Abstract
Osteoporosis in gastric cancer patients is often overlooked or even neglected despite its high prevalence in these patients. Considering that old age, malnutrition, chronic disease, chemotherapy, decreased body mass index and gastrectomy are independent risk factors for osteoporosis, it is reasonable that the prevalence of osteoporosis in gastric cancer patients would be high. Many surviving patients suffer from back pain and pathological fractures, which are related to osteoporosis. Fractures have obvious associated morbidities, negative impact on quality of life, and impose both direct and indirect costs. In the era of a >55.6% 5-year survival rate of gastric cancer and increased longevity in gastric cancer patients, it is very important to eliminate common sequelae such as osteoporosis. Fortunately, the diagnosis of osteoporosis is well established and many therapeutic agents have been shown to be effective and safe not only in postmenopausal females but also in elderly males. Recently, effective treatments of gastric cancer patients with osteoporosis using bisphosphonates, which are commonly used in postmenopausal woman, were reported.
Keywords: Stomach neoplasms, Osteoporosis, Prevalence, Diagnosis, Therapeutics
Introduction
Gastric cancer is one of the most common cancers in the world. Although relatively decreasing in prevalence, it is still the second most common cause of death from cancer in the world. The incidence of gastric cancer is very high in East Asia, and it is a major burden in countries, such as Korea.(1) Although the overall prognosis of gastric cancer is poor in Western countries, the 5-year survival rate for those who received operative treatment in Korea is 55.6%. In cases of early gastric cancer, the survival rate is more than 90% in Korea due to early diagnosis and aggressive surgical intervention.(2)
However, many surviving patients unfortunately suffer from sequelae caused by the surgical procedure. Weight loss, malnutrition, anemia and osteoporosis are known consequences of gastrectomy.(3) Old age, malnutrition, chronic disease, chemotherapy, decreased body mass index and gastrectomy are independent risk factors for osteoporosis. Most gastric cancer patients have these risk factors.(4) The prevalence of osteoporosis in gastric cancer patients who are older than 50 years old is up to 38.3% in Korea.(5) Similar to other primary and secondary forms of osteoporosis, most gastrectomy-induced osteoporosis patients suffer from back pain and pathologic fractures. Recently, a 63-year-old woman who underwent gastrectomy for a gastric malignancy spontaneously developed multiple fractures.(6) It was speculated that the synergistic effects of gastrectomy and other osteoporosis risk factors led to multiple fractures.
The American Gastroenterology Association (AGA) recommended dual-energy X-ray absorptiometry (DXA) evaluation in patients who had any of the following conditions: at least 10 years postgastrectomy, low-trauma fracture history, such as a vertebral fracture, postmenopausal female or male over 50 years of age, and hypogonadism.(4) Most of the gastric cancer patients in Korea qualified for these conditions.
In this review article, we discuss the prevalence, pathophysiology, screening, and management of osteoporosis in gastric cancer and postgastrectomy patients. The objective of this review is to help to understand a practical diagnostic strategy and to provide management recommendations regarding osteoporosis in gastric cancer patients.
The Prevalence of Osteoporosis and Fractures in Gastric Cancer or Postgastrectomy Patients
The overall prevalence of osteoporosis in Korean gastric cancer patients older than 50 years of age was 39.6%.(5) The osteoporosis rate of the lumbar spine in gastric cancer patients was 29.8% in male and 54.5% in female patients. The osteoporosis rate of the femoral neck was 11.9% in males and 26.3% in females.(5) Those rates are higher than the osteoporosis rates in a community-based Korean study. Cui et al.(7) studied 4,148 Korean adults to assess the prevalence of osteoporosis. The osteoporosis rate of the lumbar spine in patients over 50 years was 6.5% in male and 40.1% in female patients. The osteoporosis rate of the femoral neck was 5.9% in male and 12.4% in female patients.
In other DXA-based studies in postgastrectomy patients, including gastric cancer and peptic ulcer patients, the prevalence of osteoporosis of the lumbar spine was 22~37% and that of the femoral neck was 10~61%.(8,9) In a U.S. population-based study involving 355 males over age 60, 17 had a previous total gastrectomy for gastric cancer. Their bone mineral density (BMD) as measured by DXA decreased at the spine (-14.1%), total hip (-9%), and distal radius (-9.9%).(10) In Korea, mean bone loss in the lumbar spine (-5.7%), total hip (-5.4%), and femoral neck (-6.6%) was reported 1 year after gastrectomy.(11) Thus, even in the more recent studies, gastrectomy remains a significant contributor to bone disease.
In our study, 24 of 133 (18.0%) gastric cancer patients had osteoporotic fractures (such as Colles' fracture, ankle and severe vertebral fractures). Of the gastric cancer patients, 61 of 133 (45.9%) complained of back pain, and 46.6% showed vertebral bone deformity.(5) In a population-based study from the U.S. which assessed 438 subjects who underwent peptic ulcer surgery, the estimated incidence of hip fracture was 25% with a relative risk (RR) of 2.5 and that of vertebral fracture was 41% with a RR of 4.7.(12) In a Swedish male cohort, gastrectomy patients had a higher fracture rate (40% in Billroth I and 33% in a Billroth II) compared to that of the controls (12%).(13) At almost 9 years' follow-up, the postgastrectomy patients had an average of 1.7 spinal fractures compared to an average of 0.6 fractures in the controls, representing a threefold increase in risk.(14)
Pathogenesis of Osteoporosis in Gastric Cancer
The exact pathogenesis of osteoporosis in gastric cancer is unknown. Old age, female sex, malabsorption, cancer itself and its treatment are independent risk factors for osteoporosis. Malabsorption before and after gastric bypass surgery might play a major role as suspected. Deficiency of nutrients such as calcium, phosphorous, vitamin D, iron and protein is frequently noted in postgastrectomy patients. Poor absorption of vitamin D and calcium results in secondary hyperparathyroidism, which increases rates of bone loss. In addition, low vitamin D levels or hypocalcemia can lead to osteomalacia or osteoporosis. Chemotherapy can also affect bone metabolism, as it does in other cancer patients; however, evidence is limited.
1. Malabsorption and malnutrition
Removal of the gastric antrum with anastomosis to the duodenum (Billroth I) or with anastomosis to the jejunum (Billroth II) alters normal gastrointestinal (GI) physiology. Consequently, calcium malabsorption follows because calcium is absorbed primarily in the duodenum. Gastric dumping or formation of insoluble calcium soaps due to fat malabsorption may also cause calcium malabsorption. Mean serum calcium levels were found to be lower than those in controls.(15,16) One study showed 7.3% out of 342 postgastrectomy patients had subnormal serum calcium levels, compared with only 0.5% of controls.(16) However, in the clinical setting, it is difficult to find low calcium levels in postgastrectomy patients because on average, the serum calcium level still falls into the lowest levels of normal range.(17)
After gastrectomy, patients often suffer from steatorrhea, leading to malabsorption of vitamin D.(18) However, in clinical studies, vitamin D malabsorption in postgastrectomy patients was mild or normal at worst.(15) After gastrectomy, patients may alter their diet, and decreased serum 25-hydroxyvitamin D (25-OHD) level may in part reflect reduced dietary intake of vitamin D.(18) Patients who had subnormal 25-OHD levels generally had longer duration after gastrectomy.
Protein metabolism has an obvious role in the formation of the collagen matrix of bone. Impaired protein nutrition secondary to reduced intake may also play a role in postgastrectomy bone disease.(3,19) The BMD of the total hip, femoral neck, and trochanter also correlated to the change in body weight at 12 months.(11) The standard recommended daily allowances (RDAs) of nutrients are often inadequate, and gastric cancer patients require much more intake, although weight loss and nutrition are stabilized over time after gastrectomy.
2. Hyperparathyroidism
Hyperparathyroidism is associated with osteoporosis. The parathyroid hormone (PTH) activates osteoblasts, which in turn matures the osteoclasts. Osteoclasts release hydrochloric acid, which dissolves bone mineral, causing osteopenia and osteoporosis.(20)
Serum calcium, phosphate and vitamin D may be maintained at the expense of bone mass. Bone-related hormones, such as PTH, play an important role in regulation of calcium homeostasis. Secondary hyperparathyroidism could explain these results. Elevated PTH was reported in some studies,(21) although most studies report PTH levels within normal range.(17,22) However, those studies are cross-sectional and different from each other in sampling timing after gastrectomy.
Recently, Baek et al.(11) found that serum PTH levels were increased after gastrectomy in gastric cancer patients. Patients who had higher PTH levels after gastrectomy lost more bone at the lumbar spine and total hip during the first year after gastrectomy. Bone resorption markers increased without an associated increase in bone formation markers after the early gastrectomy-period. Such an uncoupling of bone metabolism suggests that postgastrectomy bone loss is a consequence of an imbalance between bone formation and bone resorption. Bone resorption markers, bone formation markers, and PTH were all normalized 1 year postgastrectomy, which indicates adequate coupling and discontinuation of active bone loss. Significant correlations were found between the percent change in the BMD at the lumbar spine and total hip and that of the PTH level from their baseline to 12 months. In an animal study, total gastrectomy resulted in reduced serum calcium and 25-OHD and increased PTH compared with sham-operated controls.(23) In secondary hyperparathyroidism, elevated 1,25(OH)2-vitamin D levels and enhanced 25-OHD clearance can be reversed with adequate nutrition such as oral calcium supplementation and higher doses of vitamin D.(24)
3. Chemotherapy
A variety of cancer therapies, including hormonal therapy, chemotherapy, and radiotherapy affect bone metabolism.(25) In vitro chemotherapeutic agents can interfere with osteoblast function, cause adverse effects on mineralization by osteoblasts, and reduce the number of osteoblasts.(26) The most commonly used chemotherapeutic agents for gastric cancer is doxifluridine and cisplatin; 5-fluorouracil was reported to induce apoptosis among osteoblasts and preosteoblasts in animal models.(27) Cisplatin treatment showed increased osteoclast activity and delayed bone formation during the remodeling phase in an animal model.(28) Bone formation marker levels were generally lower for the patients who received chemotherapy than for the patients who did not.(11) In conclusion, systemic adjuvant chemotherapy in postgastrectomy cancer patients may account for the uncoupling of the markers of bone formation during the early postgastrectomy period, and it is probable that the chemotherapeutic agents contribute to the more severe bone loss noted for patients who received chemotherapy.
4. Other
Inflammatory cytokines, such as tumor necrosis factor (TNF)-a, IL-6, and IL-1, are often increased in gastrointestinal disease patients including those with gastric cancer.(29) TNF-a inhibits differentiation of osteoblasts from pluripotent progenitor cells as well as the expression of a critical transcription factor required for osteoblast differentiation. TNF-a also induces osteoclast differentiation and increases osteoclastic bone resorption. TNF-a also inhibits bone collagen synthesis in vitro. TNF-a inhibits the action of 1,25 (OH) vitamin D through activation of a nuclear inhibitor that antagonizes the effect of vitamin D.(30) However, reports on the relationship of cytokine and bone metabolism in gastric cancer are scarce.
Calcitonin has osteoclast-inhibiting effects. Decreased calcitonin may favor bone resorption. One study found that the serum calcitonin levels were significantly reduced in postgastrectomy patients.
The destruction of gastric acid after gastrectomy also might result in osteoporosis. Epidemiologic studies suggest an association between the use of protein pump inhibitors, low bone density, and fractures.(31) The proposed mechanism of action is decreased gastric acidity causing a decrease in absorption of calcium although this theory has been questioned yet.
Screening and Diagnosis of Osteoporosis in Gastric Cancer
Gastric cancer patients over 50 years of age should be screened for the risk factors for fracture and bone mineral density by DXA. Selected patients with high risk of osteoporosis should consider blood tests, such as tests for calcium, phosphorus, 25-OHD, PTH, bone markers, estrogen in females, and testosterone in males. If specific risk factors are detected, work up by a specialist is essential.
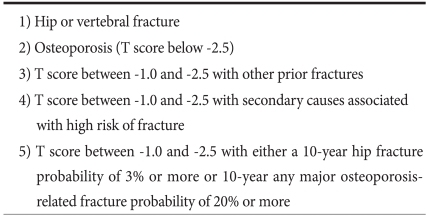
There are no specific osteoporosis screening guidelines for gastric cancer patients, but the AGA recommends DXA screening in postgastrectomy patients with any of following conditions: low-trauma fracture history, such as a vertebral fracture, postmenopausal female or male over 50 year of age, and hypogonadism.(4) The Korean Society of Bone Metabolism also recommends DXA in patients with risk factors, such as malabsorption and chemotherapy. (32) The risk factors commonly associated with fracture are listed in Table 1.(33) However, a risk factor grading system for GI disorders was not established; only low body weight, smoking, exercise, disease activity, menstrual status, corticosteroid use, dietary calcium intake, family history of osteoporosis, and personal history of fractures are candidates. Patients with one or more known risk factors probably should undergo initial screening with DXA. In this regard, most gastric cancer patients after gastrectomy should be screened for bone mineral density by DXA. Indications for BMD testing by The Korean Society of Bone Metabolism are listed in Table 2.(32)
Table 1.
Relative risk factors of fracture
Adapted from (33).
Table 2.
Indications for bone mineral density testing
Adapted from The Korean Society of Bone Metabolism.(32)
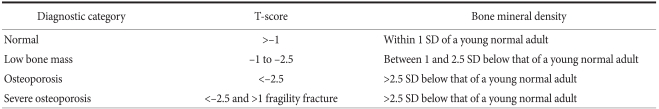
DXA is the gold standard for the non-invasive diagnosis of osteoporosis.(4,33) The World Health Organization (WHO) reported a classification of BMD for the diagnosis of osteoporosis based on DXA (Table 3).(33) T-score in DXA reports is defined as the number of standard deviations away from the mean BMD of a healthy young population. Several epidemiological studies have confirmed the association between a low BMD T-score and fracture risk in the elderly population. The fracture risk doubles for every SD decrease in BMD T-score.
Table 3.
World Health Organization (WHO) definition of osteoporosis
Adapted from WHO Technical Report Series.(33)
25-hydroxyvitamin D should be measured in patients at risk for malabsorption with a target blood level of 30 ng/ml because secondary hyperparathyroidism is initiated when vitamin D is less than this level.(24) Serum levels of the active metabolite 1,25 dihydroxyvitamin D do not correlate with nutritional stores and need not be measured.
Measuring serum PTH levels and 24-hour urine calcium collection may be useful in select patients. Secondary hyperparathyroidism and low urinary calcium levels may be seen in patients with severe malabsorption or low vitamin D and may be useful for monitoring the replacement therapy.
Management of Osteoporosis in Gastric Cancer
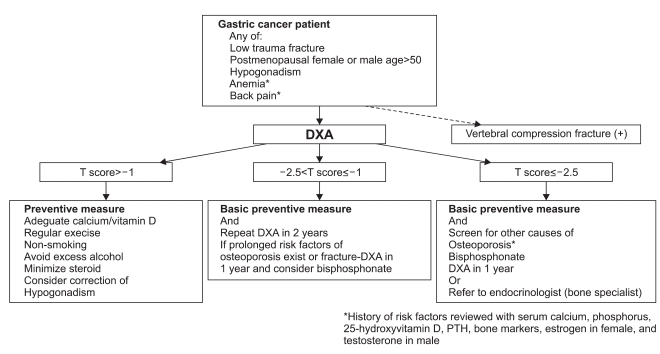
The management algorithm for osteoporosis in gastric cancer patients is shown in Fig. 1. All gastric cancer patients with T scores between -1.0 and -2.5 are associated with high risks of fracture, and pharmacologic therapy should be considered. Calcium and vitamin D supplements are generally required. In addition, in some cases such as T score lower than -2.5, bisphosphonates should be considered. Indications for pharmacologic therapy in postmenopausal women and men over 50 years of age by the Korean Society of Bone Metabolism are listed in Table 4. It is well known that postgastrectomy patients have higher risks of both hip and vertebral fractures.(13,14)
Fig. 1.
The management approach for osteoporosis in gastric cancer patients. DXA = dual-energy X-ray absorptiometry; PTH = parathyroid hormone.
Table 4.
Indications for pharmacologic therapy in postmenopausal women and men aged 50 and older
Adapted from The Korean Society of Bone Metabolism. (32)
The first goal in managing osteoporosis in gastric cancer patients is to restore adequate nutrition and prevent hyperparathyroidism to prevent further bone loss. The final goal is to prevent fractures and maintain a healthy life to improve quality of life in gastric cancer patients.
1. Physical activity
Physical activity is necessary for bone formation and maintenance throughout life. Weight training has been shown to induce a small increase in BMD at some, but not all skeletal sites. Walking has not demonstrated to increase BMD or to reduce fracture risk, but improvements in mobility, muscle function, and balance may reduce fracture risk by decreasing the risk of falling.
2. Nutrition and calcium/vitamin D supplemen tation
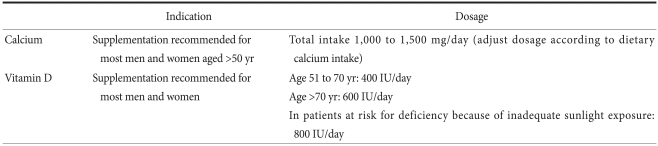
Calcium and vitamin D supplements are generally required, as the standard RDAs are rarely met with diet only in gastric cancer patients. Considering that average calcium and vitamin D intake of Korean is not sufficient,(34) it is essential to supplements all gastric cancer patients. The RDA for calcium is 1,000 mg per day for men and premenopausal women and 1,000~1,500 mg per day for postmenopausal women.(32,35) As the average daily calcium intake is less than 500 mg in people 50 years and older, most gastric cancer patients will need 500~1,000 mg daily as a supplement. The current RDA of vitamin D 400 IU per day is clearly inadequate, The National Osteoporosis Foundation recommends routine supplementation with 800~1,000 IU daily. Many patients have vitamin D malabsorption and require even higher doses. If the baseline 25-OHD level is lower than 15 ng/ml, most recommendations are to administer 50,000 units per week for 8`~12 weeks and then reevaluate.(24) The goal is to maintain the serum levels of 25-OH vitamin D at 30 ng/ml or higher. Higher levels of vitamin D have fewer side effects and are supposed to have possible additional health benefits.(24) It is particularly important to replace calcium and vitamin D in patients when beginning antiresorptive therapy, such as bisphosphonates, because treatment can induce hypocalcemia. The usual recommended doses of calcium and vitamin D are listed in Table 5.
Table 5.
Recommendations for calcium and vitamin D supplementation
Most gastric cancer patients experience a rapid weight loss during the immediate postoperative period. They also experience lack of appetite, dyspepsia, altered intestinal motility, and dysphagia, which are explanatory factors of the low food intake.(3) Low energy intake and fecal energy loss (inadequate absorption) is suggested to be an important mechanism. The mean energy imbalance is approximately 390 kcal/d 6 months after gastrectomy and about 310±50 kcal/d 5 years after the operation.(3) As a result, approximately 10% loss of the preoperative weight occurs during the early postoperative period.(11) Previous studies showed that a 10% weight loss resulted in 1~2% bone loss in various sites. Thus, proper nutrition is essential to prevent osteoporosis in gastric cancer patients.
3. Pharmacologic intervention
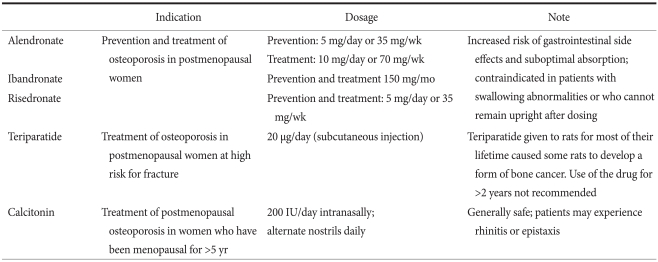
Bisphosphonates, selective estrogen receptor modulators (SERMs), calcitonin, teriparatide, and estrogen reduce the risk of fracture in osteoporosis patients, and the US Food and Drug Administration (FDA) approved these therapeutic options for treatment or prevention of osteoporosis.(32) The commonly prescribed medicines are listed on Table 6. However, only bisphosphonates showed effectiveness in a clinical trial of gastric cancer patients until now.(36)
Table 6.
Commonly used medication in osteoporosis
Adapted from The Korean Society of Bone Metabolism and National Osteoporosis Foundation.(32)
4. Bisphosphonates
Bisphosphonates are stable analogues of pyrophosphate with a strong affinity for bone apatite; these agents inhibit bone resorption by reducing the recruitment and activity of osteoclasts and increasing apoptosis of osteoclasts.(37,38) The bones formed while patients are receiving bisphosphonates treatment is histologically normal.
5. Alendronate
Iwamoto et al.(36) reported successful treatment with alendronate (5 mg daily and 35 mg weekly) in postgastrectomy osteoporotic patients. They treated 24 postgastrectomy patients (mostly gastric cancer patients) for 24 months. Lumbar BMD increased by 5.2% following reductions in urinary levels of N-telopeptides of bone type I collagen (NTx) (-27.0% at 3 months) and serum levels of ALP (-11.2% at 24 months). Alendronate sodium is indicated for the prevention (5 mg daily and 35 mg weekly) and treatment (10 mg daily and 70 mg weekly) of osteoporosis in postmenopausal women.(39) Alendronate has been shown to increase bone mass and to reduce the incidence of fractures of the hip and spine in the treatment of preexisting osteoporosis. Alendronate is also indicated to increase bone mass in men, and to treat glucocorticoid-induced osteoporosis and Paget's disease of bone in both men and women.(39)
The efficacy of alendronate 10 mg once daily in increasing bone mass was tested in postmenopausal women with osteoporosis ages 44 to 84.(39) Significant increases in BMD relative to baseline and placebo were observed at each measurement site in the lumbar spine, trochanter, femoral neck, forearm, and total body. Weekly alendronate formulations have efficacy and tolerability similar to that of the daily formulations. In the randomized, double-blind, placebo-controlled Fracture Intervention Trial (FIT), patients with one or more baseline radiographic vertebral fractures, alendronate significantly reduced the risk of recurrent vertebral fracture, symptomatic vertebral fracture, hip fracture, and wrist fracture at 3 years. (40) Among women who were 6 months into menopause, alendronate prevented bone loss in the majority of patients at the spine, hip, and total body and reduced the rate of bone loss at the forearm by approximately 50%.
The oral bioavailability of bisphosphonates is low, ranging from 1 to 3% of the ingested dose.(38) To achieve optimum absorption and tolerability, patients should take their pill with a full glass of water and avoid food and beverages for 30 minutes after the morning dose. Importantly, patients must remain upright for 30 minutes after their first meal of the day. Failure to follow these guidelines increases the risk of esophageal side effects and reduces the absorption of the medication.(39) When dosing recommendations are followed, the safety profile of bisphosphonates is generally favorable; mild gastrointestinal discomfort (e.g., dyspepsia, and abdominal pain) is the most common side effect.(38) Esophagitis has been reported with alendronate.(39) Hypocalcemia can occur if the patient has pretreatment calcium or vitamin D deficiency or renal insufficiency with secondary hyperparathyroidism. Hypocalcemia should be managed with 1,000 mg calcium and 800~1,000 IU vitamin D daily before bisphosphonates. Of particular concern are the reports of osteonecrosis of the jaw among patients receiving bisphosphonates.(38) However, it should be noted that the majority of these patients (87%) were receiving high-dose bisphosphonates for indications other than osteoporosis. Other bisphosphonates such as risedronate and ibandronate are also available in Korea and showed similar efficacy.(34) Zoledronate or pamidronate can be used as intravenous therapy because these bisphosphonates show fewer GI side effects.
We treated some gastric cancer patients with severe GI side effects with the oral bisphosphonate, pamidronate. Pamidronate was also effective and patients had fewer GI symptoms (unpublished data). Parenteral bisphosphonates can cause a minor acute-phase reaction or flu-like syndrome in up to 20~22% of patients.(38) The incidence is much lower with repeated doses. Rapid infusions of parenteral pamidronate or zoledronate can alter renal function and cause rare cases of renal failure. The FDA has approved this therapy only for patients with a creatinine clearance of greater than 35 ml/min.
Conclusions
Gastric cancer patients should be screened for their BMD levels using DXA and for other risk factor in accordance with current osteoporosis treatment guidelines. These patients have a very high prevalence of osteoporosis. Similar to other primary and secondary forms of osteoporosis, most gastric cancer patients with gastrectomy suffer from back pain and pathologic fractures. Many patients who sustain a hip fracture do not regain full mobility, often requiring nursing home care, which means the profound decrease in quality of life.
BMD assessment by DXA assists with the diagnosis of osteoporosis and monitoring of treatment effects in gastric cancer patients. The treatment algorithm summarized in this review show effective and well-tolerated treatments for GI patients, including gastric cancer patients. Calcium and vitamin D are needed separately or with bisphosphonates.
Further research is needed to help define the magnitude of the excessive risk of fracture in patients with gastric cancer. Furthermore, the best treatment or prevention schedule for osteoporosis in gastric cancer patients has not been clearly defined because there is not sufficient evidence. Thus, prospective data are needed to determine which pharmacologic intervention is more effective and safe. These efforts will lead to improving the quality of life of long term survival gastric cancer patients.
Acknowledgments
The authors wish to thank Bong sup Song, Seung Yon Kim and Hyeon Jeong Lee for proof-reading and data collection.
References
- 1.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 2.Lee HJ, Yang HK, Ahn YO. Gastric cancer in Korea. Gastric Cancer. 2002;5:177–182. doi: 10.1007/s101200200031. [DOI] [PubMed] [Google Scholar]
- 3.Liedman B. Symptoms aft er total gastrectomy on food intake, body composition, bone metabolism, and quality of life in gastric cancer patients--is reconstruction with a reservoir worthwhile? Nutrition. 1999;15:677–682. doi: 10.1016/s0899-9007(99)00123-9. [DOI] [PubMed] [Google Scholar]
- 4.Bernstein CN, Leslie WD, Leboff MS. AGA technical review on osteoporosis in gastrointestinal diseases. Gastroenterology. 2003;124:795–841. doi: 10.1053/gast.2003.50106. [DOI] [PubMed] [Google Scholar]
- 5.Lim JS, Kim SB, Bang HY, Cheon GJ, Lee JI. High prevalence of osteoporosis in patients with gastric adenocarcinoma following gastrectomy. World J Gastroenterol. 2007;13:6492–6497. doi: 10.3748/wjg.v13.i48.6492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Iba K, Wada T, Takada J, Yamashita T. Multiple insufficiency fractures with severe osteoporosis. J Orthop Sci. 2003;8:717–720. doi: 10.1007/s00776-003-0685-z. [DOI] [PubMed] [Google Scholar]
- 7.Cui LH, Choi JS, Shin MH, Kweon SS, Park KS, Lee YH, et al. Prevalence of osteoporosis and reference data for lumbar spine and hip bone mineral density in a Korean population. J Bone Miner Metab. 2008;26:609–617. doi: 10.1007/s00774-007-0847-8. [DOI] [PubMed] [Google Scholar]
- 8.Liedman B, Bosaeus I, Mellström D, Lundell L. Osteoporosis aft er total gastrectomy. Results of a prospective, clinical study. Scand J Gastroenterol. 1997;32:1090–1095. doi: 10.3109/00365529709002986. [DOI] [PubMed] [Google Scholar]
- 9.Adachi Y, Shiota E, Matsumata T, Iso Y, Yoh R, Kitano S. Osteoporosis after gastrectomy: bone mineral density of lumbar spine assessed by dual-energy X-ray absorptiometry. Calcif Tissue Int. 2000;66:119–122. doi: 10.1007/s002230010025. [DOI] [PubMed] [Google Scholar]
- 10.Orwoll ES, Bevan L, Phipps KR. Determinants of bone mineral density in older men. Osteoporos Int. 2000;11:815–821. doi: 10.1007/s001980070039. [DOI] [PubMed] [Google Scholar]
- 11.Baek KH, Jeon HM, Lee SS, Lim DJ, Oh KW, Lee WY, et al. Short-term changes in bone and mineral metabolism following gastrectomy in gastric cancer patients. Bone. 2008;42:61–67. doi: 10.1016/j.bone.2007.08.027. [DOI] [PubMed] [Google Scholar]
- 12.Melton LJ, 3rd, Crowson CS, Khosla S, O'Fallon WM. Fracture risk aft er surgery for peptic ulcer disease: a population-based cohort study. Bone. 1999;25:61–67. doi: 10.1016/s8756-3282(99)00097-6. [DOI] [PubMed] [Google Scholar]
- 13.Mellström D, Rundgren A. Long-term effects after partial gastrectomy in elderly men. A longitudinal population study of men between 70 and 75 years of age. Scand J Gastroenterol. 1982;17:433–439. doi: 10.3109/00365528209182082. [DOI] [PubMed] [Google Scholar]
- 14.Klein KB, Orwoll ES, Lieberman DA, Meier DE, McClung MR, Parfitt AM. Metabolic bone disease in asymptomatic men after partial gastrectomy with Billroth II anastomosis. Gastroenterology. 1987;92:608–616. doi: 10.1016/0016-5085(87)90008-4. [DOI] [PubMed] [Google Scholar]
- 15.Bisballe S, Eriksen EF, Melsen F, Mosekilde L, Sørensen OH, Hessov I. Osteopenia and osteomalacia after gastrectomy: interrelations between biochemical markers of bone remodelling, vitamin D metabolites, and bone histomorphometry. Gut. 1991;32:1303–1307. doi: 10.1136/gut.32.11.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eddy RL. Metabolic bone disease after gastrectomy. Am J Med. 1971;50:442–449. doi: 10.1016/0002-9343(71)90333-0. [DOI] [PubMed] [Google Scholar]
- 17.Kwon SJ, Hahm JS, Cho YJ, Ahn Y, Shin DI. The influence of gastrectomy on the change of bone metabolism and bone density. Korean J Intern Med. 2000;15:25–31. doi: 10.3904/kjim.2000.15.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thompson GR. Vitamin D deficiency after gastrectomy. Sci Basis Med Annu Rev. 1970:260–275. [PubMed] [Google Scholar]
- 19.Tovey FI, Hall ML, Ell PJ, Hobsley M. A review of postgastrectomy bone disease. J Gastroenterol Hepatol. 1992;7:639–645. doi: 10.1111/j.1440-1746.1992.tb01498.x. [DOI] [PubMed] [Google Scholar]
- 20.Holick MF. The vitamin D epidemic and its health consequences. J Nutr. 2005;135:2739S–2748S. doi: 10.1093/jn/135.11.2739S. [DOI] [PubMed] [Google Scholar]
- 21.Filipponi P, Gregorio F, Cristallini S, Mannarelli C, Blass A, Scarponi AM, et al. Partial gastrectomy and mineral metabolism: effects on gastrin-calcitonin release. Bone Miner. 1990;11:199–208. doi: 10.1016/0169-6009(90)90059-o. [DOI] [PubMed] [Google Scholar]
- 22.Nilas L, Christiansen C. Influence of PTH and 1,25(OH)2D on calcium homeostasis and bone mineral content after gastric surgery. Calcif Tissue Int. 1985;37:461–466. doi: 10.1007/BF02557827. [DOI] [PubMed] [Google Scholar]
- 23.Maier GW, Kreis ME, Zittel TT, Becker HD. Calcium regulation and bone mass loss after total gastrectomy in pigs. Ann Surg. 1997;225:181–192. doi: 10.1097/00000658-199702000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 25.Stava CJ, Jimenez C, Hu MI, Vassilopoulou-Sellin R. Skeletal sequelae of cancer and cancer treatment. J Cancer Surviv. 2009;3:75–88. doi: 10.1007/s11764-009-0083-4. [DOI] [PubMed] [Google Scholar]
- 26.Davies JH, Evans BA, Jenney ME, Gregory JW. In vitro effects of combination chemotherapy on osteoblasts: implications for osteopenia in childhood malignancy. Bone. 2002;31:319–326. doi: 10.1016/s8756-3282(02)00822-0. [DOI] [PubMed] [Google Scholar]
- 27.Xian CJ, Cool JC, Pyragius T, Foster BK. Damage and recovery of the bone growth mechanism in young rats following 5-fluorouracil acute chemotherapy. J Cell Biochem. 2006;99:1688–1704. doi: 10.1002/jcb.20889. [DOI] [PubMed] [Google Scholar]
- 28.Ehrhart N, Eurell JA, Tommasini M, Constable PD, Johnson AL, Feretti A. Effect of cisplatin on bone transport osteogenesis in dogs. Am J Vet Res. 2002;63:703–711. doi: 10.2460/ajvr.2002.63.703. [DOI] [PubMed] [Google Scholar]
- 29.Gorouhi F, Islami F, Bahrami H, Kamangar F. Tumour-necrosis factor-A polymorphisms and gastric cancer risk: a meta-analysis. Br J Cancer. 2008;98:1443–1451. doi: 10.1038/sj.bjc.6604277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fernandez-Martin JL, Kurian S, Farmer P, Nanes MS. Tumor necrosis factor activates a nuclear inhibitor of vitamin D and retinoid-X receptors. Mol Cell Endocrinol. 1998;141:65–72. doi: 10.1016/s0303-7207(98)00094-x. [DOI] [PubMed] [Google Scholar]
- 31.Ali T, Roberts DN, Tierney WM. Long-term safety concerns with proton pump inhibitors. Am J Med. 2009;122:896–903. doi: 10.1016/j.amjmed.2009.04.014. [DOI] [PubMed] [Google Scholar]
- 32.Chung HY. Osteoporosis diagnosis and treatment 2007. J Korean Endocr Soc. 2008;23:76–108. [Google Scholar]
- 33.World Health Organization. Assessment of fracture risk and its application to screening for postmenopausal osteoporosis. Geneva (Switzerland): World Health Organization; 1994 Report No.: WHO Technical Report Series 843. [PubMed] [Google Scholar]
- 34.Jang JY, Chung HY, Hwang YC, Jeong IK, Ahn KJ, Kwon MK, et al. Dietary calcium intake and bone metabolism in Korean postmenopausal women. Korean J Bone Metab. 2008;15:143–149. [Google Scholar]
- 35.NIH Consensus Conference. Optimal calcium intake. NIH Consensus Development Panel on Optimal Calcium Intake. JAMA. 1994;272:1942–1948. [PubMed] [Google Scholar]
- 36.Iwamoto J, Uzawa M, Sato Y, Takeda T, Matsumoto H. Effect of alendronate on bone mineral density and bone turnover markers in post-gastrectomy osteoporotic patients. J Bone Miner Metab. 2010;28:202–208. doi: 10.1007/s00774-009-0116-0. [DOI] [PubMed] [Google Scholar]
- 37.Rodan GA, Fleisch HA. Bisphosphonates: mechanisms of action. J Clin Invest. 1996;97:2692–2696. doi: 10.1172/JCI118722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Drake MT, Clarke BL, Khosla S. Bisphosphonates: mechanism of action and role in clinical practice. Mayo Clin Proc. 2008;83:1032–1045. doi: 10.4065/83.9.1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gass M, Dawson-Hughes B. Preventing osteoporosis-related fractures: an overview. Am J Med. 2006;119(4 Suppl 1):S3–S11. doi: 10.1016/j.amjmed.2005.12.017. [DOI] [PubMed] [Google Scholar]
- 40.Black DM, Cummings SR, Karpf DB, Cauley JA, Thompson DE, Nevitt MC, et al. Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Fracture Intervention Trial Research Group. Lancet. 1996;348:1535–1541. doi: 10.1016/s0140-6736(96)07088-2. [DOI] [PubMed] [Google Scholar]