Abstract
Constitutive activity of Bcr-abl fusion protein kinase causes chronic myeloid leukemia (CML). Inhibitors of Bcr-abl such as imatinib mesylate have replaced the cytokine IFNα as the primary treatment for the management of patients with this malignancy. We found that pretreatment of CML cells with imatinib mesylate augments the antigrowth effects of IFNα. Furthermore, introduction of Bcr-abl into non-CML cells inhibits the cellular responses to IFNα. This inhibition is mediated via a mechanism that involves activation of protein kinase D2. The latter promotes an accelerated phosphorylation-dependent degradation of the interferon-α/β receptor 1 chain of the type I interferon receptor, leading to attenuation of IFNα signaling. We discuss the relationship between Bcr-abl activity and IFNα signaling as a molecular basis of the combination of inhibitors of Bcr-abl and IFNα for CML treatment.
Introduction
Patients with chronic myeloid leukemia (CML) harbor a specific translocation t(9;22)(q34;q11), the Philadelphia chromosome, resulting in the expression of a constitutively active protein tyrosine kinase Bcr-abl that is essential for the hematopoietic cell transformation.1 This kinase exerts its oncogenic function by activating a cascade of intracellular signaling pathways that lead to increased cell survival and proliferation and limited dependence on growth factors. Among these pathways are those that mediate activation of PI3K-Akt, MAPK, and protein kinase (PK)C/PKD signaling cascades that generally stimulate cell proliferation and survival.2,3
The Bcr-abl inhibitor imatinib mesylate (IM) has replaced interferon IFNα as the standard of care for patients with newly diagnosed chronic myeloid leukemia (CML) because of higher response frequency, substantially superior molecular and cytogenetic responses, lesser toxicity, and better survival.4–7 Yet, although rapidly killing differentiated CML cells, IM is less efficient against more primitive leukemic stem cells and early progenitor cells.8,9 Thus, patients receiving IM are not cured and require life-long treatment that is often compromised by resistance to IM because of mutations in Bcr-abl tyrosine kinase.7,10 The ability of the second and third generation of tyrosine kinase inhibitors (TKIs; eg, dasatinib that might be active against mutant kinases) to eradicate quiescent early CML progenitors remains to be determined.11 It is plausible that a combination of Bcr-abl inhibitors and the agents targeting the leukemic stem cell population might be required to eradicate the disease in CML patients.
Recent evidence suggests that leukemic stem cells might undergo terminal differentiation in response to IFNα,12 a cytokine known to produce a curative effect in a small subset of patients (reviewed in Kujawski and Talpaz13). Although these results, along with reports on several cases of successful treatment with IFNα after a course of IM (or vice versa),14,15 provide new enthusiasm for the reintroduction of IFNα into the management of CML,13 the molecular mechanisms that underlie the rationale for combining Bcr-abl inhibitors and IFNα remain to be delineated.
Cellular responses to IFNα are mediated by the cell surface type I IFN receptor that consists of the interferon-α/β receptor (IFNAR)1 and IFNAR2 chains. Ligand-stimulated dimerization of these chains leads to the activation of Janus kinase (JAK) family members JAK1 and TYK2. JAK1 and TYK2 phosphorylate signal transducers and activators of transcription (STAT) family proteins at specific tyrosine residues. Tyrosine phosphorylation of STAT1 and STAT2 along with subsequent recruitment of p48/IRF9 leads to the formation of a potent transcription factor that transactivates IFN-stimulated genes and contribute to the antiproliferative effects of IFNα (for reviews, see 16–19).
A better understanding of molecular interactions between IFNα- and Bcr-abl–induced signaling pathways is required for rational approaches to CML therapy. Intriguingly, heterologous expression of Bcr-abl was shown to temper IFNα-induced expression of the IFN-stimulated genes and antiproliferative effects of IFNα; the mechanisms underlying this regulation have not been reported.20 Here, we describe studies that reveal that Bcr-abl inhibits IFNα signaling and cell responses to this cytokine via accelerating the phosphorylation-dependent degradation of the IFNAR1 chain of its receptor and that this mechanism regulates the sensitivity of CML cells to IFNα.
Methods
Cells, cell lines, and culture conditions
HeLa cells were maintained in DMEM supplemented with 10% (v/v) FBS (HyClone Laboratories). Human fibrosarcoma cell line TYK2-null 11.1-derivatives that re-express either wild-type TYK2 (WT) or catalytically inactive TYK221,22 (a kind gift of S. Pellegrini, Institut Pasteur, Paris, France) also received G418 (400 μg/mL). CML cell lines KT1 (kindly provided by Drs I. Sakai, Matsuyama University, Ehime, Japan and R. J. Jones, John Hopkins University, Baltimore, MD) and KU812 (ATCC) were grown in RPMI 1640 supplemented with 10% (v/v) FBS (HyClone Laboratories). Lentiviral-mediated knockdown was carried out as described previously.23 For stable transduction, cells were selected in the media containing either puromycin (2 μg/mL) or hygromycin (10 μg/mL). HeLa cells were transfected by Lipofectamine 2000 (Invitrogen) using standard transfection protocols. Plasmids for expression of proteins or shRNA were transfected into KT1 cells by a Nucleofector apparatus (Amaxa) following the manufacturer's guidelines for cell line transfection. Codified primary human samples from the CML patients in chronic (62, 383, 586, 773, 845, 852, 894, 954, 1000, 1067, 1276, 1953, and 2299) or blast crisis (623, 834, 967, and 1420) phase were obtained from the Stem Cell and Leukemia Core Facility, School of Medicine, University of Pennsylvania, after informed consent in accordance with the Declaration of Helsinki, with approval of the University of Pennsylvania Institutional Review Board.
Chemicals and immunotechniques
IM was obtained from LC Laboratories, and PKD inhibitor Gö6976, casein kinase (CK)1 inhibitor D4476, and JAK inhibitor 1 were from Calbiochem. Inducer of unfolded protein responses thapsigargin (TG) and protein synthesis inhibitor cycloheximide (CHX) were purchased from Sigma-Aldrich. PKD inhibitor CID755673 was purchased from Tocris Bioscience. Antibodies against FLAG, GST, and β-actin (Sigma-Aldrich), PKD3 (PKCν), phospho-STAT1 and STAT1 (Cell Signaling Technology), and PKD2 (Bethyl Laboratories) were purchased from the indicated sources. AA3, GB8, and EA12 antibodies that recognize endogenous IFNAR124 and antibodies against IFNAR1 phosphorylated on Ser53525 were described previously. Secondary antibodies conjugated to horseradish peroxidase were purchased from Millipore Bioscience Research Reagents and LI-COR Biosciences. Activation of STAT1 was assessed by determining the extent of its tyrosine phosphorylation. Immunoprecipitation and immunoblotting procedures are described previously.23
Plasmids and mRNA analyses
shRNA against PKD2 and IFNAR1 constructs based on pLKO.1-puro were purchased from Sigma-Aldrich (MISSION shRNA, SHGLY-NM_016457, SHCLNV-NM_000629). Vectors for mammalian expression of FLAG-IFNAR1, FLAG-IFNAR1S532A, and FLAG-IFNAR1S535A have been described previously.25,26 Vectors for mammalian expression of human p210-Bcr-abl (wild type or kinase-dead) were kindly provided by Dr Warren S. Pear (University of Pennsylvania). Constructs for expression of the GST-tagged PKD2 were kindly provided by Dr V. Malhotra (Centre de Regulació Genòmica, Barcelona, Spain). Silent mutations that would make GST-PKD2 insensitive to shRNA (WT*), as well as replacement of Tyr438 with Phe (YF*), were generated using the site-directed mutagenesis. All resulting mutants were verified by dideoxy sequencing.
Viability assays
KT1 or KU812 cells were grown in RPMI 1640 supplemented with 10% FBS and seeded at 5 × 106 cells/mL. In some experiments, cells were transduced with virus encoding shRNA (control or for knockdown of IFNAR1) and then selected for 48 hours in media supplemented with puromycin. Alternatively, in other experiments, cells were electroporated with plasmids encoding shRNA for knockdown of PKD2 or constructs for expression of IFNAR1WT or IFNAR1S535A along with plasmids encoding hygromycin resistance. These cells were selected in media supplemented with hygromycin. After 48 hours of selection, cells were pretreated with either IM (0.5μM) or IFN (250 IU/mL) for 4 hours followed by treatment with IM or IFN for 8 hours. The cells were then either counted in a hemocytometer after trypan blue staining or analyzed by the MTT colorimetric assay using cell proliferation colorimetric assay kit, CellTiter 96 (Promega).
Colony formation assays
Cells were thawed and placed into the RPMI 1640 containing 20% FBS and cytokines (IL-3, IL-6, and SCF at 10 ng/mL). Approximately 10 000 cells were used per 30-mm plate. In each 30-mm plate, 1 mL of methylcellulose media was used. The cells were preincubated with either vehicle, IM (0.25μM), or IFNα (1000 IU/mL) for 6 hours followed by incubation with IFNα (1000 IU/mL) for 24 hours or for duration of colony growth in methylcellulose media as indicated. Fresh media (0.5 mL) was used to refeed the cells weekly. The colonies were counted 2 weeks after plating.
Statistical analyses
Every shown quantified result is an average of at least 3 independent experiments carried out in either triplicate or quadruplicate and calcu-lated as means ± SE. The P values were calculated using the 2-tailed Student t test.
Results
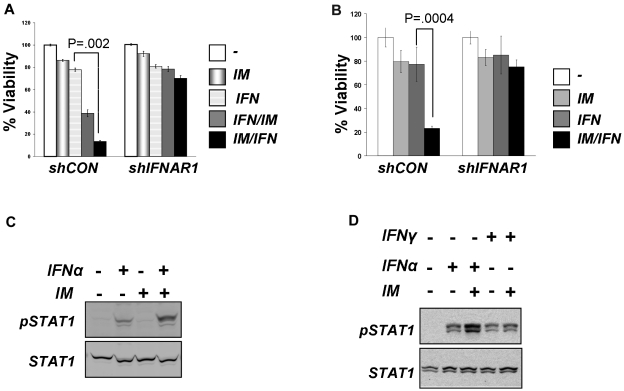
Given the encouraging reports of the use of IFNα together with IM in CML patients,14,15,27,28 we aimed to investigate the molecular basis of combining these drugs. We determined the effects of these agents and their combinations on the viability of the human CML cell line KT1 that harbors the Philadelphia chromosome and is sensitive to both IFNα and IM.12,29 Suboptimal doses of either IM or IFNα alone caused no more than 20% of growth inhibition in either naive KT1 cells (data not shown) or in cells that received irrelevant control shRNA (Figure 1A). A regimen of adding a TKI after pretreatment with IFNα would mimic the scenario in patients who were switched from this cytokine to IM during the clinical trials.30–32 When this sequence was applied to the in vitro experimental settings, we observed that the IFNα + IM combination robustly impaired the viability of KT1 cells. Notably, adding IFNα after pretreatment with IM caused a much more severe inhibition of KT1 viability compared with a vehicle-treated control (Figure 1A). Similar data were obtained in cells that did not receive any RNA interference (RNAi) reagents (data not shown). This result suggests that inhibition of Bcr-abl may augment cellular responses of CML cells to IFNα. Assessment of cell viability using MTT assay in either KT1 cells (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article) or in another CML KU812 cells33 (Figure 1B) also supported this conclusion.
Figure 1.
Inhibition of Bcr-abl activity in CML cells augments cellular responses to IFNα. (A) Viability of KT1 cells that received indicated shRNA and were treated with IM (0.5μM for 8 hours), IFNα (250 IU/mL for 8 hours), or a combination (4 hours + 4 hours) of the 2 as indicated. (B) Viability of KU812 cells that received indicated shRNA and were treated as described in panel A. (C) KT1 cells were pretreated with IM (0.5μM) for 8 hours and then treated with IFNα (250 IU/mL) for 30 minutes. Activation and total levels of STAT1 was analyzed in whole cell lysates by immunoblotting using the indicated antibodies. (D) KT1 cells were pretreated with IM (0.5μM) for 8 hours and then treated with IFNα (250 IU/mL) or IFNγ (50 IU/mL) for 30 minutes. Activation and total levels of STAT1 was analyzed in whole cell lysates by immunoblotting using the indicated antibodies.
This result is in line with data reported by Platanias and colleagues on suppression of IFN-stimulated gene expression by Bcr-abl.20 To determine whether Bcr-abl also affects proximal early events induced by IFNα, we analyzed activation of STAT1 protein (assessed by its tyrosine phosphorylation). IM treatment alone did not induce STAT1 phosphorylation in KT1 cells (Figure 1C). Whereas this phosphorylation was stimulated by IFNα, pretreatment with IM noticeably augmented the effects of IFNα (Figure 1C-D). STAT1 is phosphorylated by Janus kinases in response to both IFNα (through activation of IFNAR) and IFNγ, which uses a different receptor, IFNGR (reviewed in Platanias19). Intriguingly, IM treatment did not augment STAT1 phosphorylation induced by IFNγ (Figure 1D), suggesting that Bcr-abl activity may specifically affect IFNα signaling at the level of its receptor. Consistent with this, we observed that RNAi-mediated knockdown of IFNAR1 noticeably attenuated the sensitization of KT1 or KU812 cells to the combined treatment with IM and IFNα (Figure 1A-B).
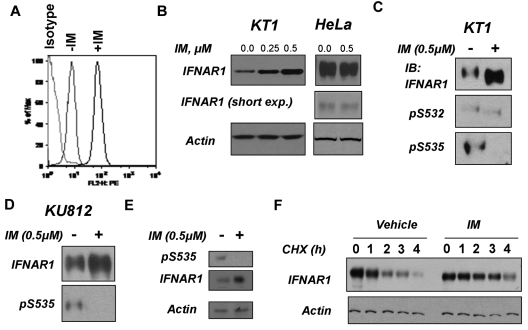
Cell surface levels of IFNAR are largely regulated via the serine phosphorylation-dependent ubiquitination of its IFNAR1 chain that leads to endocytosis of the receptor and its lysosomal degradation.34,35 Treatment of KT1 cells with IM markedly increased the cell surface levels of IFNAR1 (Figure 2A), suggesting that Bcr-abl signaling may target this receptor for down-regulation. Consistent with this observation, IM treatment up-regulated total levels of IFNAR1 in KT1 or KU812 cells but not in HeLa cells that lack Bcr-abl expression (Figure 2B-D). Furthermore, treatment of peripheral blood mononuclear cells from a CML patient with IM also up-regulated total levels of IFNAR1 (Figure 2E). These results suggest that Bcr-abl may signal to inhibit the expression of IFNAR1. We further assessed the degradation rate of IFNAR1 in KT1 cells using the cycloheximide chase (Figure 2F). Lesser amounts of lysates from IM-treated cells were taken into analysis to achieve comparable levels of IFNAR1 at time point 0. These studies showed that IM pretreatment noticeably increased the half-life of IFNAR1 in KT1 cells (Figure 2F). Taken together, these results suggest that Bcr-abl activity might affect IFNα signaling via accelerating proteolytic turnover of IFNAR1 and ensuing down-regulation of the levels of this receptor.
Figure 2.
Inhibition of Bcr-abl activity in CML cells stabilizes and up-regulates IFNAR1. (A) Cell surface levels of IFNAR1 in KT1 cells treated as indicated were analyzed by flow cytometry using anti-IFNAR1 AA3 antibody or isotype control antibody. (B) Total levels of IFNAR1 and β-actin in KT1 or HeLa cells treated with indicated doses of IM for 8 hours were analyzed by immunoblotting. Short exposure for IFNAR1 blot in HeLa cells also is provided. (C-D) KT1 or KU812 CML cells were incubated with IM (0.5μM) for 8 hours and harvested. Phosphorylation and total levels of IFNAR1 were analyzed by IFNAR1 immunoprecipitation (using anti-IFNAR1 EA12 antibody) followed by immunoblotting using anti–phospho-S535 antibody or anti-IFNAR1 GB8 antibody (as indicated). (E) Primary CML cells from a patient sample (623) were incubated with IM (0.5μM) for 8 hours and harvested. Phosphorylation and total levels of IFNAR1 were analyzed by IFNAR1 immunoprecipitation (using anti-IFNAR1 EA12 antibody) followed by immunoblotting using anti–phospho-S535 antibody or anti-IFNAR1 GB8 antibody (as indicated). Supernatants of the immunoprecipitation reactions were immunoblotted for β-actin to determine loading. (F) KT1 cells pretreated with IM (0.5μM) or vehicle for 8 hours were subjected to treatment with CHX (10μg/mL) for the indicated time points. Endogenous IFNAR1 was immunoprecipitated and levels of IFNAR1 were determined by immunoblotting. Amount of lysates was normalized to achieve comparable levels of IFNAR1 at time point 0. Levels of β-actin in reaction supernatants also were analyzed.
Degradation of IFNAR1 is controlled by phosphorylation of Ser535 within its degron.34,35 This phosphorylation can be induced by IFNα in a manner that depends on activity of JAK1 and TYK222,36 and downstream activation of PKD2.37 Alternatively, basal phosphorylation of Ser535 by CK1α38 is shown to be augmented by a priming site (Ser532) phosphorylation, which can be promoted by signaling stimulated by inducers of unfolded protein responses such as thapsigargin.23,26 Degron phosphorylation on Ser535 within IFNAR1 was noticeably decreased in the KT1 and KU812 CML cell lines (Figure 2C-D) and in primary CML cells (Figure 2E) after treatment with IM, indicating that Bcr-abl signaling may stimulate IFNAR1 turnover via triggering its degron phosphorylation.
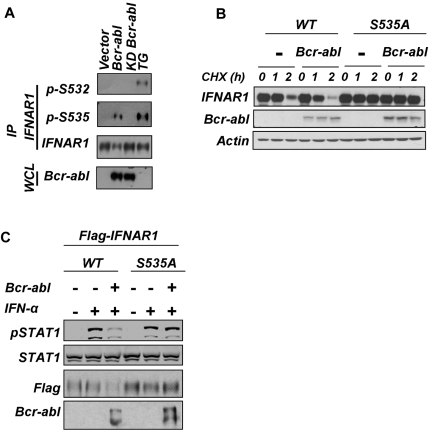
Indeed, expression of catalytically active (but not kinase-deficient) Bcr-abl in HeLa cells stimulated phosphorylation of endogenous IFNAR1 on Ser535 and decreased the total levels of endogenous IFNAR1 or coexpressed wild-type FLAG-IFNAR1 (Figure 3A; supplemental Figure 2). The latter effect was not seen on FLAG-IFNAR1S535A mutant (supplemental Figure 2), suggesting that phosphorylation of the IFNAR1 degron is required for Bcr-abl–stimulated down-regulation of this protein. Furthermore, Bcr-abl expression accelerated the degradation of wild-type FLAG-IFNAR1 but not of the S535A mutant in HeLa cells (Figure 3B). Importantly, the cells expressing FLAG-IFNAR1S535A were somewhat resistant to inhibition of IFNα-induced STAT1 phosphorylation in response to Bcr-abl coexpression (Figure 3C). These data collectively suggest that signaling driven by catalytically active Bcr-abl leads to phosphorylation of the IFNAR1 degron and accelerated degradation of IFNAR1 that results in attenuation of cellular responses to IFNα.
Figure 3.
Expression of Bcr-abl signals to promote degradation and down-regulation of IFNAR1 and to attenuate IFNα signaling. (A) HeLa cells were either transfected with plasmids expressing Bcr-abl (wild type or kinase dead [KD]) or treated with TG (1μM for 30 minutes) and harvested. Phosphorylation and total levels of endogenous IFNAR1 (in IFNAR1 immunoprecipitates) or of expressed Bcr-abl (in whole cell lysates [WCL]) were determined by immunoblotting using the indicated antibodies. (B) HeLa cells expressing (or not) Bcr-abl and either FLAG-IFNAR1 or FLAG-IFNAR1S535A as indicated were treated with CHX (10 μg/mL) for the indicated time points. Immunoblotting analyses of the levels of Flag-IFNAR1 Bcr-abl and β-actin are depicted. (C) HeLa cells transfected with plasmids for expression of Bcr-abl and FLAG-tagged IFNAR1 (wild type or the phosphorylation-deficient mutant S535A) were treated or not with IFNα (250 IU/mL) as indicated. STAT1 phosphorylation and levels as well as the levels of FLAG-IFNAR1 and Bcr-abl were analyzed by immunoblotting using indicated antibodies.
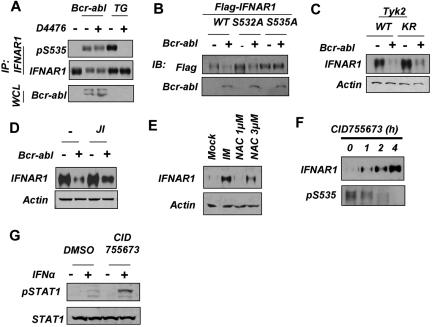
The fact that Bcr-abl is a tyrosine kinase implies that a serine kinase downstream of it must be involved in IFNAR1 Ser535 phosphorylation. Treatment of cells with the inhibitor of CK1α D4476 did not affect Bcr-abl–induced Ser535 phosphorylation (Figure 4A). Consistent with a previously published report,38 this inhibitor attenuated the thapsigargin-induced phosphorylation of the IFNAR1 degron. Neither KT1 cells treated with IM (Figure 2C) nor HeLa cells expressing Bcr-abl (Figure 3A) displayed marked changes in the levels of priming (Ser532) phosphorylation. Furthermore, expression of Bcr-abl led to a noticeable down-regulation of the IFNAR1S532A mutant in HeLa cells (Figure 4B). Thus, it is plausible that Bcr-abl does not use the ligand-independent pathway, which requires CK1α activity and Ser532 priming phosphorylation.26
Figure 4.
Down-regulation of IFNAR1 in cells that express Bcr-abl does not require CK1 or priming phosphorylation of IFNAR1. (A) HeLa cells transfected with Bcr-abl or treated with TG (1μM for 30 minutes) also were treated with CK1 inhibitor D4476 (400nM) and harvested. Phosphorylation and levels of endogenous IFNAR1 and expression of Bcr-abl was analyzed as described in Figure 3. (B) HeLa cells expressing FLAG-tagged IFNAR1 (wild type or IFNAR1S532A or IFNAR1S535A mutants) were cotransfected with either empty vector or vector expressing Bcr-abl. Total levels of FLAG-IFNAR1 and Bcr-abl were assessed by immunoblotting using the indicated antibodies. (C) Human fibrosarcoma 11.1-derivative cells expressing either wild-type TYK2 (WT) or kinase dead TYK2 (KR) were transfected with empty vector or vector expressing Bcr-abl. IFNAR1 was immunoprecipitated, and total IFNAR1 was assessed by immunoblotting. Supernatants of the immunoprecipitation reactions were immunoblotted for β-actin to determine loading. (D) HeLa cells transfected with Bcr-abl as indicated were treated with JAK inhibitor 1 (0.5μM for 24 hours). Levels of IFNAR1 after treatment was determined by immunoprecipitation and immunoblotting. Loading of the immunoprecipitation mixture was determined by immunoblotting the supernatants for β-actin. (E) KT1 cells were treated with IM (0.5μM) or N-acetyl cysteine (NAC; 1 or 3 μg/mL). Levels of IFNAR1 after treatment was determined by immunoprecipitation and immunoblotting. Loading of the immunoprecipitation mixture was determined by immunoblotting the supernatants for β-actin. (F) KT1 cells were treated with CID755673 (20μM) for the indicated time points. IFNAR1 was immunoprecipitated, and phosphorylated Ser535 (pS535) and total IFNAR1 was assessed by Western blotting. (G) KT1 cells were treated with CID755673 (20μM) for 6 hours and then treated with IFNα (250 IU/mL for 30 minutes). Levels of p-STAT1 and total STAT1 in whole cell lysates (WCL) were determined by immunoblotting using the indicated antibodies.
Bcr-abl is capable of activating Janus kinases39 whose catalytic activity is required for Ser535 phosphorylation and IFNAR1 degradation induced by IFNα.36 Although slightly attenuated, a noticeable down-regulation of IFNAR1 was seen in Bcr-abl–expressing KR cells (Figure 4C) that express kinase-dead TYK2 and are deficient in IFNAR1 degradation induced by IFNα.22 Furthermore, only a partial attenuation of IFNAR1 down-regulation in Bcr-abl–expressing HeLa cells was achieved by treating these cells with JAK inhibitor 1 (Figure 4D). Taken together, these results suggest that Bcr-abl may exert its effects, at least in part, downstream of JAK. Remarkably, the latter effects were clearly compromised by the treatment of cells with the scavenger of reactive oxidative species N-acetyl cysteine (Figure 4E). Given that accumulation of reactive oxygen species in cells that harbor Bcr-abl was shown to lead to activation of PKD240 and that activation of this kinase downstream of JAK has been implicated in IFNAR1 degron phosphorylation induced by the ligand,37 we next tested the role of PKD2 in regulation of IFNAR1 phosphorylation and levels by Bcr-abl.
Treatment of KT1 cells with PKD inhibitors Gö6973 or CID755673 robustly decreased Ser535 phosphorylation and concomitantly increased total IFNAR1 levels (Figure 4F; supplemental Figure 3). Accordingly, pretreatment of KT1 cells with CID755673 noticeably increased the extent of STAT1 phosphorylation induced by IFNα (Figure 4G). These results suggest that PKD activity is required for Bcr-abl–induced activation of IFNAR1 degron phosphorylation, down-regulation of IFNAR1, and inhibition of IFNα signaling.
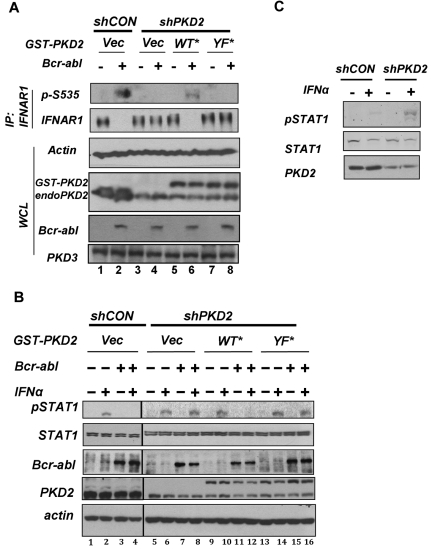
To seek further evidence that would corroborate this hypothesis, we chose to use an RNAi-based approach. In HeLa cells that do not express the related enzyme PKD1,41 the knockdown of PKD2 prevented the Bcr-abl–induced decrease in the levels of endogenous IFNAR1 (Figure 5A lane 4 vs lane 2). Accordingly, expression of GST-PKD2 construct insensitive to these particular shRNA restored IFNAR1 down-regulation (Figure 5A lane 4 vs lane 6). Activation of PKD2 by Bcr-abl was shown to involve putative phosphorylation of PKD2 on Tyr438.40 Importantly, the GST-PKD2Y438F mutant failed to rescue the phenotype of Bcr-abl expression (Figure 5A lane 6 vs lane 8), suggesting that activation of PKD2 is essential for IFNAR1 down-regulation.
Figure 5.
Role of PKD2 in Bcr-abl–induced IFNAR1 down-regulation and inhibition of IFNα signaling. (A) HeLa cells stably transduced with control shRNA or shPKD2 were transfected with either Bcr-abl or empty vector with or without cotransfection of shRNA-insensitive GST-PKD2 or GST-PKD2Y438F. Endogenous IFNAR1 was purified by immunoprecipitation and analyzed by immunoblotting using the indicated antibodies. Whole cell lysates (WCL) from these cells also were analyzed by immunoblotting for levels of PKD2, Bcr-abl, and β-actin. (B) HeLa cells stably transduced with control shRNA or shPKD2 and transfected with Bcr-abl/empty vector with or without shRNA-insensitive GST-PKD2 or GST-PKD2Y438F (as described in panel A) were treated with IFNα (250 IU/mL for 30 minutes). Activation and levels of STAT1 as well the levels of Bcr-abl, PKD2, and β-actin were analyzed by immunoblotting. (C) KT1 cells were transfected with control shRNA (shCON) or shRNA against PKD2 (shPKD2) and cotransfected with plasmid encoding hygromycin resistance. After 48 hours of selection in hygromycin, interferon signaling was investigated by immunoblotting the WCL for p-STAT1 and STAT1 after treatment with IFNα (250 IU/mL for 30 minutes).
Using the same HeLa-derived cell lines, we examined the role of PKD2 in Bcr-abl–mediated inhibition of IFNα signaling. Bcr-abl decreased IFNα-induced STAT1 phosphorylation in cells that received control shRNAs (Figure 5B lane 2 vs lane 4) but not shRNA against PKD2 (Figure 5B lane 6 vs lane 8). In addition, the inhibitory effects of Bcr-abl were restored by the re-expression of wild-type PKD2 (Figure 5B lane 12) but not of the Y438F mutant (Figure 5B lane 16). Furthermore, knockdown of PKD2 in KT1 cells also led to augmentation of IFNα-induced phosphorylation of STAT1 (Figure 5C). In all, these data suggest that activation of PKD2 by Bcr-abl is required for IFNAR1 down-regulation and inhibition of IFNα signaling.
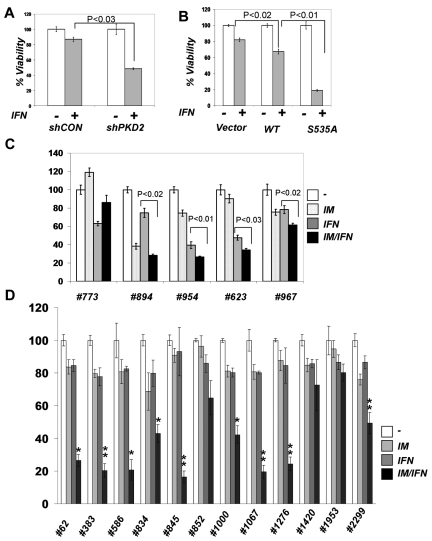
Consistent with these data, knockdown of PKD2 led to a significant sensitization of KT1 cells to anti-growth effects of IFNα assessed by trypan blue staining (Figure 6A) or MTT assay (supplemental Figure 4). Whereas we could not test the combination of IFNα with PKD inhibitors because of high levels of toxicity of the latter compounds in vitro against CML cells (data not shown), we complemented the RNAi-based analysis by experiments where KT1 cells were engineered to express IFNAR1. Under these conditions, IFNα caused a significantly greater decrease in cell viability when these cells expressed the IFNAR1S535A mutant (Figure 6B), which was insensitive to Bcr-abl–induced down-regulation (Figures 4B; supplemental Figure 2). These data collectively suggest that Bcr-abl signals through PKD2 to induce phosphorylation and down-regulation of IFNAR1 to attenuate the cellular responses to IFNα.
Figure 6.
Role of IFNAR1 down-regulation in CML cell viability and clonogenicity. (A) KT1 cells were transfected with control shRNA or shPKD2 and cotransfected with plasmid encoding hygromycin resistance. The cells were kept under hygromycin selection for 48 hours, and viability in response to IFNα was assessed by trypan blue staining and depicted as relative values. (B) KT1 cells were transfected with empty vector, plasmid expressing wild-type IFNAR1, or IFNAR1S535A. The cells were cotransfected with plasmid encoding hygromycin resistance. The hygromycin-resistant cells were selected for 48 hours and analyzed for their viability after IFNα treatment as described in panel A. (C) Colony formation assays were carried out with CML samples from peripheral blood of patients in the chronic phase (773, 894, and 954) and blast crisis (623 and 967) who were positive for Bcr-abl expression. Cells were preincubated with either vehicle, IM (0.25μM), or IFNα (1000 IU/mL) for 6 hours followed by incubation with IFNα (1000 IU/mL) for 24 hours. Cells were then washed and plated in drug-free methylcellulose media, and the colonies were scored 2 weeks later and depicted as percentage relative to untreated controls. (D) Colony formation assays were carried out with CML samples from peripheral blood of patients in the chronic phase (62, 383, 586, 845, 852, 1000, 1067, 1276, 1953, and 2299) and blast crisis (834 and 1420) who were positive for Bcr-abl expression. Cells were preincubated with either vehicle, IM (0.15μM), or IFNα (150 IU/mL) for 6 hours followed by incubation in the methylcellulose media containing or not IFNα (150 IU/mL) for 3 weeks before the colonies were scored and depicted as percentage relative to untreated controls. Asterisks denote statistically significant (compared with other treatment groups) differences, *P < .05 and **P < .001.
We then tested primary cells from CML patients. The cells were preincubated with either vehicle, IM (0.25μM), or IFNα (1000 IU/mL) for 6 hours followed by incubation with IFNα (1000 IU/mL) for 24 hours where indicated. After that, cells were washed and their ability to form colonies (in a drug-free environment) was then immediately tested by assessment of colony-forming units of granulocytes and monocytes/macrophages in methylcellulose. Under these conditions, we observed that pretreatment with IM led to a significant increase in sensitivity to IFNα in cells from a patient in the chronic phase (954) and 2 patients from the blast crisis phase (623, 967) of CML (Figure 6C). These data indicate that Bcr-abl activity can confer refractoriness to IFNα in a subset of CML patients.
Additional verification of this hypothesis was sought in experiments that would model clinical short-term treatment with low dose of IM followed by prolonged IFNα treatment for the entire period of colony growth. Under these conditions, 3 of 12 samples from CML patients (1953, 1420, and 852) did not display a statistically significant effect of IM pretreatment on inhibition of colony growth by IFNα (Figure 6D). Whereas a combination of IM with IFNα produced an additive effect in 3 samples (834, 1000, and 2299), samples from 6 other patients (62, 383, 586, 845, 1067, and 1276) exhibited a synergistic effect on combining these 2 drugs. These results strongly suggest that inhibiting Bcr-abl activity might sensitize a subset of patients to antitumorigenic effects of IFNα therapy.
Discussion
When combining IM and IFNα in vitro, the greatest effect on the viability of a human CML cell line is observed when pretreatment with TKI precedes the addition of the cytokine (Figure 1A-B). It seems that this pretreatment stabilizes and up-regulates IFNAR1 and, accordingly, augments signaling induced by IFNα (Figures 1–2). Conversely, expression of Bcr-abl in non-CML cells stimulates phosphorylation of the IFNAR1 degron and accelerates the degradation of IFNAR1, leading to an attenuation of IFNα signaling (Figure 3). The latter observation is consistent with impaired expression of IFN-stimulated genes reported by Katsoulidis et al.20 Whereas activity of CK1 and the integrity of the IFNAR1 priming site are dispensable for Bcr-abl–stimulated IFNAR1down-regulation, the latter requires PKD2 activity and phosphorylation of the IFNAR1 degron (Figures 3 and 4). Furthermore, activity of PKD2 and IFNAR1 degron phosphorylation are essential for attenuation of IFNα signaling in Bcr-abl–expressing cells (Figures 5 and 6). Pretreatment of primary cells from CML patients with a Bcr-abl inhibitor can sensitize a subset of these samples to inhibition of colony formation by IFNα (Figure 6).
Collectively, these observations suggest that Bcr-abl signaling can compromise the cellular response to IFNα via PKD2-dependent phosphorylation and degradation of IFNAR1. The exact mechanism underlying these events is yet to be determined in detail; however, a hypothetical chain of events can be envisioned as follows. It is plausible that tyrosine kinase activity of Bcr-abl promotes the phosphorylation of PKD2 on Y438, which was previously implicated in the activation of PKD2 in Bcr-abl–expressing cells.40 In turn, activated PKD2 signals to elicit phosphorylation of Ser535 within the IFNAR1 degron. PKD2 is capable of directly phosphorylating Ser535 in vitro37; however, the actual degron phosphorylation in cells could be alternatively mediated by a different PK that is activated downstream of PKD2. Regardless, phosphorylation of the IFNAR1 degron has been shown to enable the interaction with the SCFβTrcp E3 ubiquitin ligase that ubiquitinates IFNAR1 and promotes its endocytosis and lysosomal degradation.25,34,35 As a result, cells expressing Bcr-abl display decreased levels of IFNAR on their surface and are less sensitive to all effects of IFNα, including its inhibition of cell viability. The latter possibility is in line with an observation that Bcr-abl–expressing cells display an impaired defense against encephalomyocarditis virus and attenuated IFNα-induced growth suppression (20; this study). It has been suggested that low levels of IFNAR in human melanomas correlate with poor prognosis for these patients.42 Conversely, up-regulation of IFNAR levels by treatment of leukemia cells with hydroxyurea sensitized these cells to IFNα.43 Furthermore, the initial pretreatment mRNA and protein levels of IFNAR2c were predictive of outcome of IFNα therapy in CML patients.44,45
Besides these mechanistic insights, the significance of these findings may be clinically relevant for efforts to restore the sensitivity of CML cells to IFNα. This purported mechanism provides a rationale for a strategy using suboptimal doses of TKI before IFNα delivery to increase the sensitivity of the chronic phase CML patients to IFNα. Although this approach would be limited to a group of patients that do not yet display a resistance to TKI, it may have certain advantages for this group. First, the use of lower or pulsatile doses of TKI may fend off the development of Bcr-abl mutations and ensuing selection of the resistant clones. Second, the resistance associated with the life-long use of IM also might be prevented if an optimized IFNα therapy leads to a stable curative effect that enables the discontinuation of its use. Future studies also should determine the utility of adding IFNα to TKI regimens in the in blast crisis phase.
Further studies also are warranted on the possibility of targeting PKD for CML therapy. Novel inhibitors of this kinase are being actively developed46,47; some of them showed a promise in preclinical models of pancreatic and prostate cancers.48,49 It is plausible that combination of PKD inhibitors with IFNα may be helpful as the last resort in relapsed CML patients when mutations in Bcr-abl render it insensitive even to the third generation anti-CML drugs (currently proposed to overcome resistance caused by the T315I mutation50). Investigation of a specific role of PKD in the responsiveness of Bcr-abl–harboring leukemic stem cells and their normal hematopoietic counterparts to IFNα is underway.
Supplementary Material
Acknowledgments
The authors thank R. Jones, V. Malhotra, W. Pear, S. Pellegrini, and I. Sakai for the reagents. They are also grateful to W. Pear and D. E. Zhang for helpful comments and suggestions.
This work was supported by Public Health Service grant CA142425 (S.Y.F.) from the National Cancer Institute.
Footnotes
An Inside Blood analysis of this article appears at the front of this issue.
The online version of the article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: S.B., H.Z., C.T., and M.C. performed the experiments; S.B., H.Z., C.T., and S.Y.F. made the figures; and S.B., H.Z., M.C., D.P.B., and S.Y.F. designed research, analyzed data, and wrote the manuscript.
Conflict-of-interest disclosure: D.P.B. is an employee of and owns stock in Biogen Idec Inc. The remaining authors declare no competing financial interests.
Correspondence: Serge Y. Fuchs, Department of Animal Biology, University of Pennsylvania, 380 S University Ave, Hill 316, Philadelphia, PA 19104-4539; e-mail: syfuchs@vet.upenn.edu.
References
- 1.Burke BA, Carroll M. BCR-ABL: a multi-faceted promoter of DNA mutation in chronic myelogenous leukemia. Leukemia. 2010;24(6):1105–1112. doi: 10.1038/leu.2010.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kharas MG, Fruman DA. ABL oncogenes and phosphoinositide 3-kinase: mechanism of activation and downstream effectors. Cancer Res. 2005;65(6):2047–2053. doi: 10.1158/0008-5472.CAN-04-3888. [DOI] [PubMed] [Google Scholar]
- 3.Ren R. Mechanisms of BCR-ABL in the pathogenesis of chronic myelogenous leukaemia. Nat Rev Cancer. 2005;5(3):172–183. doi: 10.1038/nrc1567. [DOI] [PubMed] [Google Scholar]
- 4.Branford S, Rudzki Z, Harper A, et al. Imatinib produces significantly superior molecular responses compared to interferon alfa plus cytarabine in patients with newly diagnosed chronic myeloid leukemia in chronic phase. Leukemia. 2003;17(12):2401–2409. doi: 10.1038/sj.leu.2403158. [DOI] [PubMed] [Google Scholar]
- 5.O'Brien SG, Guilhot F, Larson RA, et al. Imatinib compared with interferon and low-dose cytarabine for newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med. 2003;348(11):994–1004. doi: 10.1056/NEJMoa022457. [DOI] [PubMed] [Google Scholar]
- 6.Kantarjian HM, O'Brien S, Cortes J, et al. Imatinib mesylate therapy improves survival in patients with newly diagnosed Philadelphia chromosome-positive chronic myelogenous leukemia in the chronic phase: comparison with historic data. Cancer. 2003;98(12):2636–2642. doi: 10.1002/cncr.11831. [DOI] [PubMed] [Google Scholar]
- 7.Deininger M, Buchdunger E, Druker BJ. The development of imatinib as a therapeutic agent for chronic myeloid leukemia. Blood. 2005;105(7):2640–2653. doi: 10.1182/blood-2004-08-3097. [DOI] [PubMed] [Google Scholar]
- 8.Graham SM, Jorgensen HG, Allan E, et al. Primitive, quiescent, Philadelphia-positive stem cells from patients with chronic myeloid leukemia are insensitive to STI571 in vitro. Blood. 2002;99(1):319–325. doi: 10.1182/blood.v99.1.319. [DOI] [PubMed] [Google Scholar]
- 9.Bhatia R, Holtz M, Niu N, et al. Persistence of malignant hematopoietic progenitors in chronic myelogenous leukemia patients in complete cytogenetic remission following imatinib mesylate treatment. Blood. 2003;101(12):4701–4707. doi: 10.1182/blood-2002-09-2780. [DOI] [PubMed] [Google Scholar]
- 10.Elrick LJ, Jorgensen HG, Mountford JC, Holyoake TL. Punish the parent not the progeny. Blood. 2005;105(5):1862–1866. doi: 10.1182/blood-2004-08-3373. [DOI] [PubMed] [Google Scholar]
- 11.Copland M, Hamilton A, Elrick LJ, et al. Dasatinib (BMS-354825) targets an earlier progenitor population than imatinib in primary CML but does not eliminate the quiescent fraction. Blood. 2006;107(11):4532–4539. doi: 10.1182/blood-2005-07-2947. [DOI] [PubMed] [Google Scholar]
- 12.Angstreich GR, Matsui W, Huff CA, et al. Effects of imatinib and interferon on primitive chronic myeloid leukaemia progenitors. Br J Haematol. 2005;130(3):373–381. doi: 10.1111/j.1365-2141.2005.05606.x. [DOI] [PubMed] [Google Scholar]
- 13.Kujawski LA, Talpaz M. The role of interferon-alpha in the treatment of chronic myeloid leukemia. Cytokine Growth Factor Rev. 2007;18(5–6):459–471. doi: 10.1016/j.cytogfr.2007.06.015. [DOI] [PubMed] [Google Scholar]
- 14.Carella AM. Interferon-alpha is able to maintain complete molecular remission induced by imatinib after its discontinuation. Leukemia. 2008;22(5):1090–1091. doi: 10.1038/leu.2008.94. [DOI] [PubMed] [Google Scholar]
- 15.Alimena G, Breccia M, Luciano L, et al. Imatinib mesylate therapy in chronic myeloid leukemia patients in stable complete cytogenic response after interferon-alpha results in a very high complete molecular response rate. Leuk Res. 2008;32(2):255–261. doi: 10.1016/j.leukres.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 16.Taniguchi T, Takaoka A. A weak signal for strong responses: interferon-alpha/beta revisited. Nat Rev Mol Cell Biol. 2001;2(5):378–386. doi: 10.1038/35073080. [DOI] [PubMed] [Google Scholar]
- 17.Aaronson DS, Horvath CM. A road map for those who know JAK-STAT. Science. 2002;296(5573):1653–1655. doi: 10.1126/science.1071545. [DOI] [PubMed] [Google Scholar]
- 18.Stark GR, Kerr IM, Williams BR, Silverman RH, Schreiber RD. How cells respond to interferons. Annu Rev Biochem. 1998;67:227–264. doi: 10.1146/annurev.biochem.67.1.227. [DOI] [PubMed] [Google Scholar]
- 19.Platanias LC. Mechanisms of type-I- and type-II-interferon-mediated signalling. Nat Rev Immunol. 2005;5(5):375–386. doi: 10.1038/nri1604. [DOI] [PubMed] [Google Scholar]
- 20.Katsoulidis E, Sassano A, Majchrzak-Kita B, et al. Suppression of interferon (IFN)-inducible genes and IFN-mediated functional responses in BCR-ABL-expressing cells. J Biol Chem. 2008;283(16):10793–10803. doi: 10.1074/jbc.M706816200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gauzzi MC, Barbieri G, Richter MF, et al. The amino-terminal region of Tyk2 sustains the level of interferon alpha receptor 1, a component of the interferon alpha/beta receptor. Proc Natl Acad Sci U S A. 1997;94(22):11839–11844. doi: 10.1073/pnas.94.22.11839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marijanovic Z, Ragimbeau J, Kumar KG, Fuchs SY, Pellegrini S. TYK2 activity promotes ligand-induced IFNAR1 proteolysis. Biochem J. 2006;397(1):31–38. doi: 10.1042/BJ20060272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu J, HuangFu WC, Kumar KG, et al. Virus-induced unfolded protein response attenuates antiviral defenses via phosphorylation-dependent degradation of the type I interferon receptor. Cell Host Microbe. 2009;5(1):72–83. doi: 10.1016/j.chom.2008.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goldman LA, Zafari M, Cutrone EC, et al. Characterization of antihuman IFNAR-1 monoclonal antibodies: epitope localization and functional analysis. J Interferon Cytokine Res. 1999;19(1):15–26. doi: 10.1089/107999099314379. [DOI] [PubMed] [Google Scholar]
- 25.Kumar KG, Krolewski JJ, Fuchs SY. Phosphorylation and specific ubiquitin acceptor sites are required for ubiquitination and degradation of the IFNAR1 subunit of type I interferon receptor. J Biol Chem. 2004;279(45):46614–46620. doi: 10.1074/jbc.M407082200. [DOI] [PubMed] [Google Scholar]
- 26.Bhattacharya S, HuangFu WC, Liu J, et al. Inducible priming phosphorylation promotes ligand-independent degradation of the IFNAR1 chain of type I interferon receptor. J Biol Chem. 2010;285(4):2318–2325. doi: 10.1074/jbc.M109.071498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nicolini FE, Hayette S, Legros L, et al. Pegylated IFN-alpha2a combined to imatinib mesylate 600mg daily can induce complete cytogenetic and molecular responses in a subset of chronic phase CML patients refractory to IFN alone or to imatinib 600mg daily alone. Leuk Res. 2011;35(1):80–86. doi: 10.1016/j.leukres.2010.04.010. [DOI] [PubMed] [Google Scholar]
- 28.Preudhomme C, Guilhot J, Nicolini FE, et al. Imatinib plus peginterferon alfa-2a in chronic myeloid leukemia. N Engl J Med. 2010;363(26):2511–2521. doi: 10.1056/NEJMoa1004095. [DOI] [PubMed] [Google Scholar]
- 29.Yanagisawa K, Yamauchi H, Kaneko M, Kohno H, Hasegawa H, Fujita S. Suppression of cell proliferation and the expression of a bcr-abl fusion gene and apoptotic cell death in a new human chronic myelogenous leukemia cell line, KT-1, by interferon-alpha. Blood. 1998;91(2):641–648. [PubMed] [Google Scholar]
- 30.Palandri F, Castagnetti F, Iacobucci I, et al. The response to imatinib and interferon-alpha is more rapid than the response to imatinib alone: a retrospective analysis of 495 Philadelphia-positive chronic myeloid leukemia patients in early chronic phase. Haematologica. 2010;95(8):1415–1419. doi: 10.3324/haematol.2009.021246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Burchert A, Muller MC, Kostrewa P, et al. Sustained molecular response with interferon alfa maintenance after induction therapy with imatinib plus interferon alfa in patients with chronic myeloid leukemia. J Clin Oncol. 2010;28(8):1429–1435. doi: 10.1200/JCO.2009.25.5075. [DOI] [PubMed] [Google Scholar]
- 32.Druker BJ, Talpaz M, Resta DJ, et al. Efficacy and safety of a specific inhibitor of the BCR-ABL tyrosine kinase in chronic myeloid leukemia. N Engl J Med. 2001;344(14):1031–1037. doi: 10.1056/NEJM200104053441401. [DOI] [PubMed] [Google Scholar]
- 33.Kishi K. A new leukemia cell line with Philadelphia chromosome characterized as basophil precursors. Leuk Res. 1985;9(3):381–390. doi: 10.1016/0145-2126(85)90060-8. [DOI] [PubMed] [Google Scholar]
- 34.Kumar KG, Barriere H, Carbone CJ, et al. Site-specific ubiquitination exposes a linear motif to promote interferon-alpha receptor endocytosis. J Cell Biol. 2007;179(5):935–950. doi: 10.1083/jcb.200706034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kumar KG, Tang W, Ravindranath AK, Clark WA, Croze E, Fuchs SY. SCF(HOS) ubiquitin ligase mediates the ligand-induced down-regulation of the interferon-alpha receptor. EMBO J. 2003;22(20):5480–5490. doi: 10.1093/emboj/cdg524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu J, Plotnikov A, Banerjee A, et al. Ligand-independent pathway that controls stability of interferon alpha receptor. Biochem Biophys Res Commun. 2008;367(2):388–393. doi: 10.1016/j.bbrc.2007.12.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zheng H, Qian J, Varghese B, Baker DP, Fuchs SY. Ligand-stimulated downregulation of the interferon alpha receptor: role of protein kinase D2. Mol Cell Biol. 2011;31(4):710–720. doi: 10.1128/MCB.01154-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu J, Carvalho LP, Bhattacharya S, et al. Mammalian casein kinase 1alpha and its leishmanial ortholog regulate stability of IFNAR1 and type I interferon signaling. Mol Cell Biol. 2009;29(24):6401–6412. doi: 10.1128/MCB.00478-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Henderson YC, Guo XY, Greenberger J, Deisseroth AB. Potential role of bcr-abl in the activation of JAK1 kinase. Clin Cancer Res. 1997;3(2):145–149. [PubMed] [Google Scholar]
- 40.Mihailovic T, Marx M, Auer A, et al. Protein kinase D2 mediates activation of nuclear factor kappaB by Bcr-Abl in Bcr-Abl+ human myeloid leukemia cells. Cancer Res. 2004;64(24):8939–8944. doi: 10.1158/0008-5472.CAN-04-0981. [DOI] [PubMed] [Google Scholar]
- 41.Bossard C, Bresson D, Polishchuk RS, Malhotra V. Dimeric PKD regulates membrane fission to form transport carriers at the TGN. J Cell Biol. 2007;179(6):1123–1131. doi: 10.1083/jcb.200703166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Messina JL, Yu H, Riker AI, Munster PN, Jove RL, Daud AI. Activated stat-3 in melanoma. Cancer Control. 2008;15(3):196–201. doi: 10.1177/107327480801500302. [DOI] [PubMed] [Google Scholar]
- 43.Tamura T, Matsuzaki M, Harada H, Ogawa K, Mohri H, Okubo T. Upregulation of interferon-alpha receptor expression in hydroxyurea-treated leukemia cell lines. J Investig Med. 1997;45(4):160–167. [PubMed] [Google Scholar]
- 44.Barthe C, Mahon FX, Gharbi MJ, et al. Expression of interferon-alpha (IFN-alpha) receptor 2c at diagnosis is associated with cytogenetic response in IFN-alpha-treated chronic myeloid leukemia. Blood. 2001;97(11):3568–3573. doi: 10.1182/blood.v97.11.3568. [DOI] [PubMed] [Google Scholar]
- 45.Ito K, Tanaka H, Ito T, et al. Initial expression of interferon alpha receptor 2 (IFNAR2) on CD34-positive cells and its down-regulation correlate with clinical response to interferon therapy in chronic myelogenous leukemia. Eur J Haematol. 2004;73(3):191–205. doi: 10.1111/j.1600-0609.2004.00275.x. [DOI] [PubMed] [Google Scholar]
- 46.Evans IM, Bagherzadeh A, Charles M, et al. Characterization of the biological effects of a novel protein kinase D inhibitor in endothelial cells. Biochem J. 2010;429(3):565–572. doi: 10.1042/BJ20100578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Monovich L, Vega RB, Meredith E, et al. A novel kinase inhibitor establishes a predominant role for protein kinase D as a cardiac class IIa histone deacetylase kinase. FEBS Lett. 2010;584(3):631–637. doi: 10.1016/j.febslet.2009.12.014. [DOI] [PubMed] [Google Scholar]
- 48.Lavalle CR, Bravo-Altamirano K, Giridhar KV, et al. Novel protein kinase D inhibitors cause potent arrest in prostate cancer cell growth and motility. BMC Chem Biol. 2010;10:5. doi: 10.1186/1472-6769-10-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Harikumar KB, Kunnumakkara AB, Ochi N, et al. A novel small-molecule inhibitor of protein kinase D blocks pancreatic cancer growth in vitro and in vivo. Mol Cancer Ther. 2010;9(5):1136–1146. doi: 10.1158/1535-7163.MCT-09-1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.O'Hare T, Shakespeare WC, Zhu X, et al. AP24534, a pan-BCR-ABL inhibitor for chronic myeloid leukemia, potently inhibits the T315I mutant and overcomes mutation-based resistance. Cancer Cell. 2009;16(5):401–412. doi: 10.1016/j.ccr.2009.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.