells. J. Biol. Chem. 1999;274:8797–8805. doi: 10.1074/jbc.274.13.8797. [DOI] [PubMed] [Google Scholar]
- 114.Hayashida T, et al. Cross-talk between ERK MAP kinase and Smad signaling pathways enhances TGF-β-dependent responses in human mesangial cells. FASEB J. 2003;17:1576–1578. doi: 10.1096/fj.03-0037fje. [DOI] [PubMed] [Google Scholar]
- 115.Yang Y, et al. Compound Astragalus and Salvia miltiorrhiza extract exerts anti-fibrosis by mediating TGF-β/Smad signaling in myofibroblasts. J. Ethnopharmacol. 2008;118:264–270. doi: 10.1016/j.jep.2008.04.012. [DOI] [PubMed] [Google Scholar]
- 116.He S, et al. Mechanisms of transforming growth factor β(1)/Smad signalling mediated by mitogen-activated protein kinase pathways in keloid fibroblasts. Br. J. Dermatol. 2010;162:538–546. doi: 10.1111/j.1365-2133.2009.09511.x. [DOI] [PubMed] [Google Scholar]
- 117.Sasseville M, et al. Growth differentiation factor 9 signaling requires ERK1/2 activity in mouse granulosa and cumulus cells. J. Cell Sci. 2010;123:3166–3176. doi: 10.1242/jcs.063834. [DOI] [PubMed] [Google Scholar]
- 118.Rachakonda G, et al. Increased cell migration and plasticity in Nrf2-deficient cancer cell lines. Oncogene. 2010;29:3703–3714. doi: 10.1038/onc.2010.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Velden JL, et al. c-Jun N-Terminal Kinase 1 promotes TGF-β1-induced epithelial to mesenchymal transition via control of linker phosphorylation and transcriptional activity of Smad3. Am. J. Respir. Cell Mol. Biol. 2011;44:571–581. doi: 10.1165/rcmb.2009-0282OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Wrighton KH, et al. Small C-terminal domain phosphatases dephosphorylate the regulatory linker regions of Smad2 and Smad3 to enhance transforming growth factor-β signaling. J. Biol. Chem. 2006;281:38365–38375. doi: 10.1074/jbc.M607246200. [DOI] [PubMed] [Google Scholar]
- 121.Chen CJ, et al. REVEAL-HBV study group. Risk of hepatocellular carcinoma across a biological gradient of serum hepatitis B virus DNA level. JAMA. 2006;295:65–73. doi: 10.1001/jama.295.1.65. [DOI] [PubMed] [Google Scholar]
- 122.Sawyers CL. The cancer biomarker problem. Nature. 2008;452:548–552. doi: 10.1038/nature06913. [DOI] [PubMed] [Google Scholar]
- 123.Thorgeirsson SS, et al. Molecular pathogenesis of human hepatocellular carcinoma. Nat. Genet. 2002;31:339–346. doi: 10.1038/ng0802-339. [DOI] [PubMed] [Google Scholar]
- 124.Felsher DW. Reversibility of oncogene-induced cancer. Curr. Opin. Genet. Dev. 2004;14:37–42. doi: 10.1016/j.gde.2003.12.008. [DOI] [PubMed] [Google Scholar]
- 125.Heldin CH, et al. Role of Smads in TGF-β signaling. Cell Tissue Res. 2011 doi: 10.1007/s00441-011-1190-x. In press. [DOI] [PubMed] [Google Scholar]
- 126.Ashcroft GS, et al. Mice lacking Smad3 show accelerated wound healing and an impaired local inflammatory response. Nat. Cell Biol. 1999;1:260–266. doi: 10.1038/12971. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.