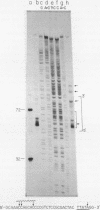
Abstract
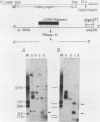
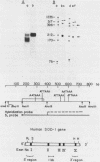
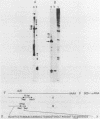
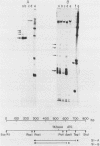
Two cytoplasmic superoxide dismutase (SOD-1) mRNAs of about 0.7 and 0.9 kilobases (Kb.) were previously found in a variety of human cells. The two SOD-1 mRNAs are transcribed from the same gene and the major 0.7 Kb. species is approximately four times more abundant than the minor 0.9 Kb. mRNA. These two mRNAs differ in the length of their 3'-untranslated region and both have multiple 5'-ends. The longer transcript contains 222 additional nucleotides beyond the 3'-polyadenylated terminus of the short mRNA. S1 nuclease mapping and sequence analysis showed that these extra 222 nucleotides are specified by sequences contiguous to those shared by the two SOD-1 mRNAs. The 5'-termini of the two SOD-1 mRNAs were identified and mapped by both primer extension and S1 mapping. The majority of SOD-1 mRNA molecules (90-95%) have a 5'-start site located 23 base pairs (b.p.) downstream of the hexanucleotide -TATAAA-. The rest of the SOD-1 mRNA molecules have 5'-termini 30, 50 and 65 b.p. upstream from the major start region.
Full text
PDF


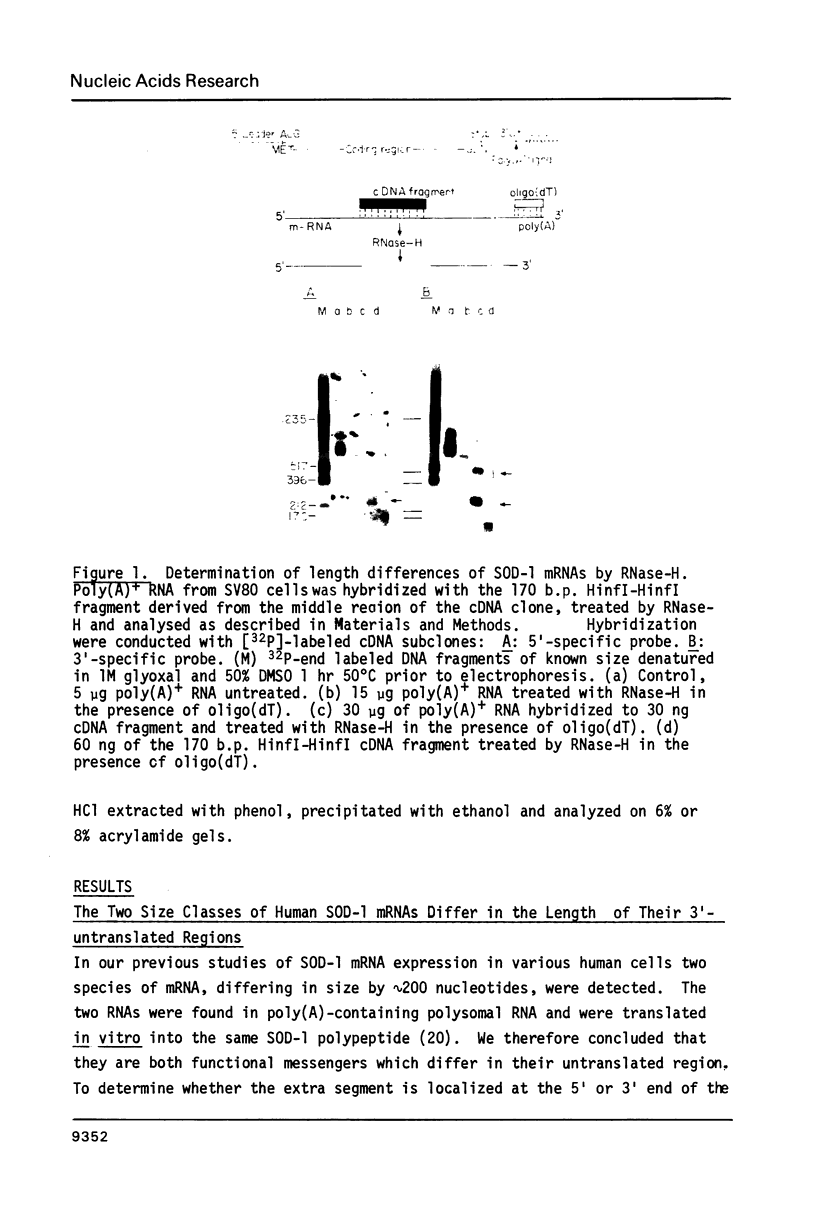

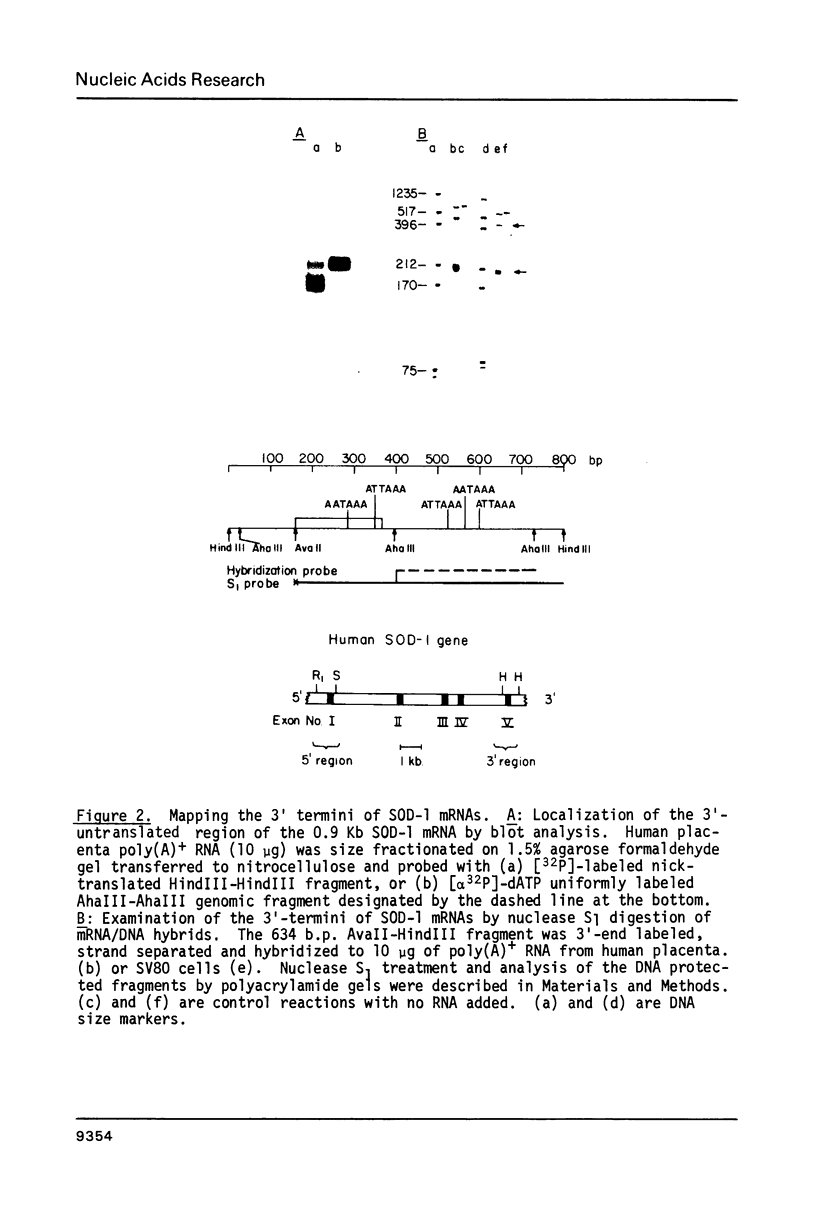

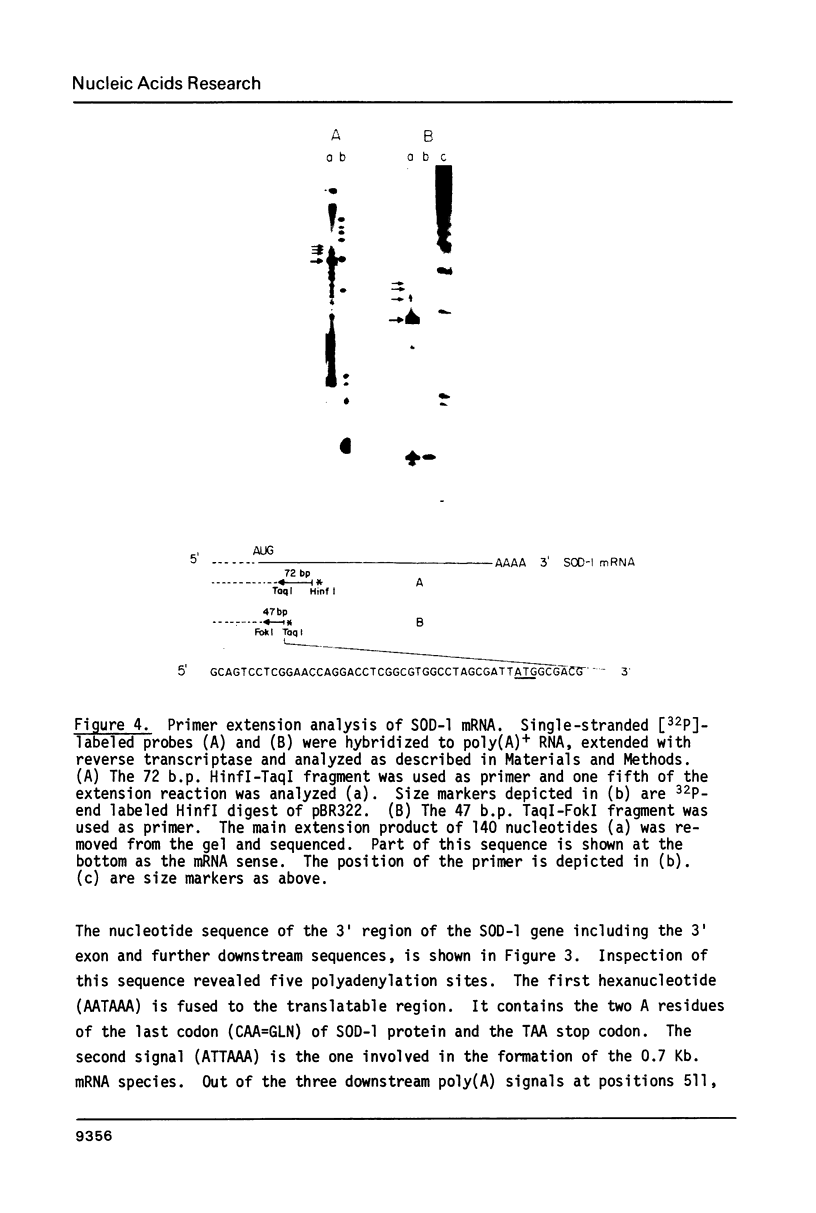

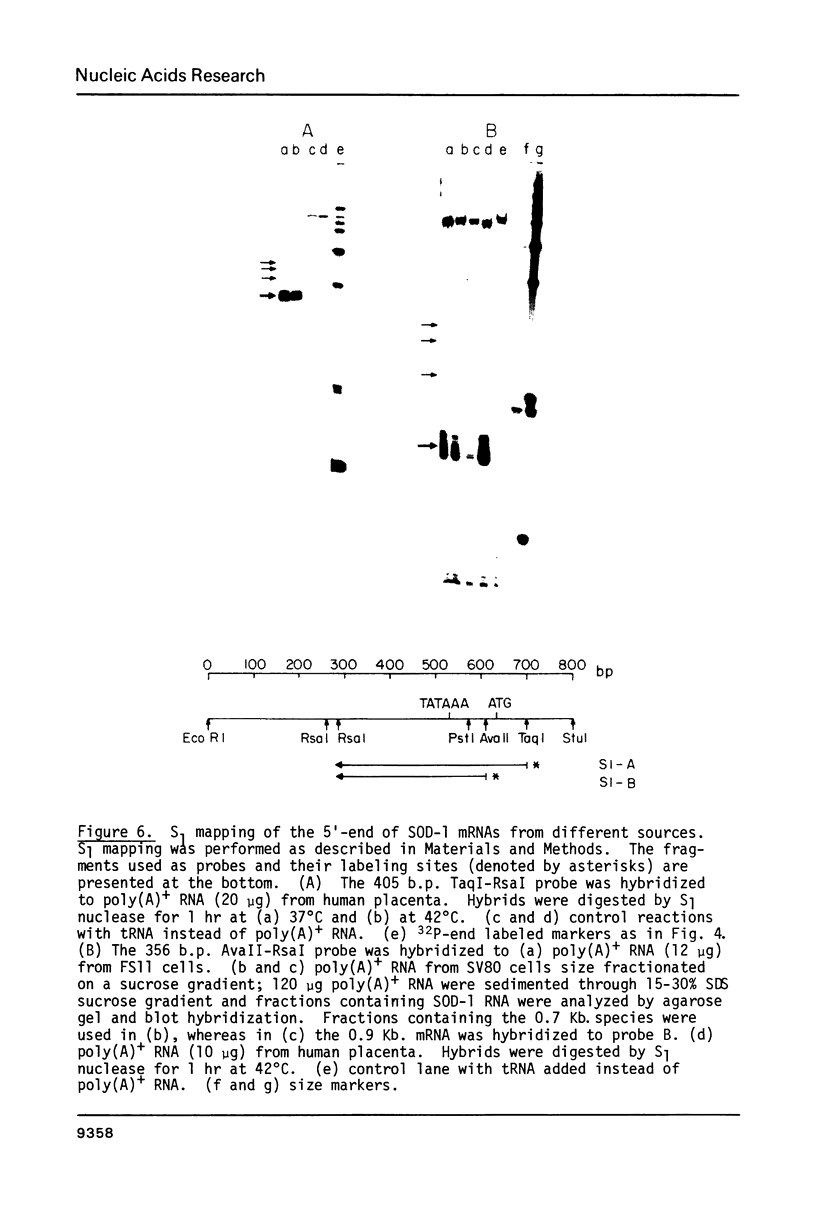
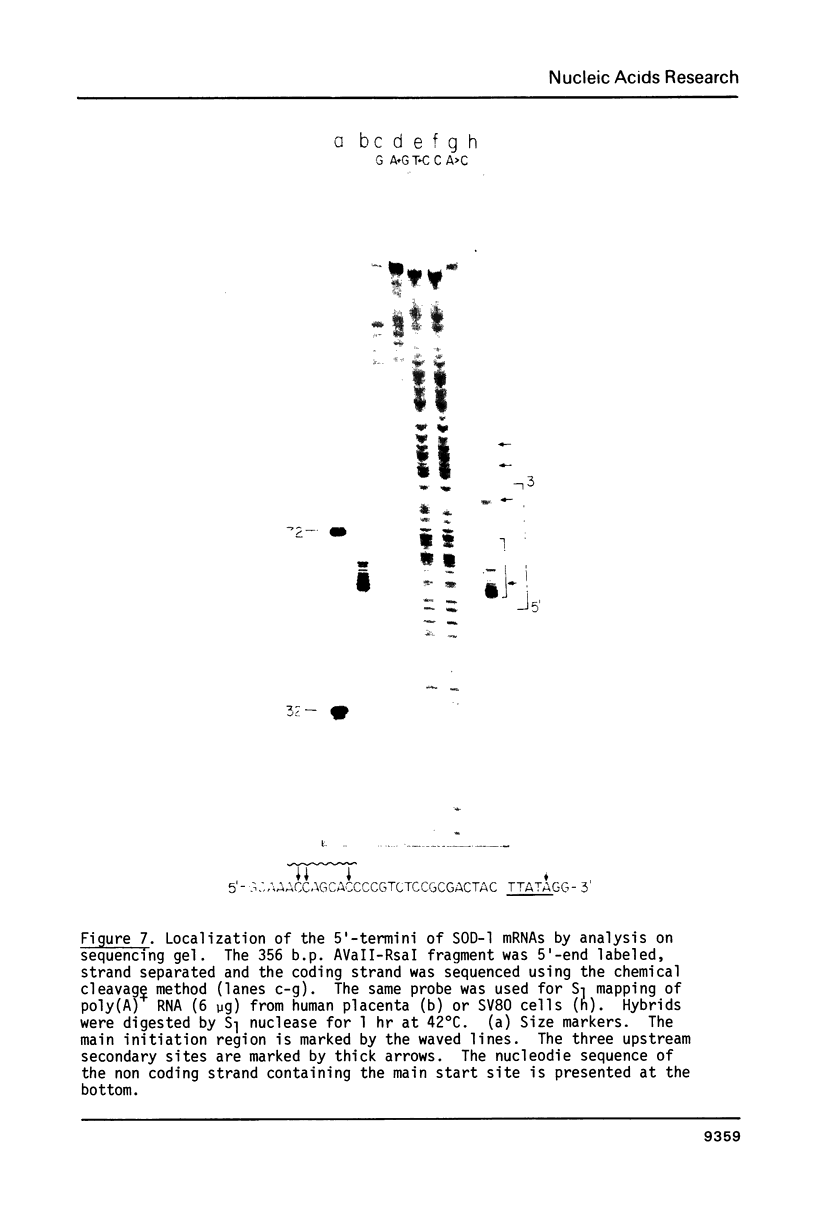






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aho S., Tate V., Boedtker H. Multiple 3' ends of the chicken pro alpha 2(I) collagen gene. Nucleic Acids Res. 1983 Aug 25;11(16):5443–5450. doi: 10.1093/nar/11.16.5443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allan M., Lanyon W. G., Paul J. Multiple origins of transcription in the 4.5 Kb upstream of the epsilon-globin gene. Cell. 1983 Nov;35(1):187–197. doi: 10.1016/0092-8674(83)90221-0. [DOI] [PubMed] [Google Scholar]
- Alt F. W., Bothwell A. L., Knapp M., Siden E., Mather E., Koshland M., Baltimore D. Synthesis of secreted and membrane-bound immunoglobulin mu heavy chains is directed by mRNAs that differ at their 3' ends. Cell. 1980 Jun;20(2):293–301. doi: 10.1016/0092-8674(80)90615-7. [DOI] [PubMed] [Google Scholar]
- Baker C. C., Herisse J., Courtois G., Galibert F., Ziff E. Messenger RNA for the Ad2 DNA binding protein: DNA sequences encoding the first leader and heterogenity at the mRNA 5' end. Cell. 1979 Oct;18(2):569–580. doi: 10.1016/0092-8674(79)90073-4. [DOI] [PubMed] [Google Scholar]
- Barra D., Martini F., Bannister J. V., Schininà M. E., Rotilio G., Bannister W. H., Bossa F. The complete amino acid sequence of human Cu/Zn superoxide dismutase. FEBS Lett. 1980 Oct 20;120(1):53–56. doi: 10.1016/0014-5793(80)81044-1. [DOI] [PubMed] [Google Scholar]
- Berk A. J., Sharp P. A. Sizing and mapping of early adenovirus mRNAs by gel electrophoresis of S1 endonuclease-digested hybrids. Cell. 1977 Nov;12(3):721–732. doi: 10.1016/0092-8674(77)90272-0. [DOI] [PubMed] [Google Scholar]
- Breathnach R., Chambon P. Organization and expression of eucaryotic split genes coding for proteins. Annu Rev Biochem. 1981;50:349–383. doi: 10.1146/annurev.bi.50.070181.002025. [DOI] [PubMed] [Google Scholar]
- Briggs R. G., Fee J. A. Further characterization of human erythrocyte superoxide dismutase. Biochim Biophys Acta. 1978 Nov 20;537(1):86–99. doi: 10.1016/0005-2795(78)90605-0. [DOI] [PubMed] [Google Scholar]
- Canaani D., Kahana C., Mukamel A., Groner Y. Sequence heterogeneity at the 5' termini of late simian virus 40 19S and 16S mRNAs. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3078–3082. doi: 10.1073/pnas.76.7.3078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capetanaki Y. G., Ngai J., Flytzanis C. N., Lazarides E. Tissue-specific expression of two mRNA species transcribed from a single vimentin gene. Cell. 1983 Dec;35(2 Pt 1):411–420. doi: 10.1016/0092-8674(83)90174-5. [DOI] [PubMed] [Google Scholar]
- Cheng H. L., Blattner F. R., Fitzmaurice L., Mushinski J. F., Tucker P. W. Structure of genes for membrane and secreted murine IgD heavy chains. Nature. 1982 Apr 1;296(5856):410–415. doi: 10.1038/296410a0. [DOI] [PubMed] [Google Scholar]
- Contreras R., Fiers W. Initiation of transcription by RNA polymerase II in permeable, SV40-infected or noninfected, CVI cells; evidence for multiple promoters of SV40 late transcription. Nucleic Acids Res. 1981 Jan 24;9(2):215–236. doi: 10.1093/nar/9.2.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crosti N., Serra A., Rigo A., Viglino P. Dosage effect of SOD-A gene in 21-trisomic cells. Hum Genet. 1976 Feb 29;31(2):197–202. doi: 10.1007/BF00296146. [DOI] [PubMed] [Google Scholar]
- Faye G., Leung D. W., Tatchell K., Hall B. D., Smith M. Deletion mapping of sequences essential for in vivo transcription of the iso-1-cytochrome c gene. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2258–2262. doi: 10.1073/pnas.78.4.2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feaster W. W., Kwok L. W., Epstein C. J. Dosage effects for superoxide dismutase-1 in nucleated cells aneuploid for chromosome 21. Am J Hum Genet. 1977 Nov;29(6):563–570. [PMC free article] [PubMed] [Google Scholar]
- Flavell A. J., Cowie A., Legon S., Kamen R. Multiple 5' terminal cap structures in late polyoma virus RNA. Cell. 1979 Feb;16(2):357–371. doi: 10.1016/0092-8674(79)90012-6. [DOI] [PubMed] [Google Scholar]
- Fridovich I. Superoxide dismutases. Annu Rev Biochem. 1975;44:147–159. doi: 10.1146/annurev.bi.44.070175.001051. [DOI] [PubMed] [Google Scholar]
- Fridovich I. The biology of oxygen radicals. Science. 1978 Sep 8;201(4359):875–880. doi: 10.1126/science.210504. [DOI] [PubMed] [Google Scholar]
- Gerlinger P., Krust A., LeMeur M., Perrin F., Cochet M., Gannon F., Dupret D., Chambon P. Multiple initiation and polyadenylation sites for the chicken ovomucoid transcription unit. J Mol Biol. 1982 Dec 5;162(2):345–364. doi: 10.1016/0022-2836(82)90531-9. [DOI] [PubMed] [Google Scholar]
- Ghosh P. K., Reddy V. B., Piatak M., Lebowitz P., Weissman S. M. Determination of RNA sequences by primer directed synthesis and sequencing of their cDNA transcripts. Methods Enzymol. 1980;65(1):580–595. doi: 10.1016/s0076-6879(80)65061-7. [DOI] [PubMed] [Google Scholar]
- Ghosh P. K., Reddy V. B., Swinscoe J., Lebowitz P., Weissman S. M. Heterogeneity and 5'-terminal structures of the late RNAs of simian virus 40. J Mol Biol. 1978 Dec 25;126(4):813–846. doi: 10.1016/0022-2836(78)90022-0. [DOI] [PubMed] [Google Scholar]
- Gidoni D., Kahana C., Canaani D., Groner Y. Specific in vitro initiation of transcription of simian virus 40 early and late genes occurs at the various cap nucleotides including cytidine. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2174–2178. doi: 10.1073/pnas.78.4.2174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grez M., Land H., Giesecke K., Schütz G., Jung A., Sippel A. E. Multiple mRNAs are generated from the chicken lysozyme gene. Cell. 1981 Sep;25(3):743–752. doi: 10.1016/0092-8674(81)90182-3. [DOI] [PubMed] [Google Scholar]
- Groner Y., Monroy G., Jacquet M., Hurwitz J. Chromatin as a template for RNA synthesis in vitro. Proc Natl Acad Sci U S A. 1975 Jan;72(1):194–199. doi: 10.1073/pnas.72.1.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haegeman G., van Heuverswyn H., Gheysen D., Fiers W. Heterogeneity of the 5' terminus of late mRNA induced by a viable simian virus 40 deletion mutant. J Virol. 1979 Aug;31(2):484–493. doi: 10.1128/jvi.31.2.484-493.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagemeijer A., Smit E. M. Partial trisomy 21. Further evidence that trisomy of band 21q22 is essential for Down's phenotype. Hum Genet. 1977 Aug 31;38(1):15–23. doi: 10.1007/BF00295803. [DOI] [PubMed] [Google Scholar]
- Hagenbüchle O., Schibler U. Mouse beta-globin and adenovirus-2 major late transcripts are initiated at the cap site in vitro. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2283–2286. doi: 10.1073/pnas.78.4.2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagenbüchle O., Tosi M., Schibler U., Bovey R., Wellauer P. K., Young R. A. Mouse liver and salivary gland alpha-amylase mRNAs differ only in 5' non-translated sequences. Nature. 1981 Feb 19;289(5799):643–646. doi: 10.1038/289643a0. [DOI] [PubMed] [Google Scholar]
- Hashimoto S., Green M. Methylated 5'-terminal caps of adenovirus type 2 early mRNA: evidence for at least six 5'-termini. Virology. 1979 Apr 30;94(2):254–272. doi: 10.1016/0042-6822(79)90460-4. [DOI] [PubMed] [Google Scholar]
- Heidecker G., Messing J., Gronenborn B. A versatile primer for DNA sequencing in the M13mp2 cloning system. Gene. 1980 Jun;10(1):69–73. doi: 10.1016/0378-1119(80)90145-6. [DOI] [PubMed] [Google Scholar]
- Hofer E., Darnell J. E., Jr The primary transcription unit of the mouse beta-major globin gene. Cell. 1981 Feb;23(2):585–593. doi: 10.1016/0092-8674(81)90154-9. [DOI] [PubMed] [Google Scholar]
- Hu N., Messing J. The making of strand-specific M13 probes. Gene. 1982 Mar;17(3):271–277. doi: 10.1016/0378-1119(82)90143-3. [DOI] [PubMed] [Google Scholar]
- Jabusch J. R., Farb D. L., Kerschensteiner D. A., Deutsch H. F. Some sulfhydryl properties and primary structure of human erythrocyte superoxide dismutase. Biochemistry. 1980 May 27;19(11):2310–2316. doi: 10.1021/bi00552a005. [DOI] [PubMed] [Google Scholar]
- Jacquet M., Groner Y., Monroy G., Hurwitz J. The in vitro synthesis of avian myeloblastosis viral RNA sequences. Proc Natl Acad Sci U S A. 1974 Aug;71(8):3045–3049. doi: 10.1073/pnas.71.8.3045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahana C., Gidoni D., Canaani D., Groner Y. Simian virus 40 early mRNA's in lytically infected and transformed cells contain six 5'-terminal caps. J Virol. 1981 Jan;37(1):7–16. doi: 10.1128/jvi.37.1.7-16.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEJEUNE J., GAUTIER M., TURPIN R. Etude des chromosomes somatiques de neuf enfants mongoliens. C R Hebd Seances Acad Sci. 1959 Mar 16;248(11):1721–1722. [PubMed] [Google Scholar]
- Lai E. C., Roop D. R., Tsai M. J., Woo S. L., O'Malley B. W. Heterogeneous initiation regions for transcription of the chicken ovomucoid gene. Nucleic Acids Res. 1982 Sep 25;10(18):5553–5567. doi: 10.1093/nar/10.18.5553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M. G., Lewis S. A., Wilde C. D., Cowan N. J. Evolutionary history of a multigene family: an expressed human beta-tubulin gene and three processed pseudogenes. Cell. 1983 Jun;33(2):477–487. doi: 10.1016/0092-8674(83)90429-4. [DOI] [PubMed] [Google Scholar]
- Lieman-Hurwitz J., Dafni N., Lavie V., Groner Y. Human cytoplasmic superoxide dismutase cDNA clone: a probe for studying the molecular biology of Down syndrome. Proc Natl Acad Sci U S A. 1982 May;79(9):2808–2811. doi: 10.1073/pnas.79.9.2808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malek L. T., Eschenfeldt W. H., Munns T. W., Rhoads R. E. Heterogeneity of the 5' terminus of hen ovalbumin messenger ribonucleic acid. Nucleic Acids Res. 1981 Apr 10;9(7):1657–1673. doi: 10.1093/nar/9.7.1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Montell C., Fisher E. F., Caruthers M. H., Berk A. J. Inhibition of RNA cleavage but not polyadenylation by a point mutation in mRNA 3' consensus sequence AAUAAA. Nature. 1983 Oct 13;305(5935):600–605. doi: 10.1038/305600a0. [DOI] [PubMed] [Google Scholar]
- Nevins J. R., Darnell J. E., Jr Steps in the processing of Ad2 mRNA: poly(A)+ nuclear sequences are conserved and poly(A) addition precedes splicing. Cell. 1978 Dec;15(4):1477–1493. doi: 10.1016/0092-8674(78)90071-5. [DOI] [PubMed] [Google Scholar]
- Niebuhr E. Down's syndrome. The possibility of a pathogenetic segment on chromosome no. 21. Humangenetik. 1974 Jan 22;21(1):99–101. doi: 10.1007/BF00278575. [DOI] [PubMed] [Google Scholar]
- Parnes J. R., Robinson R. R., Seidman J. G. Multiple mRNA species with distinct 3' termini are transcribed from the beta 2-microglobulin gene. 1983 Mar 31-Apr 6Nature. 302(5907):449–452. doi: 10.1038/302449a0. [DOI] [PubMed] [Google Scholar]
- Proudfoot N. The end of the message. Nature. 1982 Aug 5;298(5874):516–517. doi: 10.1038/298516a0. [DOI] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R., Barrell B. G., Smith A. J., Roe B. A. Cloning in single-stranded bacteriophage as an aid to rapid DNA sequencing. J Mol Biol. 1980 Oct 25;143(2):161–178. doi: 10.1016/0022-2836(80)90196-5. [DOI] [PubMed] [Google Scholar]
- Scarpulla R. C., Wu R. Nonallelic members of the cytochrome c multigene family of the rat may arise through different messenger RNAs. Cell. 1983 Feb;32(2):473–482. doi: 10.1016/0092-8674(83)90467-1. [DOI] [PubMed] [Google Scholar]
- Setzer D. R., McGrogan M., Nunberg J. H., Schimke R. T. Size heterogeneity in the 3' end of dihydrofolate reductase messenger RNAs in mouse cells. Cell. 1980 Nov;22(2 Pt 2):361–370. doi: 10.1016/0092-8674(80)90346-3. [DOI] [PubMed] [Google Scholar]
- Sherman L., Dafni N., Lieman-Hurwitz J., Groner Y. Nucleotide sequence and expression of human chromosome 21-encoded superoxide dismutase mRNA. Proc Natl Acad Sci U S A. 1983 Sep;80(18):5465–5469. doi: 10.1073/pnas.80.18.5465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinet P. M., Allard D., Lejeune J., Jérôme H. Augmentation d'activité de la superoxyde dismutase érythrocytaire dans la trisomie pour le chromosome 21. C R Acad Sci Hebd Seances Acad Sci D. 1974 Jun 17;278(25):3267–3270. [PubMed] [Google Scholar]
- Sinet P. M., Allard D., Lejeune J., Jérôme H. Augmentation d'activité de la superoxyde dismutase érythrocytaire dans la trisomie pour le chromosome 21. C R Acad Sci Hebd Seances Acad Sci D. 1974 Jun 17;278(25):3267–3270. [PubMed] [Google Scholar]
- Sinet P. M., Couturier J., Dutrillaux B., Poissonnier M., Raoul O., Rethore M. O., Allard D., Lejeune J., Jerome H. Trisomie 21 et superoxyde dismutase-1 (IPO-A). Tentative de localisation sur la sous bande 21Q22.1. Exp Cell Res. 1976 Jan;97:47–55. doi: 10.1016/0014-4827(76)90653-4. [DOI] [PubMed] [Google Scholar]
- Sinet P. M. Metabolism of oxygen derivatives in down's syndrome. Ann N Y Acad Sci. 1982;396:83–94. doi: 10.1111/j.1749-6632.1982.tb26845.x. [DOI] [PubMed] [Google Scholar]
- Sollner-Webb B., Reeder R. H. The nucleotide sequence of the initiation and termination sites for ribosomal RNA transcription in X. laevis. Cell. 1979 Oct;18(2):485–499. doi: 10.1016/0092-8674(79)90066-7. [DOI] [PubMed] [Google Scholar]
- Soreq H., Safran A., Zisling R. Variations in gene expression during development of the rat cerebellum. Brain Res. 1982 Jan;255(1):65–79. doi: 10.1016/0165-3806(82)90076-1. [DOI] [PubMed] [Google Scholar]
- Tan Y. H., Tischfield J., Ruddle F. H. The linkage of genes for the human interferon-induced antiviral protein and indophenol oxidase-B traits to chromosome G-21. J Exp Med. 1973 Feb 1;137(2):317–330. doi: 10.1084/jem.137.2.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todaro G. J., Green H., Swift M. R. Susceptibility of human diploid fibroblast strains to transformation by SV40 virus. Science. 1966 Sep 9;153(3741):1252–1254. doi: 10.1126/science.153.3741.1252. [DOI] [PubMed] [Google Scholar]
- Tosi M., Young R. A., Hagenbüchle O., Schibler U. Multiple polyadenylation sites in a mouse alpha-amylase gene. Nucleic Acids Res. 1981 May 25;9(10):2313–2323. doi: 10.1093/nar/9.10.2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J. Y., Baltimore D. Cellular RNA homologous to the Abelson murine leukemia virus transforming gene: expression and relationship to the viral sequence. Mol Cell Biol. 1983 May;3(5):773–779. doi: 10.1128/mcb.3.5.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver R. F., Weissmann C. Mapping of RNA by a modification of the Berk-Sharp procedure: the 5' termini of 15 S beta-globin mRNA precursor and mature 10 s beta-globin mRNA have identical map coordinates. Nucleic Acids Res. 1979 Nov 10;7(5):1175–1193. doi: 10.1093/nar/7.5.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissenbach J., Zeevi M., Landau T., Revel M. Identification of the translation products of human fibroblast interferon mRNA in reticulocyte lysates. Eur J Biochem. 1979 Jul;98(1):1–8. doi: 10.1111/j.1432-1033.1979.tb13153.x. [DOI] [PubMed] [Google Scholar]
- Williams J. D., Summitt R. L., Martens P. R., Kimbrell R. A. Familial Down syndrome due to t(10;21) translocation: evidence that the Down phenotype is related to trisomy of a specific segment of chromosome 21. Am J Hum Genet. 1975 Jul;27(4):478–485. [PMC free article] [PubMed] [Google Scholar]
- Young R. A., Hagenbüchle O., Schibler U. A single mouse alpha-amylase gene specifies two different tissue-specific mRNAs. Cell. 1981 Feb;23(2):451–458. doi: 10.1016/0092-8674(81)90140-9. [DOI] [PubMed] [Google Scholar]
- Zehner Z. E., Paterson B. M. Characterization of the chicken vimentin gene: single copy gene producing multiple mRNAs. Proc Natl Acad Sci U S A. 1983 Feb;80(4):911–915. doi: 10.1073/pnas.80.4.911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziff E., Fraser N. Adenovirus type 2 late mRNA's: structural evidence for 3'-coterminal species. J Virol. 1978 Mar;25(3):897–906. doi: 10.1128/jvi.25.3.897-906.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]