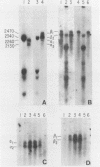
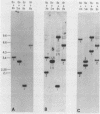
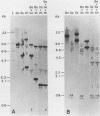
Abstract
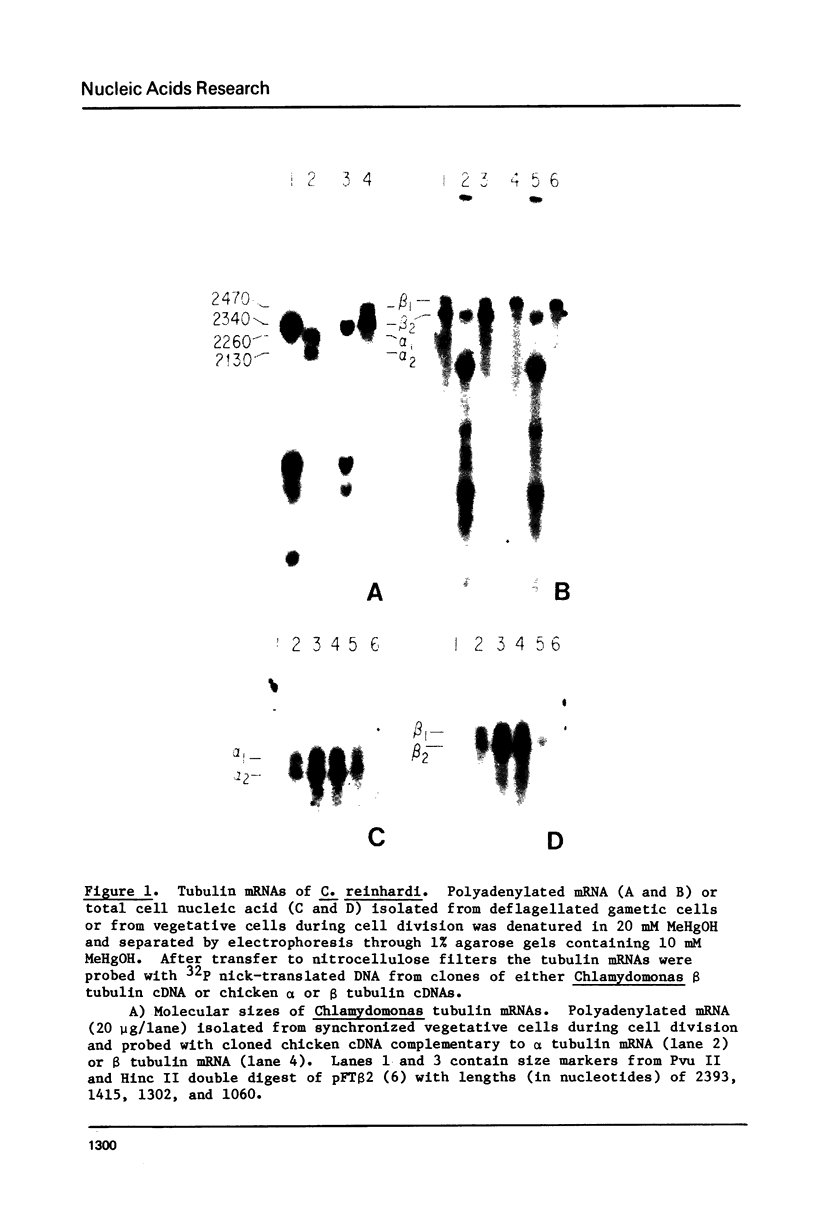
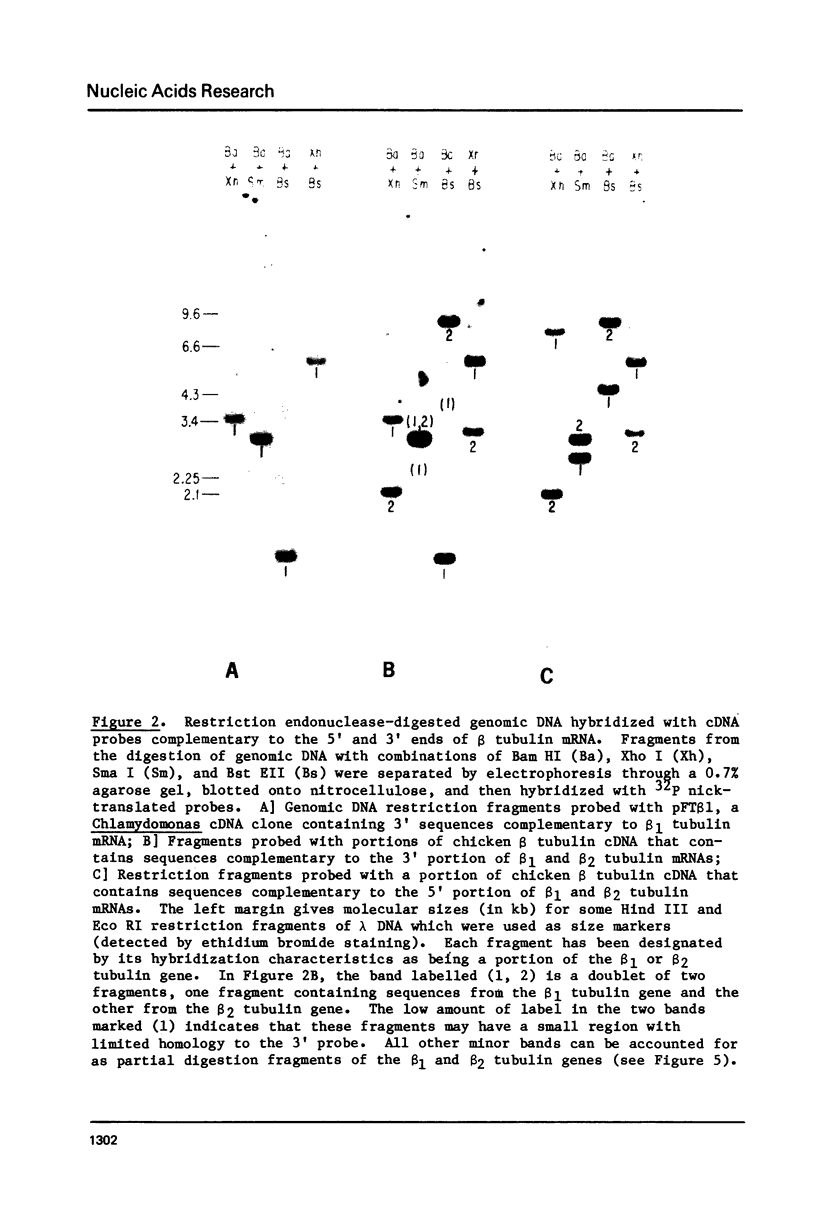
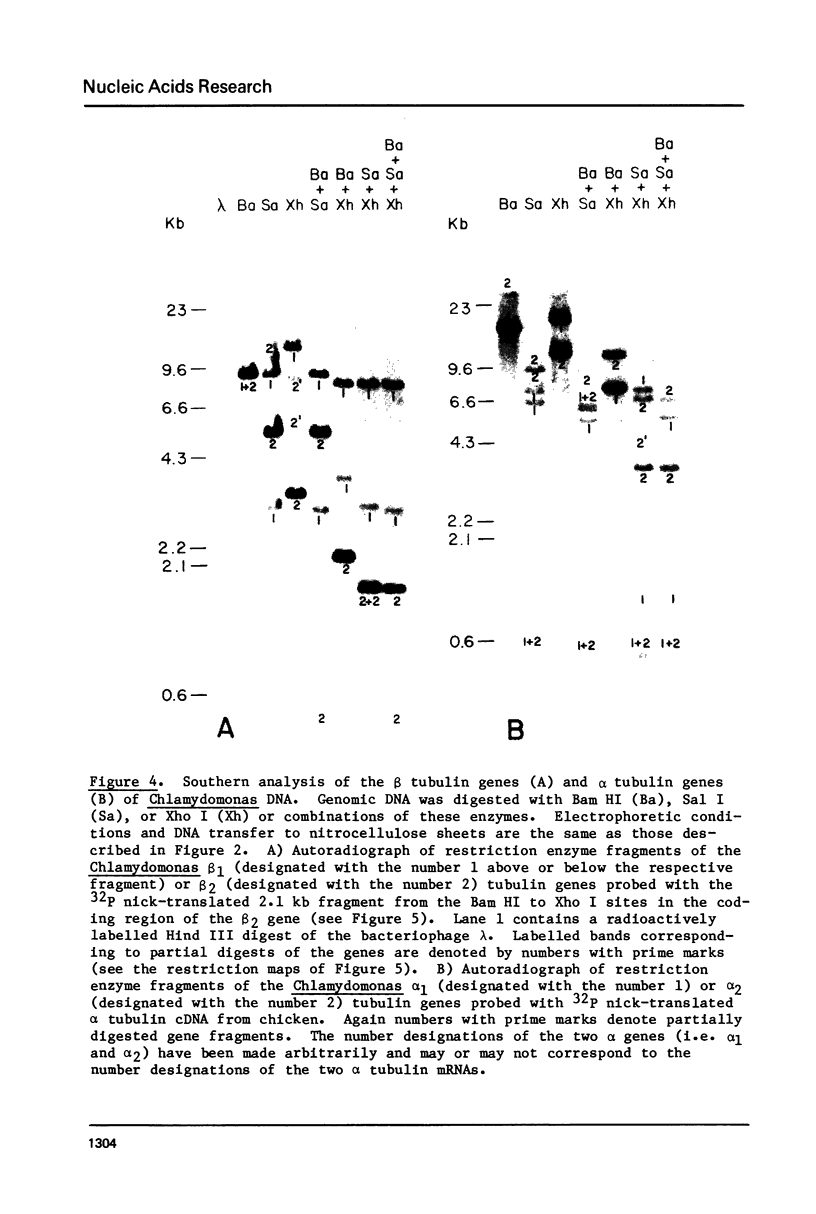
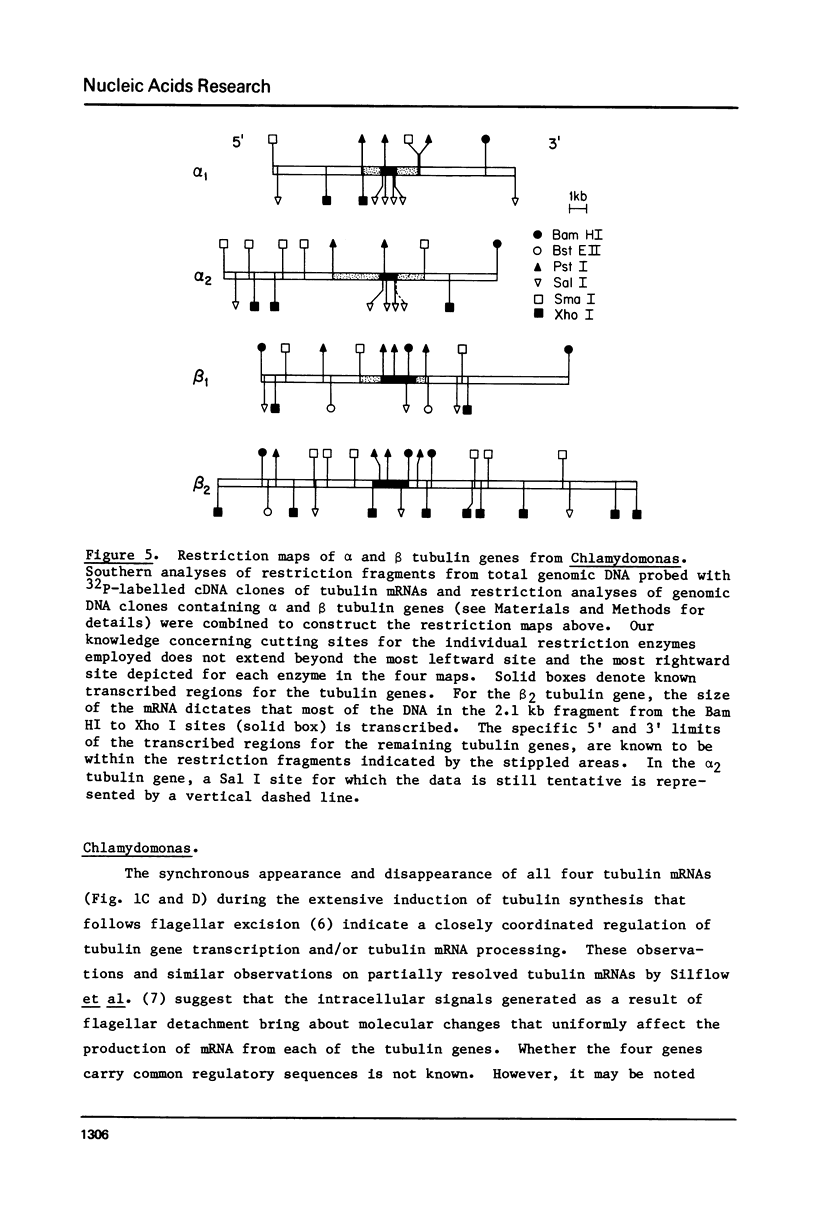
During cell division and during the induction of tubulin synthesis that accompanies flagellar regeneration in Chlamydomonas reinhardi, four tubulin mRNAs of discrete molecular sizes are produced. During induction two beta tubulin mRNAs (2.47 kb and 2.34 kb) and two alpha tubulin mRNAs (2.26 kb and 2.13 kb) are synthesized in high abundance and in a closely coordinated fashion. Combined data from restriction enzyme mapping (i.e., Southern analysis) of genomic DNA and of Charon 30 recombinant clones bearing inserts of Chlamydomonas tubulin genes provide direct evidence for four distinct tubulin genes in this organism. Dot-blot analysis of the level of hybridization of a 32p nick-translated beta tubulin cDNA to genomic DNA from gametic cells and to a clone containing the beta 1 tubulin gene indicate that each beta 1 tubulin gene is present in one copy per cell. Additional hybridization experiments employing fragments of cDNA clones which selectively anneal to either the 3' or 5' portions of the two alpha tubulin genes or to one or both of the two beta tubulin genes suggest that each tubulin gene is actively transcribed to give rise to one of the four tubulin mRNAs. These observations further suggest that at most four basic types of tubulin subunits are produced by Chlamydomonas and that the heterogeneity of tubulin subunits reported to exist in the flagellar axoneme must arise as a result of post-translational modification.
Full text
PDF











Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams G. M., Huang B., Piperno G., Luck D. J. Central-pair microtubular complex of Chlamydomonas flagella: polypeptide composition as revealed by analysis of mutants. J Cell Biol. 1981 Oct;91(1):69–76. doi: 10.1083/jcb.91.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton W. D., Davis R. W. Screening lambdagt recombinant clones by hybridization to single plaques in situ. Science. 1977 Apr 8;196(4286):180–182. doi: 10.1126/science.322279. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Lopata M. A., MacDonald R. J., Cowan N. J., Rutter W. J., Kirschner M. W. Number and evolutionary conservation of alpha- and beta-tubulin and cytoplasmic beta- and gamma-actin genes using specific cloned cDNA probes. Cell. 1980 May;20(1):95–105. doi: 10.1016/0092-8674(80)90238-x. [DOI] [PubMed] [Google Scholar]
- Cowan N. J., Wilde C. D., Chow L. T., Wefald F. C. Structural variation among human beta-tubulin genes. Proc Natl Acad Sci U S A. 1981 Aug;78(8):4877–4881. doi: 10.1073/pnas.78.8.4877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firtel R. A., Timm R., Kimmel A. R., McKeown M. Unusual nucleotide sequences at the 5' end of actin genes in Dictyostelium discoideum. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6206–6210. doi: 10.1073/pnas.76.12.6206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulton A. B., Wan K. M., Penman S. The spatial distribution of polyribosomes in 3T3 cells and the associated assembly of proteins into the skeletal framework. Cell. 1980 Jul;20(3):849–857. doi: 10.1016/0092-8674(80)90331-1. [DOI] [PubMed] [Google Scholar]
- George H. J., Misra L., Field D. J., Lee J. C. Polymorphism of brain tubulin. Biochemistry. 1981 Apr 28;20(9):2402–2409. doi: 10.1021/bi00512a006. [DOI] [PubMed] [Google Scholar]
- Ginzburg I., Behar L., Givol D., Littauer U. Z. The nucleotide sequence of rat alpha-tubulin: 3'-end characteristics, and evolutionary conservation. Nucleic Acids Res. 1981 Jun 25;9(12):2691–2697. doi: 10.1093/nar/9.12.2691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez F. J., Garrett C. T., Wiener D., Caine M. D. The use of potassium iodide equilibrium density gradient centrifugation in the purification of RNA for hybridization with nonreiterated DNA sequences. Anal Biochem. 1978 Mar;85(1):146–156. doi: 10.1016/0003-2697(78)90285-3. [DOI] [PubMed] [Google Scholar]
- Goodenough U. W., Forest C. L. Genetics of cell-cell interactions in Chlamydomonas reinhardi. Birth Defects Orig Artic Ser. 1978;14(2):429–438. [PubMed] [Google Scholar]
- Gozes I., Sweadner K. J. Multiple tubulin forms are expressed by a single neurone. Nature. 1981 Dec 3;294(5840):477–480. doi: 10.1038/294477a0. [DOI] [PubMed] [Google Scholar]
- Huang B., Piperno G., Ramanis Z., Luck D. J. Radial spokes of Chlamydomonas flagella: genetic analysis of assembly and function. J Cell Biol. 1981 Jan;88(1):80–88. doi: 10.1083/jcb.88.1.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kafatos F. C., Jones C. W., Efstratiadis A. Determination of nucleic acid sequence homologies and relative concentrations by a dot hybridization procedure. Nucleic Acids Res. 1979 Nov 24;7(6):1541–1552. doi: 10.1093/nar/7.6.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katcoff D., Nudel U., Zevin-Sonkin D., Carmon Y., Shani M., Lehrach H., Frischauf A. M., Yaffe D. Construction of recombinant plasmids containing rat muscle actin and myosin light chain DNA sequences. Proc Natl Acad Sci U S A. 1980 Feb;77(2):960–964. doi: 10.1073/pnas.77.2.960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefebvre P. A., Silflow C. D., Wieben E. D., Rosenbaum J. L. Increased levels of mRNAs for tubulin and other flagellar proteins after amputation or shortening of Chlamydomonas flagella. Cell. 1980 Jun;20(2):469–477. doi: 10.1016/0092-8674(80)90633-9. [DOI] [PubMed] [Google Scholar]
- Lu R. C., Elzinga M. The primary structure of tubulin. Sequences of the carboxyl terminus and seven other cyanogen bromide peptides from the alpha-chain. Biochim Biophys Acta. 1978 Dec 20;537(2):320–328. doi: 10.1016/0005-2795(78)90515-9. [DOI] [PubMed] [Google Scholar]
- Luduena R. F., Woodward D. O. Isolation and partial characterization of alpha and beta-tubulin from outer doublets of sea-urchin sperm and microtubules of chick-embryo brain. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3594–3598. doi: 10.1073/pnas.70.12.3594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minami S. A., Collis P. S., Young E. E., Weeks D. P. Tubulin induction in C. reinhardii: requirement for tubulin mRNA synthesis. Cell. 1981 Apr;24(1):89–95. doi: 10.1016/0092-8674(81)90504-3. [DOI] [PubMed] [Google Scholar]
- Piperno G., Luck D. J. Phosphorylation of axonemal proteins in Chlamydomonas reinhardtii. J Biol Chem. 1976 Apr 10;251(7):2161–2167. [PubMed] [Google Scholar]
- Ponstingl H., Krauhs E., Little M., Kempf T. Complete amino acid sequence of alpha-tubulin from porcine brain. Proc Natl Acad Sci U S A. 1981 May;78(5):2757–2761. doi: 10.1073/pnas.78.5.2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Rimm D. L., Horness D., Kucera J., Blattner F. R. Construction of coliphage lambda Charon vectors with BamHI cloning sites. Gene. 1980 Dec;12(3-4):301–309. doi: 10.1016/0378-1119(80)90113-4. [DOI] [PubMed] [Google Scholar]
- Schibler U., Marcu K. B., Perry R. P. The synthesis and processing of the messenger RNAs specifying heavy and light chain immunoglobulins in MPC-11 cells. Cell. 1978 Dec;15(4):1495–1509. doi: 10.1016/0092-8674(78)90072-7. [DOI] [PubMed] [Google Scholar]
- Silflow C. D., Rosenbaum J. L. Multiple alpha- and beta-tubulin genes in Chlamydomonas and regulation of tubulin mRNA levels after deflagellation. Cell. 1981 Apr;24(1):81–88. doi: 10.1016/0092-8674(81)90503-1. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Stephens R. E. Primary structural differences among tubulin subunits from flagella, cilia, and the cytoplasm. Biochemistry. 1978 Jul 11;17(14):2882–2891. doi: 10.1021/bi00607a029. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenzuela P., Quiroga M., Zaldivar J., Rutter W. J., Kirschner M. W., Cleveland D. W. Nucleotide and corresponding amino acid sequences encoded by alpha and beta tubulin mRNAs. Nature. 1981 Feb 19;289(5799):650–655. doi: 10.1038/289650a0. [DOI] [PubMed] [Google Scholar]
- Wahl G. M., Stern M., Stark G. R. Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hybridization by using dextran sulfate. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3683–3687. doi: 10.1073/pnas.76.8.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weeks D. P., Collis P. S. Induction and synthesis of tubulin during the cell cycle and life cycle of Chlamydomonas reinhardi. Dev Biol. 1979 Apr;69(2):400–407. doi: 10.1016/0012-1606(79)90300-2. [DOI] [PubMed] [Google Scholar]
- Weeks D. P., Collis P. S. Induction of microtubule protein synthesis in Chlamydomonas reinhardi during flagellar regeneration. Cell. 1976 Sep;9(1):15–27. doi: 10.1016/0092-8674(76)90048-9. [DOI] [PubMed] [Google Scholar]
- Witman G. B., Carlson K., Berliner J., Rosenbaum J. L. Chlamydomonas flagella. I. Isolation and electrophoretic analysis of microtubules, matrix, membranes, and mastigonemes. J Cell Biol. 1972 Sep;54(3):507–539. doi: 10.1083/jcb.54.3.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witman G. B., Plummer J., Sander G. Chlamydomonas flagellar mutants lacking radial spokes and central tubules. Structure, composition, and function of specific axonemal components. J Cell Biol. 1978 Mar;76(3):729–747. doi: 10.1083/jcb.76.3.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf H. A procedure for simultaneous preparation of large amounts of DNA and RNA by the use of potassium iodide gradients. Anal Biochem. 1975 Oct;68(2):505–511. doi: 10.1016/0003-2697(75)90645-4. [DOI] [PubMed] [Google Scholar]