Abstract
Ectopic fat depots may mediate local and systemic disease. Animal models of diet-induced obesity demonstrate increased fat accumulation in the renal sinus. The association of renal sinus fat with hypertension, chronic kidney disease (CKD), and other metabolic disorders has not been studied in a large, community-based sample. Participants from the Framingham Heart Study (n=2923, mean age 54 years, 51% women) underwent quantification of renal sinus fat area using computed tomography. High renal sinus fat (“fatty kidney”) was defined using sex-specific 90th percentiles in a healthy referent sub-sample. Multivariable linear and logistic regression was used to model metabolic risk factors as a function of fatty kidney and log-transformed renal sinus fat. Multivariable models were adjusted for age, sex, outcome-specific covariates, and then additionally adjusted for body mass index (BMI) or abdominal visceral adipose tissue (VAT). The prevalence of fatty kidney was 30.1% (n=879). Individuals with fatty kidney had a higher odds ratio (OR) of hypertension (OR 2.12, p<0.0001), which persisted after adjustment for BMI (OR 1.49, p<0.0001) and VAT (OR 1.24, p=0.049). Fatty kidney was also associated with an increased odds ratio for CKD (OR 2.30, p=0.005), even after additionally adjusting for BMI (OR 1.86, p=0.04) or VAT (OR 1.86, p=0.05). We observed no association between fatty kidney and diabetes after adjusting for VAT. In conclusion, fatty kidney is a common condition that is associated with an increased risk of hypertension and chronic kidney disease. Renal sinus fat may play a role in blood pressure regulation and CKD.
Keywords: renal sinus fat, hypertension, chronic kidney disease, blood pressure, computed tomography, epidemiology
Introduction
Obesity continues to be an important global public health problem. Current estimates indicate that over two-thirds of American adults1 and 1.3 billion adults worldwide2 are either overweight or obese. The impact of obesity on cardiovascular and metabolic diseases, including diabetes, hypertension, dyslipidemia, cardiovascular disease, and cardiovascular mortality is well established.3 Additionally, obesity is now recognized as a risk factor for the development of renal dysfunction, with a growing body of evidence supporting the association of higher body mass index (BMI) with chronic kidney disease (CKD).4–6
BMI is a good measure of general adiposity, but it captures both lean and fat mass and does not distinguish between patterns of fat distribution. Abdominal adiposity, independent of generalized obesity, is associated with CKD.7,8 The association of regional fat deposition with CKD suggests a potential role for ectopic fat.9 This is particularly relevant for the kidneys, which are surrounded by abdominal visceral adipose tissue (VAT) and have the potential to accumulate ectopic fat in the renal sinus.
The accumulation of renal sinus fat is important because the renal vein and artery pass through the renal sinus and may be compressed by ectopic fat. Renal vein constriction has been shown to increase kidney volume and renal interstitial pressure and decrease sodium excretion in animal models.10–13 Human studies and animal models have demonstrated fat accumulation in the renal sinus9,14,15 and renal parenchyma.16–19 Concomitant structural and functional changes in the kidney and renal vasculature have been observed in animal models.14,17–19 However, whether these renal changes are associated with diseases of the kidney in humans is uncertain. The association of renal sinus fat with hypertension, CKD, and other metabolic traits has not been previously characterized in a large, community-based sample. Thus, the aim of this study was to evaluate the association of renal sinus fat accumulation, or “fatty kidney,” quantified using computed tomography with these cardio-metabolic and renal traits in the Framingham Heart Study. We hypothesized that renal sinus fat would be independently associated with measures of blood pressure and renal function, and not with other cardio-metabolic traits, after accounting for abdominal VAT as a measure of abdominal adiposity.
Methods
Study Sample
Participants were drawn from the Framingham Multi-Detector Computed Tomography (MDCT) cohort, which consists of 3529 participants from the Framingham Offspring (n=1418) and Third Generation (n=2111) cohorts who underwent MDCT between June 2002 and March 2005, as previously described.20 Eligible participants included 2923 participants who attended the 8th Offspring or 1st Third Generation examination with an interpretable abdominal MDCT scan for the renal sinus fat measurement protocol. A sub-sample consisted of 1210 Offspring participants with serum cystatin-C measured during the 7th Offspring examination. Participants provided written informed consent and this study was approved by the Boston University Medical Center and Massachusetts General Hospital institutional review boards.
Renal Sinus Fat Quantification
Abdominal MDCT scans were captured using an 8-slice MDCT scanner (LightSpeed Ultra, General Electric; Milwaukee, WI, USA), covering 125mm in the abdomen with 25 5.0-mm slices above the S1 level (120kVp, 400mA, gantry rotation time 500ms, table feed 3:1) and were interpreted using the Aquarius 3D Workstation (TeraRecon, Inc, San Mateo, CA, USA).
Renal sinus fat was quantified in a single MDCT slice within the right kidney. Briefly, renal sinus fat (cm2) was measured by one reader manually tracing the right kidney from the abdominal MDCT scan after applying a selection rule to a set of candidate slices selected based on visual inspection. Adipose tissue was identified using MDCT pixel density in Hounsfield Units (HU) centered on −120HU with a window width of −195 to −45HU. The interclass correlation coefficients were 0.93 and 0.86 for intra- and inter-reader reproducibility, respectively. Our complete protocol appears in the supplementary methods in the Online Supplement (please see http://hyper.ahajournals.org).
Outcome Assessment
Systolic (SBP) and diastolic (DBP) blood pressure were measured by the examining clinic physician using the mean of two readings. Hypertension was defined as SBP≥140mmHg, DBP≥90mmHg, or current use of prescription hypertension medication. Imputed blood pressure values were calculated by adding 10mmHg to SBP and 5mmHg to DBP if a participant was currently using hypertension medication.21
Serum creatinine was measured using the modified Jaffe method (Inter-assay coefficient of variation[CV]=2.8%, intra-assay CV=4.0%; Roche Hitachi 911, Roche Diagnostics, Indianapolis, IN) and indirectly calibrated to the NHANES III serum creatinine values as previously described.22 Cystatin-C was measured using nephelometry on previously frozen serum samples (Inter-assay CV=3.3%, intra-assay CV=2.4%; Dade Behring Diagnostic, Marburg, Germany). The estimated glomerular filtration rate (eGFR) was determined using (1) the abbreviated Modification of Diet in Renal Disease (MDRD) Study Equation (eGFRcrea)23 and (2) the cystatin-C only Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation (eGFRcys).24 CKDcrea and CKDcys were defined as eGFRcrea or eGFRcys<60mL/min/1.73m2, respectively.
Urinary albumin and creatinine were determined using spot urine samples. Urinary albumin was quantified using a Tina-quant albumin immunoturbidometric assay (Inter-assay CV=3.1%, intra-assay CV=2.1%; Roche Diagnostics, Indianapolis, IN). Urinary creatinine was quantified using a modified Jaffe method (Inter-assay CV=1.9%, intra-assay CV=1.0%; Roche Diagnostics, Indianapolis, IN). The urinary albumin-to-creatinine ratio (UACR) was calculated by dividing the amount of urinary albumin (mg) by the amount of urinary creatinine (g). Microalbuminuria was defined as a UACR>25mg/g in women or >17mg/g in men.
Serum levels of fasting plasma glucose, total cholesterol, high-density lipoprotein (HDL) cholesterol, and triglycerides were determined using a fasting blood sample from the clinic examination. Diabetes was defined as a fasting plasma glucose ≥126mg/dL or current use of oral hypoglycemic treatment or insulin. High triglycerides was defined as serum triglycerides ≥150mg/dL or current use of lipid lowering medication. Low HDL-cholesterol was defined as HDL-cholesterol <50mg/dL in women and <40mg/dL in men.
Covariate Assessment
Height and waist circumference at the umbilicus were recorded to the nearest quarter-inch and weight to the nearest pound by trained clinic staff. BMI was defined as weight divided by height2 (kg/m2). Abdominal VAT volume was assessed by MDCT.20 Current smoking was defined as smoking ≥1 cigarette/day in the past year. High alcohol intake was defined as >7 drinks/week among women and >14 drinks/week among men based on self-report. Physical activity was determined by calculating a physical activity index based on a structured questionnaire.
Statistical Methods
Fatty kidney was defined as the presence of high renal sinus fat based on sex-specific 90th percentiles in a healthy referent sub-sample, defined using the following exclusion criteria: (1) BMI≥30 kg/m2; (2) hypertension, high triglycerides, low HDL-cholesterol, impaired fasting plasma glucose, or diabetes; (3) CKDcrea or microalbuminuria; (4) current smoking; (5) BMI<18.5kg/m2; and (6) missing covariates described in the previous exclusion steps and other model covariates. The healthy referent sample consisted of 400 women and 213 men, with 90th percentile renal sinus fat cut points of 0.445 cm2 in women and 0.71cm2 in men.
Renal sinus fat measurements below the observed lower limit of detection (0.0048cm2) were set to 0.004cm2 in statistical analyses. Age- and sex-adjusted partial Pearson correlation coefficients were used to assess the correlation of log-transformed renal sinus fat with continuous covariates. Renal sinus fat was modeled dichotomously as fatty kidney and continuously with a natural-log transformation, standardized to a sex-specific mean of 0 and standard deviation of 1. Linear and logistic regression was used to model continuous and dichotomous outcomes as functions of renal sinus fat. Models were initially adjusted for age and sex and then underwent further multivariable adjustment. Multivariable models of hypertension, imputed SBP, and imputed DBP were adjusted for age, sex, current smoking, high alcohol intake, and physical activity index. Multivariable models of eGFR and CKD were adjusted for age, sex, diabetes, hypertension medication use, SBP, current smoking, and HDL-cholesterol. Multivariable models of HDL-cholesterol were adjusted for age, sex, current smoking, high alcohol intake, and lipid lowering medication use. Multivariable models of triglycerides were adjusted for age, sex, and current smoking. Finally, multivariable models were separately adjusted for BMI and abdominal VAT. To help disentangle the potential association of renal sinus fat and abdominal VAT with blood pressure and eGFR, we examined trends across sex-specific renal sinus fat tertiles within sex-specific abdominal VAT tertiles. Statistical analyses were performed using SAS Version 9.2 (SAS Institute, Cary, NC).
Secondary Analyses
As a secondary analysis, we recreated the healthy referent sample excluding individuals with BMI≥25 kg/m2 instead of BMI≥30 kg/m2 (‘lean healthy referent’) and determined the sex-specific 90th percentile cut-points. The lean healthy referent sample included 282 women and 100 men; the 90th percentile cut points were 0.42 cm2 in women and 0.455 cm2 in men.
Results
Overall Study Sample Characteristics
Renal sinus fat ranged from the lower limit of detection in 133 participants to 4.89cm2 with a median value of 0.31cm2. The prevalence of fatty kidney (renal sinus fat ≥0.445cm2 in women and ≥0.71cm2 in men) was 30.9% in the overall sample (n=879). Individuals with fatty kidney were older, had a higher BMI, and a more adverse metabolic risk factor profile when compared to individuals without fatty kidney. The prevalence of hypertension, CKDcys, CKDcrea, and microalbuminuria were also significantly higher among those with as compared to those without fatty kidney (Table 1). Age- and sex-adjusted correlations of renal sinus fat with adiposity measures and continuous covariates are presented in Table 2. Renal sinus fat was correlated with all covariates examined (p≤0.03) except UACR (p=0.18), with the strongest correlations observed for other adiposity measures and age.
Table 1.
Study sample characteristics by fatty kidney status.* Continuous variables presented as mean ± standard deviation and dichotomous variables presented as N(%), unless otherwise specified.
| Variable | Fatty kidney* (n=879) | No fatty kidney (n=2044) | Age-and sex-adjusted p-value |
|---|---|---|---|
| Age (years) | 61 ± 13 | 51 ± 12 | <0.0001 |
| % Women | 42.7 (375) | 54.6 (1117) | <0.0001 |
| Renal sinus fat (cm2)† | 0.97 (0.73, 1.34) | 0.18 (0.06, 0.33) | <0.0001 |
| Body mass index (kg/m2) | 30.3 ± 5.7 | 26.6 ± 4.8 | <0.0001 |
| Visceral adipose tissue (cm3) | 2557 ± 1040 | 1456 ± 842 | <0.0001 |
| Waist circumference (cm) | 106 ± 14 | 94 ± 14 | <0.0001 |
| HDL-cholesterol (mg/dL) | 52 ± 15 | 57 ± 18 | <0.0001 |
| Triglycerides (mg/dL)† | 117 (82, 165) | 91 (66, 134) | <0.0001 |
| Systolic blood pressure (mm Hg) | 129 ± 17 | 120 ± 15 | <0.0001 |
| Diastolic blood pressure (mm Hg) | 75 ± 11 | 75 ± 9 | <0.0001 |
| Hypertension (%) | 57.8 (508) | 26.6 (543) | <0.0001 |
| Use of antihypertensive medication (%) | 46.2 (406) | 18.6 (381) | <0.0001 |
| Diabetes (%) | 14.6 (128) | 4.2 (86) | <0.0001 |
| Current smoking status (%) | 10.5 (92) | 12.6 (257) | 0.17 |
| High alcohol intake (%)‡ | 9.2 (123) | 11.2 (228) | 0.003 |
| Physical activity index | 36 ± 7 | 37 ± 7 | 0.002 |
| eGFRcrea (mL/min/1.73m2) | 84.9 ± 19.8 | 90.9 ± 17.8 | 0.40 |
| CKDcrea (%) | 9.2 (81) | 2.8 (57) | 0.04 |
| eGFRcys (mL/min/1.73m2)§ | 81 ± 17 | 89 ± 16 | 0.0005 |
| CKDcys (%)§ | 9.1 (53) | 2.8 (18) | 0.0005 |
| Urinary albumin to creatinine ratio (mg/g)† | 5.4 (3.1, 11.1) | 4.5 (2.8, 8.8) | 0.005 |
| Microalbumuria (%) | 13.4 (118) | 6.0 (123) | 0.01 |
Note: All participants in the study sample are Caucasian.
Abbreviations: CKDcrea=chronic kidney disease, defined as eGFRcrea<60 mL/min/1.73m2; CKDcys=chronic kidney disease, defined as eGFRcys<60 mL/min/1.73m2; eGFRcrea=estimated glomerular filtration rate using the modified MDRD study equation; eGFRcys=estimated glomerular filtration rate using the cystatin-C only CKD-EPI equation; HDL=high density lipoprotein
Sex-specific cut points for fatty kidney: ≥ 0.71 cm2 in men; ≥ 0.445 cm2 in women.
Presented as median (25th, 75th percentiles)
>7 drinks/week in women, >14 drinks/week in men
Cystatin C levels collected during the Offspring 7th examination cycle (n=1210; fatty kidney: n=561; no fatty kidney: n=649)
Table 2.
Age- and sex-adjusted partial Pearson correlations (r) with log-transformed renal sinus fat
| Variable | r | p-value |
|---|---|---|
| Age* | 0.40 | <0.0001 |
| Visceral adipose tissue | 0.48 | <0.0001 |
| Subcutaneous adipose tissue | 0.34 | <0.0001 |
| Body mass index | 0.38 | <0.0001 |
| Waist circumference | 0.38 | <0.0001 |
| Systolic blood pressure | 0.14 | <0.0001 |
| Diastolic blood pressure | 0.13 | <0.0001 |
| High density lipoprotein cholesterol | −0.14 | <0.0001 |
| Log(triglycerides) | 0.26 | <0.0001 |
| Fasting blood glucose | 0.13 | <0.0001 |
| log(UACR) | 0.02 | 0.18 |
| eGFRcrea | 0.04 | 0.03 |
| eGFRcys | −0.10 | 0.0004 |
Abbreviations: eGFRcrea=estimated glomerular filtration rate using the modified MDRD study equation; eGFRcys=estimated glomerular filtration rate using the cystatin-C only CKD-EPI equation
Sex-adjusted partial Pearson correlation
Renal Sinus Fat and Hypertension
Individuals with fatty kidney had a higher odds ratio (OR) for hypertension (Table 3, OR 2.12, p<0.0001), which persisted after adjustment for BMI (OR 1.49, p<0.0001) and VAT (OR 1.24, p=0.049). Individuals with fatty kidney also had higher imputed systolic blood pressure (SBP; 4.8mmHg) and diastolic blood pressure (DBP; 2.3mmHg) compared to those without fatty kidney (Table 3, both p<0.0001). In models with continuous renal sinus fat as the exposure, estimates were similar (Table S1; please see the Online Supplement at http://hyper.ahajournals.org).
Table 3.
Imputed blood pressure and renal function outcomes modeled as functions of fatty kidney status.* Increments in the outcome when fatty kidney is present (standard errors shown in parentheses) are presented for continuous outcomes. Odds ratios (95% confidence intervals) comparing those with fatty kidney to those without fatty kidney are presented for dichotomous outcomes.
| Model outcome of interest | Age and sex | Multivariable† | Multivariable + BMI | Multivariable + VAT |
|---|---|---|---|---|
| Continuous outcomes | ||||
| Systolic blood pressure (mmHg) | 4.9 (0.7) p<0.0001 |
4.8 (0.7) p<0.0001 |
2.2 (0.7) p=0.002 |
1.2 (0.7) p=0.11 |
| Diastolic blood pressure (mmHg) | 2.4 (0.4) p<0.0001 |
2.3 (0.4) p<0.0001 |
0.8 (0.4) p=0.07 |
0.5 (0.5) p=0.31 |
| eGFRcys (mL/min/1.73m2) | −3.27 (0.89) p=0.0002 |
−2.20 (0.89) p=0.01 |
−0.64 (0.90) p=0.48 |
−0.90 (0.96) p=0.35 |
| eGFRcrea (mL/min/1.73m2) | 0.61(0.72) p=0.40 |
0.57 (0.72) p=0.43 |
0.05 (0.75) p=0.95 |
−0.27 (0.78) p=0.72 |
| Dichotomous outcomes | ||||
| Hypertension | 2.13 (1.77 – 2.57) p<0.0001 |
2.12 (1.75 – 2.56) p<0.0001 |
1.49 (1.22 – 1.83) p<0.0001 |
1.24 (1.00 – 1.53) p=0.049 |
| CKDcys | 2.68 (1.51 – 4.75) p=0.0007 |
2.30 (1.28 – 4.14) p=0.005 |
1.86 (1.02 – 3.42) p=0.04 |
1.86 (1.00 – 3.46) p=0.05 |
| CKDcrea | 1.49 (1.01 – 2.20) p=0.04 |
1.14 (0.76 – 1.72) p=0.53 |
1.16 (0.76 – 1.78) p=0.49 |
1.20 (0.77 – 1.86) p=0.43 |
Abbreviations: BMI=body mass index; eGFRcrea=estimated glomerular filtration rate using the modified MDRD study equation; eGFRcys=estimated glomerular filtration rate using the cystatin-C only CKD-EPI equation; VAT=abdominal visceral adipose tissue volume; CKDcrea=chronic kidney disease status based on eGFRcrea; CKDcys=chronic kidney disease status based on eGFRcys.
Sex-specific cut points for fatty kidney: ≥ 0.71 cm2 in men; ≥ 0.445 cm2 in women.
Multivariable models are adjusted for age and sex as well as covariates listed below by outcome:
eGFRcys, eGFRcrea, CKDcys, CKDcrea: diabetes status, current hypertension medication use, systolic blood pressure, current smoking status, high-density lipoprotein cholesterol level
Imputed systolic blood pressure, diastolic blood pressure, hypertension: Current smoking status, high alcohol intake, physical activity index
Renal Sinus Fat and Renal Function
Among participants with cystatin-C measurements (n=1210), 5.9% (n=71) had CKDcys. Fatty kidney was associated with an increased odds ratio for CKDcys (Table 3; OR 2.30, p=0.005), which persisted after adjustment for BMI (OR 1.86, p=0.04) and VAT (OR 1.86, p=0.05). Fatty kidney was associated with an increased odds ratio for CKDcrea after age- and sex-adjustment (OR 1.49, p=0.04) but not after multivariable adjustment (Table 3, OR 1.14, p=0.53).
The prevalence of microalbuminuria was 13.4% among individuals with fatty kidney and 6.0% among those without fatty kidney (Table 1). Fatty kidney was associated with an increased odds ratio of microalbuminuria in age- and sex-adjusted models (OR 1.45 95%CI 1.08–1.94, p=0.01), which was attenuated and no longer statistically significant after multivariable adjustment (OR 1.17 95%CI 0.86–1.59, p=0.31).
Renal Sinus Fat and Additional Metabolic Risk Factors
Fatty kidney was associated with an increased odds ratio for diabetes after age-and sex- adjustment (OR 2.26, p<0.0001, Table 4), which was attenuated after adjustment for VAT (OR 1.09, p=0.62); similar results were observed for fasting blood glucose (Table 4). Similarly, fatty kidney was associated with an increased odds ratio for high triglycerides (Table 4, OR 1.88, p<0.0001) but not after adjustment for VAT (OR 1.04, p=0.69).
Table 4.
Lipid and glucose outcomes as a function of fatty kidney status.* Increments in each outcome associated with the presence of fatty kidney (standard errors shown in parentheses) is presented for continuous outcomes. Odds ratios (95% confidence interval) comparing those with fatty kidney to those without fatty kidney are presented for dichotomous outcomes.
| Model outcomes of interest | Age and sex | Multivariable† | Multivariable + BMI | Multivariable + VAT |
|---|---|---|---|---|
| Continuous outcomes | ||||
| HDL-cholesterol (mg/dL) | −4.16 (0.67) p<0.0001 |
−4.14 (0.66) p<0.0001 |
−1.10 (0.67) p=0.10 |
1.07 (0.68) p=0.12 |
| Triglycerides (mg/dL), log transformed | 0.20 (0.02) p<0.0001 |
0.20 (0.02) p<0.0001 |
0.10 (0.02) p<0.0001 |
0.01 (0.02) p=0.71 |
| Fasting blood glucose (mg/dL) | 5.66 (0.90) p<0.0001 |
‡ | 1.99 (0.92) p=0.03 |
−0.07 (0.96) p=0.94 |
| Dichotomous outcomes | ||||
| Low HDL-Cholesterol | 1.39 (1.15 – 1.69) p=0.0009 |
1.41 (1.15 – 1.72) p=0.0008 |
0.99 (0.80 – 1.23) p=0.93 |
0.74 (0.59 – 0.93) p=0.009 |
| High Triglycerides | 1.89 (1.58 – 2.26) p<0.0001 |
1.88 (1.58 – 2.25) p<0.0001 |
1·38 – (1.14 1.66) p=0.001 |
1.04 (0.85 – 1.28) p=0.69 |
| Diabetes | 2.26 (1.66 – 3.09) p<0.0001 |
‡ | 1.42 (1.02 – 1.97) p=0.04 |
1.09 (0.77 – 1.55) p=0.62 |
Abbreviations: BMI=body mass index; HDL=high density lipoprotein; VAT=abdominal visceral adipose tissue volume.
Sex-specific cut points for fatty kidney: ≥ 0.71 cm2 in men; ≥ 0.445 cm2 in women.
Multivariable models are adjusted for age and sex as well as covariates as listed below:
HDL Cholesterol, log(Triglycerides), low HDL cholesterol: Current smoking status, high alcohol intake, current use of lipid lowering medication
High Triglycerides: Current smoking status
Models of fasting blood glucose and diabetes were not adjusted beyond age and sex before further adjustment for BMI or VAT
Abdominal Fat Distribution Patterns and CKD
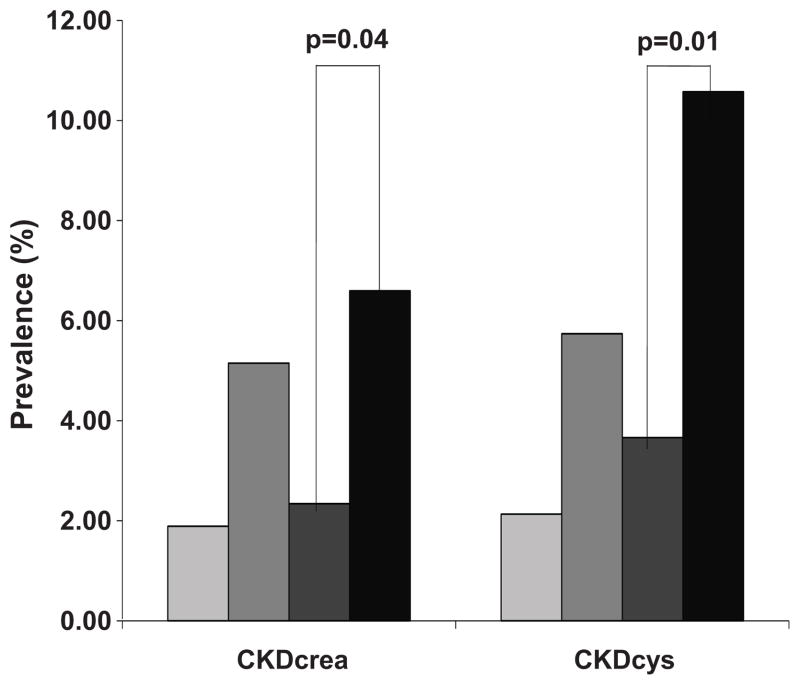
The prevalence of CKDcrea and CKDcys by fat distribution pattern category is presented in Figure 1. The highest prevalence of both conditions was observed among individuals with fatty kidney and high VAT. The prevalence of CKDcys (p=0.002), but not CKDcrea (p=0.20) varied across all four distribution categories. Among those with high VAT, the prevalence of CKDcys and CKDcrea were higher among those with fatty kidney when compared to those without fatty kidney (p=0.01 and 0.04, respectively).
Figure 1. Prevalence of Chronic Kidney Disease (CKD) by Fatty Kidney and Abdominal Visceral Adipose Tissue (VAT) Distribution Patterns.
CKDcys is defined as eGFR<60 mL/min/1.73m2, based on the Cystatin-C only CKD-EPI equation. CKDcrea is defined as eGFR<60 mL/min/1.73m2, based on the modified MDRD Study Equation.
 Not fatty kidney and normal VAT (Group 1);
Not fatty kidney and normal VAT (Group 1);
 Fatty kidney and Normal VAT (Group 2);
Fatty kidney and Normal VAT (Group 2);
 Not fatty kidney and high VAT (Group 3); ■ Fatty kidney and high VAT (Group 4). For comparison, CKDcys and CKDcrea status at the 7th Offspring exam cycle are presented. P-values are adjusted for age and sex. CKDcrea available in 2943 participants (Group 1 N=1437; Group 2 N=236; Group 3 N=605; Group 4 N=665). CKDcys available in 1206 participants with cystatin-C measures (Group 1 N=375; Group 2 N=122; Group 3 N=273; Group 4 N=436).
Not fatty kidney and high VAT (Group 3); ■ Fatty kidney and high VAT (Group 4). For comparison, CKDcys and CKDcrea status at the 7th Offspring exam cycle are presented. P-values are adjusted for age and sex. CKDcrea available in 2943 participants (Group 1 N=1437; Group 2 N=236; Group 3 N=605; Group 4 N=665). CKDcys available in 1206 participants with cystatin-C measures (Group 1 N=375; Group 2 N=122; Group 3 N=273; Group 4 N=436).
Mean imputed SBP and eGFRcys within renal sinus fat and VAT tertiles are presented in Figures S1A and S1B, respectively (please see the Online Supplement at http://hyper.ahajournals.org). When examining trends across renal sinus fat tertiles within VAT tertiles, we observed that mean SBP is higher and eGFRcys is lower as renal sinus fat increases within each VAT tertile, highlighting the association between renal sinus fat and SBP and eGFRcys.
Secondary analyses
Using lean healthy referent cut points, the prevalence of fatty kidney was 38.9% (26.9% of women and 51.4% of men). Among participants not currently using hypertension medication (n=2129), results from models of SBP and DBP as functions of fatty kidney were essentially unchanged (data not shown). Results from blood pressure, renal function, and lipid models remained consistent in diabetes-free sub-samples from the overall (n=2702) and cystatin C subgroups (n=1103).
Discussion
Fatty kidney is a common condition, present in nearly one-third of our community-based sample. Fatty kidney is associated with both hypertension and CKD based on cystatin-C. These associations persisted after accounting for measures of generalized or abdominal adiposity, suggesting that renal sinus fat may have an independent association with renal function. In contrast, the observed associations of renal sinus fat with other cardio-metabolic traits were generally attenuated after accounting for overall VAT, which is consistent with the hypothesized localized impact of renal sinus fat and provides further support for the potential unique role of this fat depot in hypertension and renal dysfunction.
The association of obesity with the development of CKD4–6 and hypertension25 is well-established, although the pathogenic mechanisms are not fully understood. Ectopic fat accumulation is one of several mechanisms proposed to explain these associations. Renal sinus fat accumulation has been observed in small imaging studies of children (n=15)26 and adults (n=6)27 with normal renal function. The association of renal sinus fat with hypertension and creatinine clearance was recently studied in 205 participants from the Pulmonary Edema and Stiffness of the Vascular System study, a study designed to assess predictors of congestive heart failure in older individuals.15 In this study, patients with SBP≥160mmHg or DBP≥100mmHg had higher levels of renal sinus fat, quantified by magnetic resonance imaging, than in those with blood pressure<160/100mmHg, although associations with SBP, DBP, and renal function were not observed,15 perhaps due to the small sample of highly selected individuals.
Animal models of diet-induced obesity provide additional insight into potential mechanisms involved in the pathogenesis of renal sinus fat. Rabbits with diet-induced obesity undergo a 61% increase in renal sinus mass, primarily driven by increases in fat within the renal sinus.14 This increase in renal sinus mass is observed concomitantly with increases in blood pressure.14 It has been hypothesized that renal sinus fat deposition leads to increases in renal interstitial pressure through the compression of vessels exiting the kidney, including the renal vein and lymph vessels.9 This potential mechanism is supported by studies in dog and rat models in which renal vein compression leads to increased renal interstitial pressure, kidney volume, and, in the presence of volume expansion, increased sodium reabsorption in the loop of Henle and decreased sodium excretion.10–13 Increased tubular reabsorption and retention of sodium is also observed in the dog model of obesity-related hypertension,28 a model for the development of obesity-related hypertension in humans.29
Alternative animal models of obesity reported lipid accumulation within the renal parenchyma, supporting proposed mechanisms of obesity leading to kidney damage and hypertension through lipotoxicity, oxidative stress, inflammation, and fibrosis.30,31 Obese mice fed a high-fat diet developed lipid accumulation in the glomeruli and proximal tubules in addition to albuminuria, increased SBP and oxidative stress, and a larger glomerular tuft area and mesangial matrix when compared to mice fed a low-fat diet.18 Zucker diabetic fatty rats exhibit greater lipid accumulation in the renal cortex when compared to pair-fed lean controls.17 Lipid accumulation within the renal parenchyma has also been described in humans.16
Overall, evidence from animal models supports the presence of obesity-related increases in renal lipid accumulation with concomitant structural and functional changes in the kidney and vasculature. However, it is uncertain whether these changes are specifically due to renal fat accumulation as compared to generalized weight gain and adiposity. Higher levels of BMI are among the strongest correlates of many ectopic fat depots.20,32,33 We have attempted to dissect the specific role of renal sinus fat as compared to generalized markers of adiposity and ectopic fat through our modeling structure and serial adjustment for BMI and VAT. While our findings are attenuated after accounting for each of these adiposity-related variables, the residual statistical significance suggests a potential independent association of renal sinus fat with hypertension and CKD. We have also addressed this issue by evaluating the trends in SBP and eGFRcys with increasing renal sinus fat within narrower ranges of abdominal VAT; these findings further support an independent association with renal sinus fat. Finally, we did not observe an association with the presence of diabetes after accounting for abdominal VAT, which is in contrast to our prior work demonstrating consistent associations with VAT,20 liver fat,34 and upper body subcutaneous fat.35 This suggests that potential mechanisms for the association of renal sinus fat with hypertension and CKD are unlikely due to high correlations among different fat depots and provides support for a unique and specific association between renal sinus fat and hypertension and CKD.
Similar to our previous findings investigating abdominal VAT and CKD,36 we observed in the present analysis that fatty kidney is associated with CKD when using cystatin-C based eGFR but not creatinine-based eGFR. One potential explanation is that cystatin-C may be a more sensitive marker for assessing renal function as compared to serum creatinine in older populations,37 given that serum creatinine is predominately derived from muscle tissue and that overall muscle mass is lower in older individuals.23,38 However, our results may also reflect potential confounding due to the independent association of cystatin-C with non-renal factors39,40 including BMI.39 Cystatin-C is secreted by adipose tissue41 and the prevalence of cystatin-C based CKD may be overestimated in overweight and obese individuals when compared to the prevalence based on the MDRD Study equation.42 While it is important to consider the role of adiposity in cystatin-C production, if our association observed for fatty kidney and cystatin-C based CKD were solely due to confounding by overall adiposity, the association would have been completely attenuated after adjusting for BMI or abdominal VAT. Conversely, we observed a significant residual association upon further adjustment. Therefore, secretion of cystatin C by adipocytes is unlikely to fully explain our observations.
The Framingham MDCT cohort is a large, well-characterized, community-based sample with multiple measures of adiposity, allowing for adjustment of several important confounders and further adjustment for generalized and central adiposity. Using computed tomography, we were able to develop a non-invasive, reproducible method to quantify renal sinus fat accumulation in a community-based setting. Some limitations warrant mention. This is an observational study, which limits our ability to assess the causality of our findings. Given the cross-sectional design, we were also unable to assess the temporality of our observed associations. Our study sample is comprised of Caucasian participants. Based on this, our findings may not be generalizable to populations consisting of other racial or ethnic groups.
Perspectives
Fatty kidney is a common condition associated with an increased risk of hypertension and CKD. Our results suggest renal sinus fat may be associated with blood pressure regulation and CKD in humans and provides additional insight into the pathophysiologic role of adiposity in renal dysfunction. Further research is necessary to evaluate the longitudinal associations of renal sinus fat with markers of renal function and metabolic risk factors.
Supplementary Material
Acknowledgments
This article was presented in part as an abstract at the AHA 50th EPI/NPAM Conference 2010; March 2–5, 2010.
Sources of Funding: The Framingham Heart Study is supported by the National Heart, Lung and Blood Institute (N01-HC-25195).
Footnotes
Conflicts of Interest/Disclosures: The authors declare no completing financial interests.
References
- 1.Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999–2008. JAMA. 2010;303:235–241. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- 2.Kelly T, Yang W, Chen CS, Reynolds K, He J. Global burden of obesity in 2005 and projections to 2030. Int J Obes (Lond) 2008;32:1431–1437. doi: 10.1038/ijo.2008.102. [DOI] [PubMed] [Google Scholar]
- 3.Malnick SD, Knobler H. The medical complications of obesity. QJM. 2006;99:565–579. doi: 10.1093/qjmed/hcl085. [DOI] [PubMed] [Google Scholar]
- 4.Fox CS, Larson MG, Leip EP, Culleton B, Wilson PW, Levy D. Predictors of new-onset kidney disease in a community-based population. JAMA. 2004;291:844–850. doi: 10.1001/jama.291.7.844. [DOI] [PubMed] [Google Scholar]
- 5.Gelber RP, Kurth T, Kausz AT, Manson JE, Buring JE, Levey AS, Gaziano JM. Association between body mass index and CKD in apparently healthy men. Am J Kidney Dis. 2005;46:871–880. doi: 10.1053/j.ajkd.2005.08.015. [DOI] [PubMed] [Google Scholar]
- 6.Foster MC, Hwang SJ, Larson MG, Lichtman JH, Parikh NI, Vasan RS, Levy D, Fox CS. Overweight, obesity, and the development of stage 3 CKD: the Framingham Heart Study. Am J Kidney Dis. 2008;52:39–48. doi: 10.1053/j.ajkd.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kurella M, Lo JC, Chertow GM. Metabolic syndrome and the risk for chronic kidney disease among nondiabetic adults. J Am Soc Nephrol. 2005;16:2134–2140. doi: 10.1681/ASN.2005010106. [DOI] [PubMed] [Google Scholar]
- 8.Lee JE, Choi SY, Huh W, Kim YG, Kim DJ, Oh HY. Metabolic syndrome, C-reactive protein, and chronic kidney disease in nondiabetic, nonhypertensive adults. Am J Hypertens. 2007;20:1189–1194. doi: 10.1016/j.amjhyper.2007.04.020. [DOI] [PubMed] [Google Scholar]
- 9.Montani JP, Carroll JF, Dwyer TM, Antic V, Yang Z, Dulloo AG. Ectopic fat storage in heart, blood vessels and kidneys in the pathogenesis of cardiovascular diseases. Int J Obes Relat Metab Disord. 2004;28 (Suppl 4):S58–S65. doi: 10.1038/sj.ijo.0802858. [DOI] [PubMed] [Google Scholar]
- 10.Ott CE, Navar LG, Guyton AC. Pressures in static and dynamic states from capsules implanted in the kidney. Am J Physiol. 1971;221:394–400. doi: 10.1152/ajplegacy.1971.221.2.394. [DOI] [PubMed] [Google Scholar]
- 11.Stolarczyk J, Carone FA. Effects of renal lymphatic occlusion and venous constriction on renal function. Am J Pathol. 1975;78:285–296. [PMC free article] [PubMed] [Google Scholar]
- 12.Burnett JC, Jr, Knox FG. Renal interstitial pressure and sodium excretion during renal vein constriction. Am J Physiol. 1980;238:F279–F282. doi: 10.1152/ajprenal.1980.238.4.F279. [DOI] [PubMed] [Google Scholar]
- 13.Burnett JC, Jr, Haas JA, Knox FG. Segmental analysis of sodium reabsorption during renal vein constriction. Am J Physiol. 1982;243:F19–F22. doi: 10.1152/ajprenal.1982.243.1.F19. [DOI] [PubMed] [Google Scholar]
- 14.Dwyer TM, Mizelle HL, Cockrell K, Buhner P. Renal sinus lipomatosis and body composition in hypertensive, obese rabbits. Int J Obes Relat Metab Disord. 1995;19:869–874. [PubMed] [Google Scholar]
- 15.Chughtai HL, Morgan TM, Rocco M, Stacey B, Brinkley TE, Ding J, Nicklas B, Hamilton C, Hundley WG. Renal sinus fat and poor blood pressure control in middle-aged and elderly individuals at risk for cardiovascular events. Hypertension. 2010;56:901–906. doi: 10.1161/HYPERTENSIONAHA.110.157370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee HS, Lee JS, Koh HI, Ko KW. Intraglomerular lipid deposition in routine biopsies. Clin Nephrol. 1991;36:67–75. [PubMed] [Google Scholar]
- 17.Bobulescu IA, Dubree M, Zhang J, McLeroy P, Moe OW. Effect of renal lipid accumulation on proximal tubule Na+/H+ exchange and ammonium secretion. Am J Physiol Renal Physiol. 2008;294:F1315–F1322. doi: 10.1152/ajprenal.00550.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Deji N, Kume S, Araki S, Soumura M, Sugimoto T, Isshiki K, Chin-Kanasaki M, Sakaguchi M, Koya D, Haneda M, Kashiwagi A, Uzu T. Structural and functional changes in the kidneys of high-fat diet-induced obese mice. Am J Physiol Renal Physiol. 2009;296:F118–F126. doi: 10.1152/ajprenal.00110.2008. [DOI] [PubMed] [Google Scholar]
- 19.do Carmo JM, Tallam LS, Roberts JV, Brandon EL, Biglane J, da Silva AA, Hall JE. Impact of obesity on renal structure and function in the presence and absence of hypertension: evidence from melanocortin-4 receptor-deficient mice. Am J Physiol Regul Integr Comp Physiol. 2009;297:R803–R812. doi: 10.1152/ajpregu.00187.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fox CS, Massaro JM, Hoffmann U, Pou KM, Maurovich-Horvat P, Liu CY, Vasan RS, Murabito JM, Meigs JB, Cupples LA, D’Agostino RB, Sr, O’Donnell CJ. Abdominal visceral and subcutaneous adipose tissue compartments: association with metabolic risk factors in the Framingham Heart Study. Circulation. 2007;116:39–48. doi: 10.1161/CIRCULATIONAHA.106.675355. [DOI] [PubMed] [Google Scholar]
- 21.Levy D, Ehret GB, Rice K, Verwoert GC, Launer LJ, Dehghan A, Glazer NL, Morrison AC, Johnson AD, Aspelund T, Aulchenko Y, Lumley T, Kottgen A, Vasan RS, Rivadeneira F, Eiriksdottir G, Guo X, Arking DE, Mitchell GF, Mattace-Raso FU, Smith AV, Taylor K, Scharpf RB, Hwang SJ, Sijbrands EJ, Bis J, Harris TB, Ganesh SK, O’Donnell CJ, Hofman A, Rotter JI, Coresh J, Benjamin EJ, Uitterlinden AG, Heiss G, Fox CS, Witteman JC, Boerwinkle E, Wang TJ, Gudnason V, Larson MG, Chakravarti A, Psaty BM, van Duijn CM. Genome-wide association study of blood pressure and hypertension. Nat Genet. 2009;41:677–687. doi: 10.1038/ng.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Coresh J, Astor BC, McQuillan G, Kusek J, Greene T, Van LF, Levey AS. Calibration and random variation of the serum creatinine assay as critical elements of using equations to estimate glomerular filtration rate. Am J Kidney Dis. 2002;39:920–929. doi: 10.1053/ajkd.2002.32765. [DOI] [PubMed] [Google Scholar]
- 23.Levey AS, Coresh J, Greene T, Stevens LA, Zhang YL, Hendriksen S, Kusek JW, Van LF. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145:247–254. doi: 10.7326/0003-4819-145-4-200608150-00004. [DOI] [PubMed] [Google Scholar]
- 24.Stevens LA, Coresh J, Schmid CH, Feldman HI, Froissart M, Kusek J, Rossert J, Van LF, Bruce RD, III, Zhang YL, Greene T, Levey AS. Estimating GFR using serum cystatin C alone and in combination with serum creatinine: a pooled analysis of 3,418 individuals with CKD. Am J Kidney Dis. 2008;51:395–406. doi: 10.1053/j.ajkd.2007.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lavie CJ, Milani RV, Ventura HO. Obesity and cardiovascular disease: risk factor, paradox, and impact of weight loss. J Am Coll Cardiol. 2009;53:1925–1932. doi: 10.1016/j.jacc.2008.12.068. [DOI] [PubMed] [Google Scholar]
- 26.Dietrich RB, Kangarloo H. Kidneys in infants and children: evaluation with MR. Radiology. 1986;159:215–221. doi: 10.1148/radiology.159.1.3952310. [DOI] [PubMed] [Google Scholar]
- 27.Hricak H, Crooks L, Sheldon P, Kaufman L. Nuclear magnetic resonance imaging of the kidney. Radiology. 1983;146:425–432. doi: 10.1148/radiology.146.2.6849088. [DOI] [PubMed] [Google Scholar]
- 28.Hall JE, Brands MW, Dixon WN, Smith MJ., Jr Obesity-Induced Hypertension: Renal Function and Systemic Hemodynamics. Hypertension. 1993;22:292–299. doi: 10.1161/01.hyp.22.3.292. [DOI] [PubMed] [Google Scholar]
- 29.Rocchini AP, Moorehead C, Wentz E, Deremer S. Obesity-induced hypertension in the dog. Hypertension. 1987;9:III64–III68. doi: 10.1161/01.hyp.9.6_pt_2.iii64. [DOI] [PubMed] [Google Scholar]
- 30.Hall JE, Brands MW, Henegar JR, Shek EW. Abnormal kidney function as a cause and a consequence of obesity hypertension. Clin Exp Pharmacol Physiol. 1998;25:58–64. doi: 10.1111/j.1440-1681.1998.tb02146.x. [DOI] [PubMed] [Google Scholar]
- 31.Reisin E, Jack AV. Obesity and hypertension: mechanisms, cardio-renal consequences, and therapeutic approaches. Med Clin North Am. 2009;93:733–751. doi: 10.1016/j.mcna.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 32.Rosito GA, Massaro JM, Hoffmann U, Ruberg FL, Mahabadi AA, Vasan RS, O’Donnell CJ, Fox CS. Pericardial fat, visceral abdominal fat, cardiovascular disease risk factors, and vascular calcification in a community-based sample: the Framingham Heart Study. Circulation. 2008;117:605–613. doi: 10.1161/CIRCULATIONAHA.107.743062. [DOI] [PubMed] [Google Scholar]
- 33.Schlett CL, Massaro JM, Lehman SJ, Bamberg F, O’Donnell CJ, Fox CS, Hoffmann U. Novel measurements of periaortic adipose tissue in comparison to anthropometric measures of obesity, and abdominal adipose tissue. Int J Obes (Lond) 2009;33:226–232. doi: 10.1038/ijo.2008.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Speliotes EK, Massaro JM, Hoffmann U, Vasan RS, Meigs JB, Sahani DV, Hirschhorn JN, O’Donnell CJ, Fox CS. Fatty liver is associated with dyslipidemia and dysglycemia independent of visceral fat: the Framingham Heart Study. Hepatology. 2010;51:1979–1987. doi: 10.1002/hep.23593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Preis SR, Massaro JM, Hoffmann U, D’Agostino RB, Sr, Levy D, Robins SJ, Meigs JB, Vasan RS, O’Donnell CJ, Fox CS. Neck circumference as a novel measure of cardiometabolic risk: the Framingham Heart study. J Clin Endocrinol Metab. 2010;95:3701–3710. doi: 10.1210/jc.2009-1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Young JA, Hwang SJ, Sarnak MJ, Hoffmann U, Massaro JM, Levy D, Benjamin EJ, Larson MG, Vasan RS, O’Donnell CJ, Fox CS. Association of visceral and subcutaneous adiposity with kidney function. Clin J Am Soc Nephrol. 2008;3:1786–1791. doi: 10.2215/CJN.02490508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Menon V, Shlipak MG, Wang X, Coresh J, Greene T, Stevens L, Kusek JW, Beck GJ, Collins AJ, Levey AS, Sarnak MJ. Cystatin C as a risk factor for outcomes in chronic kidney disease. Ann Intern Med. 2007;147:19–27. doi: 10.7326/0003-4819-147-1-200707030-00004. [DOI] [PubMed] [Google Scholar]
- 38.Levey AS. Measurement of renal function in chronic renal disease. Kidney Int. 1990;38:167–184. doi: 10.1038/ki.1990.182. [DOI] [PubMed] [Google Scholar]
- 39.Stevens LA, Schmid CH, Greene T, Li L, Beck GJ, Joffe MM, Froissart M, Kusek JW, Zhang YL, Coresh J, Levey AS. Factors other than glomerular filtration rate affect serum cystatin C levels. Kidney Int. 2009;75:652–660. doi: 10.1038/ki.2008.638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Knight EL, Verhave JC, Spiegelman D, Hillege HL, de ZD, Curhan GC, de Jong PE. Factors influencing serum cystatin C levels other than renal function and the impact on renal function measurement. Kidney Int. 2004;65:1416–1421. doi: 10.1111/j.1523-1755.2004.00517.x. [DOI] [PubMed] [Google Scholar]
- 41.Taleb S, Cancello R, Clement K, Lacasa D. Cathepsin s promotes human preadipocyte differentiation: possible involvement of fibronectin degradation. Endocrinology. 2006;147:4950–4959. doi: 10.1210/en.2006-0386. [DOI] [PubMed] [Google Scholar]
- 42.Vupputuri S, Fox CS, Coresh J, Woodward M, Muntner P. Differential estimation of CKD using creatinine- versus cystatin C-based estimating equations by category of body mass index. Am J Kidney Dis. 2009;53:993–1001. doi: 10.1053/j.ajkd.2008.12.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.