Abstract
Bile salt secretion is mediated primarily by the bile salt export pump (Bsep), a transporter on the canalicular membrane of the hepatocyte. However, little is known about the short-term regulation of Bsep activity. Ca2+ regulates targeting and insertion of transporters in many cell systems, and Ca2+ release near the canalicular membrane is mediated by the type II inositol 1,4,5-trisphosphate receptor (InsP3R2), so we investigated the possible role of InsP3R2 in modulating Bsep activity. The kinetics of Bsep activity were monitored by following secretion of the fluorescent Bsep substrate cholylglycylamido-fluorescein (CGamF) in rat hepatocytes in collagen sandwich culture, an isolated cell system in which structural and functional polarity is preserved. CGamF secretion was nearly eliminated in cells treated with Bsep siRNA, demonstrating specificity of this substrate for Bsep. Secretion was also reduced after chelating intracellular calcium, inducing redistribution of InsP3R2 by depleting the cell membrane of cholesterol, or reducing InsP3R function by either knocking down InsP3R2 expression using siRNA or pharmacologic inhibition using xestospongin C. Confocal immunofluorescence showed that InsP3R2 and Bsep are in close proximity in the canalicular region, both in rat liver and in hepatocytes in sandwich culture. However, after knocking down InsP3R2 or inducing its dysfunction with cholesterol depletion, Bsep redistributed intracellularly. Finally, InsP3R2 was lost from the pericanalicular region in animal models of estrogen- and endotoxin-induced cholestasis.
Conclusion
These data provide evidence that pericanalicular calcium signaling mediated by InsP3R2 plays an important role in maintaining bile salt secretion through post-translational regulation of Bsep, and suggest that loss or redistribution of InsP3R2 may contribute to the pathophysiology of intrahepatic cholestasis.
Keywords: Calcium Signaling, Bile Secretion, Lipid Rafts, Cholestasis, Sandwich Culture
Bile formation is an active process mediated in part by a group of ATP-Binding Cassette (ABC) transporters in the canalicular membrane of the hepatocyte, and defects in canalicular bile secretion result in cholestasis (1). Canalicular secretion of bile acids is mediated primarily by the bile salt export pump Bsep (ABCB11) (2). The hepatocyte responds to changing secretory requirements by modulating Bsep activity, both on short and longer time scales (2). Long term regulation is transcriptionally mediated, principally by farnesoid X receptor (FXR), which is activated by elevated cytosolic bile salt concentrations and translocates to the nucleus to increase Bsep expression (3). Short term regulation consists of trafficking of Bsep to the canalicular membrane to increase transporter density and thus secretory capacity. The reservoir for Bsep consists of two distinct subapical endosomal pools, one dependent on choleretic bile acids such as taurocholate (4), and the other on cAMP (5, 6). There is also a fraction of vesicular Bsep that is mobilized in response to ursodeoxycholate (UDCA) (7), requiring mitogen activated protein kinase (8) and protein kinase C-α (PKCα) (9) signaling for insertion.
Among the signaling molecules implicated in post-translational regulation of Bsep, Ca2+ is one of the least understood. It might be predicted that Ca2+ would play a critical role in bile secretion for two reasons. First, subplasmalemmal Ca2+ signals are obligatory for exocytosis in all cells (10), suggesting that Ca2+ would be necessary for canalicular Bsep insertion. Second, in polarized epithelia there is an apical enrichment of inositol 1,4,5-trisphosphate receptors (InsP3Rs) (11, 12) which initiates apical-to-basolateral Ca2+ waves (13). It has been demonstrated in certain epithelia, including pancreatic acinar cells (14) and cholangiocytes (15), that this polarized Ca2+ signaling pattern is important for secretion. Because the hepatocyte also contains a pericanalicular clustering of (type II) InsP3Rs (16), and because this ‘trigger zone’ produces polarized Ca2+ waves in the hepatocyte (16), these Ca2+ signals might be expected to promote bile secretion in an analogous manner. However, direct evidence for a role for Ca2+ in bile secretion has been limited and even contradictory. Early studies in the isolated perfused rat liver demonstrated that rises in intracellular Ca2+ induced by ionophores and SERCA pump inhibitors do not increase exocytosis (17) and in fact have a cholestatic effect (18). On the other hand, Ca2+ transients induced by the choleretic bile acid UDCA are associated with canalicular exocytosis (19). These seemingly contradictory effects of Ca2+ signals may be explained by differences in the nature of choleretic and cholestatic Ca2+ signals; it is now appreciated that the magnitude, timing and subcellular localization of Ca2+ signals all contribute to their physiological effects (20). Therefore, the purpose of this study was to investigate the machinery responsible for peri-canalicular Ca2+ signaling and determine its role in bile salt excretion.
MATERIALS AND METHODS
Animals and materials
Male Sprague-Dawley rats weighing 100–250 g (Charles River Labs, Wilmington, MA) were used for all experiments. All animal procedures were approved by the Yale Animal Care and Use Committee. Tissue culture reagents were from Invitrogen (Basel, Switzerland). Rabbit polyclonal Bsep antibodies were from Kamiga (Seattle, WA). Rabbit InsP3R-1 antibodies were from Upstate (Billerica, MA); InsP3R2 antibodies were kindly provided by Richard Wojcikiewicz (SUNY, Syracuse, NY)(21); mouse anti-InsP3R-3 was from BD Biosciences (San Jose, CA). Monoclonal Mrp2 antibodies (M2 III–6) were from Alexis Biochemicals (Plymouth Meeting, PA) and those against actin and tubulin were from Sigma-Aldrich (St. Louis, MO). Methyl-beta-cyclodextrin (mβCD) also was from Sigma-Aldrich. Cholylglycylamido-fluorescein (CGamF) was originally a gift of Alan Hoffman to James L. Boyer, who kindly provided the substrate to our lab. siRNAs against Bsep and InsP3R2 were from Ambion (Austin, TX), and Lipofectamine 2000 was from Invitrogen. All other chemicals were of the highest quality commercially available.
Isolation and collagen sandwich culture of rat hepatocytes
Hepatocytes were cultured as previously described (22). Lipid rafts were disrupted by depleting cholesterol with 5 mM mβCD for 30 min (23). Cytosolic Ca2+ was chelated by adding 1 mM 1,2-bis(o-aminophenoxy)ethane-N,N,N',N'-tetraacetic acid (BAPTA-AM) for 30 min. In select experiments, chemically synthesized siRNA duplexes against InsP3R2 or Bsep mRNA were transfected into hepatocytes 2 hr after isolation, prior to collagen gel overlay. siRNA (100 nM) and Lipofectamine (1 µl) were transfected per 800,000 hepatocytes in 35 mm dishes according to manufacturer’s instructions.
In vitro bile secretion assay
Hepatocyte bile salt secretory function was measured using a confocal microscopy-based assay, adapted from one previously reported (22), in which the canalicular secretion of CGamF, a fluorescent Bsep substrate, was quantified over time. Hepatocytes were maintained in collagen sandwich culture for 4 days on glass coverslips, then rinsed in 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) buffer at room temperature, and mounted onto a perfusion chamber on the stage of a Zeiss LSM 510 confocal microscope. Confocal fluorescence images were collected (excitation 488 nm, emission 505–550 nm) at a rate of one image/2.5 sec in a horizontal plane through the hepatocytes to visualize the canalicular spaces. After establishing a baseline level of fluorescence, 1 µM CGamF was perfused through the chamber and images were collected for 10 min. CGamF is a bile acid conjugate that relies on basolateral uptake before being transported across the canalicular membrane into the sealed canalicular vacuole of cultured hepatocytes (24, 25). The increase in fluorescence intensity in the canalicular space over time relative to baseline fluorescence served as a measure of Bsep activity.
Lipopolysaccharide (LPS) and estrogen-induced cholestasis models
For LPS-induced cholestasis, rats were anaesthetized with isoflurane and injected intravenously with 2 mg/kg LPS, or 0.9% saline as control. After 16 hr, animals were anesthetized with pentobarbital sodium (50 mg/kg) and their livers were harvested and snap frozen in liquid nitrogen for further analysis (26). For estrogen-induced cholestasis, 17 a-ethinylestradiol (EE) dissolved in 1,2- propanediol (5 mg/ml) was administered to rats subcutaneously (5mg/kg/day) for 5 consecutive days as previously described (26). Control animals received equivalent amounts of vehicle alone. After 5 days, animals were anesthetized and livers harvested as above.
Immunofluorescence
Hepatocytes on glass coverslips were washed and then fixed in ice-cold methanol for 5 min; rat liver was snap frozen under liquid nitrogen, cryo-sectioned and then fixed in 4% paraformaldehyde for 10 min. Samples were then permeabilized with 1% Triton X-100, blocked in 2% bovine serum albumin, incubated with primary antibodies for 1 hr at room temperature, washed with phosphate-buffered saline (PBS), incubated with fluorophore-conjugated secondary antibodies for 1 hr at room temperature, washed again with PBS, and then mounted. Negative controls were incubated with secondary antibodies alone. Double- and triple-labeled specimens were examined with a Zeiss LSM 510 Confocal Microscope (Thornwood, NY) (23).
Immunoblots
Liver was homogenized in protein extraction reagent from Thermo (Rockford, Il). Protein was extracted from isolated hepatocytes in the same buffer. Proteins were resolved by SDS-PAGE electrophoresis on a 4–20% gel. After transferring to PVDF using a semi-dry system, the membrane was blocked, incubated with primary antibodies overnight at 4°C, washed, incubated with horseradish peroxidase conjugated secondary antibodies for 1 hr, washed and then revealed by enhanced chemiluminescence (27).
Statistics
Values listed are mean±SEM. Comparisons were made using Student t test or analysis of variance (ANOVA). P Values < 0.05 were considered significant.
RESULTS
Expression and localization of InsP3R in rat liver matches that in hepatocytes in collagen sandwich culture
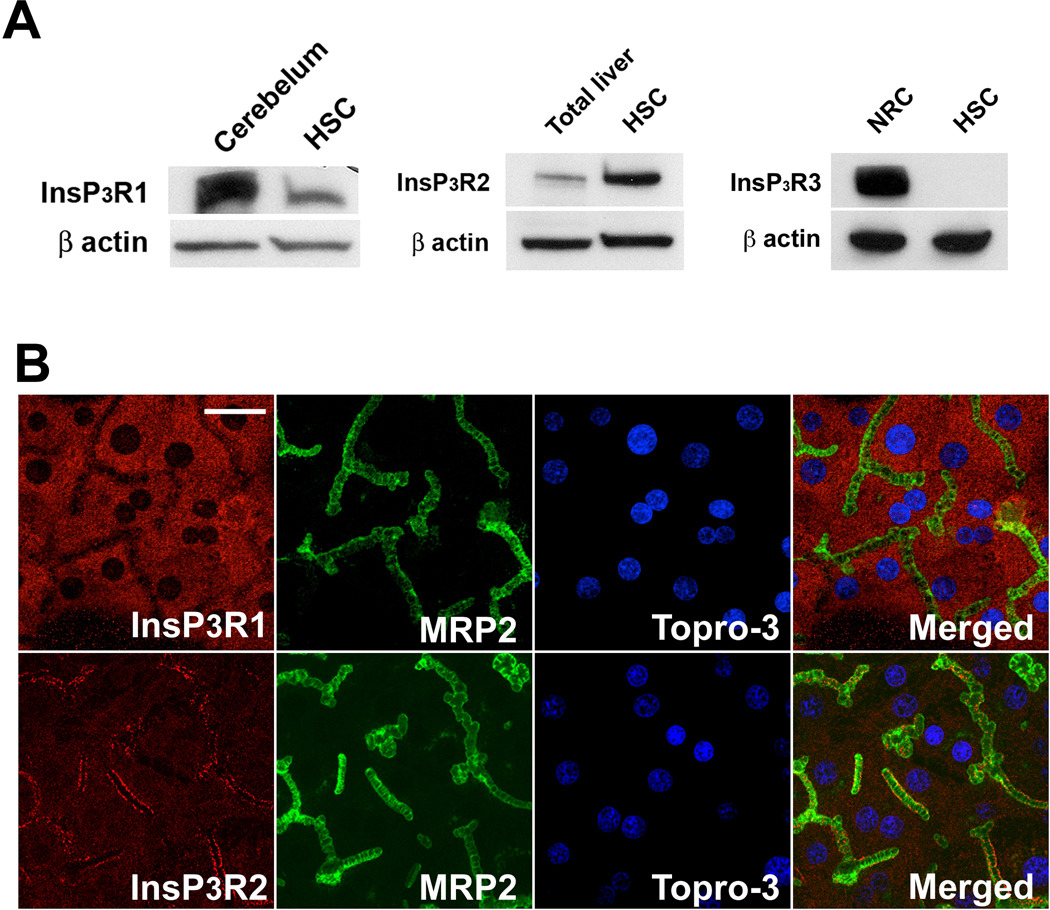
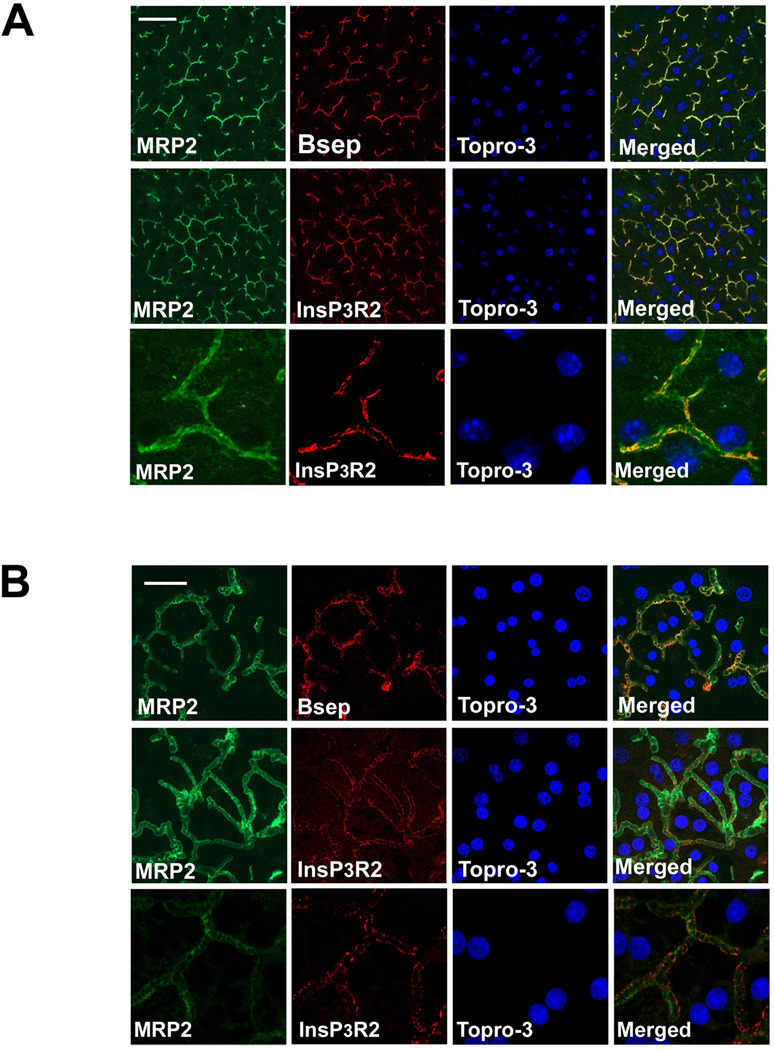
Like hepatocytes in intact rat liver, hepatocytes in collagen sandwich culture have a well-developed canalicular network to which canalicular membrane proteins appropriately traffic (28). To determine whether this culture system accurately recapitulates the Ca2+ signaling machinery in native rat liver, we examined expression and localization of InsP3R isoforms in both systems. Immunoblots of lysates from hepatocytes in culture revealed that, as in liver (16), InsP3R1 and InsP3R2 are expressed, while InsP3R-3 is absent (Figure 1a). In addition, as in liver (16), InsP3R1 is expressed diffusely throughout the hepatocyte, while InsP3R2 is concentrated in the canalicular region (Figure 1b). Both Bsep (29) and InsP3R2 (16) are expressed mainly in the canalicular region, so confocal immunofluorescence microscopy was used to compare their localization directly. Available Bsep (29) and InsP3R2 (30) antibodies are both generated in the rabbit, so each protein was co-localized with multi-drug resistance protein 2 (Mrp2), which resides in and immediately beneath the canalicular membrane (22). In rat liver Bsep and InsP3R2 were both in proximity to Mrp2 at the canalicular membrane (Figure 2a), suggesting they are in proximity to each other as well. Similarly, Bsep and InsP3R2 are expressed in close proximity in the canalicular region of cultured rat hepatocytes (Figure 2b). Therefore, the expression and localization of InsP3R isoforms is preserved in rat hepatocytes in collagen sandwich culture, similar to what has been observed in mouse hepatocytes (22). These findings provide support for using this cell culture model to study the functional relationship between InsP3R2-mediated Ca2+ release and Bsep activity in rat liver. Despite the proximity of InsP3R2 and Bsep, these proteins could not be coimmunoprecipitated in lysates from rat liver or rat hepatocytes in culture, even after treatment with cross-linking agents (not shown). This suggests that InsP3R2 and Bsep are in proximity but do not physically associate, consistent with the observation that InsP3R2 is in a specialized region of ER beneath the canalicular membrane (23), while Bsep is located instead within subapical vesicles and the canalicular membrane itself (31).
Figure 1. Isoform expression and localization of InsP3Rs in cultured hepatocytes.
(A) Immunoblot analysis of InsP3R isoforms demonstrates that InsP3R1 and InsP3R2, but not InsP3R-3 are expressed in sandwich-cultured rat hepatocytes, as in native liver. Cerebellum was used as positive control for InsP3R1, total rat liver for InsP3R2, and normal rat cholangiocytes (NRC) for InsP3R3. β-actin was used as loading control. (B) The distribution of InsP3R isoforms (red) was examined by confocal immunofluorescence in cells in culture. InsP3R1 is diffusely localized throughout the cytoplasm while InsP3R2 is concentrated in the peri-canalicular domain, just as in native liver. Mrp2 (green) was used as an apical marker and nuclei were labeled with TO-PRO3 (blue). Scale bar, 10 µm.
Figure 2. InsP3R2 is localized near Bsep at the canalicular membrane.
Confocal immunofluorescence was used to examine the distribution of Bsep and InsP3R2 (red) with respect to the apical marker Mrp2 (green). TO-PRO3 was used to label nuclei (blue). (A) In rat liver, Bsep is adjacent to Mrp2 (top row) and InsP3R2 is adjacent to Mrp2 (middle row), thus indicating that Bsep and InsP3R2 are in proximity to each other. Scale bar, 20 µm. Bottom row shows a magnified view of the upper row image demonstrating the proximity between InsP3R2 and the apical marker Mrp2. (B) In rat hepatocytes in collagen sandwich culture, Bsep and InsP3R2, respectively, localize near Mrp2, indicating that Bsep and InsP3R2 are in close proximity here as well. Scale bar, 10 µm
InsP3R2 and Ca2+ regulate Bsep activity
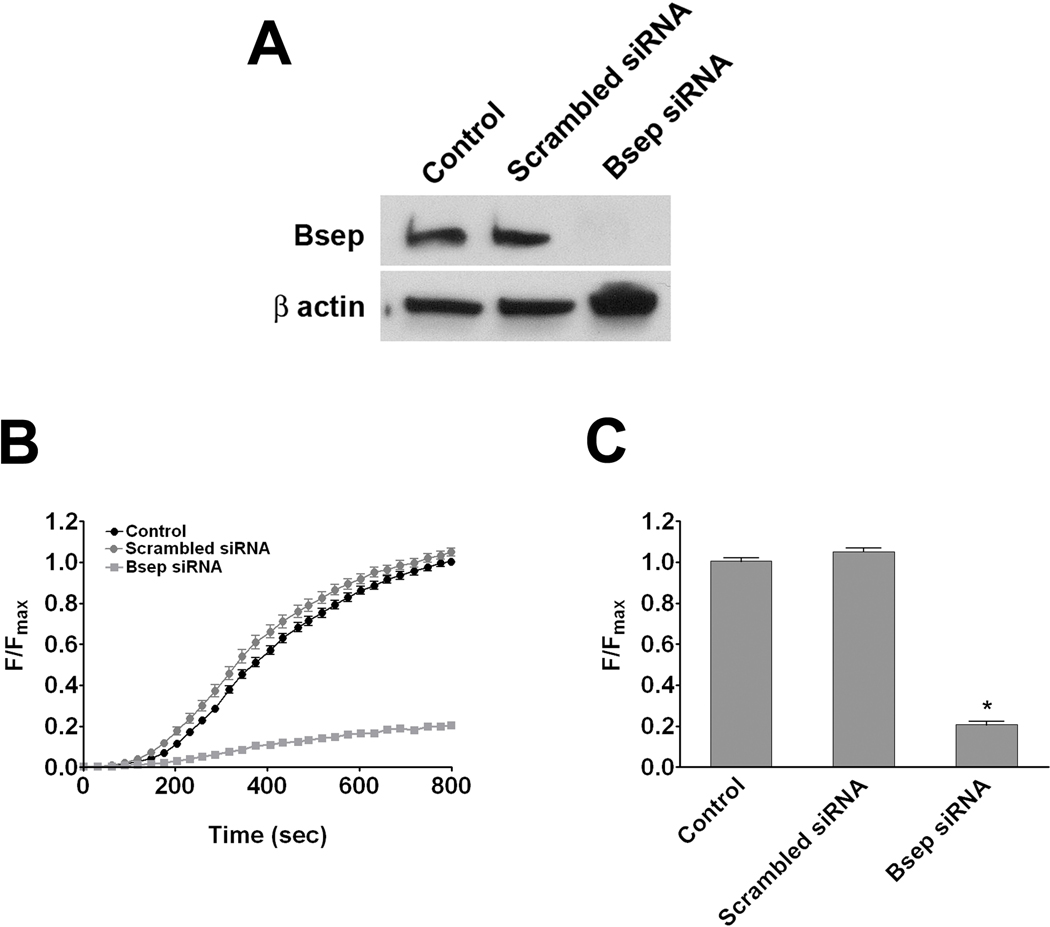
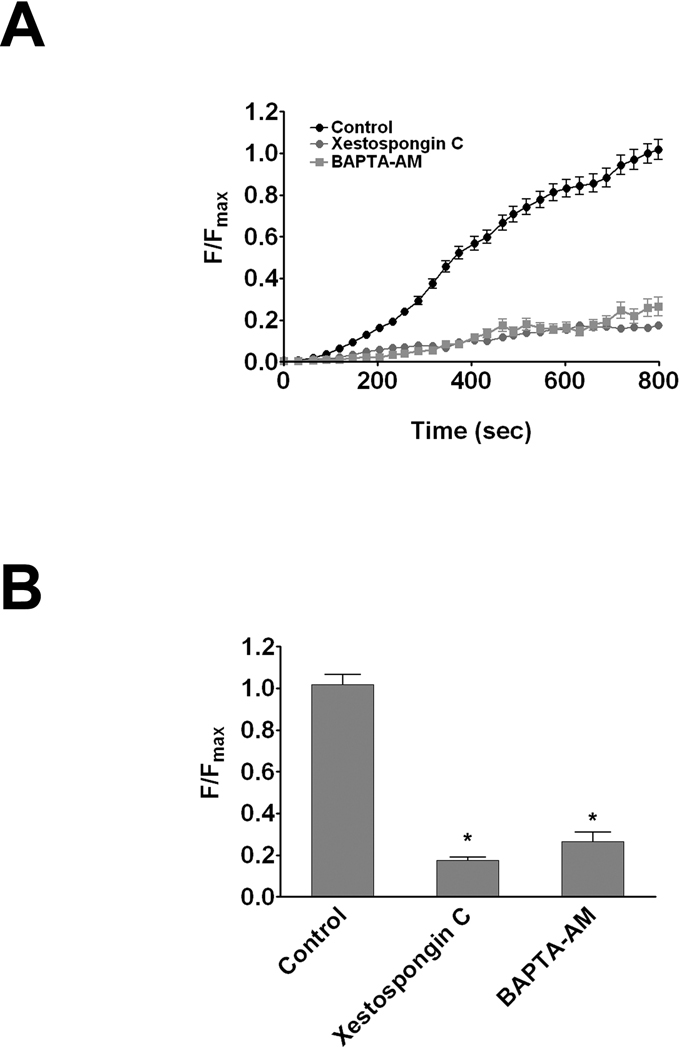
A confocal microscopy-based assay that monitors CGamF accumulation in the canalicular space was developed to detect the kinetics of bile salt secretion in hepatocytes in collagen sandwich culture. CGamF fluorescence was measured in untreated or scrambled siRNA-treated hepatocyte controls and in cells treated with siRNA to knockdown Bsep expression. Bsep siRNA reduced Bsep expression in hepatocytes by 75% while scrambled siRNA had a negligible effect (Figure 3a). Canalicular CGamF fluorescence was reduced by 80% in hepatocytes treated with Bsep siRNA, relative to hepatocytes treated with scrambled siRNA or untreated hepatocytes (Figure 3b–c). These results demonstrate the specificity of canalicular CGamF secretion for Bsep activity. Several experiments were performed to determine the role of InsP3R2 in modulating Bsep activity. First, canalicular fluorescence was measured after treatment with the cytosolic Ca2+ buffer BAPTA-AM (Figure 4a–b). This produced a 74% decrease, providing evidence that Bsep activity depends upon cytosolic Ca2+. Second, InsP3R function was inhibited by treating hepatocytes with the InsP3R inhibitor xestospongin C (32). This resulted in an 83% reduction in canalicular fluorescence of CGamF relative to controls (Figure 4a–b). Most InsP3Rs in hepatocytes are type II (21), so InsP3R2 expression was reduced by 70% (Figure 5a) using an isoform-specific siRNA validated previously (22). This resulted in a 53% reduction in canalicular CGamF fluorescence relative to controls (Figure 5c–d), similar to what was observed in matched preparations treated with BAPTA-AM (Figure 5c–d). Interestingly, in InsP3R2-depleted cells there was a 40% decrease in Bsep expression (Figure 5a). Finally, the importance of InsP3R2’s peri-canalicular localization was examined. Cells were treated with mβCD to disrupt lipid rafts, which had no effect on the amount of InsP3R2 or Bsep that was expressed (Figure 6a), but redistributed InsP3R2 and Bsep so that they were less concentrated in the canalicular region (Figure 6b–c). This reduced canalicular CGamF fluorescence by 67% relative to controls, similar to what was observed in BAPTA-treated preparations (Figure 6d–e). Together, these findings provide evidence that Bsep activity is Ca2+-dependent, and in particular depends upon expression and peri-canalicular localization of InsP3R2.
Figure 3. A CGamF secretion assay specifically measures Bsep activity.
Collagen sandwich cultures of untransfected control hepatocytes, hepatocytes transfected with scrambled siRNA, and hepatocytes transfected with Bsep siRNA were studied. (A) Immunoblot analysis demonstrates that Bsep expression is abolished only in cells treated with Bsep siRNA. β-actin was the loading control. (B) Canalicular CGamF fluorescence accumulation over time was markedly decreased in Bsep depleted cells. Results represent mean±SEM increases over time in control cells (112 canaliculi, black circles), cells transfected with scrambled siRNA (80 canaliculi, gray circles), and cells transfected with Bsep siRNA (80 canaliculi, gray squares). Data were pooled from three different transfection experiments (C) Peak canalicular CGamF fluorescence was significantly decreased in Bsep depleted cells (0.21±1.02 a.u; p < 0.0001) compared with scrambled siRNA transfected cells (1.05±0.02 a.u), and control cells (1.00±0.02 a.u).
Figure 4. Inhibition of calcium signaling impairs Bsep function.
CGamF secretion was monitored by confocal microscopy in hepatocytes treated with DMSO vehicle (black circles), the pan-InsP3R inhibitor xestospongin C (1 µM; gray circles), or the cytosolic Ca2+ chelator BAPTA-AM (50 µM; gray squares). (A) Canalicular CGamF fluorescence accumulation over time was decreased in cells treated with either xestospongin C (122 canaliculi), or BAPTA-AM (36 canaliculi), when compared with controls (179 canaliculi). Data are mean±SEM increases over time from three different experiments. (B) Peak canalicular CGamF fluorescence was significantly decreased in xestospongin (0.18±0.01 a.u; p < 0.0001) or BAPTA-AM treated cells (0.26 ± 0.06 a.u; p < 0.0001) compared with controls (1.02±0.04 a.u).
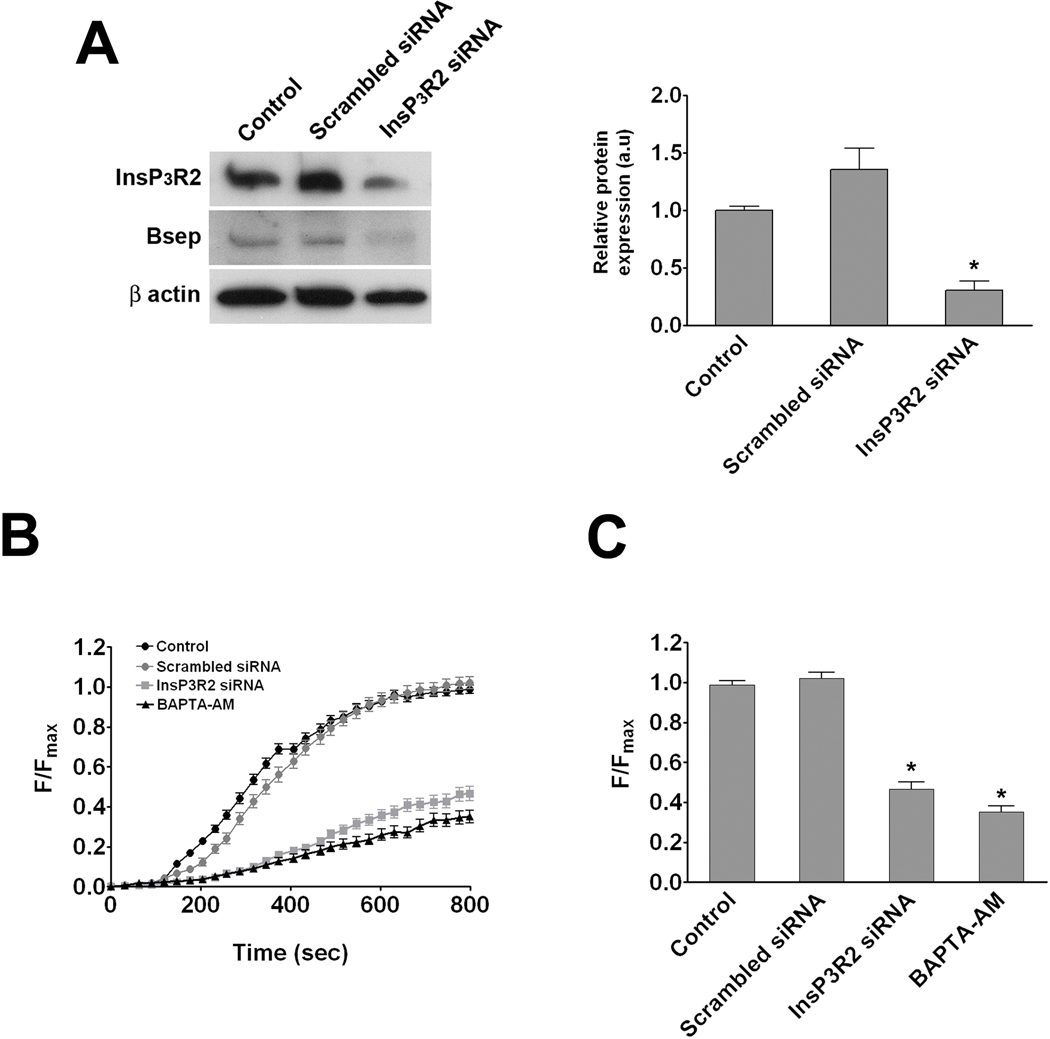
Figure 5. InsP3R2 is necessary for proper expression and function of Bsep.
Untransfected control hepatocytes, hepatocytes transfected with scrambled siRNA, and hepatocytes transfected with InsP3R2 siRNA were examined in collagen sandwich culture. (A) Immunoblot analysis demonstrates that InsP3R2 expression is decreased only in cells treated with InsP3R2 siRNA (0.30±0.08 a.u; p < 0.01) when compared to control cells (1.00±0.03 a.u). This decrease was associated with a 40% reduction in Bsep expression. β-actin was used as loading control. (B) Canalicular CGamF fluorescence accumulation over time was decreased in InsP3R2 depleted cells. Results represent mean±SEM increases over time in control cells (72 canaliculi; black circles), cells transfected with scrambled siRNA (39 canaliculi; gray circles), cells transfected with InsP3R2 siRNA (69 canaliculi; gray squares), and untransfected cells treated with BAPTA-AM (21 canaliculi; black triangles). Data were pooled from three different transfection experiments (C) Compared with scrambled siRNA transfected (1.02±0.03 a.u), and control cells (0.99±0.02 a.u), peak canalicular CGamF fluorescence was significantly decreased in InsP3R2 depleted cells (0.47±0.04 a.u.; p < 0.0001), to a similar level as untransfected cells treated with BAPTA-AM (0.35±0.03 a.u.; p < 0.0001).
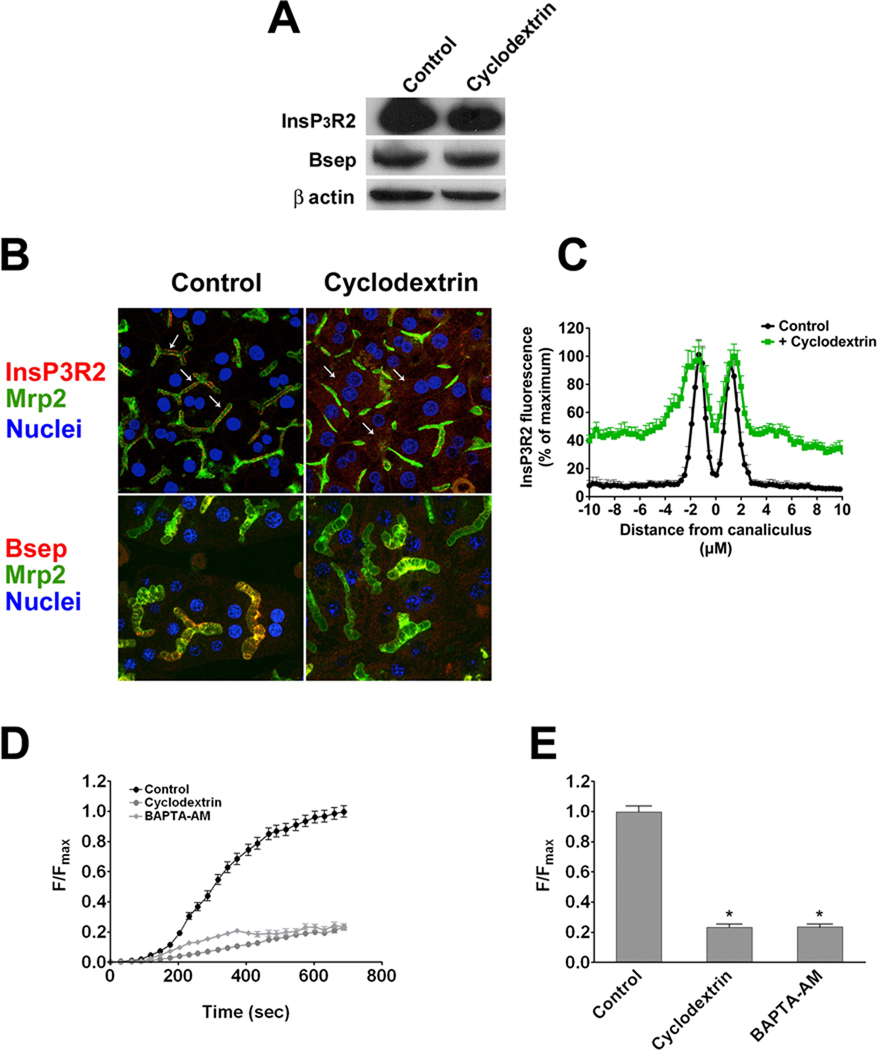
Figure 6. Cholesterol depletion induces Bsep redistribution and dysfunction.
Hepatocytes in sandwich culture were treated with 5 mM methyl-β-cyclodextrin to deplete membrane cholesterol. (A) Immunoblot analysis demonstrates that InsP3R2 and Bsep expression are unaffected by cholesterol depletion. β-actin was the loading control. (B) Partial redistribution of InsP3R2 and Bsep (red) from the canalicular membrane to the cytoplasm (arrows) was observed after cholesterol depletion using confocal immunofluorescence. Mrp2 (green) was used as an apical marker and TO-PRO3 (blue) was used to label nuclei. (C) Redistribution of InsP3R2 was quantified by its normalized fluorescence intensity along a 20 µm line perpendicular to the canalicular membrane. Sharper canalicular peaks are seen in controls, while broader peaks with more cytoplasmic labeling are observed in cholesterol depleted cells (p < 0.0001 by one-way ANOVA). (D) Canalicular CGamF fluorescence accumulation over time was decreased in cholesterol depleted cells. Results represent mean±SEM increases over time in control cells (60 canaliculi; black circles) or cells treated with cyclodextrin (56 canaliculi; gray circles) or BAPTA-AM (58 canaliculi; gray squares). Data were pooled from three experiments. (E) Compared with controls (1.00±0.04 a.u), peak canalicular CGamF fluorescence was significantly decreased in cholesterol depleted cells (0.23±0.02 a.u.; p < 0.0001), to a similar level as cells treated with BAPTA-AM (0.23 ± 0.02 a.u.; p < 0.0001).
Peri-canalicular InsP3R2 expression is lost in cholestasis
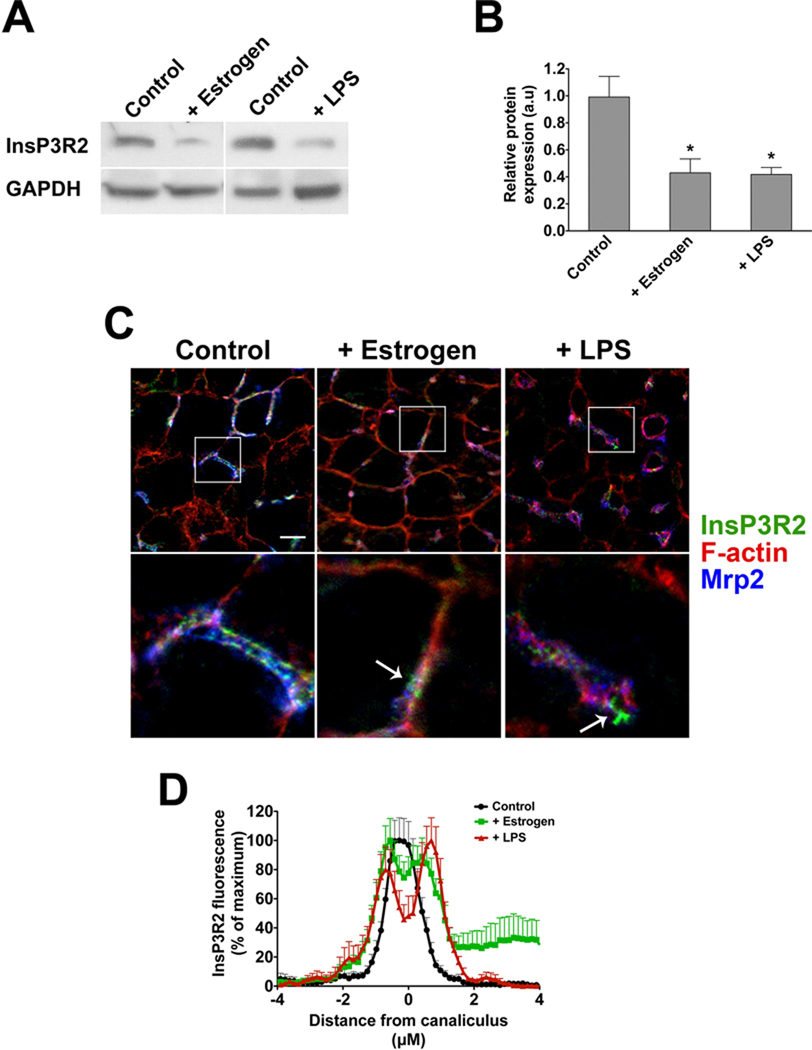
In rats treated with either LPS or estrogen, InsP3R2 expression was significantly decreased (Figure 7a–b). Moreover, InsP3R2 labeling in proximity to the canalicular membrane was decreased, and InsP3R2 labeling was seen in a punctate pattern in the peri-canaliuclar region (Figure 7c). Quantification of InsP3R2 labeling confirmed that the receptor is distributed more diffusely throughout the canalicular region in LPS- or estrogen-treated animals (Figure 7d). Together, these findings raise the possibility that the mis-targeting of canalicular transporters such as Bsep observed in canalicular cholestasis (33, 34) may be related to decreased expression and/or mis-localization of InsP3R2.
Figure 7. InsP3R2 expression is reduced in rat models of canalicular cholestasis.
Rats were treated with17α-ethinyl estradiol (5 mg/kg) for 5 days or LPS (1 mg/kg) for 16 hr. (A) Representative western blots show that InsP3R2 expression is decreased in both estradiol and LPS-treated livers compared to vehicle-treated animals. (B) InsP3R2 expression was significantly reduced in estrogen (0.43±0.10 a.u; p < 0.05, n= 4) and LPS-treated (0.42±0.05 a.u; p < 0.02, n=6) animals compared to controls (0.99±0.15 a.u; n=10). (C) Confocal immunofluorescence images of rat liver with labeling of InsP3R2 (green), Mrp2 (blue), and Factin (red). Expression of InsP3R2 becomes punctate (arrows) and less co-localized with the canalicular marker Mrp2 in rat liver after treatment with LPS or estradiol. Bottom row shows magnified views of the canalicular regions of interest marked by the white boxes. Scale bar, 10 µM. (D) Redistribution of InsP3R2 was quantified by its normalized fluorescence intensity along an 8 µm line perpendicular to the canicular membrane. A single peak centered at the canaliculus is seen in controls, while two separate, broader, peri-canalicular peaks are observed in the estrogen and LPS treated livers (p < 0.01 by one-way ANOVA).
DISCUSSION
InsP3R2 is the major intracellular Ca2+ release channel in hepatocytes (16). Ca2+ release from pericanalicular InsP3R2 promotes the activity of Mrp2, in part by increasing Mrp2 insertion into the plasma membrane (22). The present study demonstrates that InsP3R2 also promotes the activity of Bsep. Pericanalicular Ca2+ signaling likely promotes Bsep activity by enhancing exocytic insertion, as with Mrp2. Vesicle fusion depends on a localized Ca2+ increase, which must be in the range of ~10 µM for the form of exocytosis that governs transporter insertion (35). Apical clusters of InsP3R in other polarized cells are capable of producing such large amplitude, focal Ca2+ transients to regulate secretion (36). In Wif-B9 cells (37), Bsep constitutively recycles between a sub-apical endosomal pool and the canalicular membrane. Therefore it would be predicted that Bsep would accumulate intracellularly and bile secretion would decrease without InsP3R2. Our observation that Bsep redistributes to the subapical compartment when InsP3R2 is nonfunctional is consistent with this. However, we also observed a small but significant decrease in Bsep expression in the absence of InsP3R2. This may reflect that the half-life of Bsep in the intracellular compartment is shorter than in the membrane. Evidence for this comes from studies of rodent liver treated with estrogen, which induces rapid internalization of Bsep (33). In this model, total protein content of the transporter decreases without a change in mRNA levels, suggesting a posttranslational mechanism of Bsep down-regulation such as increased protein turnover (38).
Our findings suggest not only that InsP3R2 promotes bile secretion but that canalicular cholestasis (in both estrogen and LPS models) is associated with loss of InsP3R2 expression. Bsep and Mrp2 are mistargeted in both of these models and overall expression of these proteins is reduced secondary to posttranslational mechanisms (33, 34, 38–41). Moreover, in the case of Bsep this defect is reversed by UDCA (40), which increases Ca2+ (42) and stimulates exocytosis (19). Considering our current and previous findings (22), it may be that decreased expression and localization of canalicular transporters seen in canalicular cholestasis is secondary to loss of pericanalicular Ca2+ signaling. This may be analogous to what is observed in cholangiocytes, in which InsP3R3 rather than InsP3R2 is the predominant isoform (11). InsP3R3 expression is concentrated sub-apically in cholangiocytes and is lost in animal models of ductular cholestasis, as well as in patients with a variety of cholestatic disorders (27). This decreased InsP3R3 expression impairs polarized Ca2+ signaling (27) and ductular bicarbonate secretion (43). Therefore loss or redistribution of apical InsP3Rs may be a common basis for impaired secretion in polarized epithelia.
Cells in various tissues use raft-based platforms to spatially coordinate membrane proteins with the Ca2+ release machinery that regulates them (44). Rafts can promote physical and functional interactions between ER channels and membrane proteins (45–48), and also can cluster Ca2+ signaling proteins, including PLC (49) and PIP2 (44); downstream effectors such as PKCα (49) and store operated Ca2+ entry proteins (44), such as TRPC1 and Orai; and the plasma membrane Ca2+ ATPase (44). Thus, lipid rafts help generate large amplitude localized Ca2+ transients in specific membrane microdomains, and this may be ideal for regulating Bsep insertion. It is interesting to note that InsP3R2 labeling can be punctate, perhaps indicating focal densities of release channels coordinated with sites of exocytosis. The localized nature of Ca2+ signaling microdomains would also help explain the apparent inconsistency between the present results, which support a choleretic effect of Ca2+, and earlier studies, which suggest a cholestatic effect (18). In particular, a highly localized Ca2+ response in the pericanalicular domain may mediate Bsep insertion and bile formation, whereas sustained, global Ca2+ signals induced by ionophores may promote cholestasis. Answers to these questions may provide insight into basic mechanisms of cholestasis and lead to novel therapeutic approaches for this condition.
ACKNOWLEDGMENTS
We thank Kathy Harry and Albert Mennone for help with hepatocyte isolations and Carol Soroka for help with estradiol and LPS treatments. We thank Alan Hofmann for providing cholylglycylamido-fluorescein.
Financial support:
This work was supported by NIH Grants DK45710, DK34989, DK57751, DK61747 to MHN, and SG was supported by T32 GM07205.
Abbreviations
- ATP
adenosine triphosphate
- ABC transporter
ATP-binding cassette transporter
- Bsep
bile salt export pump
- FXR
farnesoid X receptor
- cAMP
cyclic adenosine monophosphate
- UDCA
ursodeoxycholate
- PKCα
protein kinase C-α
- InsP3R
inositol 1,4,5-trisphosphate receptor
- mβCD
methyl-beta-cyclodextrin
- CGamF
cholylglycylamido-fluorescein
- siRNA
small interfering ribonucleic acid
- BAPTA-AM
1,2-bis(o-aminophenoxy)ethane-N,N,N',N'-tetraacetic acid;
- HEPES
4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid
- PBS
phosphate-buffered saline
- Mrp2
multidrug resistance protein 2
- ANOVA
analysis of variance
- LPS
lipopolysaccharide.
Contributor Information
Emma A. Kruglov, Email: emma.kruglov@yale.edu.
Samir Gautam, Email: samir.gautam@yale.edu.
Mateus T. Guerra, Email: mateus.t.guerra@yale.edu.
Michael H. Nathanson, Email: michael.nathanson@yale.edu.
REFERENCES
- 1.Trauner M, Meier PJ, Boyer JL. Molecular pathogenesis of cholestasis. N Engl J Med. 1998;339:1217–1227. doi: 10.1056/NEJM199810223391707. [DOI] [PubMed] [Google Scholar]
- 2.Kullak-Ublick GA, Stieger B, Hagenbuch B, Meier PJ. Hepatic transport of bile salts. Semin Liver Dis. 2000;20:273–292. doi: 10.1055/s-2000-9426. [DOI] [PubMed] [Google Scholar]
- 3.Ananthanarayanan M, Balasubramanian N, Makishima M, Mangelsdorf DJ, Suchy FJ. Human bile salt export pump promoter is transactivated by the farnesoid X receptor/bile acid receptor. J Biol Chem. 2001;276:28857–28865. doi: 10.1074/jbc.M011610200. [DOI] [PubMed] [Google Scholar]
- 4.Kipp H, Pichetshote N, Arias IM. Transporters on demand: intrahepatic pools of canalicular ATP binding cassette transporters in rat liver. J Biol Chem. 2001;276:7218–7224. doi: 10.1074/jbc.M007794200. [DOI] [PubMed] [Google Scholar]
- 5.Boyer JL, Soroka CJ. Vesicle targeting to the apical domain regulates bile excretory function in isolated rat hepatocyte couplets. Gastroenterology. 1995;109:1600–1611. doi: 10.1016/0016-5085(95)90649-5. [DOI] [PubMed] [Google Scholar]
- 6.Misra S, Varticovski L, Arias IM. Mechanisms by which cAMP increases bile acid secretion in rat liver and canalicular membrane vesicles. Am J Physiol Gastrointest Liver Physiol. 2003;285:G316–G324. doi: 10.1152/ajpgi.00048.2003. [DOI] [PubMed] [Google Scholar]
- 7.Dombrowski F, Stieger B, Beuers U. Tauroursodeoxycholic acid inserts the bile salt export pump into canalicular membranes of cholestatic rat liver. Lab Invest. 2006;86:166–174. doi: 10.1038/labinvest.3700371. [DOI] [PubMed] [Google Scholar]
- 8.Kurz AK, Graf D, Schmitt M, Vom Dahl S, Haussinger D. Tauroursodesoxycholate-induced choleresis involves p38(MAPK) activation and translocation of the bile salt export pump in rats. Gastroenterology. 2001;121:407–419. doi: 10.1053/gast.2001.26262. [DOI] [PubMed] [Google Scholar]
- 9.Wimmer R, Hohenester S, Pusl T, Denk GU, Rust C, Beuers U. Tauroursodeoxycholic acid exerts anticholestatic effects by a cooperative cPKC alpha-/PKA-dependent mechanism in rat liver. Gut. 2008;57:1448–1454. doi: 10.1136/gut.2007.140871. [DOI] [PubMed] [Google Scholar]
- 10.Sudhof TC, Rothman JE. Membrane fusion: grappling with SNARE and SM proteins. Science. 2009;323:474–477. doi: 10.1126/science.1161748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hirata K, Dufour JF, Shibao K, Knickelbein R, O'Neill AF, Bode HP, Cassio D, et al. Regulation of Ca(2+) signaling in rat bile duct epithelia by inositol 1,4,5-trisphosphate receptor isoforms. Hepatology. 2002;36:284–296. doi: 10.1053/jhep.2002.34432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Matovcik LM, Maranto AR, Soroka CJ, Gorelick FS, Smith J, Goldenring JR. Co-distribution of calmodulin-dependent protein kinase II and inositol trisphosphate receptors in an apical domain of gastrointestinal mucosal cells. J Histochem Cytochem. 1996;44:1243–1250. doi: 10.1177/44.11.8918899. [DOI] [PubMed] [Google Scholar]
- 13.Leite MF, Burgstahler AD, Nathanson MH. Ca2+ waves require sequential activation of inositol 1,4,5-trisphosphate receptors and ryanodine receptors in pancreatic acinar cells. Gastroenterology. 2002;122:415–427. doi: 10.1053/gast.2002.30982. [DOI] [PubMed] [Google Scholar]
- 14.Nathanson MH, Padfield PJ, O'Sullivan AJ, Burgstahler AD, Jamieson JD. Mechanism of Ca2+ wave propagation in pancreatic acinar cells. J Biol Chem. 1992;267:18118–18121. [PubMed] [Google Scholar]
- 15.Fitz JG, Basavappa S, McGill J, Melhus O, Cohn JA. Regulation of membrane chloride currents in rat bile duct epithelial cells. J. Clin. Invest. 1993;91:319–328. doi: 10.1172/JCI116188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hirata K, Pusl T, O'Neill AF, Dranoff JA, Nathanson MH. The type II inositol 1,4,5-trisphosphate receptor can trigger Ca2+ waves in rat hepatocytes. Gastroenterology. 2002;122:1088–1100. doi: 10.1053/gast.2002.32363. [DOI] [PubMed] [Google Scholar]
- 17.Bruck R, Nathanson MH, Roelofsen H, Boyer JL. Effects of protein kinase C and cytosolic Ca2+ on exocytosis in the isolated perfused rat liver. Hepatology. 1994;20:1032–1040. doi: 10.1002/hep.1840200436. [DOI] [PubMed] [Google Scholar]
- 18.Nathanson MH, Gautam A, Bruck R, Isales CM, Boyer JL. Effects of Ca2+ agonists on cytosolic Ca2+ in isolated hepatocytes and on bile secretion in the isolated perfused rat liver. Hepatology. 1992;15:107–116. doi: 10.1002/hep.1840150119. [DOI] [PubMed] [Google Scholar]
- 19.Beuers U, Nathanson MH, Isales CM, Boyer JL. Tauroursodeoxycholic acid stimulates hepatocellular exocytosis by mobilization of extracellular Ca++, a mechanism defective in cholestasis. J. Clin. Invest. 1993;92:2984–2993. doi: 10.1172/JCI116921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Clapham DE. Calcium signaling. Cell. 2007;131:1047–1058. doi: 10.1016/j.cell.2007.11.028. [DOI] [PubMed] [Google Scholar]
- 21.Wojcikiewicz RJ. Type I, II, and III inositol 1,4,5-trisphosphate receptors are unequally susceptible to down-regulation and are expressed in markedly different proportions in different cell types. J Biol Chem. 1995;270:11678–11683. doi: 10.1074/jbc.270.19.11678. [DOI] [PubMed] [Google Scholar]
- 22.Cruz LN, Guerra MT, Kruglov E, Mennone A, Garcia CR, Chen J, Nathanson MH. Regulation of multidrug resistance-associated protein 2 by calcium signaling in mouse liver. Hepatology. 2010;52:327–337. doi: 10.1002/hep.23625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nagata J, Guerra MT, Shugrue CA, Gomes DA, Nagata N, Nathanson MH. Lipid rafts establish calcium waves in hepatocytes. Gastroenterology. 2007;133:256–267. doi: 10.1053/j.gastro.2007.03.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cai SY, Gautam S, Nguyen T, Soroka CJ, Rahner C, Boyer JL. ATP8B1 deficiency disrupts the bile canalicular membrane bilayer structure in hepatocytes, but FXR expression and activity are maintained. Gastroenterology. 2009;136:1060–1069. doi: 10.1053/j.gastro.2008.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Holzinger F, Schteingart CD, Ton-Nu HT, Eming SA, Monte MJ, Hagey LR, Hofmann AF. Fluorescent bile acid derivatives: relationship between chemical structure and hepatic and intestinal transport in the rat. Hepatology. 1997;26:1263–1271. doi: 10.1002/hep.510260526. [DOI] [PubMed] [Google Scholar]
- 26.Nathanson MH, Burgstahler AD, Mennone A, Dranoff JA, Rios-Velez L. Stimulation of bile duct epithelial secretion by glybenclamide in normal and cholestatic rat liver. J. Clin. Invest. 1998;101:2665–2676. doi: 10.1172/JCI2835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shibao K, Hirata K, Robert ME, Nathanson MH. Loss of inositol 1,4,5-trisphosphate receptors from bile duct epithelia is a common event in cholestasis. Gastroenterology. 2003;125:1175–1187. doi: 10.1016/s0016-5085(03)01201-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang W, Soroka CJ, Mennone A, Rahner C, Harry K, Pypaert M, Boyer JL. Radixin is required to maintain apical canalicular membrane structure and function in rat hepatocytes. Gastroenterology. 2006;131:878–884. doi: 10.1053/j.gastro.2006.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gerloff T, Steiger B, Hagenbuch B, Madon J, Landmann L, Roth J, Hofmann AF, et al. The sister of p-glycoprotein represents the canalicular bile salt export pump of mammalian liver. J. Biol. Chem. 1998;273:10046–10050. doi: 10.1074/jbc.273.16.10046. [DOI] [PubMed] [Google Scholar]
- 30.Wojcikiewicz RJ, He Y. Type I, II and III inositol 1,4,5-trisphosphate receptor co-immunoprecipitation as evidence for the existence of heterotetrameric receptor complexes. Biochem Biophys Res Commun. 1995;213:334–341. doi: 10.1006/bbrc.1995.2134. [DOI] [PubMed] [Google Scholar]
- 31.Trauner M, Arrese M, Soroka CJ, Ananthanarayanan M, Koeppel TA, Schlosser SF, Suchy FJ, et al. The rat canalicular conjugate export pump (mrp2) is down-regulated in intrahepatic and obstructive cholestasis. Gastroenterology. 1997;113:255–264. doi: 10.1016/s0016-5085(97)70103-3. [DOI] [PubMed] [Google Scholar]
- 32.Gafni J, Munsch JA, Lam TH, Catlin MC, Costa LG, Molinski TF, Pessah IN. Xestospongins: potent membrane permeable blockers of the inositol 1,4,5-trisphosphate receptor. Neuron. 1997;19:723–733. doi: 10.1016/s0896-6273(00)80384-0. [DOI] [PubMed] [Google Scholar]
- 33.Crocenzi FA, Mottino AD, Cao J, Veggi LM, Pozzi EJ, Vore M, Coleman R, et al. Estradiol-17beta-D-glucuronide induces endocytic internalization of Bsep in rats. Am J Physiol Gastrointest Liver Physiol. 2003;285:G449–G459. doi: 10.1152/ajpgi.00508.2002. [DOI] [PubMed] [Google Scholar]
- 34.Elferink MG, Olinga P, Draaisma AL, Merema MT, Faber KN, Slooff MJ, Meijer DK, et al. LPS-induced downregulation of MRP2 and BSEP in human liver is due to a posttranscriptional process. Am J Physiol Gastrointest Liver Physiol. 2004;287:G1008–G1016. doi: 10.1152/ajpgi.00071.2004. [DOI] [PubMed] [Google Scholar]
- 35.Augustine GJ, Santamaria F, Tanaka K. Local calcium signaling in neurons. Neuron. 2003;40:331–346. doi: 10.1016/s0896-6273(03)00639-1. [DOI] [PubMed] [Google Scholar]
- 36.Fadool DA, Ache BW. Plasma membrane inositol 1,4,5-trisphosphate-activated channels mediate signal transduction in lobster olfactory receptor neurons. Neuron. 1992;9:907–918. doi: 10.1016/0896-6273(92)90243-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wakabayashi Y, Lippincott-Schwartz J, Arias IM. Intracellular trafficking of bile salt export pump (ABCB11) in polarized hepatic cells: constitutive cycling between the canalicular membrane and rab11-positive endosomes. Mol Biol Cell. 2004;15:3485–3496. doi: 10.1091/mbc.E03-10-0737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee JM, Trauner M, Soroka CJ, Stieger B, Meier PJ, Boyer JL. Expression of the bile salt export pump is maintained after chronic cholestasis in the rat. Gastroenterology. 2000;118:163–172. doi: 10.1016/s0016-5085(00)70425-2. [DOI] [PubMed] [Google Scholar]
- 39.Kubitz R, Wettstein M, Warskulat U, Haussinger D. Regulation of the multidrug resistance protein 2 in the rat liver by lipopolysaccharide and dexamethasone. Gastroenterology. 1999;116:401–410. doi: 10.1016/s0016-5085(99)70138-1. [DOI] [PubMed] [Google Scholar]
- 40.Micheline D, Emmanuel J, Serge E. Effect of Ursodeoxycholic Acid on the Expression of the Hepatocellular Bile Acid Transporters (Ntcp and bsep) in Rats With Estrogen-Induced Cholestasis. J Pediatr Gastroenterol Nutr. 2002;35:185–191. doi: 10.1097/00005176-200208000-00015. [DOI] [PubMed] [Google Scholar]
- 41.Mottino AD, Cao J, Veggi LM, Crocenzi F, Roma MG, Vore M. Altered localization and activity of canalicular Mrp2 in estradiol-17beta-D-glucuronide-induced cholestasis. Hepatology. 2002;35:1409–1419. doi: 10.1053/jhep.2002.33327. [DOI] [PubMed] [Google Scholar]
- 42.Beuers U, Nathanson MH, Boyer JL. Effects of tauroursodeoxycholic acid on cytosolic Ca2+ signals in isolated rat hepatocytes. Gastroenterology. 1993;104:604–612. doi: 10.1016/0016-5085(93)90433-d. [DOI] [PubMed] [Google Scholar]
- 43.Minagawa N, Nagata J, Shibao K, Masyuk AI, Gomes DA, Rodrigues MA, Lesage G, et al. Cyclic AMP regulates bicarbonate secretion in cholangiocytes through release of ATP into bile. Gastroenterology. 2007;133:1592–1602. doi: 10.1053/j.gastro.2007.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pani B, Singh BB. Lipid rafts/caveolae as microdomains of calcium signaling. Cell Calcium. 2009;45:625–633. doi: 10.1016/j.ceca.2009.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Weaver AK, Olsen ML, McFerrin MB, Sontheimer H. BK channels are linked to inositol 1,4,5-triphosphate receptors via lipid rafts: a novel mechanism for coupling [Ca(2+)](i) to ion channel activation. J Biol Chem. 2007;282:31558–31568. doi: 10.1074/jbc.M702866200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rosado JA, Sage SO. Coupling between inositol 1,4,5-trisphosphate receptors and human transient receptor potential channel 1 when intracellular Ca2+ stores are depleted. Biochem J. 2000;350(Pt 3):631–635. [PMC free article] [PubMed] [Google Scholar]
- 47.Murata T, Lin MI, Stan RV, Bauer PM, Yu J, Sessa WC. Genetic evidence supporting caveolae microdomain regulation of calcium entry in endothelial cells. J Biol Chem. 2007;282:16631–16643. doi: 10.1074/jbc.M607948200. [DOI] [PubMed] [Google Scholar]
- 48.Prakash YS, Thompson MA, Vaa B, Matabdin I, Peterson TE, He T, Pabelick CM. Caveolins and intracellular calcium regulation in human airway smooth muscle. Am J Physiol Lung Cell Mol Physiol. 2007;293:L1118–L1126. doi: 10.1152/ajplung.00136.2007. [DOI] [PubMed] [Google Scholar]
- 49.Weerth SH, Holtzclaw LA, Russell JT. Signaling proteins in raft-like microdomains are essential for Ca2+ wave propagation in glial cells. Cell Calcium. 2007;41:155–167. doi: 10.1016/j.ceca.2006.06.006. [DOI] [PubMed] [Google Scholar]