Abstract
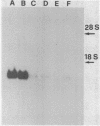
A recombinant M13 clone (O42) containing a 65 b.p. cDNA fragment from human fetal liver mRNA coding for glyceraldehyde-3-phosphate dehydrogenase has been identified and it has been used to isolate from a full-length human adult liver cDNA library a recombinant clone, pG1, which has been subcloned in M13 phage and completely sequenced with the chain terminator method. Besides the coding region of 1008 b.p., the cDNA sequence includes 60 nucleotides at the 5'-end and 204 nucleotides at the 3'-end up to the polyA tail. Hybridization of pG1 to human liver total RNA shows only one band about the size of pG1 cDNA. A much stronger hybridization signal was observed using RNA derived from human hepatocarcinoma and kidney carcinoma cell lines. Sequence homology between clone 042 and the homologous region of clone pG1 is 86%. On the other hand, homology among the translated sequences and the known human muscle protein sequence ranges between 77 and 90%; these data demonstrate the existence of more than one gene coding for G3PD. Southern blot of human DNA, digested with several restriction enzymes, also indicate that several homologous sequences are present in the human genome.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aithal H. N., Walsh-Reitz M. M., Toback F. G. Appearance of a cytosolic protein that stimulates glyceraldehyde-3-phosphate dehydrogenase activity during initiation of renal epithelial cell growth. Proc Natl Acad Sci U S A. 1983 May;80(10):2941–2945. doi: 10.1073/pnas.80.10.2941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold H. H., Domdey H., Wiebauer K., Datta K., Siddiqui M. A. Cloning, partial sequencing, and expression of glyceraldehyde-3-phosphate dehydrogenase gene in chick embryonic heart muscle cells. J Biol Chem. 1982 Aug 25;257(16):9872–9877. [PubMed] [Google Scholar]
- Cortese R., Melton D., Tranquilla T., Smith J. D. Cloning of nematode tRNA genes and their expression in the frog oocyte. Nucleic Acids Res. 1978 Dec;5(12):4593–4611. [PMC free article] [PubMed] [Google Scholar]
- Costanzo F., Castagnoli L., Dente L., Arcari P., Smith M., Costanzo P., Raugei G., Izzo P., Pietropaolo T. C., Bougueleret L. Cloning of several cDNA segments coding for human liver proteins. EMBO J. 1983;2(1):57–61. doi: 10.1002/j.1460-2075.1983.tb01380.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson B. E., Sajgò M., Noller H. F., Harris J. I. Amino-acid sequence of glyceraldehyde 3-phosphate dehydrogenase from lobster muscle. Nature. 1967 Dec 23;216(5121):1181–1185. doi: 10.1038/2161181a0. [DOI] [PubMed] [Google Scholar]
- Domdey H., Wiebauer K., Klapthor H., Arnold H. H. Sequence analysis of the cloned mRNA coding for glyceraldehyde-3-phosphate dehydrogenase from chicken heart muscle. Eur J Biochem. 1983 Mar 1;131(1):129–135. doi: 10.1111/j.1432-1033.1983.tb07239.x. [DOI] [PubMed] [Google Scholar]
- Dugaiczyk A., Haron J. A., Stone E. M., Dennison O. E., Rothblum K. N., Schwartz R. J. Cloning and sequencing of a deoxyribonucleic acid copy of glyceraldehyde-3-phosphate dehydrogenase messenger ribonucleic acid isolated from chicken muscle. Biochemistry. 1983 Mar 29;22(7):1605–1613. doi: 10.1021/bi00276a013. [DOI] [PubMed] [Google Scholar]
- Döbeli H., Schoenenberger G. A. A factor isolated from liver inhibits reassociation/reactivation of dissociated glyceraldehyde-3-phosphate dehydrogenase. FEBS Lett. 1981 Oct 12;133(1):123–126. doi: 10.1016/0014-5793(81)80486-3. [DOI] [PubMed] [Google Scholar]
- Harris J. I., Perham R. N. Glyceraldehyde 3-phosphate dehydrogenase from pig muscle. Nature. 1968 Sep 7;219(5158):1025–1028. doi: 10.1038/2191025a0. [DOI] [PubMed] [Google Scholar]
- Hocking J. D., Harris J. I. D-glyceraldehyde-3-phosphate dehydrogenase. Amino-acid sequence of the enzyme from the extreme thermophile Thermus aquaticus. Eur J Biochem. 1980 Jul;108(2):567–579. doi: 10.1111/j.1432-1033.1980.tb04752.x. [DOI] [PubMed] [Google Scholar]
- Holland J. P., Labieniec L., Swimmer C., Holland M. J. Homologous nucleotide sequences at the 5' termini of messenger RNAs synthesized from the yeast enolase and glyceraldehyde-3-phosphate dehydrogenase gene families. The primary structure of a third yeast glyceraldehyde-3-phosphate dehydrogenase gene. J Biol Chem. 1983 Apr 25;258(8):5291–5299. [PubMed] [Google Scholar]
- Holland M. J., Holland J. P. Isolation and characterization of a gene coding for glyceraldehyde-3-phosphate dehydrogenase from Saccharomyces cerevisiae. J Biol Chem. 1979 Jun 25;254(12):5466–5474. [PubMed] [Google Scholar]
- Hu N., Messing J. The making of strand-specific M13 probes. Gene. 1982 Mar;17(3):271–277. doi: 10.1016/0378-1119(82)90143-3. [DOI] [PubMed] [Google Scholar]
- Lebherz H. G., Rutter W. J. Glyceraldehyde-3-phosphate dehydrogenase variants in phyletically diverse organisms. Science. 1967 Sep 8;157(3793):1198–1200. doi: 10.1126/science.157.3793.1198. [DOI] [PubMed] [Google Scholar]
- MacLeod A. R. Expression of the mRNA coding for glyceraldehyde-3-phosphate dehydrogenase. Eur J Biochem. 1981 Oct;119(2):353–358. doi: 10.1111/j.1432-1033.1981.tb05615.x. [DOI] [PubMed] [Google Scholar]
- Milner R. J., Brow M. D., Cleveland D. W., Shinnick T. M., Sutcliffe J. G. Glyceraldehyde 3-phosphate dehydrogenase protein and mRNA are both differentially expressed in adult chickens but not chick embryos. Nucleic Acids Res. 1983 May 25;11(10):3301–3315. doi: 10.1093/nar/11.10.3301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowak K., Wolny M., Banaś T. The complete amino acid sequence of human muscle glyceraldehyde 3-phosphate dehydrogenase. FEBS Lett. 1981 Nov 16;134(2):143–146. doi: 10.1016/0014-5793(81)80587-x. [DOI] [PubMed] [Google Scholar]
- Olsen K. W., Moras D., Rossmann M. G. Sequence variability and structure of D-glyceraldehyde-3-phosphate dehydrogenase. J Biol Chem. 1975 Dec 25;250(24):9313–9321. [PubMed] [Google Scholar]
- Panabières F., Piechaczyk M., Rainer B., Dani C., Fort P., Riaad S., Marty L., Imbach J. L., Jeanteur P., Blanchard J. M. Complete nucleotide sequence of the messenger RNA coding for chicken muscle glyceraldehyde-3-phosphate dehydrogenase. Biochem Biophys Res Commun. 1984 Feb 14;118(3):767–773. doi: 10.1016/0006-291x(84)91461-x. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Rosen S. W., Weintraub B. D., Aaronson S. A. Nonrandom ectopic protein production by malignant cells: direct evidence in vitro. J Clin Endocrinol Metab. 1980 May;50(5):834–841. doi: 10.1210/jcem-50-5-834. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker J. E., Carne A. F., Runswick M. J., Bridgen J., Harris J. I. D-glyceraldehyde-3-phosphate dehydrogenase. Complete amino-acid sequence of the enzyme from Bacillus stearothermophilus. Eur J Biochem. 1980 Jul;108(2):549–565. doi: 10.1111/j.1432-1033.1980.tb04751.x. [DOI] [PubMed] [Google Scholar]