Abstract
BACKGROUND
A majority of cocaine addicts have a comorbid alcohol use disorder. Previous studies demonstrated efficacy of disulfiram in the treatment of cocaine dependence among patients with comorbid alcohol use disorder or opioid dependence. However, the cardiac risks of a disulfiram-ethanol reaction (DER) in individuals who drink, when coupled with the cardiac effects of cocaine, could result in significant toxicity or lethality due to the 3-way drug interaction.
AIMS
This study examined the safety of combining cocaine (30 mg i.v.) and ethanol (0.4 g/kg i.v.) in disulfiram-treated (0, 250, and 500 mg/d, p.o.) cocaine-dependent research volunteers.
RESULTS
The results showed that disulfiram did not enhance the cardiovascular effects of cocaine and may have reduced the subjective high from cocaine. In contrast, ethanol produced adverse ECG changes including QTc prolongation and a DER consisting of hypotension, tachycardia, nausea, and flushing in disulfiram-treated subjects. The severity of the DER was related to disulfiram dose and the trial with 500 mg/d was stopped prematurely due to safety concerns. The DER-related hypotension and tachycardia seen with ethanol infusion alone in disulfiram-treated subjects, was not exacerbated when combined with cocaine. In fact, cocaine tended to counteract the ethanol-related hypotension though it did exacerbate the tachycardia in two of seven subjects.
CONCLUSIONS
Though conclusions are limited by the moderate doses of cocaine, ethanol, and disulfiram tested, the data do suggest that the risks of the moderate use of cocaine and ethanol in individuals treated with moderate doses of disulfiram (≤250 mg/d) may not be as problematic as some may assume.
Keywords: Disulfiram, Alcohol, Cocaine, Treatment, safety, cardiac risk
1. Introduction
According to the National Survey on Drug Use and Heath, 35.2 million Americans, 12 years of age or older, have tried cocaine at least once in their lifetime (5.7 million in the past year and 2.0 million in the past month); and 1.6 million Americans are dependent on cocaine (SAMHSA 2007). The social and economic consequences of cocaine addiction are staggering, with costs upward of 45 billion dollars in the US alone (Sofuoglu and Kosten 2006). However, despite decades of preclinical and clinical research into the causes and treatment of cocaine addiction, no medication has been approved by the Food and Drug Administration (FDA) for the treatment of cocaine dependence.
Cocaine exerts its effects by binding to dopamine, serotonin, and norepinephrine transporters, thereby inhibiting their reuptake into the neurons (Withers et al., 1995; Filip et al., 2005; Sofuoglu and Sewell, 2009). Actions on dopamine and serotonin terminals are thought to contribute to the reinforcing effects of cocaine (Ritz et al., 1988); though actions at norepinephrine terminals may contribute to its sympathomimetic and cardiovascular effects (Lange and Hillis, 2001). Chronic cocaine use can cause marked cardiac and cardiovascular changes, including QT and QRS interval prolongation and arrhythmia, as well as myocardial ischemia and infarction, myocarditis, cardiomyopathy, and thrombosis (Lange and Hillis, 2001).
Disulfiram has been approved by the FDA for the treatment of alcohol abuse and dependence (Suh et al., 2006). It works by inhibiting liver aldehyde dehydrogenase preventing the breakdown of acetaldehyde, a product of ethanol metabolism (Kristenson, 1995; Beyeler et al., 1985). Increased levels of acetaldehyde are responsible for the Disulfiram-Ethanol Reaction (DER) which can produce very unpleasant effects associated with alcohol exposure while taking disulfiram. The most common and rapid onset symptoms of a DER include warmness and flushing of the skin, especially in the upper chest and face and conjunctival injection of the eyes (Kristenson, 1995). Other symptoms include pruritus, hypotension, tachycardia, nausea, vomiting, sweating, dizziness, headache, blurred vision, and confusion and electrocardiogram (ECG) changes have been noted. The intensity of these reactions varies with each individual, but is generally proportional to the doses of disulfiram and alcohol ingested.
Importantly, disulfiram also has shown promise in the treatment of cocaine dependence. Several clinical studies have reported that disulfiram reduces cocaine use and improves abstinence in patients with comorbid alcohol use disorders (Higgins et al., 1993; Carroll et al., 1998; Pettinati et al., 2008). Additionally, disulfiram efficacy to reduce cocaine use also has been observed in patients with primary opioid dependence (George et al., 2000; Petrakis et al., 2000). The mechanism by which disulfiram reduces cocaine use is unknown. It is a non-specific inhibitor of sulfhydryl-containing enzymes found in many biochemical pathways throughout the body. It is known to inhibit liver microsomal carboxylesterases and plasma cholinesterase enzymes which are involved in cocaine metabolism (Faiman, 1979). It also is an inhibitor of the dopamine-β-hydroxylase (DBH) enzyme that converts dopamine to norepinephrine (Goldstein et al., 1964; Musacchio et al., 1964) which has been proposed as one possible mechanism by which disulfiram could reduce the positive or rewarding effects of cocaine (Haile et al., 2009).
Use of disulfiram in the treatment of cocaine dependence has been met with resistance in the clinical community due to the potential for medically serious drug interactions between alcohol and disulfiram in cocaine-dependent individuals (Suh et al., 2006). Notably, the DER alone, and in the absence of cocaine, can be medically serious (Chick, 1999; Wright and Moore, 1990). Even though a placebo-controlled trial observed no significant disulfiram vs. placebo differences in adverse events (AEs) among cocaine-dependent participants who drank alcohol or used cocaine during treatment (Carroll et al., 2004) and reviews of several studies have shown the adverse events seem minimal (Malcolm et al., 2008), there remain concerns about the safety of the DER in cocaine-intoxicated individuals. In particular, cocaine-related cardiac ischemia, tachycardia, and arrhythmia represent potentially serious cardiac risks in the presence of DER-related hypotension and tachycardia. This clinical trial was initiated as part of NIDA’s medications development program in 2005 to ascertain the safety of alcohol use in cocaine-dependent subjects who may use both alcohol and cocaine during disulfiram treatment. The study was designed as a late Phase I inpatient hospital-based safety trial in cocaine-dependent research volunteers.
2. Methods
2.1 Subjects
All subjects for this study were recruited and screened under standard protocols conducted in conformance with ICH-10 guidelines and institutionally-approved by the local ethics board. A total of 22 cocaine-dependent subjects, 18 to 50 years of age, who met DSM-IV criteria for cocaine abuse or dependence and who were not seeking treatment, were randomized to receive placebo or one of two doses of disulfiram (250 or 500 mg/d). Inclusion criteria also required a history of intravenous (IV) cocaine use, twice-weekly use of cocaine (smoked or IV route), alcohol (≥2 drinks) use in 4 of the 6 weeks prior to screening, and required subjects otherwise to be in good health as determined by medical history, physical examination, and laboratory tests. Exclusion criteria included seizure disorder, history of head trauma that resulted in neurological sequelae, dependence on a drug(s) other than cocaine, or previous adverse reaction to cocaine or disulfiram. Female subjects who were pregnant or nursing were also excluded. Using the Structured Clinical Interview for Diagnosis (SCID), subjects could not meet, currently, the diagnostic criteria for the following mental disorders: psychosis, bipolar I disorder, organic brain disease, dementia, major depression, schizoaffective disorder, or schizophrenia. Serious medical conditions, including, but not limited to, heart disease, hypertension, diabetes, asthma, syphilis, AIDS, and peripheral neuropathy or other significant neurological disorders, were also exclusionary. Evidence of previous hepatitis B or C infection was not exclusionary as long as patients were asymptomatic and liver function was within 3x normal limits. Subjects using disulfiram or any medication that could interact adversely with disulfiram, including antidepressants, neuroleptics, anticoagulants, phenytoin, psychotropics, corticosteroids, and xanthenes, were also excluded.
2.2 Study Design
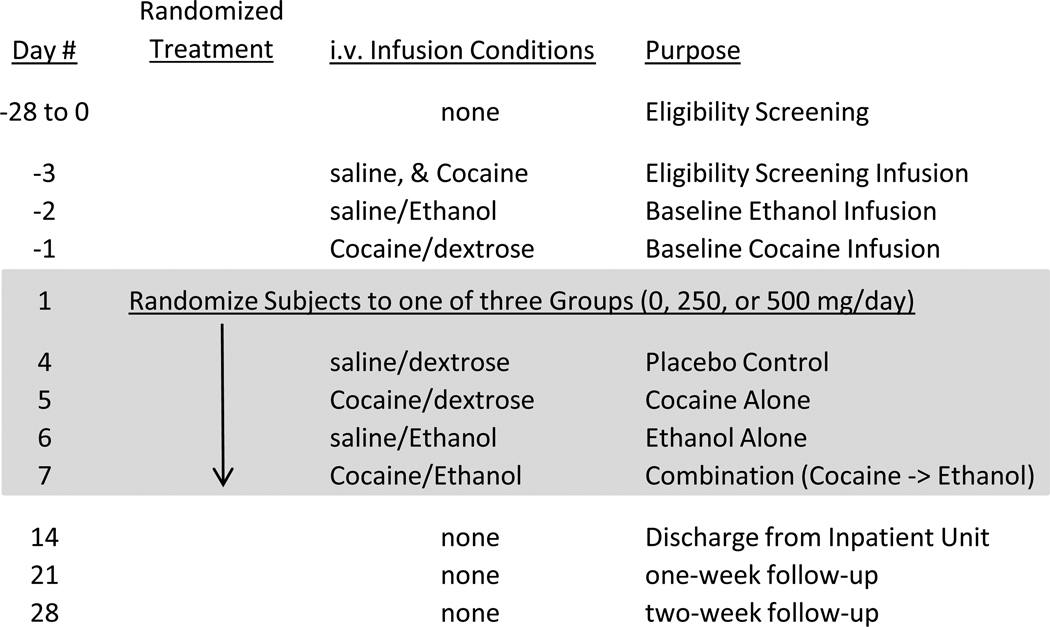
The study was conducted at two sites, the University of Texas Health Science Center at San Antonio (UTHSCSA) and the University of California, Los Angeles (UCLA), using a double-blind, placebo-controlled design. Following inpatient admission, subjects received three i.v., infusion sessions: one screening session to determine the ability of subjects to tolerate 30 mg cocaine, and two baseline sessions, one each for cocaine and ethanol (Figure 1). For safety purposes and by design, all infusion sessions were single-blind and “double-dummy”, i.e., the cocaine infusion was blinded by a “dummy” saline infusion and the alcohol infusion was blinded by a “dummy” dextrose infusion. Cocaine/saline was infused intravenously (i.v.) at 10 a.m., followed by i.v. ethanol/glucose infusions at 10:05 a.m., except on Day -3 (Screening infusion) where saline was given at 10 a.m. followed by cocaine at 11 a.m. For safety purposes, cocaine infusions were stopped if blood pressures exceeded 165 mm Hg systolic or 100 mm Hg diastolic pressure or if heart rate exceeded 130 beats per minute. Likewise, ethanol infusions were stopped if diastolic pressures were < 60 mm Hg or were decreased by more than 15% from baseline or if subjects reported distress leading to an unwillingness to continue.
Figure 1.
Study schematic describing the screening and baseline i.v. infusion conditions, 7 days of randomized oral treatment with disulfiram or placebo combined with four days of cocaine (or saline) and ethanol (or dextrose) infusion, and a two week safety follow-up period.
Following screening and baseline infusions, subjects were randomized to placebo, 250 mg/d disulfiram, or 500 mg/d disulfiram treatment groups; subjects received placebo or disulfiram once daily for 7 days (Days 1–7) under double-blind conditions. The four cocaine/ethanol treatment infusion sessions were administered on Days 4–7 in single-blind fixed order to assure that each subject could tolerate the effects of each drug alone before the combination was administered. After n=4 subjects had been randomized to 500 mg/d disulfiram, a mid-study Data and Safety Monitoring Board (DSMB) review recommended the study be amended to eliminate the 500 mg/d disulfiram group after n=2 subjects were discontinued from the study due to adverse events (AEs). Following this change, subjects were randomized using a blocked randomization schema, stratifying the subjects by clinical site into one of two arms: placebo or 250 mg/d disulfiram.
2.3 Drugs
Disulfiram tablets (250 mg/d) were cut in half and put into two Size 0 opaque gelatin capsules by research pharmacists at each site. Identical placebo tablets were prepared in a similar manner. Disulfiram (250 mg/d or 500 mg/d) was administered orally once daily. The National Institute of Drug Abuse (NIDA) provided the cocaine HCl for human use; sterile physiological saline served as the dummy for cocaine. Cocaine (30 mg/2 mL) was administered intravenously (i.v.) over 60 seconds by continuous infusion pumps. Commercially available ethanol solution (10% by volume) in 5% dextrose was used (D5E10), with 5% dextrose serving as the alcohol dummy. The ethanol/placebo was administered via an IV line infusion pump at a dose of 0.4 g/kg ethanol by varying the volume of the D5E10 solution with a pump flow rate of 30 mL/min for up to 23 minutes. The ethanol dose for women was adjusted to 85% of the dose for men to account for differential sex distributions of muscle mass. Cocaine was administered 5 minutes prior to ethanol administration, with the first cocaine administration scheduled 1.5 hours following the morning dose of disulfiram. One multivitamin tablet per day was dispensed every morning to all subjects during the inpatient phase.
2.4 Outcome Measures
Primary outcome measures for safety evaluation were blood pressure, heart rate, and ECG changes, and assessment of adverse events (AEs). Other outcomes, considered secondary, included physician observer ratings of DER and psychotic symptoms, and subject self-reported ratings of drug effects on 100 mm visual analog rating scales.
AEs were assessed at the end of each day by staff nurses and physicians who queried subjects whether or not they had medical problems during the day. Blood pressure, heart rate, and ECG were collected repeatedly during each infusion session (Sessions 1–7). For the screening infusion session (Session 1), a 12-lead ECG was measured 30 minutes prior to the start of each infusion session and 15 minutes following each infusion; continuous ECG, blood pressure, and heart rate were measured every 2 minutes for 15 minutes before and for at least 20 minutes after each infusion. For all other infusion sessions (Sessions 2–7), ECG was monitored continuously from 15 minutes before to 60 minutes following each infusion with a 12-lead ECG recorded 30 minutes prior to the start of the infusion session, and 15, 25, and 45 minutes after the cocaine infusion. ECG measures included ventricular rate (VR), QT interval corrected by the Bazette method (QTc), QRS, and PR interval. Blood pressures (systolic and diastolic) and heart rate were taken 30 prior to the cocaine infusion, and monitored continuously every 2 minutes from 15 min before until 30 min after i.v. infusion with cocaine and ethanol. Additional blood pressure and heart rate measurements were taken at 45, 60, 90, 120, 180, 240, and 360 minutes post infusion.
The study physician recorded any observed clinical signs of DER, which include conjunctival injection and flushing, using a five point (0–4) scale, which ranges from “no change from baseline” (0) to “flushing extended to the back and arms” (4) (Johnsen et al., 1992). These ratings occurred at 2-minute intervals prior to the initiation of the ethanol/dextrose infusion and continued until the infusion was completed or halted due to the development of DER. In order to measure potential psychotic reactions, the study physician also used a modified Positive Syndrome Rating Scale (mPSRS). The mPSRS was based on clinical interview at 75 min post infusion and was recorded on a on a 7-point scale (1. Not Present; 2. Very Mild; 3. Mild; 4. Moderate; 5. Moderately Severe; 6. Severe; 7. Extremely Severe) for four positive symptoms (1. Suspiciousness; 2. Unusual Thought Content; 3. Hallucinations; 4. Conceptual Disorganization).
At 15 minutes before and 4, 10, 20, 30, 45, 60, 90,120, 180, 240, 360, and 480 minutes after each infusion, subjects used a Visual Analog Scale (VAS) to self-report perceived drug effects on seven 100-mm lines labeled at the left and right-hand extremes with “not at all” to “extremely”, respectively. Subjects placed a vertical slash along the line to indicate the extent to which: “Right Now, I”:‥‥”FEEL EFFECTS of the drug”; “LIKE the DRUG effects that I feel”; “feel REALLY GOOD”; “feel a COCAINE HIGH”; “feel an ALCOHOL BUZZ”; “CRAVE COCAINE”; and “WANT COCAINE”.
2.5 Data analysis
Four subjects were randomized to 500 mg/d disulfiram before DSMB review eliminated this dose from the experimental design. One of these subjects dropped out of the study (for reasons unrelated to the study) after receiving a single randomized dose and two were discontinued from the protocol after a serious hypotensive episode on Day 6. Therefore, data from the 500 mg/d dose are presented descriptively but are excluded from all statistical analyses.
Maximum post-infusion heart rate, systolic blood pressure, and ECG measures, minimum post-infusion diastolic blood pressure, and VAS scores between the placebo and disulfiram groups were compared using repeated-measures analysis of variance (ANOVA), adjusting for average pre-infusion values, disulfiram dose, cocaine and ethanol infusion status, and the two and three-factor interactions between disulfiram dose, cocaine, and ethanol. Because of the small sample sizes, Kenwood-Rogers degrees of freedom adjustment was used. ANOVA results are presented as least squares means by disulfiram dose and cocaine/ethanol infusion status on each of the experimental days. Significant contrasts between cocaine/ethanol infusion treatment days were determined separately with repeated measures ANOVA models: 1) Day 4 (saline/dextrose) versus Day 5 (cocaine/dextrose) to determine if disulfiram modifies the effect of cocaine; 2) Day 4 (saline/dextrose) versus Day 6 (saline/ethanol) to determine if disulfiram modifies the effect of ethanol; 3) Day 5 (cocaine/dextrose) versus Day 7 (cocaine/ethanol) to determine if ethanol modifies the effect of cocaine differentially in disulfiram vs. placebo treated subjects; and 4) Day 6 (saline/ethanol) versus Day 7 (cocaine/ethanol) to determine if cocaine modifies the effect of ethanol differentially in disulfiram vs. placebo treated subjects. Results were analyzed as least squares mean disulfiram-placebo differences between days, with significant differences between days calculated using an F-test. Post-hoc t-tests were used to examine treatment group differences in pre-infusion averages, maximum (or minimum, as appropriate) values post-infusion, and changes from pre- to post-infusion.
3. Results
3.1 Subjects, Demographics, and Drug Use
A total of 22 subjects were randomized to the study (n=9 in both the placebo and 250 mg/d disulfiram group, and n=4 in the 500 mg/d disulfiram group). There were no clinically or statistically significant differences between the three groups on basic demographic, psychiatric, or drug use variables at baseline. Subjects were: mostly male (82%) African-Americans (64%), with ages ranging from 31–48 (mean 40 yrs), mostly unemployed (68%) and unmarried (91%), with 12th grade or GED equivalent education (95%). According to the SCID, all subjects currently met criteria for cocaine abuse or dependence (82% dependent) and 46% met criteria for alcohol use disorder (23% abuse and 23% dependence) though all were able to provide alcohol-free breath samples without significant withdrawal signs. Only 14% currently met criteria for cannabis abuse and only 36% ever did so in the past. There were no other psychiatric diagnoses in these subjects except for 27% showing past, but not current, amphetamine abuse (n=2) or dependence (n=2), or hallucinogen abuse (n=1) or dependence (n=1).
All subjects were current users of cocaine and alcohol (which was an inclusion criteria) and in the last 30 days, used cocaine an average of 19.1 days (range 8–30) and alcohol an average of 20.4 days (range 8–30). Most subjects were tobacco smokers (86%) who smoked most days (mean=26.7 days/30d, range 8–30 for those who smoked) though not all smoked a pack each day (mean 66.9 cigarettes per week with a range of 6–245 cigarettes per week). Only 36.4% reported using marijuana in the last 30 days, and few (13.6%) reported any other drug use.
Of the n=4 subjects randomized to the 500 mg/d disulfiram group, one was discontinued prior to any infusion due to behavior problems unrelated to the study and two subjects were discontinued following a severe decrease in diastolic blood pressure on Day 6. Thus, there was only n=1 subject who provided Day 7 data for the 3-way drug interaction of 500 mg/d disulfiram+cocaine+ethanol. Of the nine placebo subjects, one dropped out for personal reasons after receiving a single dose of randomized placebo resulting in a final n=8 for analysis. Of the nine disulfiram (250 mg/d) subjects, only n=7 were suitable for final statistical analysis because one was discontinued due to adverse ECG changes following the Day 6 infusion and one had an ethanol dosing error resulting in non-pharmacological levels of minimal ethanol exposure (all breath alcohol levels <0.02). Thus, there only n=8 placebo and n=7 disulfiram (250 mg/d) used for inferential statistical analyses which was considered sufficient for a small sample Phase I safety trial.
3.2 Effects of 250 mg/d Disulfiram and Placebo on Blood Pressure and Heart Rate
Systolic Blood Pressure
Cocaine produced post infusion increases in systolic blood pressure in the range of 20–30 mm Hg mean change on every day of cocaine infusion (i.e., Days -3, -2, 5, and 7). These effects were temporary (< 30 min) and there were no significant differences in maximum systolic blood pressure or pre to post randomization changes in systolic pressure observed between subjects receiving placebo and those receiving 250 mg/d disulfiram. Also, there were no differences in the cocaine effect across days of treatment. However, there were trends for the 250 mg/d group of subjects to have higher systolic blood pressures than the placebo subjects at the pre-infusion baseline which were evident prior to randomized treatment with disulfiram but only achieved statistical significance (p<0.05) on Day 6.
Diastolic Blood Pressure
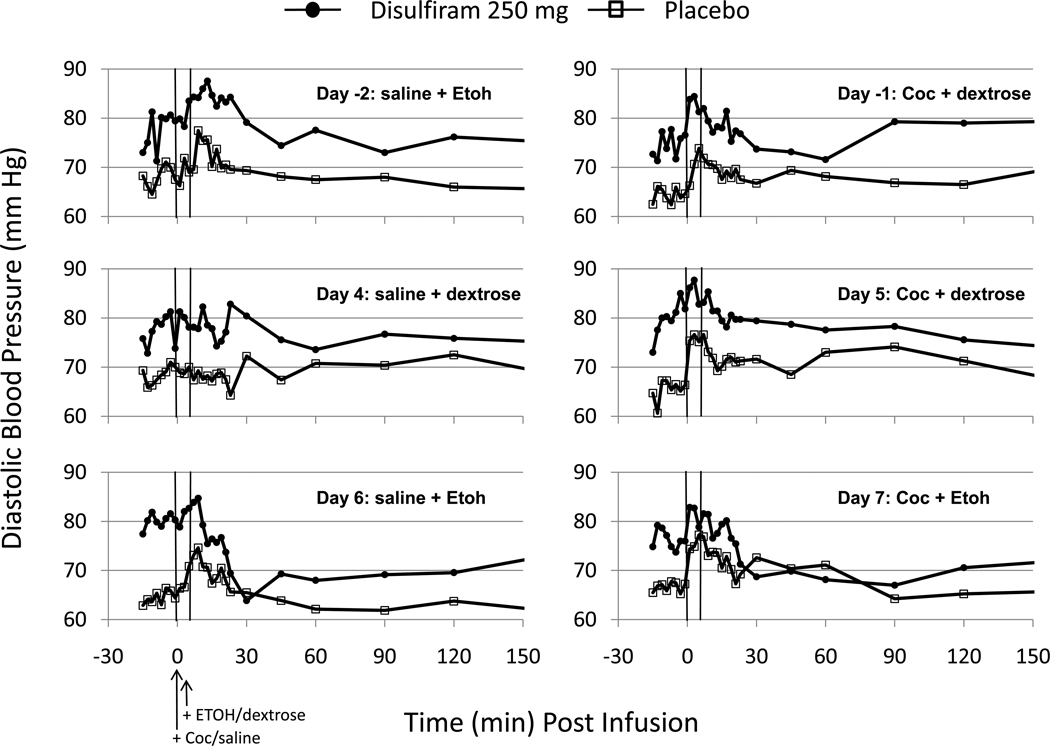
Cocaine infusion increased diastolic blood pressures on Days -1, 5 and 7 (see Fig.2). However, the 250 mg/d group of subjects had higher diastolic blood pressures than the placebo group on most days of the study. However, these were observed prior to randomization (i.e., Days -2, -1) as well as before the i.v. infusion which indicates a baseline difference in the subject groups. The significance of the baseline differences between the 250 mg/d and placebo groups prior to infusion ranged between p=0.083 on Day -2 and p=.004 on Day 6. The minimum diastolic blood pressures (i.e., decreases) were used to statistically examine the DERs in subjects treated with placebo or 250 mg/d disulfiram. The 250 mg/d group of subjects showed a Pre-Post infusion decrease in diastolic pressure that was significantly greater (p<0.005) than placebo when ethanol was infused on Day 6 - although this effect (p<0.10) was less on Day 7 when cocaine was added.
Figure 2.
Diastolic Blood Pressures (mm Hg) observed on each infusion day before (Days -2 and -1) and after (Days 4–7) randomized treatment with disulfiram (250 mg/d) or placebo. The infusion conditions for each day are shown within each panel and their timing is marked by the two vertical lines. A 2 ml volume of cocaine (Coc: 30 mg) or saline was infused over 60 sec beginning at time 0. At +5 min, ethanol (Etoh) or dextrose infusions were begun. The ethanol dose was 0.4 g/kg and was delivered at the rate of 30 ml/min of a 10% solution in dextrose.
Heart Rate
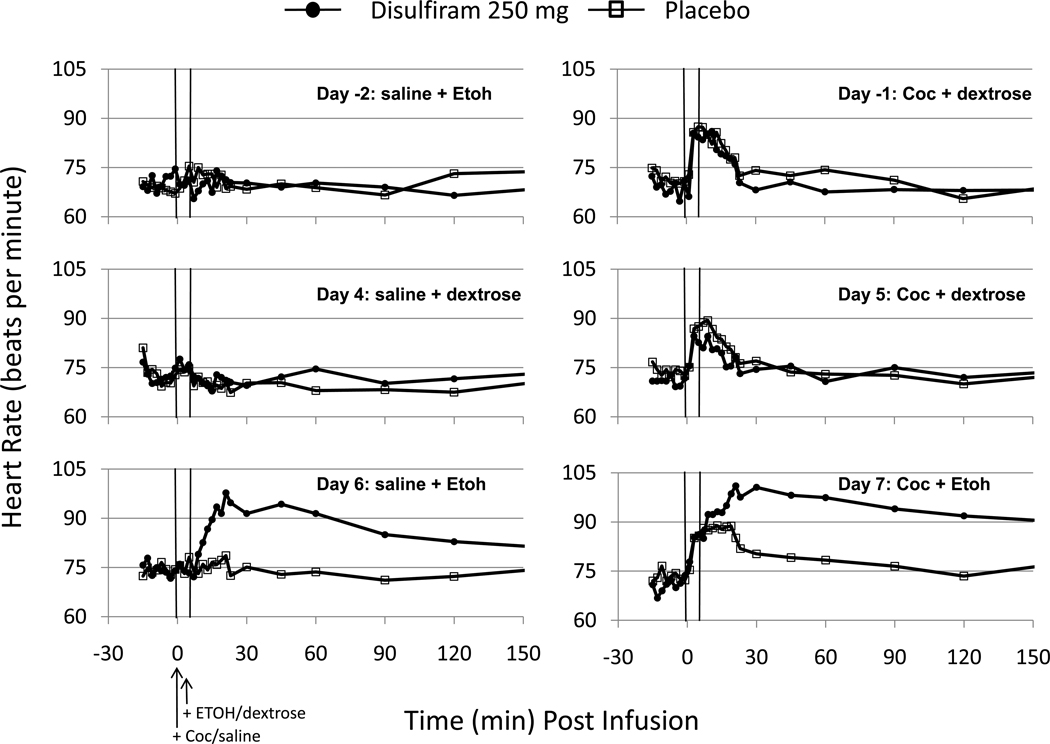
Cocaine infusion on Days -1, 5, and 7 increased heart rate temporarily over the 30 min Post-Infusion period (see Fig.3) and there was no difference between the placebo and 250 mg/d groups before (Day -1) or after (Day 5) randomization. However, disulfiram-treated subjects showed significant and sustained elevations in heart-rate (tachycardia) following ethanol infusion on Days 6 and 7. Compared to Day 6, mean heart rate increases on Day 7 showed an earlier onset and longer duration due to the cocaine infusion. Across individual subjects, all but one showed the earlier onset of heart rate increase due to cocaine infusion 5 min before the ethanol infusion. However, the apparent longer duration of heart rate increase on Day 7 vs. Day 6 was due primarily to one subject who showed a prolonged period of tachycardia from 3–240 minutes and reaching a peak of 166 beats per minute (bpm) at 30 minutes. Only two other subjects showed more brief periods of tachycardia (at 106 and 107 bpm, respectively) and for only one of those subjects heart rate increases were greater on Day 7 vs. Day 6. All other subjects showed heart rate increases which peaked below 100 bpm.
Figure 3.
Heart Rates (beats per min) observed on each infusion day before (Days -2 and -1) and after (Days 4–7) randomized treatment with disulfiram (250 mg/d) or placebo. Other details are the same as in Fig.2.
3.3 Effects of 500 mg/d Disulfiram
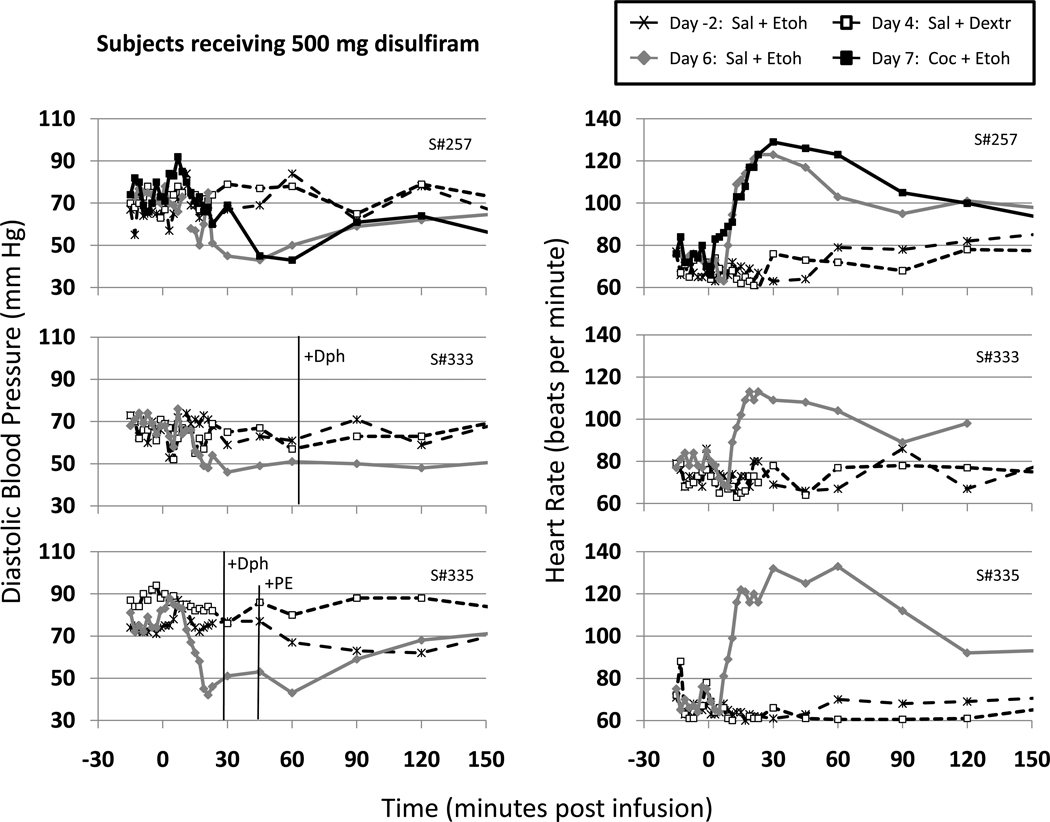
Two subjects (#333, #335) in the 500 mg/d disulfiram group had to be removed from the study following ethanol infusion on Day 6 due to sustained decreases in diastolic BP (≤60mm) lasting greater than 30 minutes (see Fig.4). Combined with the diastolic hypotension, both subjects showed QTc prolongation > 450 ms; and one subject (S#333) experienced ST/T wave changes, while the other individual (S#335) reported a +4 DER with flushing extending to the back and arms. Both of these subjects received diphenhydramine (Dph: 50 mg i.v.) to treat the DER and #335 received seven injections of phenylephrine (PE: 0.05 mg i.v. each) to maintain blood pressure. Subject #257 also showed sustained decreases in diastolic blood pressure, but there were no other clinically-significant cardiac changes, and his responses were judged to be not serious enough to discontinue protocol or to necessitate treatment. All three subjects received O2 and partial Trendelenburg position to compensate for the systemic hypotension and all showed compensatory tachycardia. In the one subject who received Day 7 infusions, cocaine only slightly extended the duration of observed ethanol-related tachycardia.
Figure 4.
Diastolic pressures (mmHg) and heart rates (beats per min) for the three subjects (#257, #333, and #335) treated with 500 mg/d disulfiram who received ethanol infusion on Day 6. Data shown are for the pre-randomization baseline ethanol infusion (Day -2: Sal+Etoh) and post-randomization control infusion (Day 4: Sal+Dextr), and ethanol infusions on Days 6 (Sal+Etoh) and 7 (Coc+Etoh). Note that only S#257 received infusions on Day 7 and that S#333 and #335 received rescue medication (+Dph=diphenhydramine and +PE=phenylephrine) on Day 6 due to DER. Other details are the same as in Fig.2.
Cocaine alone, administered on Day 5 (data not shown) to the three subjects receiving 500 mg/d disulfiram, showed typical cocaine-related increases in heart rate (30 bpm) and systolic blood pressure (30 mm Hg) but compared to the Day -1 baseline assessment, these cocaine effects were not changed (p > 0.10) by five days of disulfiram treatment.
3.4 Electrocardiogram (ECG) effects of Disulfiram
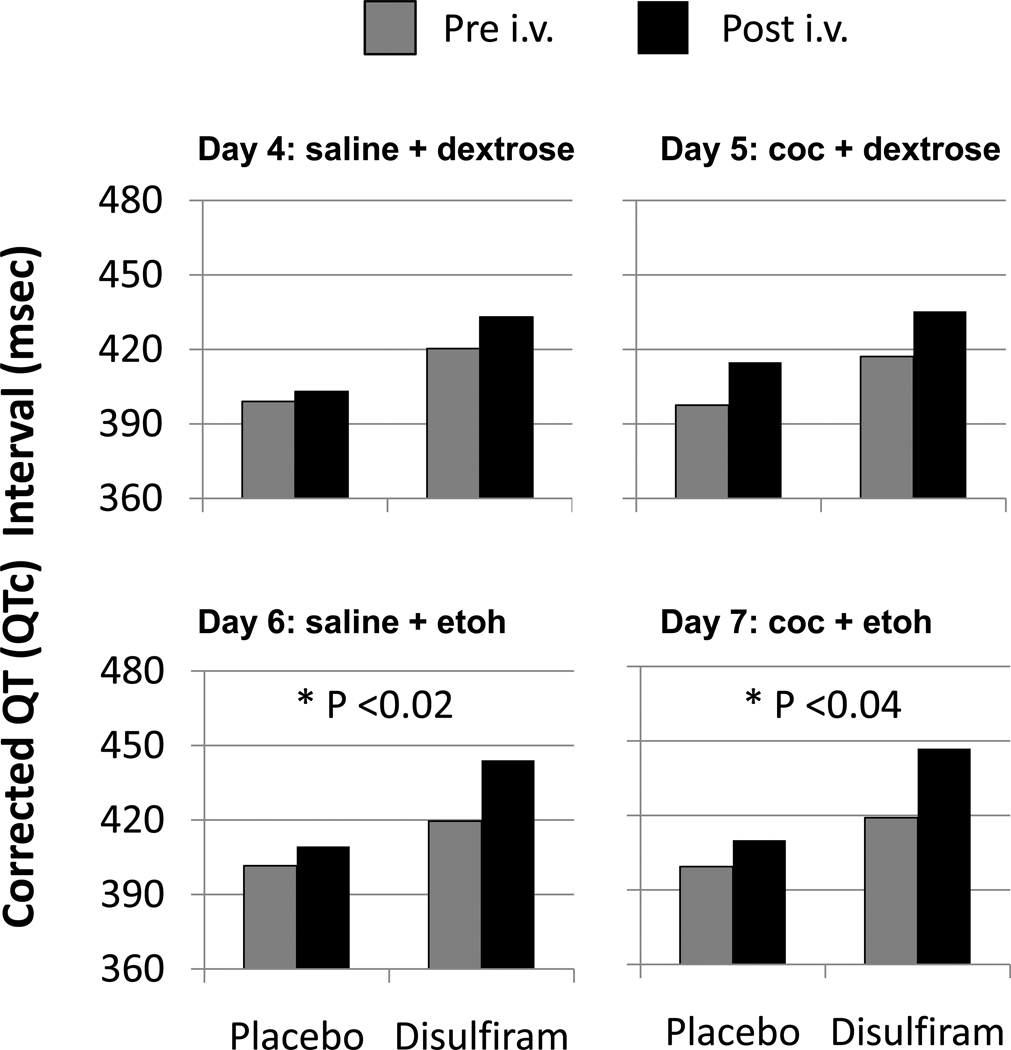
Repeated 12-lead ECG’s were collected on each of the i.v. infusion days. Prior to randomized disulfiram/placebo treatment (on Days -2 and -1, respectively), the 250 mg/d Disulfiram group had a significantly higher (p<0.03) mean corrected QT (QTc) interval and lower QRS (p<0.05) interval than the placebo group prior to any i.v. infusion. Generally, these group differences in cardiac parameters were not clinically significant or associated with symptoms and tended to be maintained on Day 4 (placebo infusion) and Day 5 (cocaine infusion) where there were no significant group differences in the response to cocaine infusion. However, following ethanol infusion on both Days 6 and 7 (see Fig.5), the 250 Disulfiram group showed significantly greater increases in QTc than did placebo subjects. On an individual subject basis, only two placebo-treated subjects ever showed an elevated QTc > 450 ms, and these occurred on Day 5 and Day 6, respectively, after cocaine administration. Five of the subjects treated with 250 mg/d showed cocaine-related elevations in QTc > 450 ms on Day -3 prior to randomized treatment with disulfiram. After treatment with 250 mg/d disulfiram, four of those five also showed elevated QTc on Day 6 (after ethanol infusion) and three of those four also showed it on Day 7 (after cocaine+ethanol) but only two of these subjects showed elevated QTc due to cocaine alone on Day 5.
Figure 5.
Corrected QT (QTc) Intervals (msec) observed before (Pre) and after (Post) the i.v. cocaine(coc)/saline and ethanol(etoh)/dextrose infusions on Days 4–7 for subjects receiving placebo (n=8) or 250 mg/d disulfiram (n=7). * P indicates the significance of the Disulfiram vs. placebo difference in the Post-Pre changes in QTc.
The most commonly observed abnormality was sinus tachycardia which was seen only in disulfiram-treated subjects following ethanol infusion. This was seen in the two subjects treated 500 mg/d disulfiram who were discontinued from the protocol after serious ethanol-related hypotension and DER on Day 6. After 250 mg/d disulfiram, ethanol-related sinus tachycardia was seen in three of nine subjects on Days 6 or 7 though none of these subjects were discontinued from the study. A fourth subject in the 250 mg/d disulfiram group exhibited transitory RSR(QR) changes and rhythm abnormalities on Day 6 and a possible ventricular conduction delay and sinus tachycardia on Day 7 which was associated with “shortness of breath”. A fifth subject receiving 250 mg/d showed a premature ventricular contraction on Day 5 following cocaine infusion, but no other ECG abnormalities on any other day. For the placebo-treated subjects, only one exhibited ECG changes and that was a supraventricular tachycardia after infusion of the cocaine + ethanol combination on Day 7. In no other subjects from any other dosage group, were the ECG changes on Day 7 any worse than observed following ethanol on Day 6.
3.5 Adverse Events
There were non-cardiac adverse events (AEs) reported during the study that were judged to be “at least possibly related” to the study procedures. A total of 15 subjects experienced a total of 77 adverse events at some point during the study. Most of these were mild or moderate in severity and only three subjects reported clinically serious reactions after disulfiram treatment. Eight subjects experienced 16 events during the Baseline Infusion period (Days -3 to -1) mostly related to the i.v. procedures (i.e., pain, tenderness). During the first four days of disulfiram/placebo dosing and before any i.v. infusion of cocaine or ethanol, five subjects reported six AEs, the most significant of which were two subjects from the 250 mg/d disulfiram group and two from the 500 mg/d group reporting “headache”. On the first day of cocaine infusion (Day 5), one subject in each of the three dosage groups reported a “headache” and one subject in each of the two disulfiram groups reported feeling “sweaty”. After ethanol infusion on Day 6, one subject receiving 250 mg/d disulfiram experienced a moderate DER including nausea, headache, chest tightness, and tachycardia symptoms. This individual was discontinued from the Day 7 protocol because of distress about his Day 6 DER in combination with ST-segment and other irregularities in his ECG prior to the Day 7 infusion. Two subjects receiving 500 mg/d disulfiram showed evidence of a DER on Day 6 – one of which was severe (+4 DER on a 4 point scale). Both of these subjects were discontinued from the Day 7 procedures because of their sinus tachycardia and diastolic hypotension and the one with the most severe reaction continued to show delayed DER symptoms into the subsequent day. Interestingly, 3 of 8 placebo-treated subjects also experienced AEs after ethanol infusion on Day 6 which even included flushing, nausea, and hypotension. On the day of cocaine + ethanol infusion (Day 7), the AE profile actually was greater in the placebo-treated subjects than in the disulfiram subjects and looked like the combination of the events seen individually with cocaine and ethanol. Of course, the three subjects (one 250 mg/d and two 500 mg/d) who were discontinued after DER on Day 6 are not represented in the Day 7 analysis.
3.6 Subject-Rated Visual Analog Scales (VAS)
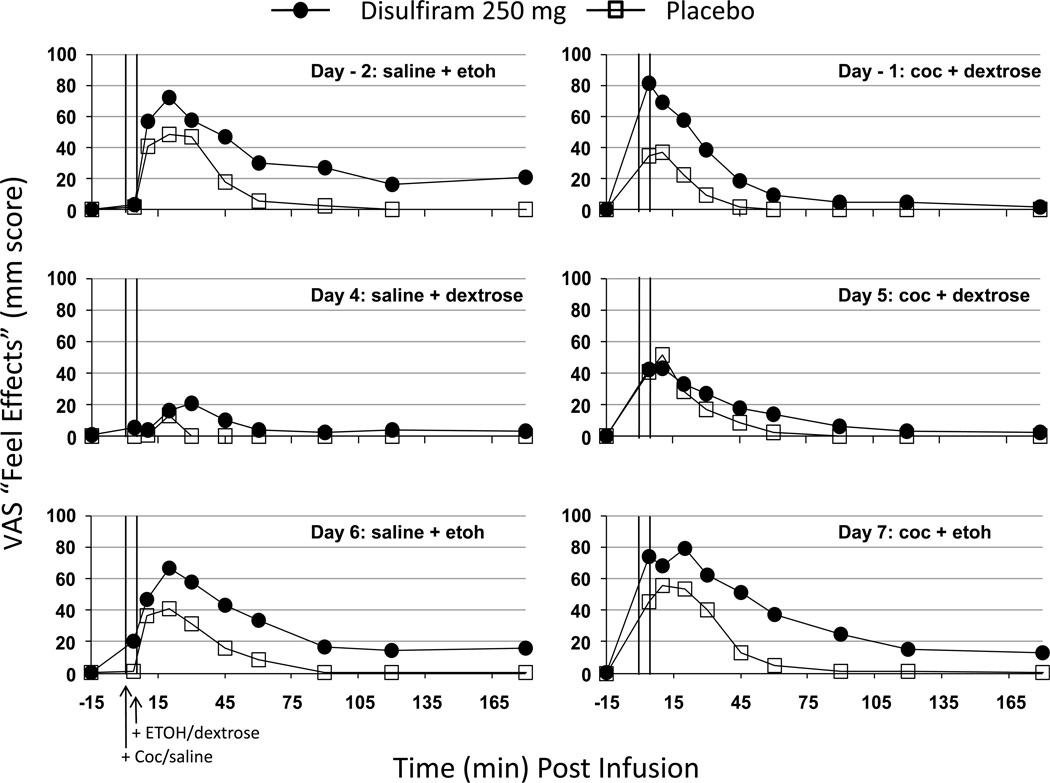
Both cocaine and alcohol showed typical drug-related increases in subjective ratings of “feel effects” and “like effects”. On days of cocaine administration (Days -1, 5, 7), cocaine increased ratings of “cocaine high” while on days of ethanol administration (Days -2, 6, 7), ethanol increased “alcohol buzz”. There were no significant effects of either cocaine or alcohol to increase ratings of “crave cocaine” or “want cocaine”. Figure 6 shows the subjective effects of cocaine and ethanol on the “feel effects” measure because it illustrates the observed effects of both ethanol and cocaine. Prior to randomized treatment with disulfiram or placebo, (Days -2 and -1), the disulfiram group tended to show larger effects of the ethanol and cocaine infusions than the placebo group which achieved statistical significance (p<0.03) following cocaine dosing on Day -1 but not after ethanol infusion on Day -2 (p>0.30). After randomized treatment with disulfiram or placebo, there clearly was no group difference in the cocaine response observed on Day 5 (p>0.70) and only a non-significant tendency (p>0.30) for the disulfiram group to show larger “feel effects” of the combined cocaine + ethanol infusion on Day 7. Similar subjective responses to cocaine in the disulfiram group, were also seen in the “cocaine high” measure (data not shown). Ethanol-related “feel effects” were the same before (i.e., Day -2) vs. after (Day 6) disulfiram or placebo treatment and the two groups were never significantly different in their ethanol response. Roughly, these same patterns of cocaine responses were observed for “like effects”, “cocaine high”, and “alcohol buzz” though the apparent disulfiram vs. placebo group differences prior to randomization were less marked and never achieved statistical significance on any day (before or after randomization).
Figure 6.
Subject-rated “Feel Effects” on each infusion day before (Days -2 and -1) and after (Days 4–7) randomized treatment with disulfiram (250 mg/d) or placebo. Other details are the same as in Fig.2.
3.7 Physician Ratings
Study physicians observed some signs of DER-related facial flushing or conjectival injection in a total of 14 subjects. Three of those were placebo-treated subjects where a +1 rating (on a 0–4 scale) was observed - though for one of these subjects, facial flushing occurred only during the Day -2 baseline. All three subjects treated with 500 mg/d disulfiram showed a flushing response on Day 6 and it reached a +4 severity on subject #335 but only a +1 severity on the other two subjects –though subject #257 showed a +2 rating on Day 7. All of the subjects treated with 250 mg/d disulfiram showed a physician-rated flushing response of at least +1 on Days 6 or 7 but only two subjects showed a +2 rating and this occurred on Day 6. Only four subjects showed any elevation on the mPSRS and all of these were observed following cocaine infusion. Two of those four subjects showed increased “suspiciousness” but it occurred during the baseline infusions of cocaine prior to but not following randomized treatment with disulfiram. A third subject showed increased “suspiciousness” on Day 5 after 250 mg/d disulfiram. The fourth subject reported “unusual thought content” following Day -1 baseline cocaine infusion prior to 250 mg/d disulfiram treatment and “conceptual disorganization” on Day 7 following the combined cocaine+ethanol infusion.
4. Discussion
The safety of combined alcohol and cocaine use in cocaine-dependent subjects who received 5–7 days of treatment with oral doses of 0, 250, or 500 mg/d disulfiram was examined by measuring the cardiovascular, cardiac, and psychiatric responses, as well as the frequency of adverse events. The results demonstrated that alcohol doses of 0.4 g/kg i v., produced a DER in subjects treated with disulfiram; and this effect was related to disulfiram dose. A few subjects in each disulfiram dose group reported discomfort due to the DER (primarily headache, and feeling hot and flushed, with some nausea). Also, statistically-significant ECG changes including increased QTc intervals were observed with ethanol administration in the 250 mg/d disulfiram group and medically serious decreases in diastolic blood pressure with compensatory increases in heart rate were observed with ethanol after 500 mg/d disulfiram. At the 500 mg/d dose of disulfiram, 2 of 3 subjects exposed to ethanol were discontinued from the study due to these effects and consequently never received the drug combination of cocaine+ethanol. Only one subject received the 3-way combination of 500 mg/d disulfiram+cocaine+ethanol and this individual was asymptomatic except for the hypotension and tachycardia which were supportively managed and were not substantially different than the effects observed with ethanol alone. Cocaine doses of 30 mg i.v. given alone produced increases in blood pressure and heart rate but there were no significant ECG changes and there was no evidence that disulfiram potentiated any of these effects of cocaine. Other adverse events were noted in the study, but these generally were mild to moderate in severity and primarily were related to the ethanol and cocaine i.v. infusion procedures with no apparent changes introduced by disulfiram treatment.
Our results indicate that 30 mg i.v. cocaine was safely administered to cocaine-dependent research volunteers in the presence of 250 and 500mg disulfiram. Although we did observe cocaine-related increases in heart rate and blood pressure which are typical for this dose, significant differences between the placebo- and disulfiram-treated groups were not found indicating that disulfiram did not potentiate the cardiovascular effects of cocaine in any clinically-significant way. We also observed cocaine-related increases in subjective effects measured on a visual analog scale, and disulfiram did not potentiate those effects either. Comparisons of cocaine effects before vs. after randomized treatment suggested that disulfiram may have reduced the magnitude of self-reported “feel effects” and “cocaine high”. However, this finding does not generalize broadly to other subjective or cardiovascular effects of cocaine. Disulfiram has been reported to modestly increase the cardiovascular effects of intranasal cocaine without enhancing subjective euphoria (McCance-Katz et al., 1998a,b). However, others have reported that disulfiram did not alter the cardiovascular effects of i.v. cocaine infusion (Hameedi et al., 1995; Baker et al., 2007) and Baker and colleagues (2007) reported reductions in cocaine-related “high” and “rush” due to low doses of disulfiram. Disulfiram is known to inhibit cocaine metabolism and increase cocaine plasma levels (Baker et al., 2007; McCance-Katz et al., 1998a) and we found evidence of increased cocaine plasma levels in this study (data not shown), however, this effect clearly did not potentiate the cardiovascular effects of cocaine. We did not test higher doses of cocaine or disulfiram to determine whether or not cocaine-related effects would have been potentiated under higher dose conditions. However, previous work with cocaine-related binge dosing patterns resulting in much higher cocaine levels (Foltin et al., 1991) observed tachyphylaxis which could mitigate against observing increased effects when cocaine metabolism is inhibited. Though paranoid or psychotic-like reactions to cocaine have been reported following disulfiram treatment (Mutschler et al., 2009), we did not observe any clinically-significant adverse psychiatric disturbances using a clinician-rated psychotic rating scale (mPSRS).
Ethanol administration (0.4 g/kg, i.v.) to disulfiram-treated subjects resulted in an observable and subjectively uncomfortable DER involving nausea and flushing in only a minority of subjects. However, ethanol produced statistically-significant hypotension and compensatory tachycardia in subjects treated with 250 mg/d disulfiram and which were clinically serious at the 500 mg/d disulfiram dose. Though our sample sizes were small (i.e., n=7 for 250 mg/d and n=3 for 500 mg/d) and idiosyncratic sensitivity can produce substantial between subject differences, both the frequency and severity of the DER and associated cardiovascular changes appeared to be related to disulfiram dose. Previous studies have shown that the probability and severity of the DER is related to disulfiram and ethanol dose (Beyeler et al., 1985; Christensen et al., 1991; Johnsen et al., 1992) and disulfiram doses greater than 500 mg/d may be required to reliably obtain a DER in some alcohol dependent outpatients (Brewer, 1984). These data suggest that doses of 250 mg/d disulfiram or less may have a reduced, but not zero risk of DER for subjects who drink. In the current study, ethanol administration also increased QTc intervals in the ECG after disulfiram treatment and the moderate doses of cocaine tested did not affect this risk. Serious QTc prolongation and potentially lethal cardiac effects of alcohol in disulfiram-treated subjects have been known for a long time (Chick, 1999; Wright and Moore, 1990). Importantly though, clinical trials with disulfiram in the treatment of cocaine dependence with comorbid alcohol use disorder has not evidenced serious adverse or cardiac events (Carroll et al., 1998; Malcolm et al., 2008; Pettinati et al., 2008).
The present study did not demonstrate any cocaine enhancement of the adverse effects of the DER. Although there was a tendency for cocaine to enhance the duration of ethanol-related tachycardia, it also significantly reduced the blood pressure reductions produced by ethanol in subjects treated with 250 mg/d disulfiram. There was also a tendency for ECG abnormalities to be more frequently observed after ethanol on Day 6 than after the cocaine-ethanol combination on Day 7. Of course, the discontinuation of 2 out of 3 of the subjects treated with 500 mg/d disulfiram and the resultant change in study design by the DSMB eliminated any further exploration of how cocaine may or may not alter the DER at the higher disulfiram dose. Certainly, the greatest concern and the primary purpose of this study was to determine how the ethanol-related DER might alter the cardiac risks of cocaine in cocaine dependent individuals. Again, we didn’t find evidence that the DER increased adverse effects of cocaine. Though cocaine increased blood pressure and heart rate, the pressor effects were counteracted and the tachycardia was enhanced by the DER in ways that appeared simply additive and not of great clinical significance. Although the subject population tended to have several ECG abnormalities at baseline, the administration of 30 mg i.v. cocaine alone did not produce many clinically-significant changes in the ECG and neither disulfiram treatment alone nor the combination with ethanol on Day 7 showed an enhancement of cocaine-related ECG disturbance beyond the effects of the ethanol alone. Specifically, ethanol infusion resulted in QTc prolongation in disulfiram-treated subjects and the addition of cocaine did not alter that effect. Nonetheless, the questions of whether or not DER-related hypotensive crises, or compensatory tachycardia, or QTc prolongation are dangerous changes that put cocaine-intoxicated subjects at increased risk of other cardiac events including serious arrhythmia or infarction are not answered questions. Conclusions regarding these medical concerns are limited by our inability to test higher doses of disulfiram or ethanol or cocaine due to safety concerns for human subjects in clinical research studies.
In summary, 5–7 days of oral dosing with 250 or 500 mg/d disulfiram in a double-blind placebo-controlled, Phase I safety trial resulted in few side effects or problems in cocaine-dependent research volunteers. A moderate dose of cocaine (30 mg i.v.) produced typical cocaine-related increases in subjective effects, blood pressure, and heart rate with no evidence that disulfiram treatment exacerbated adverse effects of cocaine. In contrast, a moderate dose of ethanol (0.4 g/kg, i.v.) produced DERs related to disulfiram dose involving flushing, nausea, clinically-significant hypotension, tachycardia, and statistically-significant QTc prolongation. These DER symptoms were serious enough to preclude further study at the 500 mg/d dose of disulfiram but in the few subjects who received either 250 or 500 mg/d disulfiram combined with both cocaine and ethanol, there was no evidence that cocaine exacerbated the adverse effects of ethanol and there was evidence the hypertensive effects of cocaine and the hypotensive effects of ethanol tend to counteract one another. Generally, we found that at the doses tested, the combinations of cocaine and ethanol in disulfiram-treated individuals were no worse than either cocaine or ethanol alone. These conclusions are limited by the moderate doses of cocaine and ethanol tested in this Phase I safety trial. Additionally, the small group sample sizes in whom we conducted multiple assessments of possible safety outcomes, limits the generality or reliability of both our positive and negative results for the broader population of cocaine dependent patients treated with disulfiram. Nonetheless, the data are consistent with the suggestion (Malcolm et al., 2008) that the risks of the moderate use of cocaine combined with ethanol in patients treated with a moderate dose of disulfiram (≤250 mg/d) may be clinically manageable with few serious side effects. Further, we would suggest that if higher doses of disulfiram were to be considered, then the risks of a more serious DER would increase.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baker JR, Jatlow P, McCance-Katz EF. Disulfiram effects on responses to intravenous cocaine administration. Drug Alcohol Depend. 2007;87:202–209. doi: 10.1016/j.drugalcdep.2006.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyeler C, Fisch HU, Preisig R. The disulfiram-alcohol reaction: factors determining and potential tests predicting severity. Alcohol. Clin. Exp. Res. 1985;9:118–124. doi: 10.1111/j.1530-0277.1985.tb05531.x. [DOI] [PubMed] [Google Scholar]
- Brewer C. How effective is the standard dose of disulfiram? A review of the alcohol-disulfiram reaction in practice. Br. J. Psychol. 1984;144:200–202. doi: 10.1192/bjp.144.2.200. [DOI] [PubMed] [Google Scholar]
- Carroll KM, Fenton LR, Ball SA, Nich C, Frankforter TL, Shi J, Rounsaville BJ. Efficacy of disulfiram and cognitive behavior therapy in cocaine-dependent outpatients: a randomized placebo-controlled trial. Arch. Gen. Psychiatry. 2004;61:264–272. doi: 10.1001/archpsyc.61.3.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll KM, Nich C, Ball SA, McCance E, Rounsaville BJ. Treatment of cocaine and alcohol dependence with psychotherapy and disulfiram. Addiction. 1998;93:713–727. doi: 10.1046/j.1360-0443.1998.9357137.x. [DOI] [PubMed] [Google Scholar]
- Chick J. Safety issues concerning the use of disulfiram in treating alcohol dependence. Drug Safety. 1999;20:427–435. doi: 10.2165/00002018-199920050-00003. [DOI] [PubMed] [Google Scholar]
- Christensen JK, Moller IW, Ronsted P, Angelo HR, Johansson B. Dose-effect relationship of disulfiram in human volunteers. I: clinical studies. Pharmacol. Toxicol. 1991;68:163–165. doi: 10.1111/j.1600-0773.1991.tb01215.x. [DOI] [PubMed] [Google Scholar]
- Filip M, Frankowska M, Zaniewska M, Golda A, Przegalinski E. The serotonergic system and its role in cocaine addiction. Pharmacol. Rep. 2005;57:685–700. [PubMed] [Google Scholar]
- Faiman MD. Biochemical pharmacology of disulfiram. In: Majchowicz E, Nobel EP, editors. Biochemistry and Pharmacology of Ethanol. Vol. 2. New York: Plenum Press; 1979. pp. 325–348. [Google Scholar]
- Foltin RW, Fischman MW. Smoked and intravenous cocaine in humans: acute tolerance, cardiovascular and subjective effects. J. Pharmacol. Exp. Ther. 1991;257:247–261. [PubMed] [Google Scholar]
- George TP, Chawarski MC, Pakes J, Carroll KM, Kosten TR, Schottenfeld RS. Disulfiram versus placebo for cocaine dependence in buprenorphine-maintained subjects: a preliminary trial. Biol. Psychiatry. 2000;47:1080–1086. doi: 10.1016/s0006-3223(99)00310-8. [DOI] [PubMed] [Google Scholar]
- Goldstein M, Anagnoste B, Lauber E, McKeregham MR. Inhibition of dopamine-beta-hydroxylase by disulfiram. Life Sci. 1964;3:763–767. doi: 10.1016/0024-3205(64)90031-1. [DOI] [PubMed] [Google Scholar]
- Haile CN, Kosten TR, Kosten TA. Pharmacogenetic treatments of drug addiction: cocaine, amphetamine, and methamphetamine. Am. J. Drug Alcohol Abuse. 2009;35:161–177. doi: 10.1080/00952990902825447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hameedi FA, Rosen MI, McCance-Katz EF, McMahon TJ, Price LH, Jatlow PI, Woods SW, Kosten TR. Behavioral, physiological, and pharmacological interaction of cocaine and disulfiram in humans. Biol. Psychiatry. 1995;37:560–563. doi: 10.1016/0006-3223(94)00361-6. [DOI] [PubMed] [Google Scholar]
- Higgins ST, Budney AJ, Bickel WK, Hughes JR, Foerg F. Disulfiram therapy in patients abusing cocaine and alcohol. Am. J. Psychiatry. 1993;150:675–676. doi: 10.1176/ajp.150.4.675b. [DOI] [PubMed] [Google Scholar]
- Johnsen J, Stowell A, Morland J. Clinical responses in relation to blood acetaldehyde levels. Pharmacol. Toxicol. 1992;70:41–45. doi: 10.1111/j.1600-0773.1992.tb00423.x. [DOI] [PubMed] [Google Scholar]
- Kristenson H. How to get the best out of antabuse. Alcohol Alcohol. 1995;30:775–783. [PubMed] [Google Scholar]
- Lange RA, Hillis LD. Cardiovascular complications of cocaine use. N. Engl. J. Med. 2001;345:351–358. doi: 10.1056/NEJM200108023450507. [DOI] [PubMed] [Google Scholar]
- Malcolm R, Olive MF, Lechner W. The safety of disulfiram for the treatment of alcohol and cocaine dependence in randomized clinical trials: guidance for clinical practice. Expert Opin.Drug Saf. 2008;7:459–472. doi: 10.1517/14740338.7.4.459. [DOI] [PubMed] [Google Scholar]
- McCance-Katz EF, Kosten TR, Jatlow P. Disulfiram effects on acute cocaine administration. Drug Alcohol Depend. 1998a;52:27–39. doi: 10.1016/s0376-8716(98)00050-7. [DOI] [PubMed] [Google Scholar]
- McCance-Katz EF, Kosten TR, Jatlow P. Chronic disulfiram treatment effects on intranasal cocaine administration: initial results. Biol. Psychiatry. 1998b;43:540–543. doi: 10.1016/S0006-3223(97)00506-4. [DOI] [PubMed] [Google Scholar]
- Musacchio J, Kopin IJ, Snyder S. Effects of disulfiram on tissue norepinephrine content and subcellular distribution of dopamine, tyramine and their beta-hydroxylated metabolites. Life Sci. 1964;3:769–775. doi: 10.1016/0024-3205(64)90032-3. [DOI] [PubMed] [Google Scholar]
- Mutschler J, Diehl A, Kiefer F. Pronounced paranoia as a result of cocaine-disulfiram interaction: case report and mode of action. J. Clin. Psychopharm. 2009;29:99–101. doi: 10.1097/JCP.0b013e3181934451. [DOI] [PubMed] [Google Scholar]
- Pettinati HM, Kampman KM, Lynch KG, Xie H, Dackis C, Rabinowitz AR, O'Brien CP. A double-blind, placebo-controlled trial that combines disulfiram and naltrexone for treating co-occurring cocaine and alcohol dependence. Addict. Behav. 2008;33:651–667. doi: 10.1016/j.addbeh.2007.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrakis IL, Carroll KM, Nich C, Gordon LT, McCance-Katz EF, Frankforter T, Rounsaville BJ. Disulfiram treatment for cocaine dependence in methadone-maintained opioid addicts. Addiction. 2000;95:219–228. doi: 10.1046/j.1360-0443.2000.9522198.x. [DOI] [PubMed] [Google Scholar]
- Ritz MC, Lamb RJ, Goldberg SR, Kuhar MJ. Cocaine self-administration appears to be mediated by dopamine uptake inhibition. Prog. Neuro-Psy. Biol. Psy. 1988;12:233–239. doi: 10.1016/0278-5846(88)90040-1. [DOI] [PubMed] [Google Scholar]
- SAMHSA. National Survey on Drug Use and Health. Rockville, MD: 2007. [Google Scholar]
- Sofuoglu M, Kosten TR. Emerging pharmacological strategies in the fight against cocaine addiction. Expert Opin. Emerg. Drugs. 2006;11:91–98. doi: 10.1517/14728214.11.1.91. [DOI] [PubMed] [Google Scholar]
- Sofuoglu M, Sewell RA. Norepinephrine and stimulant addiction. Addict. Biol. 2009;14:119–129. doi: 10.1111/j.1369-1600.2008.00138.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh JJ, Pettinati HM, Kampman KM, O'Brien CP. The status of disulfiram: a half of a century later. J. Clin. Psychopharm. 2006;26:290–302. doi: 10.1097/01.jcp.0000222512.25649.08. [DOI] [PubMed] [Google Scholar]
- Withers NW, Pulvirenti L, Koob GF, Gillin JC. Cocaine abuse and dependence. J. Clin. Psychopharmacol. 1995;15:63–78. doi: 10.1097/00004714-199502000-00010. [DOI] [PubMed] [Google Scholar]
- Wright C, Moore RD. Disulfiram treatment of alcoholism. Am. J. Med. 1990;88:647–655. doi: 10.1016/0002-9343(90)90534-k. [DOI] [PubMed] [Google Scholar]