Abstract
Isothiocyanates, derived from glucosinolates, are thought to be responsible for the chemoprotective actions conferred by higher cruciferous vegetable intake. Evidence suggests that isothiocyanates exert their effects through a variety of distinct but interconnected signaling pathways important for inhibiting carcinogenesis, including those involved in detoxification, inflammation, apoptosis, and cell cycle and epigenetic regulation, among others. This article provides an update on the latest research on isothiocyanates and these mechanisms, and points out remaining gaps in our understanding of these events. Given the variety of ITC produced from glucosinolates, and the diverse pathways on which these compounds act, a systems biology approach, in vivo, may help to better characterize their integrated role in cancer prevention. In addition, the effects of dose, duration of exposure, and specificity of different ITC should be considered.
Keywords: Isothiocyanates, cruciferous vegetables, chemoprevention, glucosinolates, glutathione S-transferase, NF-κB, biotransformation, detoxification, inflammation, apoptosis, cell cycle, epigenetic regulation
INTRODUCTION
Higher consumption of cruciferous vegetables (from the Brassicaceae plant family; e.g., broccoli, cabbage, Brussels sprouts, watercress, kale, cauliflower) is associated with a reduced risk of several cancers, particularly cancers of the gastrointestinal tract, lung and prostate.1–4 Similarly to many other plant foods, crucifers contain various compounds associated with reduced cancer risk including fiber, carotenoids, lutein, flavonoids, phytosterols, folic acid and vitamin C.5 In contrast to other plants however, cruciferous vegetables also contain substantial amounts of sulfur-containing glucosinolates, which, on hydrolysis by the enzyme myrosinase in the crucifers or β-thioglucosidases in certain gut bacteria, are converted to biologically active compounds such as indoles and isothiocyanates (ITC), and less active nitriles.6 These bioactive compounds are hypothesized to be responsible for the chemoprotective effects conferred by cruciferous vegetable consumption above and beyond the protective effects of higher intake of fruits and vegetables in general.
GLUCOSINOLATE PROFILES
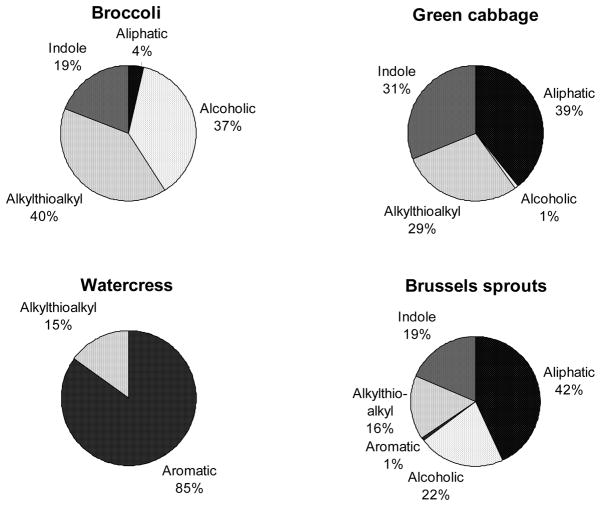
Glucosinolates are a class of sulfur-rich secondary plant metabolites. Although derived from the same botanical family, the glucosinolate composition of different cruciferous vegetables varies substantially (Figure 1).7–10 For example, broccoli is a rich source of the glucosinolate glucoraphanin, cabbage is rich in sinigrin, and watercress is high in gluconasturtiin. The type and amount of glucosinolates produced by the plants depend on environmental factors such as temperature, hydration, presence of iron, insects, and soil pH (sulfur and nitrogen content), although the ratio of individual glucosinolates remains relatively constant within each specific plant.2 There are over 150 known glucosinolates,2 which all share a common sulfur-linked β-D-glucopyranose structure, but differ in side chains. These side chains are derived from different amino acids during glucosinolate synthesis in plant cells. The glucosinolates can be divided into several sub-groups based on the chemical structure of the side chains (Figure 1).8, 11, 12 For example, the alkylthioalkyl side-chain of glucoraphanin contains a sulfur group, whereas, the aromatic side-chain of gluconasturtiin contains a phenethyl group.
Figure 1.
Glucosinolates in cruciferous vegetables can be divided into several sub-groups based on the chemical structure of the side chains.
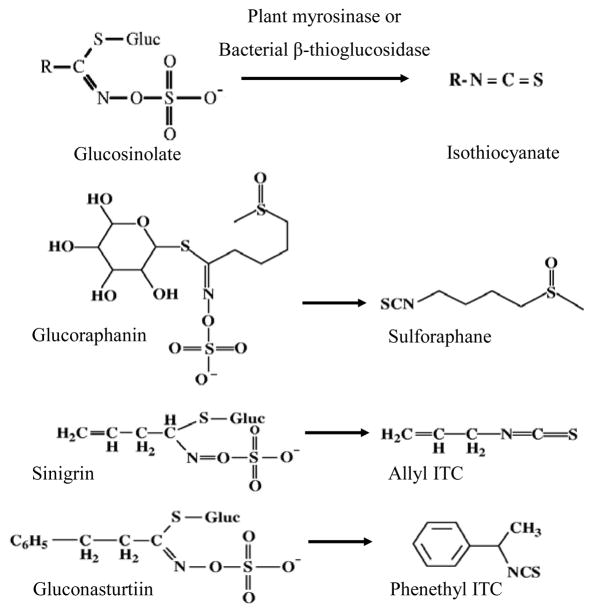
Following metabolism of glucosinolates into ITCs in vivo, the structural difference of the glucosinolate is conferred to that of the cognate ITC [e.g, glucoraphanin to sulforaphane (SFN), sinigrin to allyl ITC (AITC), and gluconasturtiin to phenethyl ITC (PEITC) Figure 2]. The biological effect of ITCs varies due to the side-chain structure. For instance, in vitro studies have shown that SFN is taken into cells faster, kept intracellularly longer, and at higher accumulations than several other ITCs,13, 14 and has the highest potency of inducing the expression of two phase 2 enzymes – glutathione S-transferase (GST) and quinone reductase (QR).13, 15 In contrast, Jakubikova et al.16 showed that AITC was most effective in causing HL60 cell cycle arrest, while PEITC and benzyl ITC (BITC)17 were the most effective in inducing apoptosis, among six different ITCs. Prawan, et al.18 studied the effect of ten synthetic ITC analogs on pro-inflammatory NF-κB activity in vitro, and reported that subtle changes in ITC structure had a profound impact on inhibition potential. Therefore, in addition to the amount consumed, the variety of cruciferous vegetables ingested may also influence biologic response. To date, differential effects of cruciferous vegetables with diverse glucosinolate profiles have not been directly compared in vivo in humans.
Figure 2.
Conversion of select glucosinolates to their corresponding isothiocyanates.
MECHANISMS OF ACTION
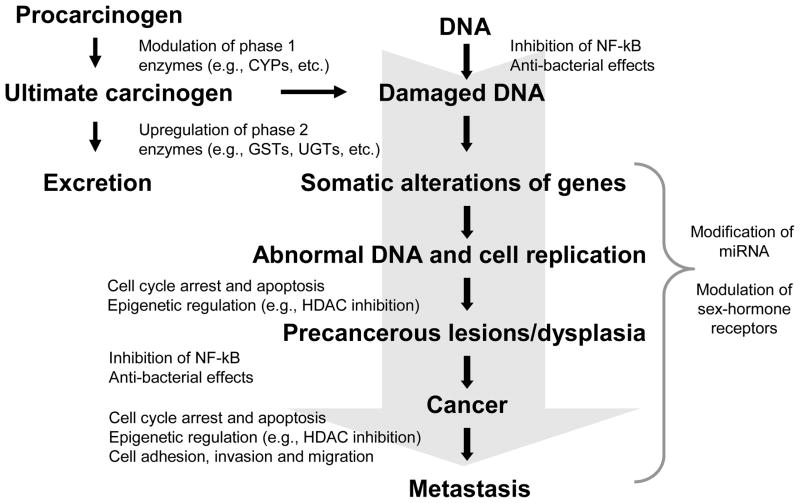
Enthusiasm for the study of the chemoprotective effects of ITCs was initially generated by early work in animal models demonstrating inhibition of carcinogen-induced tumor formation at various sites with administration of ITCs.19–23 These studies supported the epidemiologic literature suggesting a cancer protective effect of cruciferous vegetable consumption. Subsequently, evidence has emerged to suggest that ITCs may be involved in a number of other distinct but interconnected signaling pathways important for inhibiting carcinogenesis, including those involved in detoxification, inflammation, apoptosis, and cell cycle and epigenetic regulation, among others (Figure 3). In addition to ITCs, other glucosinolate hydrolysis products include indoles and nitriles, which have also been purported to have chemoprotective activity. An in-depth discussion of other glucosinolate products is beyond the scope of the present review and the reader is referred to other work on the topic.24–27 Numerous comprehensive reviews have been published on the biologic activities of ITCs over the past several years, however, this field continues to evolve and remains an active area of research.
Figure 3.
Mechanisms of action of isothiocyanates in the modulation of signaling pathways involved in cancer chemoprevention. (CYP=cytochrome P450; GST=glutathione S-transferase; UGT=UDP-glucuronosyl transferase; NF-κB=nuclear factor kappa B; HDAC=histone deacetylase; miRNA=micro RNA)
Modulation of Phase 1 and Phase 2 Biotransformation Enzymes
In studies designed to elucidate the mechanisms of cancer prevention by ITCs, Wattenberg21, 28 found that ITC administration increased carcinogen metabolism and detoxification, resulting in decreased initiation and promotion of tumors in carcinogen-challenged rats. Based on this seminal work, it was hypothesized that the major mechanism of chemoprevention by ITCs was through modulation of biotransformation enzymes [phase 1, e.g., cytochrome P450 (CYP) family, and phase 2, e.g., glutathione S-transferases (GST), UDP-glucuronosyl transferases (UGT), etc.].29, 30 GST and UGT are multigene families of enzymes involved in conjugation of exogenous compounds (e.g., various carcinogens and other xenobiotics) and endogenous compounds (e.g., sex steroid hormones) implicated in cancer risk.31
Most in vitro evidence suggests that up-regulation of phase 2 biotransformation enzymes by ITCs occurs through interaction of ITCs with the cytoplasmic-anchoring protein Kelch-like ECH-associated protein 1 (Keap 1), which represses the transcription factor NF-E2-related factor 2 (Nrf2).32, 33 ITC interaction with critical cysteine residues of Keap1 results in dissociation of Keap1 from Nrf2, allowing Nrf2 translocation to the nucleus and gene activation via the antioxidant response element (ARE) located upstream of the promoter region of the genes of many antioxidant and phase 2 biotransformation enzymes,32, 33 including GST,34 UGT,35 and NAD(P)H:quinone oxidoreductase-1 (NQO1),36 among others. Participation of Nrf2 in regulation of genes involved in carcinogen metabolism and antioxidant activity has been extensively reviewed.37–41
The effects of ITC-mediated upregulation of phase 2 enzymes have been corroborated in feeding studies in humans. For example, we showed previously that consumption of cruciferous vegetables for two weeks compared to a diet devoid of fruits and vegetables increased GST-α concentrations, and decreased serum bilirubin concentrations, indicative of increased UGT1A1 activity.42, 43 Gasper and colleagues44 observed increased expression of several xenobiotic metabolizing enzymes in gastric mucosa after individuals consumed high-glucosinolate broccoli.
There is also some evidence to suggest that ITCs directly inhibit phase 1 enzymes, although to a lesser extent, via both competitive inhibition as well as direct covalent modification (reviewed in 22, 45). Phase 1 enzymes are involved in metabolic activation of endogenous and exogenous compounds, including potential carcinogens. However, in addition to ITCs, glucosinolate hydrolysis leads to the production of indoles and nitriles. Indoles induce both phase 1 and 2 enzymes through binding with the aryl-hydrocarbon receptor (AhR) and subsequent interaction with the xenobiotic response element (XRE).27, 46, 47 Crambene, a nitrile, has been shown to act similarly to ITCs and interact with the ARE.27 Generally, induction of both phase 1 and phase 2 enzymes is thought to accelerate metabolism of carcinogens toward elimination.
As cruciferous vegetables contain a mixture of ITCs, indoles and nitriles, consumption of the whole food, versus single, isolated compounds, may confer protection beyond that of individual compounds. Thus, translation of studies based on isolated compounds to intake data in humans is very complex, and points to the need for further in vivo studies evaluating the effects of these vegetables on biologic outcomes in humans. The impact of ITCs on biotransformation has been extensively reviewed.22, 24, 45, 48–52
Inhibition of Nuclear Factor kappa B (NF-κB) Pathways
Further study on the chemopreventive potential of ITCs led to the discovery that ITCs may play a role in several other signaling pathways critical to carcinogenesis, including inhibition of NF-κB.24 NF-κB is a transcription factor and central mediator in the activation of genes involved in the inflammatory process, e.g., cytokines, chemokines, adhesion molecules and other soluble factors involved in the immune response.53 While acute inflammation is beneficial in some instances (e.g., injury or infection), persistent low-level inflammation is associated with predisposition to several chronic diseases.54–57 ITCs have been shown to inhibit NF-κB-mediated processes in vitro 24, 50, 58–60 and in vivo, in animal models,61, 62 and may therefore reduce inflammation — a well-recognized risk factor in carcinogenesis.63 For instance, constitutive activation of NF-κB is common in colon, liver and prostate cancer, and leads to upregulation of a number of cytokines, growth factors and anti-apoptotic genes.50, 64, 65 ITC-mediated inhibition of NF-κB-regulated pathways is mentioned in several reviews,24, 50, 66, 67 however, a dedicated work on the subject is still lacking.
The NF-κB family of transcription factors is composed of heterodimeric complexes of proteins from the Rel family, the most abundant of which is the p65/p50 heterodimer. 61 NF-κB is retained in an inactive form in the cytoplasm by an inhibitor molecule, inhibitor kappa B (IκB).68 Following a pro-inflammatory stimulus or oxidative event, IκB is phosphorylated via the IκB kinase complex (IKK) and ubiquitinated resulting in degradation and subsequent liberation of the NF-κB complex. ITC-mediated inhibition of NF-κB has been attributed to repression of IKK phosphorylation, preventing IκB degradation and thereby inhibiting transcriptional induction by NF-κB.59 This inhibition results in decreased expression of numerous NF-κB target genes, most notably inducible nitric oxide synthase (iNOS), cyclooxygenase-2 (COX-2), interleukin 6 (IL-6), and tumor necrosis factor alpha (TNF-α).50, 59, 69 There is also some evidence that SFN binds directly to essential thiol groups of p50, a functionally active subunit of NF-κB, thereby inhibiting NF-κB-binding to DNA.70
In addition to direct inhibition of NF-κB activity, it has been proposed that ITC-activation of Nrf2 may also lead to inhibition of NF-κB indirectly via crosstalk between Nrf2 and NF-κB transcription factors.71–73 This hypothesis is based on several observations. Nair et al.74 proposed a regulatory network for coordinated modulation of Nrf2 and NF-κB through a common mitogen-activated protein kinase (MAPK) cascade, based on common regulatory sequences in the transactivation domains of Nrf2 and NF-κB, and key regulatory genes in inflammatory signatures. Additionally, Khor et al.75 reported decreased levels of anti-oxidant/phase 2 enzymes with simultaneous up-regulation of the NF-κB-regulated pro-inflammatory mediators COX-2, iNOS, IL-1, IL-6, and TNF-α in Nrf2 knock-out mice with chemically-induced colitis. Ma et al.76 also observed a lupus-like autoimmune syndrome in Nrf2-deficient mice, marked by multi-organ inflammatory lesions and premature death due to rapid progression of nephritis. Taken together, these data suggest that Nrf2 is involved not only in the activation of antioxidant/phase 2 gene transcription machinery, but also in the suppression of pro-inflammatory signaling.
This finding is consistent with previous observations of an anti-inflammatory role for Nrf2.71, 77 Although the mechanism for this inhibition has yet to be worked out, direct evidence of the reverse (i.e., inhibition of Nrf2 by NF-κB) has been well-documented. Liu et al.78 demonstrated antagonism of Nrf2 by NF-κB at the transcriptional level through competition for co-activator CREB-binding protein (CBP) required for translocation, and concomitant recruitment of histone deacetylase (HDAC), a co-repressor. The potential cross-talk between NF-κB and Nrf2 requires further study, but may explain part of the cancer-protective effects of ITCs. Thus, Nrf2 may be a key mediator through which both phase 2 biotransformation and inflammation pathways are modulated.
Epigenetic Regulation
Epigenetic regulation is a modification of DNA without a change in the sequence, that results in a change in gene expression or phenotype. Unlike mutations in the genetic code, these epigenetic alterations, e.g., histone modification and methylation, may be modifiable. Recent evidence suggests that constituents in the diet, including ITCs, have the potential to alter a number of these epigenetic events (reviewed in 24, 50, 79–82).
One form of epigenetic regulation is the modification of histone proteins. When histones are acetylated, DNA is accessible to transcription factors. Conversely, when acetyl groups are removed from histones through the action of histone deacetylases (HDAC), access to DNA by transcription factors is restricted, and transcription is suppressed. As such, coordination of histone acetylation and deacetylation is an important regulatory mechanism for gene expression. In cancer, the balance between acetylation and deacetylation is often dysregulated, and tumor suppressor genes are frequently silenced.83 Inhibition of HDAC has been shown for ITC both in vitro and in vivo in animal models and may alter tumorigenesis.84–88 Associations between inhibition of HDAC activity and increases in gene expression have been demonstrated with the tumor suppressor gene p2189, 90 and the pro-apoptotic gene Bax.84 Interestingly, it appears that SFN metabolites (sulforaphane-cysteine and sulforaphane-N-acetyl cysteine) rather than the parent compound may be responsible for the inhibition, possibly by acting as competitive inhibitors.86
ITCs can also exert an epigenetic effect via modulation of DNA methylation. Meeran et al.91 showed that DNA methyltransferases were down-regulated by SFN in breast cancer cells, which led to site-specific CpG island demethylation in the telomerase reverse transcriptase gene. Another study by Wang et al.92 showed a CpG island demethylation effect of PEITC on the GSTP1 gene in prostate cancer cells, resulting in a significant increase in enzyme expression and activity. Lu et al.88 examined the effect of phenylhexyl ITC on myeloma cells. This ITC not only inhibited HDAC, but also induced DNA demethylation of the p16 gene, which led to reactivation of this tumor suppressor gene in myeloma cells. While potential modification of epigenetic events may be a significant chemoprotective property, how ITCs are interacting in these pathways is, thus far, poorly understood. This is currently an active area of research however, and we can anticipate further characterization of these interactions in the near future.
Micro RNA (miRNA) Regulation
miRNAs, are a recently discovered class of post-transcriptional regulators that bind to target messenger RNA transcripts, usually resulting in gene silencing.93 This is an exciting new area of research, particularly in the area of cancer prevention. Two recently published studies in animal models suggest that ITCs may have the potential to modulate miRNA regulation.94, 95 Using microarray and quantitative PCR methods, investigators found that the altered expression of a number of miRNA molecules, triggered by environmental cigarette smoke, can be attenuated by PEITC.94, 95 A variety of these small noncoding RNA molecules are known to have regulatory functions in cell proliferation, differentiation and apoptosis, Ras activation, p53 signaling, NF-κB inhibition, etc. Therefore, modulation of miRNA expression by ITCs may be another avenue through which ITCs exert a chemoprotective effect.
Stimulation of Cell Cycle Arrest and Apoptosis
Suppression of tumor cell growth in culture and animal models, has been reported with several ITCs, and while the molecular processes of this suppression are still uncertain, investigations have provided some potential ITC targets. In prostate cancer cells, treatment with either SFN or PEITC resulted in cell cycle arrest in the G2/M phase of the cell cycle and concomitant decrease in concentrations of a number of cell division cycle (Cdc) regulators (e.g., Cyclin B1, Cdc25B and Cdc25C), required for progression into M-phase.96, 97 Similarly, AITC has been shown to inhibit survival of brain malignant glioma GBM 8401 cells in a dose-dependent manner via G2/M arrest with parallel reduction in cyclin dependent kinase 1 (CDK1)/Cyclin B activity.98 Mi et al.99 reported inhibition of proteosomal protein degradation and dysruption of microtubule formation in multiple myeloma cells. In vivo, mice with mutations in one allele of the APC gene (adenomatous polyposis coli; APCnull/+) treated with 300 or 600 ppm SFN in their diet for three weeks developed fewer and smaller intestinal adenomas compared to mice on a control diet.100 The investigators also reported increased apoptotic activity and lower cell proliferation in mice fed the ITC-containing diet.
Apoptosis can be achieved through mitochondrial/intrinsic pathways, death-receptor cascades/extrinsic pathways, which converge on down-stream effector caspase-3, or caspase-independent pathways.51 In vitro studies have reported apoptosis in response to treatment with ITC through a number of targets, at different points in these pathways. These include down-regulation of anti-apoptotic Bcl-2 and up-regulation of pro-apoptotic Bax expression,101 proteolytic activation of caspase-3,101 decrease in mitochondrial potential with subsequent release of apoptotic generating proteins cytochrome c and inhibitor of apoptosis (IAP) family member Smac/DIABLO,102 activation of parallel MAPK cascades, (e.g., extracellular signal-regulated kinase (ERK), c-Jun N-terminal kinase (JNK), and p38),51 as well as others. It is not entirely clear how ITCs initiate intracellular signaling leading to apoptosis. One mechanism put forth to explain these observations is that administration of ITCs to cells in high concentrations results in the generation of reactive oxygen species (ROS) and consequent depletion of intracellular glutathione.24 Alternatively, Xiao, et al.103 proposed ITC-inhibition of oxidative phosphorylation as a means of ROS production, based on observations of PEITC interaction with mitochondrial complex III in prostate cancer cells. Mi and colleagues99 recently reported ITC-induced apoptosis through inhibition of proteasome activity with marked accumulation of the tumor suppressor p53 in multiple myeloma cells, independent of ROS generation. These results indicate that ITCs likely elicit apoptosis through a variety of pathways. Moreover, there is evidence to suggest that the cytotoxic effects of ITCs may selectively target cancer, rather than normal cell types.104 Numerous papers exist on the molecular basis of cell cycle regulation and induction of apoptosis.51, 67, 105–108
Modulation of Hormone Receptor Expression
The complexing of androgen or estrogen with their receptors can activate transcription of numerous genes involved in cell proliferation. Certain types of cancers, such as breast and prostate, have been linked to dysregulation of sex hormone-mediated gene expression.109, 110 Although modulation of sex steroid metabolism by crucifers, mainly indoles, has been documented,26, 111 few studies have investigated the effects of ITCs on the expression of sex hormones and their receptors. In an in vitro prostatic cell study, Beklemisheva et al.112 showed that PEITC suppressed testosterone-induced cell growth by down-regulating Sp1-mediated androgen receptor transcription. More recent work by Kang et al.113 found that ITCs can repress the expression of estrogen receptor (ER)-α in human breast cancer cells. These investigators found that the expression of an estrogen responsive gene, pS2, was significantly reduced after ITC treatment due to abrogation of estrogen and receptor interaction.113 Ramirez et al.114 reported that progesterone receptor expression, in addition to ER-α expression, was also inhibited by SFN. Interestingly, Telang et al.115 demonstrated up-regulation of the ER-β gene in human mammary cells by both SFN and PEITC, indicating that ITCs may serve as a modulator for the ratio of ER-α- to -β-subtype concentrations. ER subtypes have been shown to have opposing actions on AP1-dependent genes, including those involved in mammary proliferation and cell growth.116 ER-α generally enhances proliferation whereas ER-β has been shown to reduce proliferation by stimulating cell cycle arrest in the G2.115 In the work by Telang et al.,115 changes in the ER subtype ratio paralleled increases in the pro-apoptotic gene BAD, and tumor suppresser genes, p21 and p27.115
Anti-Angiogenic and Anti-Metastatic Effects
Angiogenesis and metastasis are key steps in the development of malignant cancer. The growth of new blood vessels is essential for the formation of large tumors because of the high demand for oxygen and nutrient import, and the necessity of waste export for the fast-growing tumor cells. The anti-angiogenic effects of ITCs have been reviewed recently by Cavell et al.117 Studies have shown that ITCs modulate tumor angiogenesis via several different pathways. ITCs can down-regulate the expression of pro-angiogenic genes such as vascular endothelial growth factor (VEGF) by targeting hypoxia inducible factor (HIF) transcription factors. ITCs can also inhibit other factors involved in tumor angiogenesis such as NF-κB, activator protein-1 (AP1) and MYC, independent of the HIF pathway. Tubulin, a protein required for cell morphogenesis and migration during the tumor angiogenesis process, has also been recognized as a target for ITCs. ITCs not only inhibit tubulin polymerization118, 119 but also promote tubulin degradation in cancer cells.120, 121
Metastasis, or the spread of tumor cells, both locally and to distant places via lymphatic or blood vessels, is another indication of cancer progression. ITCs and derivatives were shown to inhibit cell adhesion, invasion and migration in vitro, and suppress metastasis in vivo through down-regulation of matrix metalloproteinase (MMP) and up-regulation of tissue inhibitors of matrix metalloproteinase (TIMP). Several metastatic biomarkers were suppressed after SFN treatment in animal models.122, 123 Epidemiologic studies also indicate that crucifer intake is inversely associated with survival of many cancers.124–126
Anti-bacterial Effects
From the point of view of the plant, ITCs act as a deterrent for insects, suppress the growth of other nearby plants and provide antibiotic properties, warding off invading pathogens.2 The antibacterial effects of ITCs in humans were first described by Fahey et al.127 with Helicobacter pylori (H. pylori). SFN was shown to inhibit the growth of this bacterium, which is an established risk factor for gastric cancer. This inhibitory effect was observed irrespective of the antibiotic resistance status of the bacterium. Another study found that H. pylori colonization in mice and humans was alleviated after a two-month feeding of broccoli sprouts.128 In addition to direct antibacterial effects on H. pylori, cytoprotective responses in human cells may play a role, as the protective effects of ITCs were not observed in Nrf2 knockout mice. The transcription factor Nrf2 is involved in the induction of a battery of antioxidant and phase 2 biotransformation enzymes.37–41 These enzymes protect the mucosal cells from oxidative stress and subsequent DNA damage.129 ITCs have also been shown to have bactericidal effects on food borne pathogens, including Escherichia coli,130 Pseudomonas aeruginosa, Listeria monocytogenes and Staphylococcus aureus,131 supporting a broader anti-bacterial effect for these compounds.
Given their anti-bacterial effects, ITCs may also modulate the human gut microbial community. Glucosinolates reach the large intestine and gut bacteria play an important role in metabolizing these dietary constituents to ITCs and other compounds in vivo when cruciferous vegetables are ingested.6, 132 Aires et al.133 examined the effects of four ITCs on 17 human gut-associated bacteria. All ITCs had some anti-bacterial effects and SFN and BITC were the strongest inhibitors. These effects were dose-dependent with the strongest effects observed at high concentrations (3 μM). In humans, Shapiro et al.132 reported that urinary ITC excretion with cruciferous vegetable consumption decreased significantly when participants were pretreated with antibiotics and bowel cleansing. Further details on the anti-bacterial effects of ITCs can be found in other publications.134, 135
INTERINDIVIDUAL VARIATION IN BIOLOGIC RESPONSE IN HUMANS
By way of necessity, much of the mechanistic work on ITCs has been conducted in vitro, in cell systems, or in well-controlled animal models using isolated compounds. In humans consuming cruciferous vegetables, biologic response may vary due to differences in types and amounts of crucifers consumed, as well as genetic and other factors that influence exposure, metabolism and disposition of the ITCs, and interaction of the ITCs with target genes. Exposure to ITCs in vivo may be influenced by the environment in the digestive tract (e.g., hydrolysis by gut microbiota, microbiota composition, pH, nutrient interactions, etc.), how well food is chewed, and genetic variation in enzymes involved in metabolism of ITCs.47 These factors may translate into interindividual differences in the protective effects of cruciferous vegetables.
ITCs are metabolized by GST through the addition of a glutathione moiety, producing dithiocarbamates; then they are further metabolized to N-acetylcysteine conjugates and broken down via the mercapturic acid pathway.136 Null genotypes of GSTM1 and GSTT1 result in a complete lack of their respective enzymes and considerable variability in the modifying effect of GST genotypes on cancer risk is observed.137–144 Based on pharmacokinetic studies, this variability does not appear to be the result of slower ITC metabolism, as ITCs appear to be excreted at a greater rate among GSTM1-null individuals.44, 145 This observation may reflect ITC regulation of other signaling pathways that affect GST activity or differences in the type or amounts of ITCs consumed. It may also be that variants in N-acetyltransferase enzymes which produce N-acetylcysteine conjugates, further down the ITC metabolic pathway after conjugation with glutathione, are responsible, in part, for these observed differences.146
As discussed in the context of the anti-bacterial effects of ITCs, metabolism by gut bacteria may be another source of variation in ITC exposure. Typically, most crucifers eaten by humans are consumed cooked, and plant myrosinase is largely destroyed. In in vitro incubations of fecal or pure bacterial samples with select glucosinolates, several gut bacterial species have been shown to degrade glucosinolates.147–150 Feeding studies have also shown significant interindividual differences in urinary ITC recovery (1%–50% of original glucosinolate ingested) after participants consumed the same amount of cooked cruciferous vegetables.132, 151–155 The variation was smaller when raw vegetables or ITCs were consumed directly, indicating individual differences in the ability to metabolize glucosinolates.132, 151–155 A recent study by our group showed that fecal bacteria obtained from persons who had higher urinary ITC excretion after broccoli feeding, degraded more glucoraphanin in vitro after a two day incubation compared to the bacteria from the low excreters. However, no overall fecal microbiota composition differences were detected between the two groups, possibly due to the complexity of micriobiota structure, and functional redundancy of glucosinolate metabolism within the community.156
Other factors in the digestive tract may also contribute to the variation of ITC exposure in vivo. For example, a small percentage of glucosinolates may undergo acidic hydrolysis in stomach.157 In addition, the presence of iron and acidic environment in the intestine were found to lead to nitrile formation instead of ITCs from glucosinolates.12, 158
SUMMARY AND FUTURE DIRECTIONS
Accumulated evidence from studies conducted in cell culture, animal models, and epidemiologic cohorts over the past several decades, have demonstrated an important role for ITCs in dietary prevention of several cancers. The biological effects of ITCs are diverse, involving multiple signaling pathways as well as cross-talk between pathways, and characterization of these continues to evolve. Given the variation in chemical structure of ITCs produced from different glucosinolates, this diversity is not unexpected. However, even with the wealth of information we have on the chemoprotective effects of ITCs to date, gaps in our understanding of their function remain.
While there is value in elucidating the effects of ITCs on isolated pathways looking at a small number of targets, there is a need to understand systemic response of cells to ITCs from a whole-body perspective. Use of a systems biology approach, employing metagenomic, transcriptomic, proteomic and metabolomic technology may offer more comprehensive information on the actions of ITCs. For example, Traka, et al.159 examined gene expression profiles in prostate tissue before and after men consumed 400 g broccoli/week for 12 months. Using pathway analyses, they found diet-induced changes in insulin signaling, transforming growth factor-β1 and epidermal growth factor signaling pathways. Interestingly, these changes were greater among carriers of a GSTM1+ genotype.159 We recently utilized high throughput proteomics methods to determine how human serum peptides changed in response to cruciferous vegetables in a controlled feeding trial. We reported significant changes in circulating levels of several peptides, including GSTM1 genotype-dependent decreases in transthyretin (TTR), a carrier protein for retinol and the thyroid hormone thyroxine, and zinc α-glycoprotein, an adipokine involved in lipid metabolism.160
These previously unrecognized responses to cruciferous vegetables point to the complexity of the mechanisms involved in crucifer-mediated effects, and modulation by GST genotype. Further, the effects of dose, duration of exposure, specific ITCs, and other host genetic factors should be considered. Because precursor glucosinolates, rather than ITCs, are present in cruciferous vegetables, investigation of glucosinolate metabolism by gut microbiota may also be an important link between cruciferous vegetable consumption with ITC exposure. Finally, in addition to systems biology approaches, future investigations should examine the effects of ITCs in vivo, in humans, through study of intermediate biomarkers of cancer-related processes.
Acknowledgments
Sponsorship: This work was supported by grants R01CA142695, R56CA70913, and R25CA94880 from the National Institutes of health, National Cancer Institute
References
- 1.Keck AS, Finley JW. Integr Cancer Ther. 2004;3:5–12. doi: 10.1177/1534735403261831. [DOI] [PubMed] [Google Scholar]
- 2.International Agency for Research on Cancer. Cruciferous Vegetables, Isothiocyanates and Indoles. International Agency for Research on Cancer; Lyon France: 2004. [Google Scholar]
- 3.Murillo G, Mehta RG. Nutr Cancer. 2001;41:17–28. doi: 10.1080/01635581.2001.9680607. [DOI] [PubMed] [Google Scholar]
- 4.Cohen JH, Kristal AR, Stanford JL. J Natl Cancer Inst. 2000;92:61–68. doi: 10.1093/jnci/92.1.61. [DOI] [PubMed] [Google Scholar]
- 5.Steinmetz KA, Potter JD. J Am Diet Assoc. 1996;96:1027–1039. doi: 10.1016/S0002-8223(96)00273-8. [DOI] [PubMed] [Google Scholar]
- 6.Shapiro TA, Fahey JW, Dinkova-Kostova AT, Holtzclaw WD, Stephenson KK, Wade KL, Ye L, Talalay P. Nutr Cancer. 2006;55:53–62. doi: 10.1207/s15327914nc5501_7. [DOI] [PubMed] [Google Scholar]
- 7.Kushad MM, Brown AF, Kurilich AC, Juvik JA, Klein BP, Wallig MA, Jeffery EH. J Agric Food Chem. 1999;47:1541–1548. doi: 10.1021/jf980985s. [DOI] [PubMed] [Google Scholar]
- 8.Mithen RF, Dekker M, Verkerk R, Rabot S, Johnson IT. J Sci Food Agric. 2000;80:967–984. [Google Scholar]
- 9.Hecht SS, Carmella SG, Kenney PM, Low SH, Arakawa K, Yu MC. Cancer Epidemiol Biomarkers Prev. 2004;13:997–1004. [PubMed] [Google Scholar]
- 10.Vermeulen M, Van den Berg R, Freidig AP, Van Bladeren PJ, Vaes WHJ. J Agric Food Chem. 2006;54:5350–5358. doi: 10.1021/jf060723n. [DOI] [PubMed] [Google Scholar]
- 11.Fahey JW, Zalcmann AT, Talalay P. Phytochemistry. 2001;56:5–51. doi: 10.1016/s0031-9422(00)00316-2. [DOI] [PubMed] [Google Scholar]
- 12.Williams DJ, Critchley C, Pun S, Chaliha M, O'Hare TJ. Phytochemistry. 2009;70:1401–1409. doi: 10.1016/j.phytochem.2009.07.035. [DOI] [PubMed] [Google Scholar]
- 13.Zhang Y, Talalay P. Cancer Res. 1998;58:4632–4639. [PubMed] [Google Scholar]
- 14.Ye L, Zhang Y. Carcinogenesis. 2001;22:1987–1992. doi: 10.1093/carcin/22.12.1987. [DOI] [PubMed] [Google Scholar]
- 15.Vermeulen M, Boerboom AM, Blankvoort BM, Aarts JM, Rietjens IM, van Bladeren PJ, Vaes WH. Toxicol In Vitro. 2009;23:617–621. doi: 10.1016/j.tiv.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 16.Jakubikova J, Bao Y, Sedlak J. Anticancer Res. 2005;25:3375–3386. [PubMed] [Google Scholar]
- 17.Munday R, Zhang Y, Munday CM, Bapardekar MV, Paonessa JD. Pharm Res. 2008;25:2164–2170. doi: 10.1007/s11095-008-9595-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Prawan A, Saw CL, Khor TO, Keum YS, Yu S, Hu L, Kong AN. Chem Biol Interact. 2009;179:202–211. doi: 10.1016/j.cbi.2008.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lopez M, Mazzanti L. J Pathol Bacteriol. 1955;69:243–250. doi: 10.1002/path.1700690132. [DOI] [PubMed] [Google Scholar]
- 20.Mc LM, Rees KR. J Pathol Bacteriol. 1958;76:175–188. doi: 10.1002/path.1700760120. [DOI] [PubMed] [Google Scholar]
- 21.Wattenberg LW. J Natl Cancer Inst. 1977;58:395–398. doi: 10.1093/jnci/58.2.395. [DOI] [PubMed] [Google Scholar]
- 22.Hecht SS. Drug Metab Rev. 2000;32:395–411. doi: 10.1081/dmr-100102342. [DOI] [PubMed] [Google Scholar]
- 23.Zhang Y, Talalay P. Cancer Res. 1994;54:1976s–1981s. [PubMed] [Google Scholar]
- 24.Hayes JD, Kelleher MO, Eggleston IM. Eur J Nutr. 2008;47(Suppl 2):73–88. doi: 10.1007/s00394-008-2009-8. [DOI] [PubMed] [Google Scholar]
- 25.Minich DM, Bland JS. Nutr Rev. 2007;65:259–267. doi: 10.1301/nr.2007.jun.259-267. [DOI] [PubMed] [Google Scholar]
- 26.Higdon JV, Delage B, Williams DE, Dashwood RH. Pharmacol Res. 2007;55:224–236. doi: 10.1016/j.phrs.2007.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nho CW, Jeffery E. Toxicol Appl Pharmacol. 2004;198:40–48. doi: 10.1016/j.taap.2004.02.012. [DOI] [PubMed] [Google Scholar]
- 28.Wattenberg LW. Cancer Res. 1981;41:2991–2994. [PubMed] [Google Scholar]
- 29.Wattenberg LW. Proc Nutr Soc. 1990;49:173–183. doi: 10.1079/pns19900022. [DOI] [PubMed] [Google Scholar]
- 30.Talalay P, Fahey JW, Holtzclaw WD, Prestera T, Zhang Y. Toxicol Lett. 1995;82–83:173–179. doi: 10.1016/0378-4274(95)03553-2. [DOI] [PubMed] [Google Scholar]
- 31.Eaton DL, Bammler TK. Toxicol Sci. 1999;49:156–164. doi: 10.1093/toxsci/49.2.156. [DOI] [PubMed] [Google Scholar]
- 32.Dinkova-Kostova AT, Holtzclaw WD, Cole RN, Itoh K, Wakabayashi N, Katoh Y, Yamamoto M, Talalay P. Proc Natl Acad Sci USA. 2002;99:11908–11913. doi: 10.1073/pnas.172398899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hayes JD, McMahon M. Trends Biochem Sci. 2009;34:176–188. doi: 10.1016/j.tibs.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 34.Talalay P. Biofactors. 2000;12:5–11. doi: 10.1002/biof.5520120102. [DOI] [PubMed] [Google Scholar]
- 35.Basten GP, Bao Y, Williamson G. Carcinogenesis. 2002;23:1399–1404. doi: 10.1093/carcin/23.8.1399. [DOI] [PubMed] [Google Scholar]
- 36.Gao X, Talalay P. Proc Natl Acad Sci USA. 2004;101:10446–10451. doi: 10.1073/pnas.0403886101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Slocum SL, Kensler TW. Arch Toxicol. 2011;85:273–284. doi: 10.1007/s00204-011-0675-4. [DOI] [PubMed] [Google Scholar]
- 38.Surh YJ, Kundu JK, Na HK. Planta Med. 2008;74:1526–1539. doi: 10.1055/s-0028-1088302. [DOI] [PubMed] [Google Scholar]
- 39.Valgimigli L, Iori R. Environ Mol Mutagen. 2009;50:222–237. doi: 10.1002/em.20468. [DOI] [PubMed] [Google Scholar]
- 40.Nakamura Y, Miyoshi N. Biosci Biotechnol Biochem. 2010;74:242–255. doi: 10.1271/bbb.90731. [DOI] [PubMed] [Google Scholar]
- 41.Baird L, Dinkova-Kostova AT. Arch Toxicol. 2011;85:241–272. doi: 10.1007/s00204-011-0674-5. [DOI] [PubMed] [Google Scholar]
- 42.Navarro SL, Peterson S, Chen C, Makar KW, Schwarz Y, King IB, Li SS, Li L, Kestin M, Lampe JW. Cancer Prev Res. 2009;2:345–352. doi: 10.1158/1940-6207.CAPR-08-0178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Navarro SL, Chang J, Peterson S, Chen C, King IB, Schwarz Y, Li SS, Potter JD, Lampe JW. Cancer Epidemiol Biomarkers Prev. 2009;18:2974–2978. doi: 10.1158/1055-9965.EPI-09-0701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gasper AV, Al-Janobi A, Smith JA, Bacon JR, Fortun P, Atherton C, Taylor MA, Hawkey CJ, Barrett DA, Mithen RF. Am J Clin Nutr. 2005;82:1283–1291. doi: 10.1093/ajcn/82.6.1283. [DOI] [PubMed] [Google Scholar]
- 45.Conaway CC, Yang YM, Chung FL. Curr Drug Metab. 2002;3:233–255. doi: 10.2174/1389200023337496. [DOI] [PubMed] [Google Scholar]
- 46.Verhoeven DT, Verhagen H, Goldbohm RA, van den Brandt PA, van Poppel G. Chem Biol Interact. 1997;103:79–129. doi: 10.1016/s0009-2797(96)03745-3. [DOI] [PubMed] [Google Scholar]
- 47.Lampe JW, Peterson S. J Nutr. 2002;132:2991–2994. doi: 10.1093/jn/131.10.2991. [DOI] [PubMed] [Google Scholar]
- 48.Talalay P, Fahey JW. J Nutr. 2001;131:3027S–3033S. doi: 10.1093/jn/131.11.3027S. [DOI] [PubMed] [Google Scholar]
- 49.Myzak MC, Dashwood RH. Cancer Lett. 2006;233:208–218. doi: 10.1016/j.canlet.2005.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Clarke JD, Dashwood RH, Ho E. Cancer Lett. 2008;269:291–304. doi: 10.1016/j.canlet.2008.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Juge N, Mithen RF, Traka M. Cell Mol Life Sci. 2007;64:1105–1127. doi: 10.1007/s00018-007-6484-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Keum YS, Jeong WS, Kong ANT. Mutat Res. 2004;555:191–202. doi: 10.1016/j.mrfmmm.2004.05.024. [DOI] [PubMed] [Google Scholar]
- 53.Pahl HL. Oncogene. 1999;18:6853–6866. doi: 10.1038/sj.onc.1203239. [DOI] [PubMed] [Google Scholar]
- 54.Rath E, Haller D. Eur J Nutr. 2011;50:219–233. doi: 10.1007/s00394-011-0197-0. [DOI] [PubMed] [Google Scholar]
- 55.Sathyapalan T, Atkin SL. Minerva Endocrinol. 2011;36:147–156. [PubMed] [Google Scholar]
- 56.Maskrey BH, Megson IL, Whitfield PD, Rossi AG. Arterioscler Thromb Vasc Biol. 2011;31:1001–1006. doi: 10.1161/ATVBAHA.110.213850. [DOI] [PubMed] [Google Scholar]
- 57.Grivennikov SI, Karin M. Ann Rheum Dis. 2011;70(Suppl 1):i104–108. doi: 10.1136/ard.2010.140145. [DOI] [PubMed] [Google Scholar]
- 58.Nair S, Li W, Kong AN. Acta Pharmacol Sin. 2007;28:459–472. doi: 10.1111/j.1745-7254.2007.00549.x. [DOI] [PubMed] [Google Scholar]
- 59.Gilmore TD, Herscovitch M. Oncogene. 2006;25:6887–6899. doi: 10.1038/sj.onc.1209982. [DOI] [PubMed] [Google Scholar]
- 60.Jeong WS, Kim IW, Hu R, Kong AN. Pharm Res. 2004;21:661–670. doi: 10.1023/b:pham.0000022413.43212.cf. [DOI] [PubMed] [Google Scholar]
- 61.Youn HS, Kim YS, Park ZY, Kim SY, Choi NY, Joung SM, Seo JA, Lim KM, Kwak MK, Hwang DH, Lee JY. J Immunol. 2010;184:411–419. doi: 10.4049/jimmunol.0803988. [DOI] [PubMed] [Google Scholar]
- 62.Wu L, Noyan Ashraf MH, Facci M, Wang R, Paterson PG, Ferrie A, Juurlink BH. Proc Natl Acad Sci USA. 2004;101:7094–7099. doi: 10.1073/pnas.0402004101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ohshima H, Bartsch H. Mutat Res. 1994;305:253–264. doi: 10.1016/0027-5107(94)90245-3. [DOI] [PubMed] [Google Scholar]
- 64.Culig Z. Biochim Biophys Acta. 2011;1813:308–314. doi: 10.1016/j.bbamcr.2010.12.010. [DOI] [PubMed] [Google Scholar]
- 65.Mantovani A, Allavena P, Sica A, Balkwill F. Nature. 2008;454:436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 66.Keum YS, Jeong WS, Kong AN. Drug News Perspect. 2005;18:445–451. doi: 10.1358/dnp.2005.18.7.939350. [DOI] [PubMed] [Google Scholar]
- 67.Cheung KL, Kong AN. Aaps J. 2010;12:87–97. doi: 10.1208/s12248-009-9162-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gilmore TD. Oncogene. 2006;25:6680–6684. doi: 10.1038/sj.onc.1209954. [DOI] [PubMed] [Google Scholar]
- 69.Karin M. Nature. 2006;441:431–436. doi: 10.1038/nature04870. [DOI] [PubMed] [Google Scholar]
- 70.Heiss E, Herhaus C, Klimo K, Bartsch H, Gerhauser C. J Biol Chem. 2001;276:32008–32015. doi: 10.1074/jbc.M104794200. [DOI] [PubMed] [Google Scholar]
- 71.Li W, Khor TO, Xu C, Shen G, Jeong WS, Yu S, Kong AN. Biochem Pharmacol. 2008;76:1485–1489. doi: 10.1016/j.bcp.2008.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Surh YJ, Na HK. Genes Nutr. 2008;2:313–317. doi: 10.1007/s12263-007-0063-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yu M, Li H, Liu Q, Liu F, Tang L, Li C, Yuan Y, Zhan Y, Xu W, Li W, Chen H, Ge C, Wang J, Yang X. Cell Signal. 2011;23:883–892. doi: 10.1016/j.cellsig.2011.01.014. [DOI] [PubMed] [Google Scholar]
- 74.Nair S, Doh ST, Chan JY, Kong AN, Cai L. Br J Cancer. 2008;99:2070–2082. doi: 10.1038/sj.bjc.6604703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Khor TO, Huang MT, Kwon KH, Chan JY, Reddy BS, Kong AN. Cancer Res. 2006;66:11580–11584. doi: 10.1158/0008-5472.CAN-06-3562. [DOI] [PubMed] [Google Scholar]
- 76.Ma Q, Battelli L, Hubbs AF. Am J Pathol. 2006;168:1960–1974. doi: 10.2353/ajpath.2006.051113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yoh K, Itoh K, Enomoto A, Hirayama A, Yamaguchi N, Kobayashi M, Morito N, Koyama A, Yamamoto M, Takahashi S. Kidney Int. 2001;60:1343–1353. doi: 10.1046/j.1523-1755.2001.00939.x. [DOI] [PubMed] [Google Scholar]
- 78.Liu GH, Qu J, Shen X. Biochim Biophys Acta. 2008;1783:713–727. doi: 10.1016/j.bbamcr.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 79.Nian H, Delage B, Ho E, Dashwood RH. Environ Mol Mutagen. 2009;50:213–221. doi: 10.1002/em.20454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Dashwood RH, Ho E. Semin Cancer Biol. 2007;17:363–369. doi: 10.1016/j.semcancer.2007.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wang LG, Chiao JW. Int J Oncol. 2010;37:533–539. doi: 10.3892/ijo_00000702. [DOI] [PubMed] [Google Scholar]
- 82.Dashwood RH, Ho E. Nutr Rev. 2008;66(Suppl 1):S36–38. doi: 10.1111/j.1753-4887.2008.00065.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Fraga MF, Esteller M. Cell Cycle. 2005;4:1377–1381. doi: 10.4161/cc.4.10.2113. [DOI] [PubMed] [Google Scholar]
- 84.Myzak MC, Hardin K, Yan M, Tong P, Dashwood R, Ho E. FASEB J. 2006;20:A150–A150. [Google Scholar]
- 85.Lea MA, Rasheed M, Randolph VM, Khan F, Shareef A, desBordes C. Nutr Cancer. 2002;43:90–102. doi: 10.1207/S15327914NC431_11. [DOI] [PubMed] [Google Scholar]
- 86.Myzak MC, Dashwood RH. Curr Drug Targets. 2006;7:443–452. doi: 10.2174/138945006776359467. [DOI] [PubMed] [Google Scholar]
- 87.Batra S, Sahu RP, Kandala PK, Srivastava SK. Mol Cancer Ther. 2010;9:1596–1608. doi: 10.1158/1535-7163.MCT-09-1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lu Q, Lin X, Feng J, Zhao X, Gallagher R, Lee MY, Chiao JW, Liu D. J Hematol Oncol. 2008;1:6. doi: 10.1186/1756-8722-1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Myzak MC, Hardin K, Wang R, Dashwood RH, Ho E. Carcinogenesis. 2006;27:811–819. doi: 10.1093/carcin/bgi265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Fimognari C, Hrelia P. Mutat Res. 2007;635:90–104. doi: 10.1016/j.mrrev.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 91.Meeran SM, Patel SN, Tollefsbol TO. PLoS One. 2010;5:e11457. doi: 10.1371/journal.pone.0011457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wang LG, Beklemisheva A, Liu XM, Ferrari AC, Feng J, Chiao JW. Mol Carcinog. 2007;46:24–31. doi: 10.1002/mc.20258. [DOI] [PubMed] [Google Scholar]
- 93.Bartel DP. Cell. 2009;136:215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Izzotti A, Larghero P, Cartiglia C, Longobardi M, Pfeffer U, Steele VE, De Flora S. Carcinogenesis. 2010;31:894–901. doi: 10.1093/carcin/bgq037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Izzotti A, Calin GA, Steele VE, Cartiglia C, Longobardi M, Croce CM, De Flora S. Cancer Prev Res (Phila) 2010;3:62–72. doi: 10.1158/1940-6207.CAPR-09-0202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Singh AV, Xiao D, Lew KL, Dhir R, Singh SV. Carcinogenesis. 2004;25:83–90. doi: 10.1093/carcin/bgg178. [DOI] [PubMed] [Google Scholar]
- 97.Xiao D, Johnson CS, Trump DL, Singh SV. Mol Cancer Ther. 2004;3:567–575. [PubMed] [Google Scholar]
- 98.Chen NG, Chen KT, Lu CC, Lan YH, Lai CH, Chung YT, Yang JS, Lin YC. Oncol Rep. 2010;24:449–455. doi: 10.3892/or_00000878. [DOI] [PubMed] [Google Scholar]
- 99.Mi L, Gan N, Chung FL. Carcinogenesis. 2011;32:216–223. doi: 10.1093/carcin/bgq242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hu R, Khor TO, Shen G, Jeong WS, Hebbar V, Chen C, Xu C, Reddy B, Chada K, Kong AN. Carcinogenesis. 2006;27:2038–2046. doi: 10.1093/carcin/bgl049. [DOI] [PubMed] [Google Scholar]
- 101.Park SY, Kim GY, Bae SJ, Yoo YH, Choi YH. Oncol Rep. 2007;18:181–187. [PubMed] [Google Scholar]
- 102.Choi S, Singh SV. Cancer Res. 2005;65:2035–2043. doi: 10.1158/0008-5472.CAN-04-3616. [DOI] [PubMed] [Google Scholar]
- 103.Xiao D, Powolny AA, Moura MB, Kelley EE, Bommareddy A, Kim SH, Hahm ER, Normolle D, Van Houten B, Singh SV. J Biol Chem. 2010;285:26558–26569. doi: 10.1074/jbc.M109.063255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Clarke JD, Hsu A, Yu Z, Dashwood RH, Ho E. Mol Nutr Food Res. 2011 doi: 10.1002/mnfr.201000547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Gamet-Payrastre L. Curr Cancer Drug Targets. 2006;6:135–145. doi: 10.2174/156800906776056509. [DOI] [PubMed] [Google Scholar]
- 106.Nakamura Y. Forum Nutr. 2009;61:170–181. doi: 10.1159/000212749. [DOI] [PubMed] [Google Scholar]
- 107.Zhang Y, Yao S, Li J. Proc Nutr Soc. 2006;65:68–75. doi: 10.1079/pns2005475. [DOI] [PubMed] [Google Scholar]
- 108.Wu X, Zhou QH, Xu K. Acta Pharmacol Sin. 2009;30:501–512. doi: 10.1038/aps.2009.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Heinlein CA, Chang C. Endocr Rev. 2004;25:276–308. doi: 10.1210/er.2002-0032. [DOI] [PubMed] [Google Scholar]
- 110.Deroo BJ, Korach KS. J Clin Invest. 2006;116:561–570. doi: 10.1172/JCI27987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kim YS, Milner JA. J Nutr Biochem. 2005;16:65–73. doi: 10.1016/j.jnutbio.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 112.Beklemisheva AA, Feng J, Yeh YA, Wang LG, Chiao JW. Prostate. 2007;67:863–870. doi: 10.1002/pros.20472. [DOI] [PubMed] [Google Scholar]
- 113.Kang L, Ding L, Wang ZY. Oncol Rep. 2009;21:185–192. [PMC free article] [PubMed] [Google Scholar]
- 114.Ramirez MC, Singletary K. J Nutr Biochem. 2009;20:195–201. doi: 10.1016/j.jnutbio.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 115.Telang U, Brazeau DA, Morris ME. Exp Biol Med (Maywood) 2009;234:287–295. doi: 10.3181/0808-RM-241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Paech K, Webb P, Kuiper GG, Nilsson S, Gustafsson J, Kushner PJ, Scanlan TS. Science. 1997;277:1508–1510. doi: 10.1126/science.277.5331.1508. [DOI] [PubMed] [Google Scholar]
- 117.Cavell BE, Syed Alwi SS, Donlevy A, Packham G. Biochem Pharmacol. 2011;81:327–336. doi: 10.1016/j.bcp.2010.10.005. [DOI] [PubMed] [Google Scholar]
- 118.Jackson SJ, Singletary KW. J Nutr. 2004;134:2229–2236. doi: 10.1093/jn/134.9.2229. [DOI] [PubMed] [Google Scholar]
- 119.Smith TK, Lund EK, Parker ML, Clarke RG, Johnson IT. Carcinogenesis. 2004;25:1409–1415. doi: 10.1093/carcin/bgh149. [DOI] [PubMed] [Google Scholar]
- 120.Mi L, Gan N, Cheema A, Dakshanamurthy S, Wang X, Yang DC, Chung FL. J Biol Chem. 2009;284:17039–17051. doi: 10.1074/jbc.M901789200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Yin P, Kawamura T, He M, Vanaja DK, Young CY. Cell Biol Int. 2009;33:57–64. doi: 10.1016/j.cellbi.2008.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Hwang ES, Lee HJ. Exp Biol Med (Maywood) 2006;231:421–430. doi: 10.1177/153537020623100408. [DOI] [PubMed] [Google Scholar]
- 123.Thejass P, Kuttan G. Immunopharmacol Immunotoxicol. 2006;28:443–457. doi: 10.1080/08923970600928049. [DOI] [PubMed] [Google Scholar]
- 124.Tang L, Zirpoli GR, Guru K, Moysich KB, Zhang Y, Ambrosone CB, McCann SE. Cancer Epidemiol Biomarkers Prev. 2010;19:1806–1811. doi: 10.1158/1055-9965.EPI-10-0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Dolecek TA, McCarthy BJ, Joslin CE, Peterson CE, Kim S, Freels SA, Davis FG. J Am Diet Assoc. 2010;110:369–382. doi: 10.1016/j.jada.2009.11.014. [DOI] [PubMed] [Google Scholar]
- 126.Chan R, Lok K, Woo J. Mol Nutr Food Res. 2009;53:201–216. doi: 10.1002/mnfr.200800113. [DOI] [PubMed] [Google Scholar]
- 127.Fahey JW, Haristoy X, Dolan PM, Kensler TW, Scholtus I, Stephenson KK, Talalay P, Lozniewski A. Proc Natl Acad Sci USA. 2002;99:7610–7615. doi: 10.1073/pnas.112203099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Yanaka A, Fahey JW, Fukumoto A, Nakayama M, Inoue S, Zhang S, Tauchi M, Suzuki H, Hyodo I, Yamamoto M. Cancer Prev Res (Phila) 2009;2:353–360. doi: 10.1158/1940-6207.CAPR-08-0192. [DOI] [PubMed] [Google Scholar]
- 129.Yanaka A. Curr Pharm Des. 2011 [Google Scholar]
- 130.Luciano FB, Holley RA. Int J Food Microbiol. 2009;131:240–245. doi: 10.1016/j.ijfoodmicro.2009.03.005. [DOI] [PubMed] [Google Scholar]
- 131.Saavedra MJ, Borges A, Dias C, Aires A, Bennett RN, Rosa ES, Simoes M. Med Chem. 2010;6:174–183. doi: 10.2174/1573406411006030174. [DOI] [PubMed] [Google Scholar]
- 132.Shapiro TA, Fahey JW, Wade KL, Stephenson KK, Talalay P. Cancer Epidemiol Biomarkers Prev. 1998;7:1091–1100. [PubMed] [Google Scholar]
- 133.Aires A, Mota VR, Saavedra MJ, Rosa EA, Bennett RN. J Appl Microbiol. 2009;106:2086–2095. doi: 10.1111/j.1365-2672.2009.04180.x. [DOI] [PubMed] [Google Scholar]
- 134.Moon JK, Kim JR, Ahn YJ, Shibamoto T. J Agric Food Chem. 2010;58:6672–6677. doi: 10.1021/jf1003573. [DOI] [PubMed] [Google Scholar]
- 135.Haristoy X, Fahey JW, Scholtus I, Lozniewski A. Planta Med. 2005;71:326–330. doi: 10.1055/s-2005-864098. [DOI] [PubMed] [Google Scholar]
- 136.Kolm RH, Danielson H, Zhang Y, Talalay P, Mannervik B. Biochem J. 1995;311:453–459. doi: 10.1042/bj3110453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.London SJ, Yuan JM, Chung FL, Gao YT, Coetzee GA, Ross RK, Yu MC. Lancet. 2000;356:724–729. doi: 10.1016/S0140-6736(00)02631-3. [DOI] [PubMed] [Google Scholar]
- 138.Zhao B, Seow A, Lee EJD, Poh W-T, Teh M, Eng P, Wang Y-T, Tan W-C, Yu MC, Lee H-P. Cancer Epidemiol Biomarkers Prev. 2001;10:1063–1067. [PubMed] [Google Scholar]
- 139.Seow A, Yuan JM, Sun CL, Van Den Berg D, Lee HP, Yu MC. Carcinogenesis. 2002;23:2055–2061. doi: 10.1093/carcin/23.12.2055. [DOI] [PubMed] [Google Scholar]
- 140.Fowke JH, Shu XO, Dai Q, Shintani A, Conaway CC, Chung FL, Cai Q, Gao YT, Zheng W. Cancer Epidemiol Biomarkers Prev. 2003;12:1536–1539. [PubMed] [Google Scholar]
- 141.Spitz MR, Duphorne CM, Detry MA, Pillow PC, Amos CI, Lei L, de Andrade M, Gu X, Hong WK, Wu X. Cancer Epidemiol Biomarkers Prev. 2000;9:1017–1020. [PubMed] [Google Scholar]
- 142.Joseph MA, Moysich KB, Freudenheim JL, Shields PG, Bowman ED, Zhang Y, Marshall JR, Ambrosone CB. Nutr Cancer. 2004;50:206–213. doi: 10.1207/s15327914nc5002_11. [DOI] [PubMed] [Google Scholar]
- 143.Wang LI, Giovannucci EL, Hunter D, Neuberg D, Su L, Christiani DC. Cancer Causes Control. 2004;15:977–985. doi: 10.1007/s10552-004-1093-1. [DOI] [PubMed] [Google Scholar]
- 144.Steck SE, Gaudet MM, Britton JA, Teitelbaum SL, Terry MB, Neugut AI, Santella RM, Gammon MD. Carcinogenesis. 2007;28:1954–1959. doi: 10.1093/carcin/bgm141. [DOI] [PubMed] [Google Scholar]
- 145.Steck SE, Gammon MD, Hebert JR, Wall DE, Zeisel SH. J Nutr. 2007;137:904–909. doi: 10.1093/jn/137.4.904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Munday R, Mhawech-Fauceglia P, Munday CM, Paonessa JD, Tang L, Munday JS, Lister C, Wilson P, Fahey JW, Davis W, Zhang Y. Cancer Res. 2008;68:1593–1600. doi: 10.1158/0008-5472.CAN-07-5009. [DOI] [PubMed] [Google Scholar]
- 147.Brabban AD, Edwards C. FEMS Microbiol Lett. 1994;119:83–88. doi: 10.1111/j.1574-6968.1994.tb06871.x. [DOI] [PubMed] [Google Scholar]
- 148.Rabot S, Guerin C, Nugon-Boudon L, Szylit O. Proc 9th Int Rapeseed Congress; 1995. pp. 212–214. [Google Scholar]
- 149.Elfoul L, Rabot S, Khelifa N, Quinsac A, Duguay A, Rimbault A. FEMS Microbiol Lett. 2001;197:99–103. doi: 10.1111/j.1574-6968.2001.tb10589.x. [DOI] [PubMed] [Google Scholar]
- 150.Cheng DL, Hashimoto K, Uda Y. Food Chem Toxicol. 2004;42:351–357. doi: 10.1016/j.fct.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 151.Shapiro TA, Fahey JW, Wade KL, Stephenson KK, Talalay P. Cancer Epidemiol Biomarkers Prev. 2001;10:501–508. [PubMed] [Google Scholar]
- 152.Getahun SM, Chung FL. Cancer Epidemiol Biomarkers Prev. 1999;8:447–451. [PubMed] [Google Scholar]
- 153.Conaway CC, Getahun SM, Liebes LL, Pusateri DJ, Topham DKW, Botero-Omary M, Chung FL. Nutr Cancer. 2000;38:168–178. doi: 10.1207/S15327914NC382_5. [DOI] [PubMed] [Google Scholar]
- 154.Rouzaud G, Young SA, Duncan AJ. Cancer Epidemiol Biomarkers Prev. 2004;13:125–131. doi: 10.1158/1055-9965.epi-085-3. [DOI] [PubMed] [Google Scholar]
- 155.Li F, Hullar MA, Beresford SA, Lampe JW. Br J Nutr. 2011;106:408–416. doi: 10.1017/S0007114511000274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Li F, Hullar MA, Schwarz Y, Lampe JW. J Nutr. 2009;139:1685–1691. doi: 10.3945/jn.109.108191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Tiedink HG, de Haan LH, Jongen WM, Koeman JH. Cell Biol Toxicol. 1991;7:371–386. doi: 10.1007/BF00124072. [DOI] [PubMed] [Google Scholar]
- 158.Ludikhuyze L, Rodrigo L, Hendrickx M. J Food Prot. 2000;63:400–403. doi: 10.4315/0362-028x-63.3.400. [DOI] [PubMed] [Google Scholar]
- 159.Traka M, Gasper AV, Melchini A, Bacon JR, Needs PW, Frost V, Chantry A, Jones AM, Ortori CA, Barrett DA, Ball RY, Mills RD, Mithen RF. PLoS One. 2008;3:e2568. doi: 10.1371/journal.pone.0002568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Brauer HA, Libby TE, Mitchell BL, Li L, Chen C, Randolph TW, Yasui YY, Lampe JW, Lampe PD. Nutr J. 2011;10:11. doi: 10.1186/1475-2891-10-11. [DOI] [PMC free article] [PubMed] [Google Scholar]