Abstract
The identification of resident stem cells in the mouse gallbladder is to date, unexplored. In addition, the relationship between adult gallbladder stem cells and intrahepatic bile duct (IHBD) cells is not well understood. The goal of this study was to isolate stem cells from an adult mouse gallbladder and determine if they were unique compared to IHBD cells. By limiting dilution analyses and index sorts, we found that an EpCAM+CD49fhi sub-population from primary gallbladder is enriched in colony forming cells compared to EpCAM+CD49flo cells. EpCAM+CD49fhi cells expressed CD29, CD133 and Sca1 but were negative for lineage markers CD31, CD45 and F4/80. Using a novel feeder cell culture system, we observed long-term (>passage 20) and clonal expansion of the EpCAM+CD49fhi cells in vitro. In a matrigel differentiation assay, EpCAM+CD49f+ cells expanding in vitro underwent organotypic morphogenesis forming ductular structures and cysts. These structures are similar to, and recapitulate a transport function of primary gallbladder. EpCAM+CD49f+ cells also engraft into the subcutaneous space of recipient mice. We compared primary gallbladder and IHBD cells by flow cytometry and found phenotypic differences in expression of CD49f, CD49e, CD81, CD26, CD54 and CD166. In addition, oligonucleotide microarrays showed that the expanded EpCAM+CD49f+ gallbladder cells and IHBD cells exhibit differences related to lipid and drug metabolism. Notable genes that were different are cytochrome P450, glutathione-S-transferase, Indian hedgehog and solute carrier family genes.
Conclusion
we have isolated an epithelial cell population from primary mouse gallbladder with stem cell characteristics and found it to be unique compared to IHBD cells.
Keywords: Intrahepatic bile duct cells, EpCAM, CD49f, biliary cells, gallbladder cells
Understanding the resident stem cell populations of the biliary system has great importance for basic biology and biliary diseases. The biliary tree is divided into the intrahepatic and extrahepatic biliary systems. The latter consists of the gallbladder, cystic duct, and the common bile duct (1). The biliary system is a conduit for bile to be transported from the liver to the intestine. The gallbladder in turn stores the bile and regulates its content and concentration, playing an important role in the digestive process (2, 3).
While there has been a lot of recent interest in the liver stem cell field (4) there is still a paucity of data regarding gallbladder stem cells. The biliary system, hepatocytes and ventral pancreas develop from the ventral foregut endoderm (5, 6). Histological evidence suggesting that both intra- and extrahepatic systems originate from the hepatic diverticulum has led to the hypothesis that they descend from the same progenitor cell. However, the cell-intrinsic factors that result in their specification have heretofore been unclear. Recently, it has been shown that the progenitor cells that give rise to each system separate out during development (7). Using a Pdx1-Cre mouse Spence et al. (7) demonstrated that the hepatocytes and intrahepatic bile duct (IHBD) cells derive from Pdx1- cells while the extrahepatic bile duct (EHBD) cells and ventral pancreas derive from Pdx1+ cells. Sox17 controls the specification of the EHBD and pancreatic cells. Sox17 loss-of-function embryos exhibit gallbladder agenesis and the presence of ectopic pancreatic tissue in the extrahepatic bile duct. Conversely Sox17 gain-of-function results in ectopic ductal tissue in the developing pancreas. In both cases, the intrahepatic system is not affected. It appears that the IHBD and EHBD cells descend from separate progenitor cells governed by separate transcriptional cascades. It is therefore possible that adult IHBD and EHBD cells could be distinct as well.
The aims of this study were to isolate and characterize stem cells from the adult mouse gallbladder and compare their phenotypic and expression profiles with IHBD cells. In addition to basic biology, an understanding of gallbladder stem cells would be vital to the study of gallbladder carcinoma, a rare but poorly understand malignancy (8) and congenital diseases involving biliary dysmorphogenesis, such as biliary atresia (9). It would also elucidate the ontogeny of cells in the biliary system.
Stem cells are defined as undifferentiated cells that can self-renew at the single cell level and form lineage committed progeny (10). In this study, we use colony forming and single cell assays along with a morphogenesis assay to characterize an EpCAM+CD49fhi epithelial sub-population within primary mouse gallbladder that has stem cell characteristics. The gallbladder stem cells can be propagated in vitro through long-term passage (>passage 20) and can engraft in the subcutaneous space of recipient mice. Last the gallbladder stem cells and IHBD cells have distinct expression profiles. These data represent one of the first reports to isolate and characterize the resident stem cell population in the adult mouse gallbladder.
Material and Methods
Gallbladder cell isolation and culture
Gallbladder cells were isolated from C57BL/6-Tg (UBC-GFP) 30Scha/J mice (Jackson Laboratory, ME). For further details see Supplementary Methods.
Fluorescence-Activated Cell Sorting (FACS) Analysis
Single cell suspensions were stained with appropriate antibodies (Supplementary Table 1) at 1e6 cells/tube and analyzed on the BD FACSCanto or BD FACSAriaII. For further details see Supplementary Materials.
Oligonucleotide Microarrays
Expanded gallbladder and IHBD cells were stained with EpCAM-Biotin and eluted through two sequential MS MACS® separation columns (Supplementary Figure 1). For further details see Supplementary Materials.
Results
EpCAM is a gallbladder epithelial marker
Gallbladder cells were isolated from GFP donor mice and the epithelial cells separated by flow cytometry. EpCAM, an epithelial surface marker, is expressed on simple epithelial cells such as keratinocytes and thymic epithelial cells (11) as well as on IHBD cells but not hepatocytes, mesenchymal or hematopoietic cells (12). Analysis of mouse gallbladder showed that most epithelial cells are EpCAM+ (Fig. 1A). No expression was detected on the mesenchymal cells. To confirm epithelial identity, we performed co-localization studies with EpCAM and CK19, a pan biliary marker (13). Epifluorescence and confocal microscopy performed on acetone-fixed sections show that most CK19+ cells were EpCAM+ (Fig. 1B). Therefore, EpCAM marks most gallbladder epithelial cells.
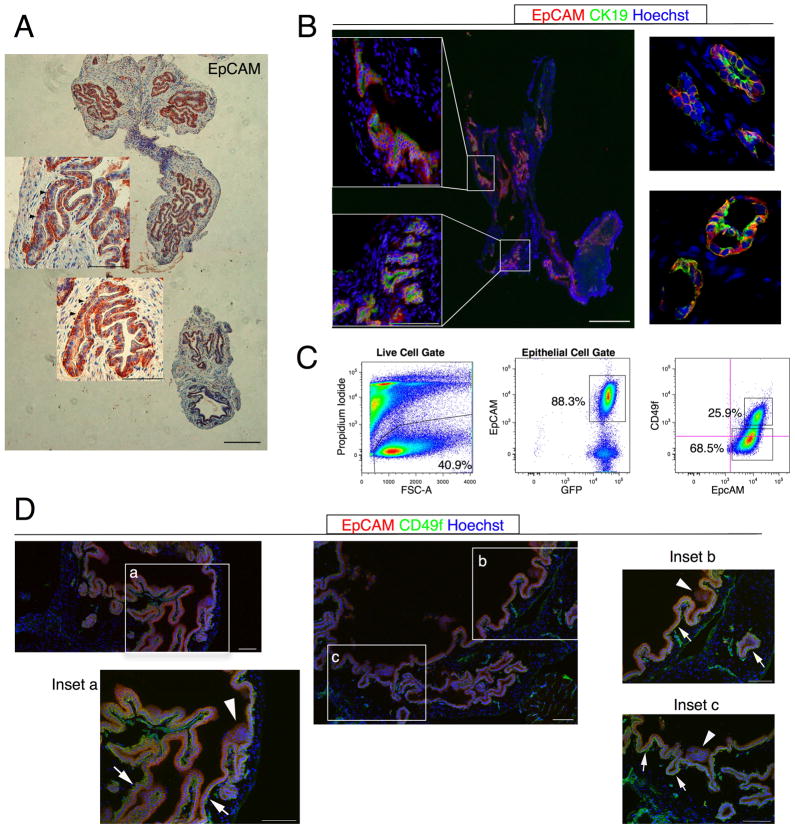
Figure 1. CD49f is heterogeneous in primary gallbladder epithelium.
(A) Paraffin sections of mouse gallbladder were stained with EpCAM. EpCAM stained only gallbladder epithelial cells. Basolateral membranes of epithelial cells were EpCAM+ (arrowheads). (B) Acetone-fixed sections were stained with CK19 and EpCAM. Epifluorescent (left panel) and confocal (right panel) micrographs indicate that CK19 co-stains with EpCAM. (C) Flow cytometric analyses on primary gallbladder indicate CD49f heterogeneity (labeled percentages are frequencies of parent population). Crosshairs on the flow plot indicate autofluorescence of unstained cells determined by the control population. (D) Confocal micrographs of acetone-fixed sections stained with EpCAM and CD49f. White arrows: EpCAM+CD49fhi cells. White arrowheads: EpCAM+CD49flo cells. Scale bars: 100 μm.
CD49f is heterogeneously expressed on Primary Gallbladder epithelial cells
Since there is a paucity of cell surface markers for gallbladder cells, we began screening primary gallbladder for general markers of stem and progenitor cells (Supplementary Table 1). Of the 38 markers we considered, 3 markers - CD49f, DBA and Sca1- were heterogeneously expressed on primary gallbladder epithelial cells (Fig. 1C and Supplementary Figure 2). However, we were only able to separate functionally distinct populations - EpCAM+CD49fhi and EpCAM+CD49flo- with CD49f. Function in this case is defined by a colony forming assay (see below). Heterogeneous expression of CD49f was confirmed by immunohistochemistry (Fig. 1D). Various reports have identified CD49f, integrin α-6, as a stem cell marker in fetal and adult liver (14–16) and other ductal epithelial tissue such as the breast (17, 18). EpCAM+CD49fhi cells expressed markers associated with epithelial stem cells such as CD29, CD133 and Sca1, but not mesenchymal or hematopoietic markers CD31, CD45 and F4/80 (Supplementary Table 1). These data led us to hypothesize that CD49f is a candidate gallbladder stem cell marker.
Gallbladder cells expanded in vitro are CD49f+
Gallbladder cells were cultured in vitro in conditions that select for epithelial cell growth (19). Briefly, total cell isolate from primary gallbladder was plated on irradiated rat mammary tumor cell line LA7 that served as feeder cells. Transmission Electron Microscopy (TEM) and flow cytometric analyses indicated that there was no fusion between the gallbladder and feeder cells (Supplementary Figure 3). As stem cells have the capacity for self-renewal, we predicted that expansion in vitro would enrich for primitive or stem cells. Flow cytometry analyses of cells after one expansion (p0) showed that only epithelial cells (EpCAM+) expand on the feeders (Fig. 2A). EpCAM- cells that were sorted from primary gallbladder did not proliferate (data not shown). Importantly, we found that all gallbladder cells at p0 were CD49f+ (Fig. 2B) supporting the notion that in vitro expansion selects for EpCAM+CD49f+ primitive epithelial cells.
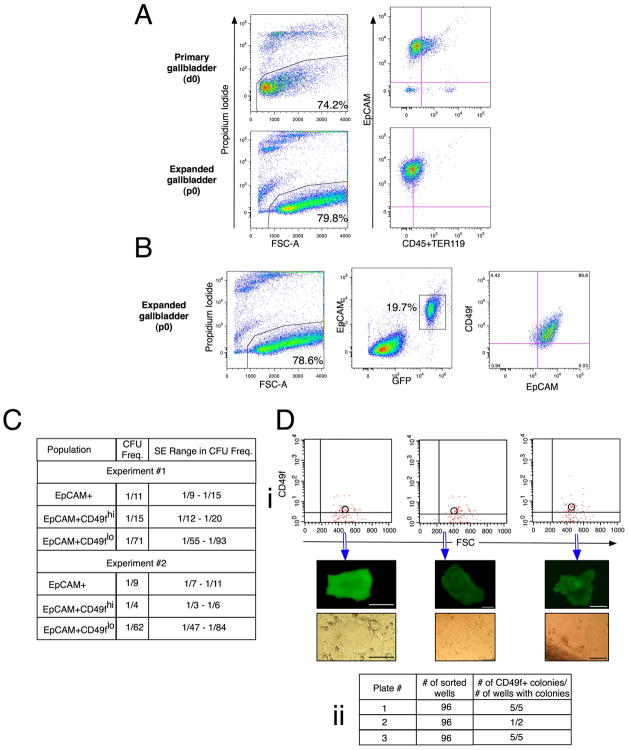
Figure 2. CD49f expression enriches for gallbladder stem cells.
Gallbladders (n≥5) from male and female mice were pooled and analyzed or sorted. (A) Flow cytometric analyses on primary and expanded mouse gallbladder cells show that only epithelial cells expand in the LA7 culture system. d0: primary cells; p0: cells at first expansion. Feeder cells (EpCAM-GFP−) have been gated out of EpCAM vs. CD45/TER119 plot for the cells at first expansion. (B) Flow cytometric analyses of expanded gallbladder cells (p0) show that all EpCAM+ cells are CD49f+. LA7 feeder cells are the EpCAM-GFP− cells in middle plot. (C) Limiting Dilution Analyses were carried out on EpCAM+CD49fhi and EpCAM+CD49lo cells from primary gallbladder. The Colony Forming Unit (CFU) frequency±SE (L-Calc®) indicates that CD49f enriches for stem cells. Pearson’s χ2 statistic was calculated on the 49fhi and 49flo groups by ELDA. Exp#1: χ2:17.8; p-value: 2.43e-5. Exp#2: χ2:42.9; p-value: 5.79e-11. Crosshairs on the flow plot in figures A to C indicate where the control population was present in the lower left hand corner. (D) Index sorts from primary gallbladder. Single EpCAM+ cells were sorted into each well of 3 96-well plates. (i) Data from 3 representative wells that grew showing CD49f expression and subsequent colony morphology. (ii) Table showing index sort results. 11/12 (91.7%) of the wells that grew originated from CD49fhi cells. Scale bars: 100 μm.
CD49f enriches for gallbladder stem cells
To evaluate CD49f as a gallbladder stem cell marker, we performed Limiting Dilution Analyses (LDAs) and Index Sorts. The LDA quantifies the frequency of a specific sub-population of cells with a biological activity (20) and was key to the isolation of hematopoietic (21) and neural (22) stem cells. In the evaluation of stem cells, biological activity is typically defined as the ability to form a colony and the LDA serves to quantify stem and progenitor cells. We separated EpCAM+CD49fhi and EpCAM+CD49flo cells from primary gallbladder and performed LDAs. EpCAM+CD49fhi cells exhibited a significantly higher enrichment in colony forming unit (CFU) frequency (ranging from 1/15 to 1/4) compared to EpCAM+CD49flo cells (1/71 to 1/62) (Fig. 2C). χ2 tests confirmed that the ranges in CFU frequency±SE were significantly different between EpCAM+CD49fhi and EpCAM+CD49flo cells (p<0.001).
We then performed index sorts to confirm these data. An index sort records the phenotype and well number of each deposited cell during a single cell sort. In this manner, the specific surface marker profile of cells that form colonies can be determined retrospectively. In our experiment, 288 single EpCAM+ cells were sorted and the CD49f profile of the each sorted cell was recorded. Retrospective analyses indicated that 11/12 (91.7%) of the colonies that formed originated from CD49fhi cells (Fig. 2D). These data together with the LDA results definitively demonstrate that CD49f enriches for candidate gallbladder stem cells.
Expanded Gallbladder cells Exhibit Morphological Heterogeneity
We observed the formation of two distinct types of colonies in EpCAM+CD49f+ gallbladder cultures at p0. The first type consisted of large colonies with an undifferentiated phenotype comprising small cells with a large nuclear-cytoplasmic ratio (red arrowheads, Fig 3A; Fig. 3B). We termed these the “flat colonies”. The second type was smaller more organized colonies called “glandular colonies” with an organotypic phenotype consisting of cells organized around a lumen (white arrowheads, Fig 3A; Fig 3B). Flat colonies were more numerous than glandular ones. TEM on the flat colonies revealed a single layer of cuboidal epithelial cells (Fig 3C). These cells have defined apical-basolateral polarity, apical microvilli and appear to secrete basement membrane at their basolateral surface. They also have interdigitating lateral membranes and junctional apparatus typical of gallbladder epithelial cells.
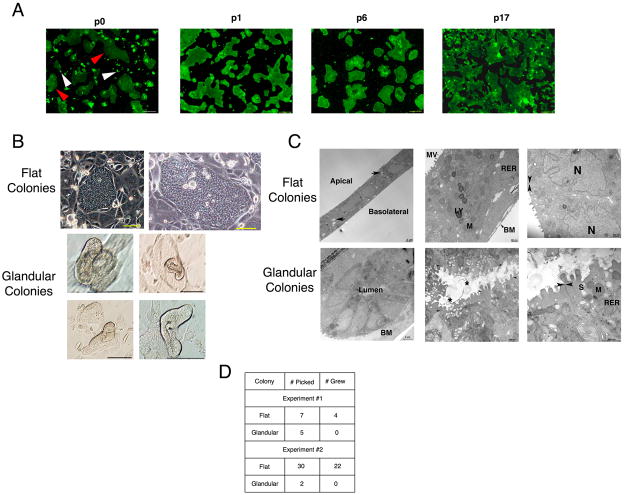
Figure 3. EpCAM+CD49f+ gallbladder cells exhibit morphological heterogeneity in vitro.
(A) At first passage (p0) in vitro, gallbladder cells exhibit two distinct morphologies. Flat colonies (red arrowheads) and glandular colonies (arrowheads) are observed. Only flat colonies are observed at later passages (p1, p6, p17). (B and C) Light and electron micrographs showing flat colonies and glandular colonies. Flat colonies consist of cuboidal cells with defined apical to basolateral polarity, show putative fluid transport (arrows) and have junctional apparatus typical of gallbladder epithelial cells. Glandular colonies consist of columnar cells organized around a central lumen. Secreted product (*) is observed in the lumen. (D) To determine if glandular colonies can expand past first passage, colonies were picked from first expansion (p0) culture. Only flat colonies expand past p0. MV: Microvilli, RER: Rough Endoplasmic Reticulum, LY: Lysosome, M: Mitochondria, S: Secretory granule, N: Nucleus, BM: Basement membrane. Arrowheads: Tight Junctions. * Glycocalyceal substance. Unless specified otherwise, scale bars: 100 μm.
Conversely, the glandular colonies consist of columnar epithelial cells organized around a central lumen (Fig. 3C) and exhibit junctional apparatus. Unlike flat colonies, numerous secretory granules are seen in their apical cytoplasm and secretory products are present in their lumen (Fig 3C). The flat and glandular colonies are distinct by morphology and ultrastructure. Importantly, only the flat colonies are observed at late passages (Fig. 3A), indicating that the glandular colonies are not capable of long-term self-renewal (>p3). To test this hypothesis, we passaged single colonies from p0 cultures. None of the glandular colonies could be successfully re-passaged (Fig 3D). This suggests that serial passage of the gallbladder cells past the first expansion enriches for EpCAM+CD49f+ cells that form flat colonies. As we found no additional markers to further purify gallbladder stem cells, we hypothesized that the cells past the first expansion are candidate stem cells. To determine their stemness, we tested whether the expanded EpCAM+CD49f+ gallbladder cells could satisfy the stem cell criteria of clonogenic self-renewal and lineage commitment.
EpCAM+CD49f+ cells differentiate into gallbladder-like structures in vitro
We developed a novel in vitro differentiation assay by utilizing the basement membrane extracellular matrix Matrigel. Matrigel has been shown to promote or maintain the differentiation or 3D morphogenesis of numerous cell lines and primary cells, including hepatocytes and IHBD cells (23–25). In our assay, expanded EpCAM+CD49f+ gallbladder cells (>p1) were mixed with serum-free media and layered above with matrigel (Fig. 4A). Within one week, we noticed the formation of two distinct morphogenetic structures – ductular structures that adhered to the plastic (Fig. 4B) and cysts that were suspended in the matrigel (Fig. 4C). Both structures persisted for over six weeks in culture. The matrigel was removed from the plastic to confirm that the cysts were suspended in it. Similar morphogenesis was observed with primary gallbladder cells (data not shown).
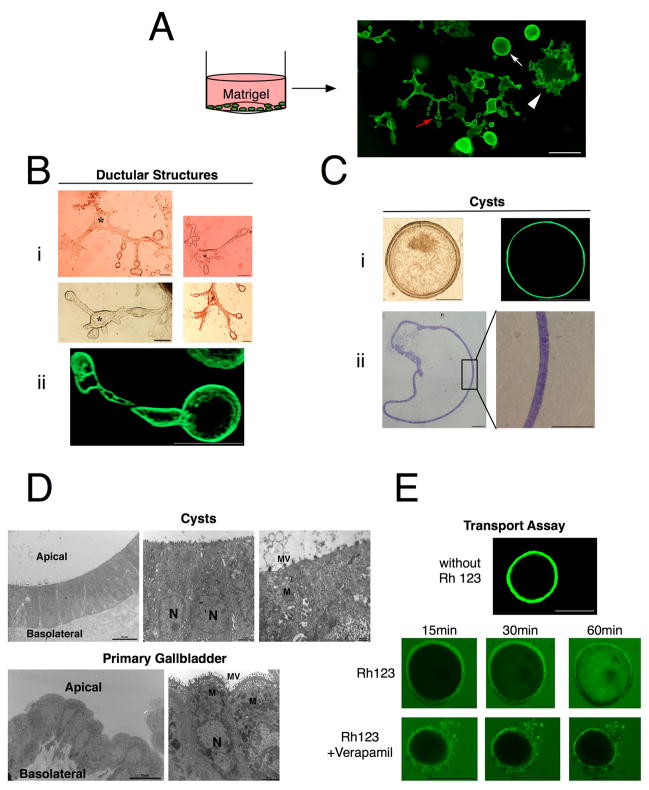
Figure 4. Expanded EpCAM+CD49f+ cells form in vitro gallbladders.
(A) Schematic of in vitro differentiation assay. EpCAM+CD49f+ gallbladder cells (>p1) were plated on tissue culture treated dishes in serum-free media and layered on top with matrigel. Two different forms of morphogenesis were observed: ductular structures (red arrow) and cysts (white arrow). Also colonies of cells were observed growing on the plastic (white arrowhead). (B) Ductular structures are luminal, consisting of long interconnecting ducts lined by a single or double layer of cells. (i) Light micrographs showing interconnecting ducts. * Lumen. (ii) Confocal micrograph of live ductular structure in situ in the matrigel. (C) Cysts similarly consist of a single or double layer of cells surrounding a hollow lumen. (i) Light and confocal micrographs of live cysts in situ in the matrigel, showing a hollow lumen lined by cells. (ii) Cysts were isolated from matrigel and stained with toluidine blue. (D) Electron micrographs of cysts (21 days post plating) and primary gallbladder indicating similar ultrastructure. Both consist of columnar epithelial cells with apical to basolateral polarity, basal nuclei, microvilli and junctional apparatus. (E) Cysts were incubated with 100 μM Rhodamine 123 (Rh 123) ± 10 μM Verapamil. Confocal micrographs showing time-lapse images of optical sections at indicated times. Rh 123 accumulates in lumen over 1 hr. Transport is blocked with Verapamil. MV: Microvilli, M: Mitochondria, N: Nucleus. Unless specified otherwise, scale bars: 100 μm.
The ductular structures consisted of ball shaped interconnecting ducts. Confocal microscopy on ductular structures in matrigel showed that they are hollow (Fig. 4Bii). The cysts similarly consisted of the hollow ball shaped structures, but lack interconnecting ducts and had much larger lumen (Fig. 4C). They appeared early on in culture – around two or three days post plating – and expanded over time (Supplementary Figure 4). TEM studies show that the cysts exhibit similar ultrastructure as primary mouse gallbladder (Fig. 4D).
We then tested if the ductular structures and cysts represent two different morphogenetic programs. Ductular structures and cysts were separated and LDAs were performed where the cells were sorted back into matrigel or on the LA7 feeder cells. When sorted back into matrigel, ductular structures could re-form ductular structures and cysts, and cysts were able to re-form both structures as well (data not shown). In addition, both expanded equally well on the feeders and no differences in LDA were observed (data not shown). Last, we performed the same assay in borosilicate dishes that inhibit cell attachment. We found that only cysts formed, which when passaged, could form both cysts and ductular structures. Therefore, ductular structures and cysts do not represent separate morphogenetic programs. Their appearance might be a function more of their microenvironment – attached to plastic vs. suspended in the matrigel – than intrinsic differences.
The physiological function of the gallbladder is to concentrate the bile and regulate its content by secretory processes (2, 3). These functions are in part due to multidrug resistance (MDR) proteins. Rhodamine 123, an MDR substrate, has been shown to accumulate in the lumen of cysts formed by a hepatic progenitor cell line grown in matrigel (23). We reasoned that such a transport assay would also be indicative of function for the gallbladder cells. Rhodamine 123 was added to the media of the matrigel cultures and confocal images were taken at various time points. We observed the steady accumulation of the dye in the lumen of the cysts over the course of 1 hour (Fig. 4E). This transport was blocked by addition of Verapamil, an MDR inhibitor. These data indicate that the cysts transport the dye from their basal side into the lumen, thereby recapitulating a transport function of the gallbladder.
EpCAM+CD49f+ gallbladder cells can self-renew and differentiate from single cells
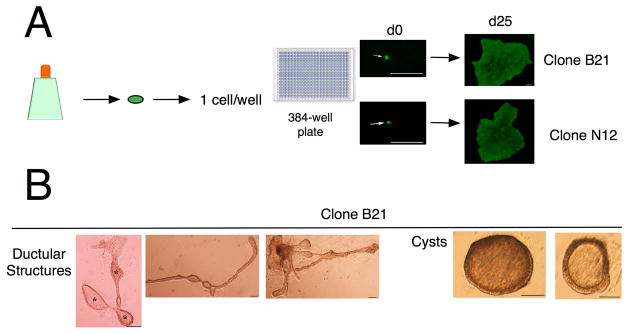
In order to test clonogenic self-renewal of EpCAM+CD49f+ gallbladder cells, we sorted single cells into 384-well plates seeded with LA7 feeders and imaged every well to confirm the presence of the single cell (Fig. 5A). In this manner, 5 clonal gallbladder cultures were generated. Further analyses were carried out with Clone B21 and Clone N12 (Fig. 5A). Surface marker analyses indicated that both Clone B21 and N12 have the same phenotype as the parent gallbladder cell population (Supplementary Table 1) suggesting that the clones are able to recapitulate the heterogeneity of the parent. In addition, both Clone B21 and N12 exhibit the same morphogenesis in matrigel as the parent (Fig. 5B).
Figure 5. Expanded EpCAM+CD49f+ cells undergo clonogenic self-renewal.
(A) Schematic of single-cell clonogenic assay. EpCAM+CD49f+ gallbladder cells at first passage (p0) were sorted into 384-well plates at one cell/well. In this manner, 8 clones were generated. Representative images of CloneB21 and CloneN12 are depicted. (B) Clonal gallbladder cultures were expanded further and differentiation assays were carried out. Clones exhibit similar morphogenesis compared to parent cultures. * Lumen, Scale bars: 100 μm.
Expanded EpCAM+CD49f+ cells can engraft in vivo
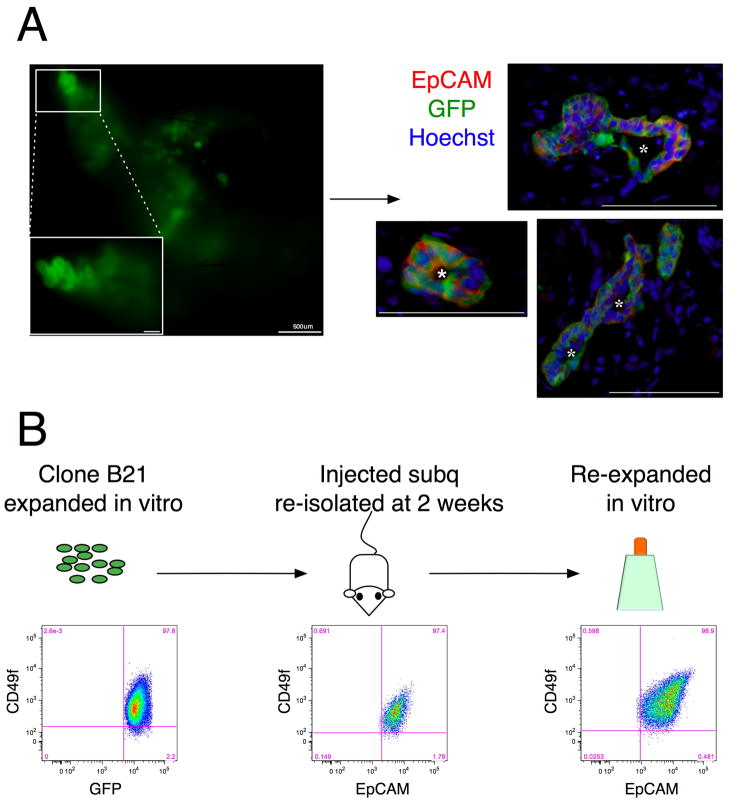
We then determined if the expanded EpCAM+CD49f+ cells could survive and engraft in vivo. An ideal location for engraftment would be the native gallbladder. However, as there currently are no protocols that allow for the injection and maintenance of cells in the gallbladder, we attempted engraftment at an ectopic location. Okumura et al. (26) have reported the long-term engraftment of in vitro explants of human gallbladder in the subcutaneous space of athymic nude mice. We injected the expanded EpCAM+CD49f+ cells mixed with matrigel into the subcutaneous neck region of immunodeficient mice. We observed the formation of cyst-like structures in the subcutaneous space one week post-injection (Fig. 6A). These cysts consisted of cells organized around a central lumen. Seven out of seven (100%) mice injected formed cysts. However, engraftment was short term. Only one out of three (33%) mice exhibited cyst formation at two weeks. Similar results were obtained with clonal cultures.
Figure 6. Expanded EpCAM+CD49f+ cells engraft in vivo.
Parent or clonal gallbladder cultures were mixed with matrigel and injected into the subcutaneous (subq) space of Rag2−/−γC−/− mice. Cysts were observed at 1 week and 2 weeks post injection. (A) (Left Panel) Macroscopic view of matrigel isolated at 1week from mouse injected with parent EpCAM+CD49f+ cells (right panel) Sections of same matrigel stained with EpCAM and GFP, showing that cysts are hollow and EpCAM+. Cysts were seen in at one week in 7/7 and 2/3 mice injected with parent and clonal cultures respectively. 1/5 and 1/3 mice respectively, exhibited engraftment at 2 weeks. (B) Clonal gallbladder cells remain EpCAM+CD49f+ in vivo. Expanded gallbladder cells from CloneB21 were injected subq. At 2 weeks, the matrigel was removed and cells re-isolated. Flow cytometric analysis shows that re-isolated cells are EpCAM+CD49f+. These cells were expanded in vitro on feeder cells. Flow cytometric analysis at p1 (second passage) showing that they remain EpCAM+CD49f+. Crosshairs on the flow plot in figures A to C indicate where the control population was present in the lower left hand corner. Debris and cell aggregates were gated out in each plot. In the middle plot, GFP-cells were gated out as well. *: Lumen. Unless specified otherwise, scale bars: 100 μm.
We then isolated the cells from the cysts in vivo two weeks post injection and cultured them in vitro to test their ability to reinitiate cultures with stem cell properties. Flow cytometry analyses showed that the cells isolated from cysts in vivo were EpCAM+CD49f+. These cells re-expanded in vitro on the feeder cells forming colonies that were morphologically identical to the parent and clonal cultures (data not shown) and remained EpCAM+CD49f+ (Fig. 6B). These data indicate that the expanded EpCAM+CD49f+ cells survive and engraft in vivo, while retaining their proliferative ability in vitro.
Gallbladder cells are unique compared to Intrahepatic bile duct cells
There is evidence indicating that intra- and extrahepatic bile duct cells develop separately (7). To date there are no reports of the molecular differences –if any- between IHBD and gallbladder cells. We first screened primary IHBD cells with the same antibody panel used for the primary gallbladder cells (Supplementary Table 1). Most IHBsD cells express EpCAM (12), and we used EpCAM expression to separate IHBD cells from other liver cells. Briefly, following liver perfusion of GFP+ mice, the high spin fraction was separated and used to isolate IHBD cells.
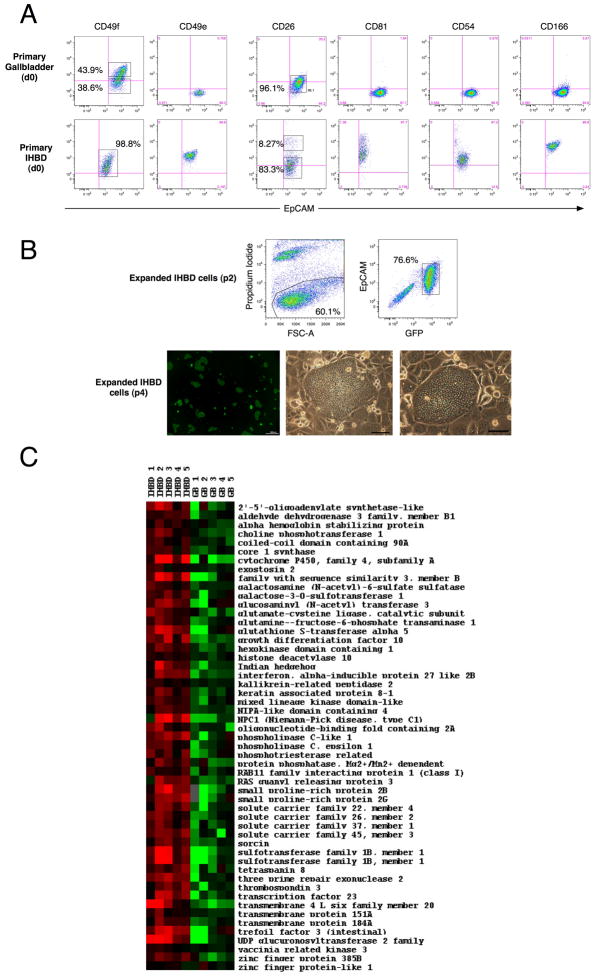
Interestingly, we found differences in integrin expression, including CD49f (Fig. 7A). Other notable markers that showed differences between IHBD and gallbladder cells were CD49e, CD81, CD54, CD26 and CD166 (Fig. 7A). We then determined if expanded gallbladder cells and IHBD cells were different. The IHBD cells are capable of expansion on the LA7 feeders and the feeder cells select for EpCAM+ cells (Fig. 7B). In addition, IHBD cells form flat colonies similar to the gallbladder cells. The phenotypic profiles of the IHBD and gallbladder cells converged in culture and we did not detect any differences using the foregoing panel of antibodies. Consequently, we used oligonucleotide microarrays to test for expression differences between the two cell types.
Figure 7. Expanded EpCAM+CD49f+ cells are unique compared to intra-hepatic biliary cells.
(A) Flow cytometric analyses of EpCAM+ (epithelial) cells from primary gallbladder and primary liver (see Methods). d0: primary cells. Phenotypic differences with select cell surface markers suggest that gallbladder and intrahepatic bile duct (IHBD) cells are different. Crosshairs on the flow plot in figures A to C indicate where the control population was present in the lower left hand corner. (B) IHBD cells expand on LA7 feeders and exhibit similar morphology to expanded gallbladder cells. (Upper panel) Flow cytometric analyses of expanded IHBD cells (p2) indicate that all GFP+ cells are EpCAM+. (Lower panel) Expanded IHBD cells exhibit flat colony appearance. (C) Heat map of 53 genes with known annotations and fold changes (≥2) differentially expressed between IHBD and gallbladder cells (Supplementary Table 3). Data were imported in Cluster and heat maps were generated with TreeView (http://rana.lbl.gov/EisenSoftware.htm). Black represents genes whose expression was at the mean intensity; red represents intensities that are greater than the mean; green represents intensities that are less than the mean. Unless specified otherwise, scale bars: 100μm.
We have shown that the expanded gallbladder cells or EpCAM+CD49f+ cells are capable of self-renewal and lineage commitment. It is possible that the expanded IHBD cells might satisfy these requirements as well. However, the evaluation of IHBD stem cells belongs to a different study and we focused on the differences in the transcriptomes of the expanded gallbladder and IHBD cells. Briefly, expanded gallbladder cells and IHBD cells were separated from LA7 feeder cells using magnetic activated cell sorting (Supplementary Figure 1). Differential gene expression between expanded gallbladder and IHBD cells (fold change ≥2) were calculated by Significance Analysis of Microarrays (SAMs) (27) using a false discovery rate of 10%. In this manner, we found 64 genes to be upregulated in IHBD cells (Fig. 7C) including those involving lipid metabolism (8 genes), stem cell proliferation (3 genes) and drug metabolism (2 genes) (Supplementary Table 2). Notable genes or groups of genes that were different were cytochrome P450, Indian hedgehog, glutathione-S-transferase and solute carrier families 22, 26, 37 and 45 (Supplementary Table 3). These differences indicate that the expanded gallbladder and IHBD cells have distinct transcriptomes and suggest functional differences as well.
Discussion
Little is known about the resident stem cells in gallbladder and the relationship between the stem cells of the hepato-biliary system. Our goal here was to identify and characterize stem cells in the adult mouse gallbladder. We found that an EpCAM+CD49ffhi sub-population from primary mouse gallbladder can expand from single cells, and exhibits morphogenesis in organotypic culture in vitro. Both parent and clonal cultures were capable of survival and short-term morphogenesis in an adapted in vivo assay. We therefore concluded that EpCAM+CD49ffhi gallbladder cells satisfy the stem cell criteria of clonogenic self-renewal and lineage commitment and represent a gallbladder stem cell population. Last we determined that gallbladder stem cells and IHBD cells expanded in vitro have distinct transcriptomes, suggesting that the cells of the IHBD and EHBD systems are different.
This study is the first to describe the identification and prospective isolation of stem cells from an uninjured mouse gallbladder. Previous reports of stem cells in the EHBD system have focused on injury models (28) or disease conditions such as biliary atresia (29, 30). Furthermore, these studies do not distinguish epithelial from non-epithelial cells in their isolation protocols. We used EpCAM to isolate gallbladder epithelial cells thereby preventing contamination by non-epithelial cells. This is especially important as we detect EpCAM-CD49f+ cells in primary gallbladder by both immunohistochemistry and flow cytometry. The isolation and subsequent expansion of EpCAM+CD49f+ cells thereby allows for the definitive identification of resident epithelial stem cells.
We confirmed CD49f as a gallbladder stem cell marker by LDAs and index sorts from primary gallbladder. EpCAM+CD49fhi cells have a significantly higher CFU readout relative to EpCAM+CD49flo cells. The low enrichment in CFU readout indicates that additional markers are required to further purify stem cells such that single cells can be isolated and expanded (31). Therefore, expression of EpCAM and CD49f enriches, but does not select for stem cells. All gallbladder epithelial cells expanded in vitro were EpCAM+CD49f+. However, these cells exhibited morphological heterogeneity at first expansion, forming flat and glandular colonies. Interestingly, none of the glandular colonies and only a fraction of the flat colonies were capable of serial passage. It appears that the EpCAM+CD49fhi population in primary gallbladder is itself heterogeneous with only a sub-population of cells capable of self-renewal. We could not identify any additional markers to select for this specific sub-population directly from primary tissue and therefore characterized the stemness of the EpCAM+CD49f+ cells expanded past p0. We determined that the expanded EpCAM+CD49f+ cells can self-renew clonogenically. However, defined protocols for gallbladder epithelial cell differentiation do not exist. In the past, researchers have used collagen gel sandwich culture to observe cyst morphogenesis with rabbit gallbladder epithelial cells (32, 33). The collagen gel is supplemented with exogenous growth factors such as EGF and TGFβ1. We postulate that our 3D culture system is similar to collagen gel culture in that matrigel is an appropriate growth factor containing extracellular matrix that supports morphogenesis. Cyst formation in our culture is similar in morphology and ultrastructure to that observed before (32, 33). We also observed dye transport that is reminiscent of a transport function of the gallbladder. In addition, we observed similar morphogenesis in vivo after transplantation. We chose an ectopic location, as engraftment in the native gallbladder would be technically challenging and the subcutaneous space has been shown to engraft human gallbladder cells (26). Lee et al. (34) have shown that gallbladder cells can engraft into the native liver of SCID mice. However, engraftment was significant only with tremendous injury to the liver (retrorsine and partial hepatectomy or carbon tetrachloride treatment) and required very large numbers of cells. For these reasons, we concluded that subcutaneous rather than liver engraftment would be a more apt in vivo assay. In our hands, the EpCAM+CD49f+ cells only engraft in the short term (2 weeks post transplantation). This short-term engraftment might be due to a lack of growth stimulus in the recipient. Also under physiological conditions, the rate of cell proliferation in the gallbladder epithelium is low (35). Future studies will determine if long-term engraftment of EpCAM+CD49f+ cells is possible. For now, these data conclusively show that parent and clonal EpCAM+CD49f+ cells can organize into organotypic structures that mimic the morphology, ultrastructure and function of the native gallbladder, both in vitro and in vivo.
Spence et al. (7) have recently showed that IHBD and EHBD cells develop from separate precursors. However, there are no reports describing their similarities or differences in the adult. We found that expression of CD49f, CD49e, CD81, CD26, CD54 and CD166 was different between primary IHBD and gallbladder cells. The goal of our experiment was to evaluate the differences if any between gallbladder stem cells and IHBD cells. Expanded EpCAM+CD49f+ gallbladder cells (>p0) represent a purer stem cell population than primary EpCAM+CD49fhi cells. The latter forms both flat and glandular colonies and only a fraction of the flat can self-renew. Therefore, we ran microarray analyses on expanded EpCAM+CD49f+ cells (>p1) and expanded IHBD cells. The major groups of differentially expressed genes were cytochrome P450 genes, glutathione-S-transferase and the solute carrier family genes. Also interferon (IFN)-inducible protein 27 was differentially expressed between gallbladder and IHBD cells. Interestingly, expression of CD54 is known to be immunologically mediated (36). The immunologic properties of bile duct cells have long been considered. They are the primary site of damage in inflammatory diseases such as primary biliary cirrhosis (37) and biliary atresia (9) and in liver allograft rejection (38). The differential expression of an IFN-inducible protein and CD54 indicates that the immunologic properties of IHBD and gallbladder cells could be different. Studies of IHBD cells are hindered by a technical inability to isolate and expand them from primary tissue (2, 39). We circumvented this hurdle by using LA7 feeder cells that allow for a robust expansion of IHBD epithelial (EpCAM+) cells. This expansion assay along with the 3D matrigel assay could serve as interchangeable and technically easy tools to study bile duct cells. In all, the complete elucidation of the differences between the IHBD and gallbladder cells belongs to another study, as does the evaluation of the stem cell characteristics of the expanded IHBD cells. The focus of this manuscript was to isolate and characterize gallbladder stem cells.
We postulate that this study will have important clinical significance. Gallbladder stem cells could be used to treat biliary atresia as has been noted with hepatic progenitor cells (40). These cells could also be reprogrammed into hepatocytes or endocrine cells. There have been recent reports of the differentiation of gallbladder epithelial cells into hepatocytes (34, 41) and ectopic endocrine cells have been observed in the EHBD cells of Hes1−/− deficient mice (42). Gallbladder stem cells might be capable of such plasticity, which along with the ready availability of donor tissue would make them an attractive candidate for cell-based therapy.
Supplementary Material
(A) Representative flow data showing separation of gallbladder cells from feeder cells with two sequential MACS® separation columns (see Methods). Similar results were obtained with IHBD cells. (B) Data showing purity of gallbladder and IHBD cells after MACS separation. GB#1–4 represent replicates at different passages in vitro of a pool of mouse gallbladder. GB#5 represents a separate pool of mouse gallbladders. IHBD#1–4 represent replicates at different passages in vitro from a single mouse liver. IHBD#5 represents a different mouse liver.
Sca1 and DBA were screened on primary mouse gallbladder. Debris and cell aggregates were gated out (data not shown). Sca1 and DBA are heterogeneous in EpCAM+ cells from primary mouse gallbladder.
(A) Flow cytometric analyses of gallbladder cells (passage 2). RT1a is the Class I MHC antigen for the rat feeder cells. The middle and right plots are different representations of the live cells in the left plot. The data indicate two populations: GFP+RT1a− and GFP-RTa1+ or EpCAM+RT1a− and EpCAM-RT1a+. (B) Ultrastructual analysis of feeder cell. Feeder cells are large and multi-nucleated indicative of an irradiated cell and are distinct compared to gallbladder cells.
The morphogenesis assay was performed (see Methods) and images of cysts taken over the indicated times. Scale bars: 100 μm.
Primary, expanded and clonal gallbladder (GB) cells and primary IHBD cells were screened against the 38 antibodies in the panel. For primary GB and IHBD cells, primary cell isolates were co-stained with EpCAM to identify epithelial cells. For expanded cells, GFP expression was used to gate out feeder cells. Representative data for clones B21 and clones N12. +: positive expression; −: negative expression; Het: heterogeneous expression with negative/positive or high/low sub-populations; Low: weak yet distinct staining.
The 64 genes with fold changes greater than or equal to 2 were analyzed in Ingenuity Pathway Analysis (Ingenuity® Systems, www.ingenuity.com). The functional analysis identified the biological functions and/or diseases that were most significant to the dataset. Right-tailed Fisher’s exact test was used to calculate a p-value determining the probability that each biological function and/or disease assigned to that dataset is due to chance alone. In this analysis only p<0.0025 were considered.
SAMs were run on expanded gallbladder and IHBD cells at a false discovery rate of 10%. The fold changes as shown are calculated in the SAM software on log transformed data and are representative of IHBD/GB cells. A total of 267 genes were differentially expressed, of which 64 genes had fold changes greater than or equal to 2 and 203 genes had fold changes less than 2.
Acknowledgments
We would like to thank Mara Sullivan and Ming Sun for tissue processing for Electron Microscopy, Michael Burger and Christin Sciulli at the Clinical Genomics Facility for sample processing and initial data analysis, and Kelly Quesnelle for help with Ingenuity Pathway Analysis. We would also like to thank Aaron DeWard for editorial assistance and Drs. Michalopoulos, Fox, Orwig, Demetris and Strom for their valuable advice and input.
Financial Support: This work was supported in part by the Pittsburgh Tissue Engineering Initiative, the Commonwealth of Pennsylvania, and by National Institutes of Health grant R01 DK085711.
Abbreviations
- IHBD
Intrahepatic bile duct
- EHBD
Extrahepatic bile duct
- ITS
Insulin-Transferrin Selenium
- LDA
Limiting Dilution Analysis
- TEM
Transmission Electron Microscopy
- MDR
multidrug resistance protein
- SAM
Significance Analysis of Microarray
References
- 1.Strazzabosco M, Fabris L. Functional anatomy of normal bile ducts. Anat Rec (Hoboken) 2008;291:653–660. doi: 10.1002/ar.20664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nakanuma Y, Katayanagi K, Kawamura Y, Yoshida K. Monolayer and three-dimensional cell culture and living tissue culture of gallbladder epithelium. Microsc Res Tech. 1997;39:71–84. doi: 10.1002/(SICI)1097-0029(19971001)39:1<71::AID-JEMT6>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 3.Frizzell RA, Heintze K. Transport functions of the gallbladder. Int Rev Physiol. 1980;21:221–247. [PubMed] [Google Scholar]
- 4.Si-Tayeb K, Lemaigre FP, Duncan SA. Organogenesis and development of the liver. Dev Cell. 2010;18:175–189. doi: 10.1016/j.devcel.2010.01.011. [DOI] [PubMed] [Google Scholar]
- 5.Shiojiri N. Development and differentiation of bile ducts in the mammalian liver. Microsc Res Tech. 1997;39:328–335. doi: 10.1002/(SICI)1097-0029(19971115)39:4<328::AID-JEMT3>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 6.Zaret KS, Grompe M. Generation and regeneration of cells of the liver and pancreas. Science. 2008;322:1490–1494. doi: 10.1126/science.1161431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Spence JR, Lange AW, Lin SC, Kaestner KH, Lowy AM, Kim I, Whitsett JA, et al. Sox17 regulates organ lineage segregation of ventral foregut progenitor cells. Dev Cell. 2009;17:62–74. doi: 10.1016/j.devcel.2009.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miller G, Jarnagin WR. Gallbladder carcinoma. Eur J Surg Oncol. 2008;34:306–312. doi: 10.1016/j.ejso.2007.07.206. [DOI] [PubMed] [Google Scholar]
- 9.Bassett MD, Murray KF. Biliary atresia: recent progress. J Clin Gastroenterol. 2008;42:720–729. doi: 10.1097/MCG.0b013e3181646730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wagers AJ, Weissman IL. Plasticity of adult stem cells. Cell. 2004;116:639–648. doi: 10.1016/s0092-8674(04)00208-9. [DOI] [PubMed] [Google Scholar]
- 11.Trzpis M, McLaughlin PM, de Leij LM, Harmsen MC. Epithelial cell adhesion molecule: more than a carcinoma marker and adhesion molecule. Am J Pathol. 2007;171:386–395. doi: 10.2353/ajpath.2007.070152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Boer CJ, van Krieken JH, Janssen-van Rhijn CM, Litvinov SV. Expression of Ep-CAM in normal, regenerating, metaplastic, and neoplastic liver. J Pathol. 1999;188:201–206. doi: 10.1002/(SICI)1096-9896(199906)188:2<201::AID-PATH339>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 13.Moll R, Franke WW, Schiller DL, Geiger B, Krepler R. The catalog of human cytokeratins: patterns of expression in normal epithelia, tumors and cultured cells. Cell. 1982;31:11–24. doi: 10.1016/0092-8674(82)90400-7. [DOI] [PubMed] [Google Scholar]
- 14.Zheng YW, Taniguchi H. Diversity of hepatic stem cells in the fetal and adult liver. Semin Liver Dis. 2003;23:337–348. doi: 10.1055/s-2004-815557. [DOI] [PubMed] [Google Scholar]
- 15.Tsuchiya A, Heike T, Baba S, Fujino H, Umeda K, Matsuda Y, Nomoto M, et al. Long-term culture of postnatal mouse hepatic stem/progenitor cells and their relative developmental hierarchy. Stem Cells. 2007;25:895–902. doi: 10.1634/stemcells.2006-0558. [DOI] [PubMed] [Google Scholar]
- 16.Kamiya A, Kakinuma S, Yamazaki Y, Nakauchi H. Enrichment and clonal culture of progenitor cells during mouse postnatal liver development in mice. Gastroenterology. 2009;137:1114–1126. 1126, e1111–1114. doi: 10.1053/j.gastro.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 17.Stingl J, Eirew P, Ricketson I, Shackleton M, Vaillant F, Choi D, Li HI, et al. Purification and unique properties of mammary epithelial stem cells. Nature. 2006;439:993–997. doi: 10.1038/nature04496. [DOI] [PubMed] [Google Scholar]
- 18.Shackleton M, Vaillant F, Simpson KJ, Stingl J, Smyth GK, Asselin-Labat ML, Wu L, et al. Generation of a functional mammary gland from a single stem cell. Nature. 2006;439:84–88. doi: 10.1038/nature04372. [DOI] [PubMed] [Google Scholar]
- 19.Ehmann UK, Peterson WD, Jr, Misfeldt DS. To grow mouse mammary epithelial cells in culture. J Cell Biol. 1984;98:1026–1032. doi: 10.1083/jcb.98.3.1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fazekas de St G. The evaluation of limiting dilution assays. J Immunol Methods. 1982;49:R11–23. doi: 10.1016/0022-1759(82)90269-1. [DOI] [PubMed] [Google Scholar]
- 21.Baum CM, Weissman IL, Tsukamoto AS, Buckle AM, Peault B. Isolation of a candidate human hematopoietic stem-cell population. Proc Natl Acad Sci U S A. 1992;89:2804–2808. doi: 10.1073/pnas.89.7.2804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Uchida N, Buck DW, He D, Reitsma MJ, Masek M, Phan TV, Tsukamoto AS, et al. Direct isolation of human central nervous system stem cells. Proc Natl Acad Sci U S A. 2000;97:14720–14725. doi: 10.1073/pnas.97.26.14720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tanimizu N, Miyajima A, Mostov KE. Liver progenitor cells develop cholangiocyte-type epithelial polarity in three-dimensional culture. Mol Biol Cell. 2007;18:1472–1479. doi: 10.1091/mbc.E06-09-0848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kleinman HK, Martin GR. Matrigel: basement membrane matrix with biological activity. Semin Cancer Biol. 2005;15:378–386. doi: 10.1016/j.semcancer.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 25.Zegers MM, O’Brien LE, Yu W, Datta A, Mostov KE. Epithelial polarity and tubulogenesis in vitro. Trends Cell Biol. 2003;13:169–176. doi: 10.1016/s0962-8924(03)00036-9. [DOI] [PubMed] [Google Scholar]
- 26.Okumura H, Sakamoto K, Nakano G, Ikeda H, Nagashima K, Nagamachi Y, Yuasa Y. Long-term maintenance of human gall-bladder epithelia as xenografts following explant organ culture. Nippon Geka Gakkai Zasshi. 1988;89:388–393. [PubMed] [Google Scholar]
- 27.Tusher VG, Tibshirani R, Chu G. Significance analysis of microarrays applied to the ionizing radiation response. Proc Natl Acad Sci U S A. 2001;98:5116–5121. doi: 10.1073/pnas.091062498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Irie T, Asahina K, Shimizu-Saito K, Teramoto K, Arii S, Teraoka H. Hepatic progenitor cells in the mouse extrahepatic bile duct after a bile duct ligation. Stem Cells Dev. 2007;16:979–987. doi: 10.1089/scd.2007.0037. [DOI] [PubMed] [Google Scholar]
- 29.Baumann U, Crosby HA, Ramani P, Kelly DA, Strain AJ. Expression of the stem cell factor receptor c-kit in normal and diseased pediatric liver: identification of a human hepatic progenitor cell? Hepatology. 1999;30:112–117. doi: 10.1002/hep.510300140. [DOI] [PubMed] [Google Scholar]
- 30.Xiao JC, Ruck P, Kaiserling E. Small epithelial cells in extrahepatic biliary atresia: electron microscopic and immunoelectron microscopic findings suggest a close relationship to liver progenitor cells. Histopathology. 1999;35:454–460. doi: 10.1046/j.1365-2559.1999.035005454.x. [DOI] [PubMed] [Google Scholar]
- 31.Christ O, Lucke K, Imren S, Leung K, Hamilton M, Eaves A, Smith C, et al. Improved purification of hematopoietic stem cells based on their elevated aldehyde dehydrogenase activity. Haematologica. 2007;92:1165–1172. doi: 10.3324/haematol.11366. [DOI] [PubMed] [Google Scholar]
- 32.Kawamura Y, Yoshida K, Nakanuma Y. Primary culture of rabbit gallbladder epithelial cells in collagen gel matrix. Lab Invest. 1989;61:350–356. [PubMed] [Google Scholar]
- 33.Mori M, Miyazaki K. Factors affecting morphogenesis of rabbit gallbladder epithelial cells cultured in collagen gels. Cell Tissue Res. 2000;300:331–344. doi: 10.1007/s004410000205. [DOI] [PubMed] [Google Scholar]
- 34.Lee SP, Savard CE, Kuver R. Gallbladder epithelial cells that engraft in mouse liver can differentiate into hepatocyte-like cells. Am J Pathol. 2009;174:842–853. doi: 10.2353/ajpath.2009.080262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lamote J, Willems G. DNA synthesis, cell proliferation index in normal and abnormal gallbladder epithelium. Microsc Res Tech. 1997;38:609–615. doi: 10.1002/(SICI)1097-0029(19970915)38:6<609::AID-JEMT5>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 36.Auth MK, Keitzer RA, Scholz M, Blaheta RA, Hottenrott EC, Herrmann G, Encke A, et al. Establishment and immunological characterization of cultured human gallbladder epithelial cells. Hepatology. 1993;18:546–555. [PubMed] [Google Scholar]
- 37.Hirschfield GM, Heathcote EJ, Gershwin ME. Pathogenesis of cholestatic liver disease and therapeutic approaches. Gastroenterology. 2010;139:1481–1496. doi: 10.1053/j.gastro.2010.09.004. [DOI] [PubMed] [Google Scholar]
- 38.Nakanuma Y, Tsuneyama K, Harada K. Pathology and pathogenesis of intrahepatic bile duct loss. J Hepatobiliary Pancreat Surg. 2001;8:303–315. doi: 10.1007/s005340170002. [DOI] [PubMed] [Google Scholar]
- 39.Reynoso-Paz S, Coppel RL, Mackay IR, Bass NM, Ansari AA, Gershwin ME. The immunobiology of bile and biliary epithelium. Hepatology. 1999;30:351–357. doi: 10.1002/hep.510300218. [DOI] [PubMed] [Google Scholar]
- 40.Khan AA, Parveen N, Mahaboob VS, Rajendraprasad A, Ravindraprakash HR, Venkateswarlu J, Rao P, et al. Management of hyperbilirubinemia in biliary atresia by hepatic progenitor cell transplantation through hepatic artery: a case report. Transplant Proc. 2008;40:1153–1155. doi: 10.1016/j.transproceed.2008.03.110. [DOI] [PubMed] [Google Scholar]
- 41.Kuver R, Savard CE, Lee SK, Haigh WG, Lee SP. Murine gallbladder epithelial cells can differentiate into hepatocyte-like cells in vitro. Am J Physiol Gastrointest Liver Physiol. 2007;293:G944–955. doi: 10.1152/ajpgi.00263.2006. [DOI] [PubMed] [Google Scholar]
- 42.Sumazaki R, Shiojiri N, Isoyama S, Masu M, Keino-Masu K, Osawa M, Nakauchi H, et al. Conversion of biliary system to pancreatic tissue in Hes1-deficient mice. Nat Genet. 2004;36:83–87. doi: 10.1038/ng1273. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) Representative flow data showing separation of gallbladder cells from feeder cells with two sequential MACS® separation columns (see Methods). Similar results were obtained with IHBD cells. (B) Data showing purity of gallbladder and IHBD cells after MACS separation. GB#1–4 represent replicates at different passages in vitro of a pool of mouse gallbladder. GB#5 represents a separate pool of mouse gallbladders. IHBD#1–4 represent replicates at different passages in vitro from a single mouse liver. IHBD#5 represents a different mouse liver.
Sca1 and DBA were screened on primary mouse gallbladder. Debris and cell aggregates were gated out (data not shown). Sca1 and DBA are heterogeneous in EpCAM+ cells from primary mouse gallbladder.
(A) Flow cytometric analyses of gallbladder cells (passage 2). RT1a is the Class I MHC antigen for the rat feeder cells. The middle and right plots are different representations of the live cells in the left plot. The data indicate two populations: GFP+RT1a− and GFP-RTa1+ or EpCAM+RT1a− and EpCAM-RT1a+. (B) Ultrastructual analysis of feeder cell. Feeder cells are large and multi-nucleated indicative of an irradiated cell and are distinct compared to gallbladder cells.
The morphogenesis assay was performed (see Methods) and images of cysts taken over the indicated times. Scale bars: 100 μm.
Primary, expanded and clonal gallbladder (GB) cells and primary IHBD cells were screened against the 38 antibodies in the panel. For primary GB and IHBD cells, primary cell isolates were co-stained with EpCAM to identify epithelial cells. For expanded cells, GFP expression was used to gate out feeder cells. Representative data for clones B21 and clones N12. +: positive expression; −: negative expression; Het: heterogeneous expression with negative/positive or high/low sub-populations; Low: weak yet distinct staining.
The 64 genes with fold changes greater than or equal to 2 were analyzed in Ingenuity Pathway Analysis (Ingenuity® Systems, www.ingenuity.com). The functional analysis identified the biological functions and/or diseases that were most significant to the dataset. Right-tailed Fisher’s exact test was used to calculate a p-value determining the probability that each biological function and/or disease assigned to that dataset is due to chance alone. In this analysis only p<0.0025 were considered.
SAMs were run on expanded gallbladder and IHBD cells at a false discovery rate of 10%. The fold changes as shown are calculated in the SAM software on log transformed data and are representative of IHBD/GB cells. A total of 267 genes were differentially expressed, of which 64 genes had fold changes greater than or equal to 2 and 203 genes had fold changes less than 2.