Abstract
Dopamine denervation gives rise to abnormal corticostriatal plasticity; however, its role in the symptoms and progression of Parkinson’s disease (PD) has not been articulated or incorporated into current clinical models. The ‘integrative selective gain’ framework proposed here integrates dopaminergic mechanisms known to modulate basal ganglia throughput into a single conceptual framework: (1) synaptic weights, the neural instantiation of accumulated experience and skill modulated by dopamine-dependent plasticity and (2) system gain, the operating parameters of the basal ganglia, modulated by dopamine’s on-line effects on cell excitability, glutamatergic transmission and the balance between facilitatory and inhibitory pathways. Within this framework and based on recent work, a hypothesis is presented that prior synaptic weights and established skills can facilitate motor performance and preserve function despite diminished dopamine; however, dopamine denervation induces aberrant corticostriatal plasticity that degrades established synaptic weights and replaces them with inappropriate, inhibitory learning that inverts the function of the basal ganglia resulting in ‘anti-optimization’ of motor performance. Consequently, mitigating aberrant corticostriatal plasticity represents an important therapeutic objective, as reflected in the long-duration response to levodopa, reinterpreted here as the correction of aberrant learning. It is proposed that viewing aberrant corticostriatal plasticity and learning as a provisional endophenotype of PD would facilitate investigation of this hypothesis.
Keywords: corticostriatal plasticity, models of basal ganglia, motor learning, dopamine, dopamine denervation, PITx3
1. Introduction
1.1 The many faces of the basal ganglia
Associated with numerous neurological and psychiatric disorders—from Parkinson’s disease to addiction-- the basal ganglia have been the subject of intense and growing research for decades. Historically there has been a ‘great divide’ between those who focus on the role of the basal ganglia in motor control, associated with primarily with the dorsal striatum and neurological disorders, and those who focus on motivational processes, reward and reinforcement, commonly associated with the ventral striatum. Reading older literature one might get the impression these two groups are investigating two different basal ganglia. This impression is diminishing as contemporary models, to an increasing extent, bridge this apparent gulf (eg., Bar-Gad and Bergman, 2001; Humphries and Prescott, 2010; Nicola, 2007). Nonetheless, a remnant of this divide persists as a lack of integration between the learning and performance functions of the basal ganglia. There is no widely accepted model describing the relationship between these two basal ganglia functions, though both are seen as crucial. This gap is nowhere more evident than in the investigation of Parkinson’s disease. Though demonstrated that dopamine denervation/depletion induces abnormal corticostriatal plasticity in the striatum (Kreitzer and Malenka, 2007; Shen et al., 2008), no hypothesis has been put forth on the contribution of this pathology to the symptoms and progression of PD. In contrast, though learning—presumably mediated by corticostriatal plasticity-- has been extensively examined in PD patients, it has been treated primarily as an associated feature distinct from the core motor symptoms of the disease. It seems improbable that pathological corticostriatal plasticity would only affect learning and play no role in motor performance; however; how learning and performance aspects of the basal ganglia are related remains a substantial conceptual gap in the field. Here, focusing on the dorsolateral, sensorimotor circuit, I develop a framework for conceptually integrating learning and performance functions of the basal ganglia. Using this framework, I propose that abnormalities in corticostriatal plasticity are part of the core pathophysiology of PD and give rise to aberrant learning that contributes to the cardinal symptoms of the disease, its progression and its treatment. The focus here is on the role of corticostriatal plasticity in behavior and PD. Excellent reviews of the cellular mechanisms of corticostriatal plasticity can be found elsewhere (Lerner and Kreitzer, 2011; Lovinger, 2010).
1.2 The ever growing complexity of Parkinson’s disease
Parkinson’s disease (PD) is the second most common neurodegenerative disorder after Alzheimer’s (Hirtz et al., 2007), affecting over 4 million worldwide (Dorsey et al., 2007). The cardinal symptoms of PD are motor, including rigidity, bradykinesia/akinesia, postural instability, and resting tremor (Gelb et al., 1999; Jankovic, 2008). Without therapeutics to stop or slow the degenerative process, treatment focuses on mitigating motor symptoms and preserving function. Dopamine replacement with levodopa (L-DOPA) continues to be the mainstay of treatment (Poewe et al.; Schapira et al., 2009; Sethi, 2010). Though highly effective initially, over time its use becomes complicated with motor fluctuations and the development of dyskinesia (Espay; Marsden and Parkes, 1977; Nutt, 2003; Poewe et al., 2010). PD continues to be a devastating disease and the development of new therapeutic strategies remains a high priority.
PD is a complex disease. For decades perceived as primarily a motor disorder arising from the loss of dopamine cells, there is a growing appreciation for the involvement of other brain regions and neurotransmitters (Barone, 2010; Braak et al., 2003) and non-motor symptoms, including cognitive disturbances (Aarsland et al., 2010; Chaudhuri and Schapira, 2009; Cools, 2006; Cools et al., 2010; Frank, 2005; Levin and Katzen, 2005; Zgaljardic et al., 2003). Even the cardinal motor symptoms, such as bradykinesia and tremor, cannot be construed as necessarily arising from a single underlying pathophysiology (Rivlin-Etzion et al., 2010; Zaidel et al., 2009). A comprehensive model that captures all these aspects of PD has not emerged and is not likely in the near future. Instead, most models focus on particular aspects of the disease and incrementally expand and improve with further investigation. The hypothesis proposed here elaborates one such model, the classic-- and still most widely held-- model linking motor symptoms to dopamine denervation in the dorsal striatum. Though the present hypothesis may expand this model, it will not eliminate all its shortcomings, and like any hypothesis represents a partial step toward fuller understanding.
2. Models of the basal ganglia and the pathophysiology of PD
Current understanding of how PD symptoms arise from dopamine denervation are based on models of the basal ganglia proposed in the late 1980s (Albin et al., 1989; Chevalier and Deniau, 1990; DeLong, 1990; Gerfen et al., 1990; Penney and Young, 1986). These models continue to evolve with on-going research (Albin et al., 1995; DeLong and Wichmann, 2009; DeLong and Wichmann, 2007; Mink, 1996). In much of the basic research on the basal ganglia, synaptic plasticity and learning are central to their function. However, as Graybiel pointed out in 1995, it is surprising that the role of learning and plasticity in the basal ganglia has not significantly entered into clinical models (Graybiel, 1995). This remains true today.
Models of the basal ganglia and PD pathophysiology can be roughly grouped into two types: aggregate output and transformative function models. The former are models that focus on the overall activity and output of the basal ganglia without regard to the information content being processed. The latter are models that characterize the neural transformations and information processing the basal ganglia is putatively implementing. In these models, the transformations yielded by the basal ganglia are critically mediated by synaptic plasticity and its behavioral correlate, learning. Each class of model implicitly assumes the other while diminishing its importance. Aggregate output models allow that plasticity and learning occur, but this is not viewed as critical in the context of a pathological output system. Conversely, transformational function models allow that the output mechanics of the system may be altered, but that it is the loss of specific learning-based functions that impairs behavior.
I propose a simple ‘integrative selective gain’ framework capturing both perspectives. It is well known that corticostriatal plasticity, modulation of glutamatergic corticostriatal transmission and medium spiny neuron (MSN) excitability all contribute to the modulation of behavior by the basal ganglia. What is less clear is the relationship between these neural processes; that is, how they interact and jointly contribute to final behavioral output. In the proposed framework, corticostriatal throughput and behavioral output arise as a composite of two functions: (a) selectivity, synaptic weights that enhance or diminish throughput of particular afferent inputs and arise as accumulating experience adjusts synaptic strengths through plasticity and (b) system gain, the on-line operating parameters of the basal ganglia, described below. Dopamine plays a crucial role in both (Beeler et al., 2010b; Braver et al., 1999).
2.1 Aggregate output perspective: classic direct/indirect motor control models
In the classic models of the basal ganglia, afferent input, chiefly cortical, enters the striatum and is processed through two distinct pathways, the direct and indirect (Albin et al., 1989; Chevalier and Deniau, 1990; DeLong, 1990; Gerfen et al., 1990; Mink, 1996; Penney and Young, 1986). Activation of the striatonigral, dopamine D1 receptor expressing direct pathway facilitates cortical activity while activation of the striatopallidal, dopamine D2 receptor expressing indirect pathway inhibits cortical activity. Dopamine facilitates the emission of behavioral responses through dual action on these pathways, enhancing direct pathway activity through D1 activation and inhibiting indirect pathway activity through D2 activation (Hernandez-Lopez et al., 1997; Hernandez-Lopez et al., 2000). In PD, gradual dopaminergic denervation is believed to result in an imbalance between the direct, facilitatory and indirect, inhibitory pathways. The result is that the inhibitory pathway predominates, impairing the ability to select, initiate and perform motor movements (Albin et al., 1989; Chevalier and Deniau, 1990; Day et al., 2008; DeLong, 1990; Filion and Tremblay, 1991; Gerfen et al., 1990; Mallet et al., 2006; Mink, 1996; Obeso et al., 2000; Penney and Young, 1986). Both dopamine replacement therapy (L-DOPA) and treatment with dopamine agonists are believed to temporarily restore the balance between facilitatory and inhibitory pathways, preserving motor function.
These models continue to evolve (Chesselet and Delfs, 1996; DeLong and Wichmann, 2009; DeLong and Wichmann, 2007; Mink, 1996). Initially focused on dopaminergic control over the relative firing rate and aggregate activity of the two pathways (Albin et al., 1989; Chevalier and Deniau, 1990; DeLong, 1990; Gerfen et al., 1990; Mink, 1996; Penney and Young, 1986), more recent iterations focus on dopaminergic effects on the synchrony/patterns of activity (Bergman et al., 1998; Bevan et al., 2002; Costa et al., 2006; Costa, 2007; DeLong and Wichmann, 2009; Hammond et al., 2007; Israel and Bergman, 2008). These models focus on the ‘online’ output of the system and do not incorporate a role for synaptic plasticity. Learning and motor control are implicitly viewed as distinct processes where pathological motor control output overshadows putative learning functions. These models remain agnostic on the contribution of prior experience-- established synaptic weights and skills (ie., synaptic potentiation and depression established through corticostriatal plasticity during learning)-- to basal ganglia output and motor performance.
Consistent with these models, dopamine has been demonstrated to modulate striatal activity, differentially modulating the excitability and response properties of direct and indirect pathway medium spiny neurons (Hernandez-Lopez et al., 1997; Hernandez-Lopez et al., 2000; Lovinger, 2010; Nicola et al., 2000) as well as modulating corticostriatal glutamatergic transmission (Bamford et al., 2004; Cepeda et al., 2001; Flores-Hernandez et al., 1997; Yin and Lovinger, 2006). By increasing and decreasing corticostriatal throughput in the direct and indirect pathways, dopamine modulates the operating parameters of the system and has direct, on-line effect on motor performance: system gain. The classic, aggregate output models capture this on-line modulatory, gain function of dopamine (Servan-Schreiber et al., 1990). ‘System gain’ is intended to capture modulatory effects of dopamine that (a) directly modulate processing as it occurs, for example, shifting the balance between the direct and indirect pathways and (b) exert effects non-selectively across the system, for example, increasing D1-expressing MSN excitability in the entire circuit rather than synapse selective modulation.
2.2 Transformational function perspective: incorporating experience into behavior
Historically associated with motor control (Calabresi et al., 1997a; Verschueren et al., 1997)—the so-called extrapyramidal system—anatomical structure and connectivity suggests the basal ganglia may subserve a more global information processing function critically modulating cortical processing and output (DeLong and Wichmann, 2009; DeLong and Wichmann, 2007; Graybiel, 2005; Parent and Hazrati, 1995). The striatum, a principle input structure of the basal ganglia (Bolam et al., 2000; Wilson, 1998), receives massive cortical afferents that are segregated into parallel loops with different regions of the cortex projecting to specific regions in the striatum which in turn ultimately project back to the same cortical regions (Alexander et al., 1986; Alexander and Crutcher, 1990; Alexander et al., 1990; Doron and Goelman; Draganski et al., 2008; Hoover and Strick, 1993; Lehericy et al., 2004; McGeorge and Faull, 1989; Middleton and Strick, 2000; Wiesendanger et al., 2004). In its simplest characterization, these projections are segregated into limbic, associative and sensorimotor loops; the latter represented by afferents from the sensory and motor cortical regions to the dorsal lateral striatum (posterior putamen in primates, DLS/PP hereafter). Evidence is accumulating that these loops are interconnected, facilitating progressive processing and learning across striatal compartments (Doron and Goelman, 2010; Draganski et al., 2008; Haber, 2003; Joel and Weiner, 1994; McGeorge and Faull, 1989; Parent, 1990). Though the larger selective gain framework proposed is relevant to the entire basal ganglia system, the current discussion will be limited to the sensorimotor circuit as the DLS/PP is a primary site of dopamine denervation in PD (Bernheimer et al., 1973; Graybiel et al., 1990; Hornykiewicz, 2001; Kish et al., 1988).
The DLS/PP has been widely associated with sensorimotor integration (Aosaki et al., 1994; Bailey and Mair, 2006; Carli et al., 1985; Cavaco et al., 2010; Dunnett and Robbins, 1992; Fornaguera et al., 1994; Iversen, 1984; Konczak et al., 2009; Lidsky et al., 1985; Mahon et al., 2004; Manetto and Lidsky, 1986; Messier et al., 2007; Poldrack et al., 2001; Ramanathan et al., 2002; Schwarz et al., 1984; Seidler et al., 2006), representing a key neural substrate for acquiring, optimizing and deploying motor responses integrated with on-going sensory information. Critically, this is experience- and learning- dependent. Associated with implicit, procedural learning (Barnes et al., 2005; Foerde et al., 2006; Jog et al., 1999; Packard and White, 1991; Packard and Knowlton, 2002; Poldrack et al., 2001; Willingham et al., 2002), the DLS/PP is widely believed to acquire stimulus-response (S-R) associations (Balleine et al., 2007; Balleine et al., 2009; Balleine and O’Doherty, 2010; Divac et al., 1967; Faure et al., 2005; Graybiel, 1998; Haruno and Kawato, 2006; Kimchi et al., 2009; Knowlton et al., 1996; Konorski, 1967; Mahon et al., 2004; Packard and Knowlton, 2002; Tang et al., 2007; Yin et al., 2004; Yin and Knowlton, 2006) that provide a substrate for skill learning (Benecke et al., 1987; Boyd et al., 2009; Graybiel, 1998; Hikosaka et al., 1999; Jog et al., 1999; Kermadi et al., 1993; Sakai et al., 2003; Seidler et al., 2007; Yin et al., 2009), especially automated and habitual responses (Balleine and O’Doherty, 2010; Costa, 2007; Doyon et al., 2009; Faure et al., 2005; Graybiel, 2008; Jog et al., 1999; Knowlton et al., 1996; Miyachi et al., 1997; Miyachi et al., 2002; Packard and Knowlton, 2002; Poldrack et al., 2005; Puttemans et al., 2005; Redgrave et al., 2010; Tang et al., 2007; Yin and Knowlton, 2006) that underlie rapid expression of behaviors under stimulus control to optimally match action selection and motor execution to on-going stimuli. As a putative substrate for action selection, the DLS/PP provides a mechanism for initiating, terminating and switching responses (Benecke et al., 1987; Cameron et al., 2009; Cools et al., 2004; Cools et al., 2006a; Cools et al., 2006b; Hikosaka and Isoda, 2010; Jin and Costa, 2010). S-R associations in which the ‘stimulus’ could be external sensory information (eg., stop sign or sensory feedback in on-going movement), internal sensory information (eg., proprioceptive feedback) or efferent copies of immediately preceding actions/movements, can be chained together facilitating sequence learning, including the chunking of discrete actions into a single fluid movement (Benecke et al., 1987; Boyd et al., 2009; Ghilardi et al., 2009; Graybiel, 1998; Jog et al., 1999; Sakai et al., 2003).
This summary characterization collapses many distinct models to capture in broad strokes the basic transformation of cortical processing commonly ascribed to the DLS/PP. These models all share a basic feature: the output of the system modulates behavioral responding based on prior experience and learning in order to optimize current and future behavior. Corticostriatal plasticity is not a secondary or collateral function, but rather is itself the primary function: selectivity based on accumulated experience. Insofar as these models provide an account of PD symptoms, they suggest a failure of motor learning and/or an extinction process. Attempting to account for all symptomatology through learning mechanisms—abnormal selectivity—these models do not incorporate on-line, gain functions of dopamine and have not been integrated into current clinical models.
Dopamine plays a critical role inducing and regulating synaptic changes in the striatum. A three-factor Hebbian rule (Berke and Hyman, 2000; Calabresi et al., 1997b; Calabresi et al., 2007; Kerr and Wickens, 2001; Kreitzer and Malenka, 2008; Reynolds et al., 2001; Reynolds and Wickens, 2002; Shen et al., 2008) has been proposed for striatal plasticity in which synaptic potentiation or depression require coincident pre- and post- synaptic activity as well as dopamine, with dopamine concentration differentially regulating the direction of plasticity (Calabresi et al., 2007; Centonze et al., 2001; Lovinger et al., 2003; Reynolds and Wickens, 2002; Thivierge et al., 2007; Wickens et al., 1996) in the direct and indirect pathways (Kreitzer and Malenka, 2008; Shen et al., 2008; Surmeier et al., 2007). Thus, dopamine is positioned to modulate the accumulation and integration of experience as synaptic weights in the striatum: selectivity. The transformational function models capture this selective, learning/acquisition function of dopamine.
2.3 Synthesis: an integrative selective gain framework
I adopt the perspective that the basal ganglia are modulating cortical processing in the service of optimizing behavioral expression based on cumulative prior experience. The DLS/PP contributes specifically to integrating rapid motor responses with on-going sensory stimuli—sensorimotor integration by—mediating S-R learning that can be chained together into fluid sequences in which the response(s), under stimulus control, are emitted automatically, facilitating optimal performance without engaging conscious, deliberative mechanisms thereby facilitating implicit, procedural learning and habit.
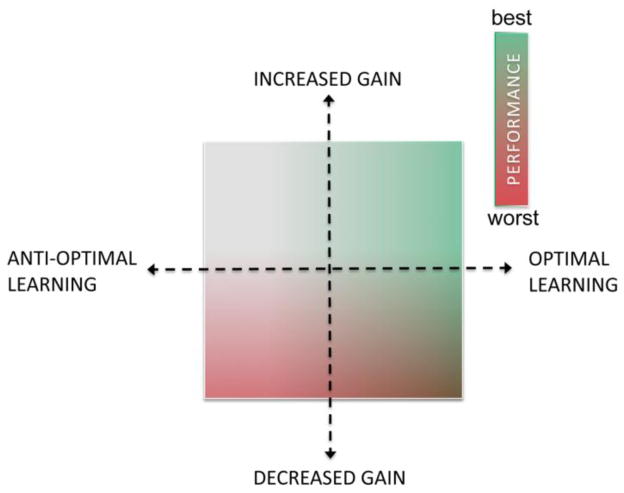
In the selective gain framework, the final corticostriatal throughput and behavioral output arises as a consequence of two dopamine-dependent functions: (1) selectivity, the modulation of corticostriatal throughput by synaptic weights (ie., synaptic strength) established through accumulated experience and learning (ie, synaptic plasticity) and (2) system-gain, modulation of the operating parameters of the basal ganglia, including the balance between the direct and indirect pathway, modulation of MSN excitability and glutamatergic corticostriatal transmission. Thus, dopamine is in a position to critically shape corticostriatal throughput by modulating both selectivity and gain, the accumulation and expression of experience. Conversely, dopamine denervation may potentially induce abnormal behavior and motor deficits through either mechanism. The integrative selective gain model provides an explicit framework for investigating the relative contribution and interaction of these two dopaminergic mechanisms to basal ganglia control over behavior in normal and pathological states. In this framework (conceptually illustrated in Fig 1), performance is a function of both learning (represented by the x-axis), which can be optimal or, as proposed below ‘anti-optimal,’ and system gain (represented by the y-axis and construed here as dopamine-dependent). The aberrant learning hypothesis proposed below arises within this framework.
Fig 1. Conceptual mapping of performance onto dual axes of learning/synaptic weights and output/system gain.
Color mapping of performance on the two axes of synaptic weight optimization (selectivity, horizontal axis) and system gain (vertical axis). Normal/increased dopamine with optimized weights (upper right quadrant) results in optimal performance (green shading). In contrast, sub- or anti-optimal weights in combination with diminished gain from dopamine denervation (lower left quadrant) results in impaired performance (red shading). In the lower right quadrant, performance is contingent upon both factors such that optimal weights will support performance even with diminished gain associated with dopamine loss, to a degree. Moreover, the less weights are optimal, the less protection is provided. In the upper left quadrant, with normal dopamine but sub- or anti-optimal learning, the effects are unknown but likely favor impairment. According to the hypothesis advanced here, the upper right and lower left quadrants are clearly defined as healthy and Parkinsonian. However, during early and middle stages of the disease, particularly under dopamine replacement therapy, most patients will likely function alternatingly in the less understood upper left and lower right quadrants.
3. Consensus and controversy: pinning down learning deficits in PD
Consistent with functions commonly attributed to the DLS/PP, studies of PD and learning have observed deficits and abnormalities in implicit and sequence learning (Carbon and Eidelberg, 2006; Jackson et al., 1995; Krebs et al., 2001; Siegert et al., 2006; Wilkinson and Jahanshahi, 2007; Wilkinson et al., 2009), automization (Ghilardi et al., 2009; Lehericy et al., 2005; Seidler et al., 2007; Wu and Hallett, 2005)-- particularly evident with increasing task difficulty or multi-tasking (Mentis et al., 2003a)-- and sensory-motor mapping (Helmich et al., 2010; Krebs et al., 2001; Marinelli et al., 2009; Smith and McDowall, 2006; Stefanova et al., 2000). Findings, however, are not uniform (Abbruzzese et al., 2009; Doyon, 2008; Nieuwboer et al., 2009; Siegert et al., 2006) with some studies reporting spared learning (eg., (Behrman et al., 2000; Cohen and Pourcher, 2007; Jordan and Sagar, 1994). Several factors contribute to variability across studies. First, learning occurs in stages (Doyon and Benali, 2005; Floyer-Lea and Matthews, 2005; Lehericy et al., 2005; Miyachi et al., 1997; Miyachi et al., 2002). Most studies examine only early stages of learning (Agostino et al., 2004; Doyon, 2008); however, deficits in striatal function may be more relevant to later stages of learning, consolidation and automization (Agostino et al., 2004; Cohen and Pourcher, 2007; Marinelli et al., 2009; Wu and Hallett, 2005). Second, observable learning impairments will depend upon the stage of disease and symptom severity. Though some studies report that learning is only impaired during later stages (Harrington et al., 1990; Muslimovic et al., 2007), considerable evidence suggest that learning abnormalities arise early in PD (Baglio et al., 2009; Carbon et al., 2004; Carbon and Eidelberg, 2006; Ghilardi et al., 2003; Marinelli et al., 2009; Mentis et al., 2003a; Mentis et al., 2003b; Nakamura et al., 2001), possibly preceding frank motor symptoms (see Ogura et al., 2005). Third, methodological and conceptual differences further complicate assessing learning in PD. For example, though we draw a distinction between implicit and explicit learning, the relationship and role of these two putative types of learning in performing particular tasks is often unclear (Ghilardi et al., 2003; Ghilardi et al., 2009; Wilkinson and Jahanshahi, 2007; Wilkinson et al., 2009). Critically, as most tests associated with striatum based learning are primarily based on measuring speed of responding, potential confounds with motor control impairments remain challenging to control. In short, though accumulating evidence indicates learning impairments associated with PD, these studies provide a rudimentary map of a complex, poorly understood landscape, consistent with our evolving and increasingly complex understanding of the role of the basal ganglia in behavior. As difficult as behaviorally characterizing learning deficits in PD has proven to be, linking those deficits to specific underlying neural mechanisms and pathologies is fraught with even greater hazard.
3.2 Neural compensation: masking pathophysiology
Assessing putative learning impairments in PD is further complicated by compensations that mask underlying neural abnormalities. Numerous studies have demonstrated that early stage PD patients show activation patterns different than controls during various learning tasks, requiring greater activation of the regions engaged by controls as well as recruitment of additional areas not utilized by controls in the same tasks (Appel-Cresswell et al., 2010; Baglio et al., 2009; Helmich et al., 2007; Helmich et al., 2010; Mentis et al., 2003a; Mentis et al., 2003b; Nakamura et al., 2001; Wu et al., 2007). These studies suggest that behavioral performance does not necessarily index underlying neural pathophysiology. Further, these studies highlight the difficulty in mapping a learning typology onto distinct brain regions (eg., implicit learning -> striatum) as anatomically distinct regions function interdependently in functional circuits (DeLong and Wichmann, 2007).
3.3 Reframing corticostriatal plasticity and learning
Rather than equating corticostriatal plasticity with a specific phenomenological type of learning, such as implicit learning-- which belies our incomplete understanding of the neural complexity underlying this typology-- the question may be how an underlying pathology in corticostriatal plasticity progressively alters basal ganglia throughput affecting an array of functions, including learning commonly ascribed to the striatum, putatively ‘cortical’ functions as well as direct, motor control functions. The pattern of learning deficits associated with progressive dopamine denervation, in the context of neural compensations, is likely to be complex rather than a simple, monotonic worsening of a single type(s) of learning. More importantly, the effects of pathological corticostriatal plasticity may not be limited to traditionally defined learning but may exert pervasive effects on all functions subserved by the basal ganglia. The integrative selective gain framework proposed here conceptually dissociates corticostriatal plasticity from the traditionally defined typology of learning and instead situates plasticity and the resulting synaptic weights (ie., potentiated/depressed synaptic strengths) as a primary determinant of basal ganglia throughput, providing a foundation for a more comprehensive and fundamental view of its role in generating and modulating behavioral output. Throughout the remainder of the text, I use ‘learning’ to generically denote experience-induced neural changes, reflecting both acquisition and expression of those changes (with expression sometimes denoted as ‘skill’). Synaptic plasticity refers to the physiological processes that mediate neural modification.
4. Dissociating two sides of a coin: discerning the dual function of dopamine
From this perspective, dopamine’s role in motor learning and motor control are not two distinct functions but rather two sides of a coin: regulating the accumulation (plasticity, learning, synaptic weights) and influence (system gain) of prior experience on current behavior, presumably in the service of behavioral optimization. In the hypothesis below, I argue that altered corticostriatal plasticity secondary to dopamine denervation is a core pathophysiology that not only impairs corticostriatal processing, but inverts it, transforming basal ganglia output from optimizing cortical processing and performance to actively degrading performance: anti-optimizing. The challenge in investigating such a hypothesis is to dissociate learning and plasticity effects from on-line, gain functions in assessing performance deficits associated with dopamine denervation. The studies described next represent an initial step in addressing this challenge.
4.1 Established learning, dopamine denervation and performance
To assess the contribution of dopamine mediated learning (selectivity in the proposed framework) to the motor performance deficits associated with dopamine denervation, we conducted a series of recent experiments using genetic and pharmacological mouse models (Beeler et al., 2010a). It is well established that diminished dopamine impairs motor performance, but can prior, established learning—established synaptic weights—mitigate the performance decrement associated with decreased dopamine? We approached this question with two animal models.
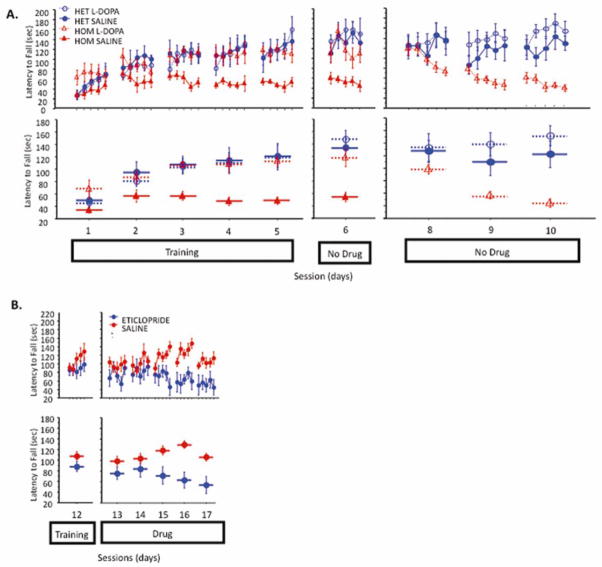
First, we used the PITx3-deficient mouse line (aka, aphakia, Varnum and Stevens, 1968) that exhibits a 90% denervation of dorsal striatal dopamine (Beeler et al., 2009; Hwang et al., 2003; Nunes et al., 2003; Smits et al., 2005; van den Munckhof et al., 2003). These mice perform extremely poorly on the accelerating rotarod (Ardayfio et al., 2008; Beeler et al., 2010a), but their performance can be rescued with L-DOPA treatment (Beeler et al., 2010a). We trained these mice under L-DOPA administration and after waiting minimally 72 hours, continued testing without L-DOPA. The traditional view of PD pathophysiology—that dopamine critically facilitates motor control and performance—would predict that upon discontinuation of L-DOPA and a return to a dopamine deficient state, the mice will not be able to perform the task, similar to untreated PITx3-deficient mice. This is not what we observed. Initially, their performance is preserved—even in the face of dopamine depletion that grossly impairs naïve PITx3 mice (Fig 2A). However, this preserved performance gradually declines (Fig 2A).
Fig 2. Effect of discontinuation of L-DOPA treatment in PITx3-deficient mice and administration of D2 antagonist in wild-type mice on established skills.
(A) Mice were trained on the rotarod with either saline or L-DOPA (25 mg/kg) for 5 sessions (sessions 1–5). After a 3-day treatment discontinuation break the mice were again tested without treatment (session 6). After one refresher training session on either saline or L-DOPA (session 7, not shown) and a 5-day treatment discontinuation break, mice were run for 3 sessions without any treatment (sessions 8–10). Top panel shows latency to fall for each trial and lower panel shows the average latency to fall during each session. (N = 6 per genotype/treatment). (B) Wild-type C57BL/6 mice were trained on the rotarod for 12 days without injections (last training session, session 12, is shown). Animals were then given a D2 blocker (eticlopride, 0.16 mg/kg) and tested on the rotarod for 5 consecutive days (sessions 13–17).
Further experiments demonstrate this is not a residual effect of L-DOPA (Beeler et al., 2010a). We can extend the period between L-DOPA discontinuation from 3 to 10 days without a loss of the observed preservation of function. We can administer L-DOPA daily 6 hours before training and observe no rescue, though presumably putative residual effects of L-DOPA treatment would still be present. We can introduce training on a similar task after L-DOPA discontinuation (ie., treadmill) before retesting without diminishing the observed preservation of function. Finally, we can demonstrate (high performance liquid chromotagraphy, HPLC) that tissue dopamine content 3 and 10 days following discontinuation is identical to untreated PITx3-deficient mice. In short, the initial retention of performance does not appear to be the result of residual L-DOPA effects and the gradual decline is dependent upon experience with the task in a dopamine depleted state.
To validate that the above observations were specifically mediated by changes in dopamine, we conducted experiments with wild-type mice. We observe a similar effect of prior learning in initially preserving function in the face of diminished dopamine signaling. After being trained to asymptotic performance, we administered a dopamine D2 antagonist and continued daily training sessions. Similar to the PITx3 study, after blockade of D2 signaling, performance is initially retained and shows a gradual decline (Fig 2B).
These data suggest two crucial points: 1. That prior learning-- established synaptic weights (ie., pattern of potentiated/depressed synaptic strengths induced by experience-dependent corticostriatal plasticity)-- can initially sustain performance counteracting the direct effects of diminished dopamine on motor control and performance and 2. that dopamine is crucial to maintaining prior learning. Together, these data suggest that learning, in the form of synaptic plasticity and weights, contributes critically to motor performance and that dopamine depletion actively degrades established learning accelerating motor decline.
4.2 The long-duration response to L-DOPA
L-DOPA treatment induces two therapeutic components, a short- and long-duration response (Muenter and Tyce, 1971; Nutt et al., 1995; Nutt and Holford, 1996). The short-duration response is reflected in the rapid improvement and subsequent return of symptoms that correlate with the pharmacokinetic rise and fall of L-DOPA concentrations (Muenter and Tyce, 1971; Nutt and Holford, 1996; Nutt, 2008; Tedroff et al., 1996), generally believed to arise directly from restoring the balance between the direct and indirect pathways through increased dopamine concentrations in the striatum. The long-duration response, in contrast, is poorly understood.
The long-duration response is observed as a gradual improvement in symptoms not correlated with pharmacokinetics of L-DOPA (Fahn et al., 2004; Hauser and Holford, 2002; Muenter and Tyce, 1971; Nutt et al., 1995; Nutt et al., 1997; Quattrone et al., 1995; Zappia et al., 1997). As a consequence, the worsening of symptoms associated with troughs in L-DOPA concentration between doses is diminished. That is, L-DOPA gradually induces symptomatic improvement independent of the actual drug being in the system. This LDR can be sustained for days to weeks after discontinuation of treatment with motor performance declining only gradually (Hauser and Holford, 2002). The physiological basis of the LDR remains unknown with arguments focusing on long-term changes in either pre- (Doller and Connor, 1980; Nutt and Holford, 1996; Quattrone et al., 1995; Zappia et al., 1997) or post-synaptic function at corticostriatal synapses (Barbato et al., 1997; Cotzias et al., 1969; Metman et al., 1997; Nutt and Carter, 2000). To date, however, neither theory has gained widespread support or conclusive empirical validation.
4.3 The long-duration response reinterpreted
An alternative hypothesis is that the LDR reflects the correction of aberrant corticostriatal plasticity associated with dopamine denervation (Beeler et al., 2010a). The PITx3-deficient mouse study described above suggests that established skills can initially preserve function in the face of dopamine depletion. It also demonstrates that dopamine is essential for the maintenance of prior skill. I hypothesize (detailed below) that dopamine denervation induces aberrant, inhibitory learning which actively ‘unlearns’ established skill replacing previously established synaptic weights (potentiated/depressed synaptic strengths) with new weights that inhibit rather than facilitate movement and performance.
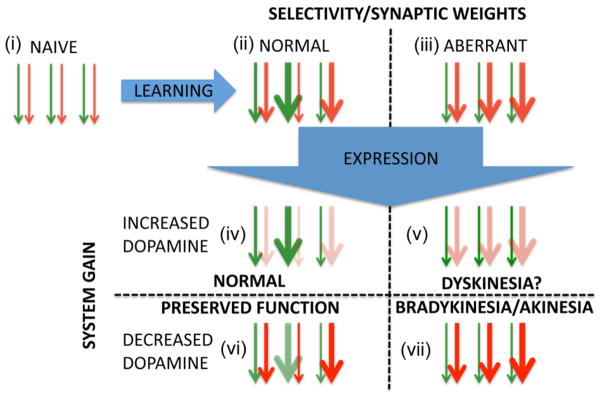
From this perspective, the LDR can be reinterpreted. The SDR directly alleviates the imbalance between the direct and indirect pathways and restores the ‘gain’ associated with corticostriatal processing allowing the striatum to process volitional movement efficiently and fully exploit prior learning. However, prior to diagnosis and initiation of treatment, an on-going aberrant learning process has degraded the synaptic weights associated with established skills, reducing the protective potential of prior learning. Consequently, when treatment is first initiated, the difference in symptoms between peak and trough L-DOPA concentrations is pronounced as this reflects strictly ‘on-line’ restoration of direct and indirect pathway balance. With continued L-DOPA treatment, however, the underlying aberration in corticostriatal plasticity is corrected. Consequently, the process reverses and appropriate learning replaces aberrant learning, giving rise to synaptic weights that facilitate appropriate striatal function and are protective against decreases in dopamine concentration. This results in reduced symptoms during the trough periods of the SDR. Moreover, analogous to the mouse model, when L-DOPA treatment is discontinued, established skills—appropriately calibrated synaptic weights—initially continue to support facilitatory corticostriatal throughput and effective movement despite diminished dopamine. However, without sufficient dopamine, aberrant learning sets in gradually replacing performance optimizing synaptic weights with performance degrading weights. This hypothesis is schematically illustrated in Figure 3 using the same two axes presented in Figure 1 (ie., learning represented by the x-axis and system gain by the y-axis). In this schematic, normal and disturbed performance are parsed to different quadrants and arrows are used to represent the effect of synaptic weight on corticostriatal throughput.
Fig 3. Schematic mapping corticostriatal plasticity onto integrated selective gain framework.
Arrows represent corticostriatal synapses with green and red representing direct/facilitatory and indirect/inhibitory pathways, respectively. Synaptic weight is represented by the thickness of the line. Learning: (top portion of panel): (i) shows undifferentiated weights prior to learning. Subsequent to normal learning (ii) weights are variously adjusted reflecting the facilitation and inhibition of different responses. In aberrant learning (iii), inhibitory weights in the indirect pathway are inappropriately increased while direct pathway weights are not. System gain: (bottom portion of panel): increase or decrease in throughput of direct/indirect pathways are denoted by the intensity of color. (iv) under normal conditions, an increase in dopamine is synergistic with weights facilitating selection of previously reinforced/strengthened responses. (v) Increased dopamine under conditions of aberrant learning are hypothesized to result in favoring direct over indirect pathway activity (diminishing inhibitory throughput); however, the weights inappropriately favor inhibitory activity. The behavioral output under these circumstances is unknown, but speculatively could contribute to dyskinesia. (vi) Under conditions of diminished dopamine but normal learning, the inhibitory pathway is inappropriately enhanced but the weights still favor appropriate actions. The behavioral output here, also, is unknown but we hypothesize that normal function may be preserved initially by the established synaptic weights. (vii) when dopamine is diminished under conditions of aberrant learning, both synaptic weights and the system gain favor the inhibitory pathway, resulting in impaired corticostriatal output and behavioral deficits.
If ‘normal’ and ‘aberrant’ plasticity fluctuates with dopamine concentration and treatment, the question arises how one predominates over the other giving rise to sustained improvement. That is, why aren’t the learning effects we associate with the LDR susceptible to the same pharmacokinetics fluctuations associated with the SDR? The temporal dynamics of L-DOPA restoration of on-line performance (gain) and corticostriatal plasticity (selectivity) likely differ. Synaptic plasticity engages multiple cellular mechanisms, including gene expression, that occur over time. L-DOPA treatment may allow the initiation of these processes of plasticity that then continue on their own time course independent of L-DOPA concentration. The LDR, then, may be decoupled from the pharmacokinetics of L-DOPA by virtue of activating cellular and genetic processes that once initiated are dopamine independent, introducing a secondary set of temporal dynamics in the mechanisms of L-DOPA efficacy. Such a ‘permissive’ or ‘initiating’ mechanisms may explain the observation that the LDR requires lower doses (Quattrone et al., 1995; Wider et al., 2006; Zappia et al., 1999; Zappia et al., 2000), suggesting aberrant corticostriatal plasticity may be more sensitive to treatment. Moreover, it is only during activity that aberrant and corrected learning are initiated. Consequently, the extent to which patients modify their activities according to their symptoms— do more during peaks and less during troughs— could potentially contribute to the balance between corrected and aberrant learning.
4.4 Role of learning in other PD treatments
If this hypothesis is correct, the question arises as to the role of learning processes in other treatments for PD. The LDR has been observed in dopamine agonist treatment (Nutt and Carter, 2000), consistent with the current hypothesis. Whether deep brain stimulation (DBS) is associated with an LDR response is uncertain. The question is difficult to address as patients frequently continue L-DOPA treatment after stereotaxic surgery, usually at a lower dose (Nutt et al., 2001). One study found that DBS reduced symptoms during the ‘trough’ between L-DOPA dosing. This might reflect an LDR response to DBS as improvement during ‘trough’ is associated with the LDR (Nutt et al., 2001). However, it could equally be argued that this represents direct, ‘on-line’ performance improvement arising from DBS that improves symptoms independent of L-DOPA fluctuations across the board. A later study specifically compared DBS patients who did or did not continue L-DOPA treatment and found that when both the stimulator and L-DOPA treatment were discontinued, “off off,” those receiving concurrent L-DOPA demonstrated less symptoms than those treated with DBS alone, suggesting that the LDR to L-DOPA continues to play an important role and that DBS itself does not provide an LDR response (Wider et al., 2006). In a recent study, Zaidel et al (Zaidel et al., 2010) demonstrate that DBS efficacy does not correlate to preoperative L-DOPA responsiveness and argue that L-DOPA and DBS act through different therapeutic mechanisms. In the framework proposed here, these data suggest the difference might lie in the effects on corticostriatal plasticity: DBS may improve system-gain only with no efficacy against aberrant corticostriatal plasticity and learning while L-DOPA corrects abnormal corticostriatal plasticity inducing an LDR. Clearly this requires further investigation.
5. Aberrant Learning: a hypothesis
5.1 Established skill can mitigate performance decline in DA denervation
Synaptic weights (potentiated/depressed synaptic strengths) facilitate some inputs into the striatum while diminishing others, by definition. That corticostriatal synaptic changes correlate with motor learning and performance has been clearly demonstrated in mice (Costa et al., 2004; Jin and Costa, 2010; Yin et al., 2009) and strongly suggested in human imaging studies (Doyon et al., 2009; Lehericy et al., 2005). What has not been established is the relative contribution of these changes in synaptic strengths (weights) to performance compared to direct dopaminergic effects on motor control. The question is novel and has not been systematically investigated. Notably, within the field of dopamine, distinguishing learning (or motivation) from performance and viewing the two as potential confounds has a long history (see Salamone, 2007 for excellent discussion), but historically the objective is to eliminate or control the effects of one or the other, not to assess their relative contribution to performance. The studies described in section 4.1 attempt to ask this question and the data suggest that established, prior learning (presumably in the form of synaptic weights) can diminish the performance deficit associated with dopamine denervation. Notably, studies by Horvitz and colleagues have demonstrated that overtrained, habitual responding can become dopamine independent (Choi et al., 2005), consistent with the current hypothesis, though these authors offer a different interpretation (Ashby et al.) suggesting the behavior becomes striatum-independent. Importantly, when they administered D1 or D2 antagonists to assess the dopamine-dependence of behavior, they did so for only a single day before resuming drug-free training. The present hypothesis would suggest that overtraining would initially result in apparent dopamine-independence (ie., Fig2A) but that continued training would induce aberrant learning and result in gradual degradation of responding. Interestingly, in their studies, blocking D2 had no effect on the overtrained behavior, but again D2 blockade was not sustained over time. Their studies, like ours, provoke interesting questions about the precise role of the D1 and D2 expressing pathways in the acquisition and maintenance of an overtrained response/skill, but dissecting the role of the two pathways in detail is beyond the scope of the present review.
5.2 Dopamine denervation induces aberrant, inhibitory learning
The protective effects of established learning, however, appear to decline in an experience-dependent manner (section 4.1), suggesting that dopamine is necessary to maintain these synaptic weights. Dopamine is widely believed to modulate the direction of plasticity in corticostriatal synapses (Calabresi et al., 2007; Centonze et al., 2001; Lovinger et al., 2003; Reynolds and Wickens, 2002; Thivierge et al., 2007; Wickens et al., 1996). Evidence indicates that dopamine denervation/depletion inverts this directional control in the indirect pathway, resulting in LTP where normally LTD would be observed (Centonze et al., 2004; Kreitzer and Malenka, 2007; Shen et al., 2008). Moreover, insofar as Hebbian plasticity arises probabilistically as a function of correlated activity, changes in synaptic weight are more likely in pathways that are more active. As a consequence, the decreased and increased activity in the direct and indirect pathways, respectively, that results from dopamine denervation (Hernandez-Lopez et al., 1997; Hernandez-Lopez et al., 2000; Mallet et al., 2006) would favor greater potential for plasticity in the indirect pathway (Wiecki et al., 2009; Wiecki and Frank, 2010). These two factors, increased probability of plasticity in the indirect pathway together with inverted direction of plasticity provides an empirical basis for aberrant learning in the striatum where plasticity in the inhibitory, indirect pathway dominates and establishes inhibitory learning where facilitatory learning should occur. Thus, although established skills may initially facilitate motor performance in the face of declining dopamine, the synaptic weights that underlie these skills are, under diminished dopamine, actively modified degrading their protective contribution.
5.3 Aberrant learning impedes rather than optimizes performance
Associated with habit and automaticity (Balleine and O’Doherty, 2010; Graybiel, 2008; Poldrack et al., 2005; Puttemans et al., 2005; Redgrave et al., 2010; Yin and Knowlton, 2006), the DLS/PP appears to be an important in providing stability and long-term retention of acquired motor skills. However, under conditions of dopamine denervation and aberrant corticostriatal plasticity, the dopaminergic mechanisms and conditions that control and maintain this stability are altered. As a consequence, new ‘rules’ of plasticity (ie., inverted direction of plasticity) are instantiated and the currently stable constellation of synaptic weights—prior learning and established skills—are actively revised in accordance to aberrant processes. As a consequence, performance optimizing facilitatory weights arising from prior learning (ie., established patterns of potentiated/depressed synaptic strengths) are actively replaced with performance impeding, inhibitory weights. Though the role of corticostriatal plasticity in motor learning has been demonstrated (section 5.1) and aberrant corticostriatal plasticity under dopamine denervation has been demonstrated (section 5.2), no electrophysiological study of aberrant corticostriatal plasticity in awake, behaving animals has yet been conducted. The studies described in section 4.1 suggest a behavior paradigm in which such studies might yield invaluable insight but this remains a task for future investigations. The notion of aberrant learning that inverts the optimizing function of the dorsal striatum and impedes motor control fits well with a recent hypothesis published by Redgrave et al. (Redgrave et al., 2010). They suggest that the automatic/habitual system is not functioning properly in PD patients (but see de Wit et al., 2011). This, in turn, necessitates greater reliance on volitional, goal-directed processing, eliminating the motor performance benefits arising from automization and habit. They further suggest that, in addition to the failure of habit and automization, pathophysiology in this system may actually interfere with compensatory goal-directed processing by generating pathological signals that actively impede volitional motor control. The hypothesis proposed here links such interference and resulting performance deficits directly to altered corticostriatal plasticity and provides a clear framework for further experimental investigation.
5.4 Remediating aberrant learning to preserve function
Remediating putative aberrant learning and plasticity may protect the performance preserving capacity of established skills by blocking aberrant, anti-optimal inhibitory learning. At present, the relative contribution of established learning and synaptic weights to motor performance in comparison to direct, on-line modulation of motor control by dopamine remains a novel and open question. Moreover, the degree to which current PD treatments correct a putative abnormality in learning/plasticity and the role that may play in therapeutic efficacy is also unknown. The data presented in section 4.1 (Beeler et al., 2010a) suggests that the poorly understood LDR in L-DOPA treatment may arise from correcting an underlying learning process. Consistent with this, abnormal LTP in D2 MSNs induced through dopamine depletion is corrected and normal LTD restored with the activation of D2 receptors (Kreitzer and Malenka, 2007; Shen et al., 2008). Dopamine replacement through L-DOPA would activate D2 receptors and presumably correct abnormalities in plasticity as observed in these studies. Though both the behavioral and physiological data are consistent with the proposal that the LDR arises through the correction of aberrant corticostriatal plasticity and learning, like other theories of the LDR this remains to be definitively proved. Though currently unrecognized, changes in underlying learning processes may contribute significantly to therapeutic efficacy in a number of current treatments (section 4.3/4.4) and may represent a target for the development of novel therapeutics.
6. Aberrant corticostriatal plasticity and aberrant learning: an endophenotype
Investigation of putative aberrant learning in PD is challenging because it is masked by direct motor control impairments on one hand and compensatory mechanisms on the other. The predominant animal model (Fleming et al., 2005; Fleming and Chesselet, 2006; Taylor et al., 2010)—lesions of dopaminergic fibers with 6-OHDA or MPTP (Sedelis et al., 2001; Ungerstedt, 1968)—induces acute and severe dopamine denervation, inconsistent with the gradual loss of dopamine cells and progressive onset of symptoms observed in PD (Betarbet et al., 2002; Brooks, 1998; Dawson et al., 2010; Fleming and Chesselet, 2006; Taylor et al. 2010 but see Lang and Lozano, 1998). Grossly impaired motor function in these models make studying learning nearly impossible (Bove et al., 2005). Genetic models, in contrast, have been criticized as none, to date, recapitulate dopamine cell loss, few show phenotypic characteristics comparable to PD and concern arises over potential unrecognized developmental compensations (Betarbet et al., 2002; Dawson et al., 2010; Jenner, 2008).
Finding an ideal animal model in PD research remains elusive (Betarbet et al., 2002; Dawson et al., 2010; Fleming et al., 2005; Fleming and Chesselet, 2006; Taylor et al., 2010). Increasingly, it is suggested that the relative utility of particular models, each with inherent strengths and weaknesses, depends upon the objectives of the investigation (Betarbet et al., 2002; Dawson et al.); that is, a model is useful to the extent that it captures what an investigator wants to study. To date, though work is beginning (Ardayfio et al., 2008; Beeler et al., 2010a; Ogura et al., 2005), there has been no fully developed animal model designed to investigate learning deficits in PD. Borrowing from psychiatric research the concept of endophenotype (Gottesman and Gould, 2003; Gould and Gottesman, 2006)—the notion that a complex disorder cannot be modeled in its entirety but subsets of pathology can be tackled individually—I propose that aberrant learning be treated as a provisional endophenotype in PD. Though the endophenotype approach has not taken root in PD research generally (but see Racette et al., 2006; van Nuenen et al., 2009), the increasing recognition of PD as a complex disease extending beyond the traditional view of dopamine cell loss and impaired motor control (Aarsland et al., 2010; Bosboom et al., 2004; Chaudhuri et al., 2006; Chaudhuri and Schapira, 2009; Frank, 2005; Jankovic, 2008; Levin and Katzen, 2005; Owen et al., 1992; Wiecki and Frank, 2010; Zgaljardic et al., 2004) suggests that this approach is likely to be increasingly germane to future research.
The etiology of PD remains unknown and evidence points to complexity in genetic contributions (Dauer and Przedborski, 2003). In favor of a plasticity/learning endophenotype, genetic mutations associated with familial PD (DJ-1, Parkin, PINK1), produce abnormalities in corticostriatal plasticity in mice despite a lack of observed degeneration in dopamine cells (Goldberg et al., 2005; Kitada et al., 2007; Kitada et al., 2009). Moreover, considerable evidence suggests that putative learning abnormalities in PD may be an early feature, preceding significant motor decline (Carbon et al., 2004; Carbon and Eidelberg, 2006; Ghilardi et al., 2003; Marinelli et al., 2009; Mentis et al., 2003a; Nakamura et al., 2001; Ogura et al., 2005). The degree to which such learning impairments may serve as a biomarker for genetic studies has not been established. Nor has learning been significantly investigated in non-affected family members in familial PD (but see van der Vegt et al., 2009). Directly investigating synaptic plasticity in humans is not practical, except as inferred from deficits in learning and through imaging. Whether alterations in learning ultimately represent a useful biomarker for genetic research and clinical assessment remains to be determined (see Maetzler et al., 2009). Adopting a provisional endophenotype approach using animal models to explore aberrant plasticity, its pathogenesis and its functional consequences in behavior can facilitate basic understanding of the contribution of altered corticostriatal plasticity to the progression, pathophysiology and treatment of PD and contribute to developing a potentially useful endophenotype for human genetic and clinical studies.
7. Testing the hypothesis
The proposed hypothesis can be specifically investigated in several ways. First, Costa and colleagues (Costa et al., 2004; Jin and Costa, 2010; Yin et al., 2009) have conducted numerous elegant studies correlating changes in MSN activity and synaptic plasticity to motor learning and performance using the accelerating rotorod. The data presented above indicate that after overtraining (ie., established skill), the administration of dopamine blocking drugs will result in a gradual decline in performance that reflects an underlying alteration in D2 mediated synaptic plasticity rather than direct effects of dopamine on MSN firing rates. This can be empirically tested using the same procedures and techniques Costa et al have used to correlate MSN activity and plasticity to the acquisition of learning under normal conditions. The current hypothesis predicts that the gradual decline in behavior, like acquisition, will correlate with changes in plasticity not overall firing rate. Specifically, this will be observed with blockade of D2 while blocking D1 will result in an immediate performance decrement correlated with immediate changes in firing rate rather than synaptic plasticity. There are multiple approaches to this line of investigation, including using ex-vivo slice electrophysiology to examine the differential effect of behavioral experience on synaptic plasticity under conditions of normal and depleted dopamine.
Second, genetic manipulations targeting subcellular signaling molecules regulating plasticity (Kheirbek et al., 2008; Kheirbek et al., 2009) can be applied to animal models of dopamine denervation to isolate the contribution of learning and plasticity to performance following dopamine dysfunction. The hypothesis predicts that blocking abnormal corticostriatal plasticity and aberrant learning will be partially protective against dopamine denervation and prolong retention of established skills.
Finally, in a human clinical population, the difference in performance between ON/OFF medication states is obvious; however, what has not been investigated is whether or not extended practice (ie., repetition of a specific motor task/skill) under ON/OFF will induce a change in performance independent of medication status. Our interpretation of LDR suggest that extended practice ON medication induces gradual improvement independent of L-DOPA pharmacokinetics (ie., whether L-DOPA is in the system or not). Conversely, we predict that extended practice OFF medication will degrade subsequent performance ON medication; that is, the degree to which L-DOPA restores performance will be dependent upon the extent of practice under normal (ON) or aberrant (OFF) learning conditions.
8. Clinical Implications: recognizing and targeting aberrant learning
Insofar as the hypothesis put forth here is supported by further evidence, it captures an important, previously unrecognized dimension of the therapeutic mechanisms and efficacy of current treatments in PD. Further investigation may yield important insights into the best use of current therapies to optimize their treatment benefits and minimize adverse side-effects, including a new perspective on dosage and timing of medication regimens, use of concurrent medications and when to start treatment, as well as a better understanding of the interaction between dopamine replacement therapies and deep brain stimulation. In addition, this hypothesis may have interesting implications in understanding the development of motor fluctuations and L-DOPA induced dyskinesia, though this is not explored here.
Moreover, this hypothesis suggests a novel strategy for identifying pharmacological targets for the development of new therapies in which the objective is to block abnormal corticostriatal plasticity and aberrant learning. Though not expected to slow the underlying degenerative process, such therapies may slow the progression of symptoms contributing to the preservation of function and may enable delayed and reduced use of dopamine replacement therapy thereby diminishing risk of motor fluctuations and dyskinesia.
Finally, this hypothesis has important implications for rehabilitation therapies. The literature on rehabilitation in PD is equivocal, generally showing such treatment has value but that it is limited, particularly by putative learning deficits associated with PD (Abbruzzese et al., 2009; Deane et al., 2002; Keus et al., 2009; Nieuwboer et al., 2009). Rehabilitation is essentially based on practice and learning, consequently understanding when and how learning is impaired in PD and, more importantly, how to remediate aberrant learning, would be critical to the success of rehabilitative approaches.
9. Conclusion
Since the introduction of L-DOPA and despite profound advances in research on the basal ganglia-- famously described as ‘mysterious’ (Marsden, 1982)-- there has been only limited innovation in PD treatment in the last half century. Dopamine replacement therapy, specifically L-DOPA, remains the mainstay of treatment and the problems of motor fluctuations and dyskinesia with prolonged treatment continue to significantly limit long-term clinical efficacy. An on-going challenge is integrating advances in our understanding of the basal ganglia into clinical models and the development of new therapeutics. The framework and hypothesis proposed here contributes to addressing this challenge by integrating corticostriatal plasticity into a prevalent clinical model and in the process suggesting a perspective that unites learning-based, transformational function models of the basal ganglia in basic research with the traditional, aggregate output models widely accepted in clinical research. The veracity and utility of this framework and hypothesis awaits further research. Though such investigation is likely to be difficult, the potential rewards are significant.
Acknowledgments
Thank you to Xiaoxi Zhuang and Nathaniel Daw for helpful feedback on the manuscript. This work was supported by grant DA025875.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aarsland D, Bronnick K, Williams-Gray C, Weintraub D, Marder K, Kulisevsky J, Burn D, Barone P, Pagonabarraga J, Allcock L, Santangelo G, Foltynie T, Janvin C, Larsen JP, Barker RA, Emre M. Mild cognitive impairment in Parkinson disease: a multicenter pooled analysis. Neurology. 2010;75:1062–9. doi: 10.1212/WNL.0b013e3181f39d0e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abbruzzese G, Trompetto C, Marinelli L. The rationale for motor learning in Parkinson’s disease. Eur J Phys Rehabil Med. 2009;45:209–14. [PubMed] [Google Scholar]
- Agostino R, Curra A, Soldati G, Dinapoli L, Chiacchiari L, Modugno N, Pierelli F, Berardelli A. Prolonged practice is of scarce benefit in improving motor performance in Parkinson’s disease. Mov Disord. 2004;19:1285–93. doi: 10.1002/mds.20247. [DOI] [PubMed] [Google Scholar]
- Albin RL, Young AB, Penney JB. The functional anatomy of basal ganglia disorders. Trends Neurosci. 1989;12:366–75. doi: 10.1016/0166-2236(89)90074-x. [DOI] [PubMed] [Google Scholar]
- Albin RL, Young AB, Penney JB. The functional anatomy of disorders of the basal ganglia. Trends Neurosci. 1995;18:63–4. [PubMed] [Google Scholar]
- Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci. 1986;9:357–81. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- Alexander GE, Crutcher MD. Functional architecture of basal ganglia circuits: neural substrates of parallel processing. Trends Neurosci. 1990;13:266–71. doi: 10.1016/0166-2236(90)90107-l. [DOI] [PubMed] [Google Scholar]
- Alexander GE, Crutcher MD, DeLong MR. Basal ganglia-thalamocortical circuits: parallel substrates for motor, oculomotor, “prefrontal” and “limbic” functions. Prog Brain Res. 1990;85:119–46. [PubMed] [Google Scholar]
- Aosaki T, Graybiel AM, Kimura M. Effect of the nigrostriatal dopamine system on acquired neural responses in the striatum of behaving monkeys. Science. 1994;265:412–5. doi: 10.1126/science.8023166. [DOI] [PubMed] [Google Scholar]
- Appel-Cresswell S, de la Fuente-Fernandez R, Galley S, McKeown MJ. Imaging of compensatory mechanisms in Parkinson’s disease. Curr Opin Neurol. 2010;23:407–12. doi: 10.1097/WCO.0b013e32833b6019. [DOI] [PubMed] [Google Scholar]
- Ardayfio P, Moon J, Leung KK, Youn-Hwang D, Kim KS. Impaired learning and memory in Pitx3 deficient aphakia mice: a genetic model for striatum-dependent cognitive symptoms in Parkinson’s disease. Neurobiol Dis. 2008;31:406–12. doi: 10.1016/j.nbd.2008.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashby FG, Turner BO, Horvitz JC. Cortical and basal ganglia contributions to habit learning and automaticity. Trends Cogn Sci. 14:208–15. doi: 10.1016/j.tics.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baglio F, Blasi V, Falini A, Farina E, Mantovani F, Olivotto F, Scotti G, Nemni R, Bozzali M. Functional brain changes in early Parkinson’s disease during motor response and motor inhibition. Neurobiol Aging. 2009 doi: 10.1016/j.neurobiolaging.2008.12.009. [DOI] [PubMed] [Google Scholar]
- Bailey KR, Mair RG. The role of striatum in initiation and execution of learned action sequences in rats. J Neurosci. 2006;26:1016–25. doi: 10.1523/JNEUROSCI.3883-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balleine BW, Delgado MR, Hikosaka O. The role of the dorsal striatum in reward and decision-making. J Neurosci. 2007;27:8161–5. doi: 10.1523/JNEUROSCI.1554-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balleine BW, Liljeholm M, Ostlund SB. The integrative function of the basal ganglia in instrumental conditioning. Behav Brain Res. 2009;199:43–52. doi: 10.1016/j.bbr.2008.10.034. [DOI] [PubMed] [Google Scholar]
- Balleine BW, O’Doherty JP. Human and rodent homologies in action control: corticostriatal determinants of goal-directed and habitual action. Neuropsychopharmacology. 2010;35:48–69. doi: 10.1038/npp.2009.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bamford NS, Zhang H, Schmitz Y, Wu NP, Cepeda C, Levine MS, Schmauss C, Zakharenko SS, Zablow L, Sulzer D. Heterosynaptic dopamine neurotransmission selects sets of corticostriatal terminals. Neuron. 2004;42:653–63. doi: 10.1016/s0896-6273(04)00265-x. [DOI] [PubMed] [Google Scholar]
- Bar-Gad I, Bergman H. Stepping out of the box: information processing in the neural networks of the basal ganglia. Curr Opin Neurobiol. 2001;11:689–95. doi: 10.1016/s0959-4388(01)00270-7. [DOI] [PubMed] [Google Scholar]
- Barbato L, Stocchi F, Monge A, Vacca L, Ruggieri S, Nordera G, Marsden CD. The long-duration action of levodopa may be due to a postsynaptic effect. Clin Neuropharmacol. 1997;20:394–401. doi: 10.1097/00002826-199710000-00003. [DOI] [PubMed] [Google Scholar]
- Barnes TD, Kubota Y, Hu D, Jin DZ, Graybiel AM. Activity of striatal neurons reflects dynamic encoding and recoding of procedural memories. Nature. 2005;437:1158–61. doi: 10.1038/nature04053. [DOI] [PubMed] [Google Scholar]
- Barone P. Neurotransmission in Parkinson’s disease: beyond dopamine. Eur J Neurol. 2010;17:364–76. doi: 10.1111/j.1468-1331.2009.02900.x. [DOI] [PubMed] [Google Scholar]
- Beeler JA, Cao ZF, Kheirbek MA, Zhuang X. Loss of cocaine locomotor response in Pitx3-deficient mice lacking a nigrostriatal pathway. Neuropsychopharmacology. 2009;34:1149–61. doi: 10.1038/npp.2008.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beeler JA, Cao ZF, Kheirbek MA, Ding Y, Koranda J, Murakami M, Kang UJ, Zhuang X. Dopamine-dependent motor learning: insight into levodopa’s long-duration response. Ann Neurol. 2010a;67:639–47. doi: 10.1002/ana.21947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beeler JA, Daw ND, Frazier CR, Zhuang X. Tonic dopamine modulates exploitation of reward learning. Frontiers in behavioral neuroscience. 2010b;4 doi: 10.3389/fnbeh.2010.00170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrman AL, Cauraugh JH, Light KE. Practice as an intervention to improve speeded motor performance and motor learning in Parkinson’s disease. J Neurol Sci. 2000;174:127–36. doi: 10.1016/s0022-510x(00)00267-7. [DOI] [PubMed] [Google Scholar]
- Benecke R, Rothwell JC, Dick JP, Day BL, Marsden CD. Disturbance of sequential movements in patients with Parkinson’s disease. Brain. 1987;110(Pt 2):361–79. doi: 10.1093/brain/110.2.361. [DOI] [PubMed] [Google Scholar]
- Bergman H, Feingold A, Nini A, Raz A, Slovin H, Abeles M, Vaadia E. Physiological aspects of information processing in the basal ganglia of normal and parkinsonian primates. Trends Neurosci. 1998;21:32–8. doi: 10.1016/s0166-2236(97)01151-x. [DOI] [PubMed] [Google Scholar]
- Berke JD, Hyman SE. Addiction, dopamine, and the molecular mechanisms of memory. Neuron. 2000;25:515–32. doi: 10.1016/s0896-6273(00)81056-9. [DOI] [PubMed] [Google Scholar]
- Bernheimer H, Birkmayer W, Hornykiewicz O, Jellinger K, Seitelberger F. Brain dopamine and the syndromes of Parkinson and Huntington. Clinical, morphological and neurochemical correlations. J Neurol Sci. 1973;20:415–55. doi: 10.1016/0022-510x(73)90175-5. [DOI] [PubMed] [Google Scholar]
- Betarbet R, Sherer TB, Greenamyre JT. Animal models of Parkinson’s disease. Bioessays. 2002;24:308–18. doi: 10.1002/bies.10067. [DOI] [PubMed] [Google Scholar]
- Bevan MD, Magill PJ, Terman D, Bolam JP, Wilson CJ. Move to the rhythm: oscillations in the subthalamic nucleus-external globus pallidus network. Trends Neurosci. 2002;25:525–31. doi: 10.1016/s0166-2236(02)02235-x. [DOI] [PubMed] [Google Scholar]
- Bolam JP, Hanley JJ, Booth PA, Bevan MD. Synaptic organisation of the basal ganglia. J Anat. 2000;196(Pt 4):527–42. doi: 10.1046/j.1469-7580.2000.19640527.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosboom JL, Stoffers D, Wolters E. Cognitive dysfunction and dementia in Parkinson’s disease. J Neural Transm. 2004;111:1303–15. doi: 10.1007/s00702-004-0168-1. [DOI] [PubMed] [Google Scholar]
- Bove J, Prou D, Perier C, Przedborski S. Toxin-induced models of Parkinson’s disease. NeuroRx. 2005;2:484–94. doi: 10.1602/neurorx.2.3.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd LA, Edwards JD, Siengsukon CS, Vidoni ED, Wessel BD, Linsdell MA. Motor sequence chunking is impaired by basal ganglia stroke. Neurobiol Learn Mem. 2009;92:35–44. doi: 10.1016/j.nlm.2009.02.009. [DOI] [PubMed] [Google Scholar]
- Braak H, Del Tredici K, Rub U, de Vos RA, Jansen Steur EN, Braak E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging. 2003;24:197–211. doi: 10.1016/s0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- Braver TS, Barch DM, Cohen JD. Cognition and control in schizophrenia: a computational model of dopamine and prefrontal function. Biol Psychiatry. 1999;46:312–28. doi: 10.1016/s0006-3223(99)00116-x. [DOI] [PubMed] [Google Scholar]
- Brooks DJ. The early diagnosis of Parkinson’s disease. Ann Neurol. 1998;44:S10–8. doi: 10.1002/ana.410440704. [DOI] [PubMed] [Google Scholar]
- Calabresi P, De Murtas M, Bernardi G. The neostriatum beyond the motor function: experimental and clinical evidence. Neuroscience. 1997a;78:39–60. doi: 10.1016/s0306-4522(96)00556-8. [DOI] [PubMed] [Google Scholar]
- Calabresi P, Pisani A, Centonze D, Bernardi G. Synaptic plasticity and physiological interactions between dopamine and glutamate in the striatum. Neurosci Biobehav Rev. 1997b;21:519–23. doi: 10.1016/s0149-7634(96)00029-2. [DOI] [PubMed] [Google Scholar]
- Calabresi P, Picconi B, Tozzi A, Di Filippo M. Dopamine-mediated regulation of corticostriatal synaptic plasticity. Trends Neurosci. 2007;30:211–9. doi: 10.1016/j.tins.2007.03.001. [DOI] [PubMed] [Google Scholar]
- Cameron IG, Coe BC, Watanabe M, Stroman PW, Munoz DP. Role of the basal ganglia in switching a planned response. Eur J Neurosci. 2009;29:2413–25. doi: 10.1111/j.1460-9568.2009.06776.x. [DOI] [PubMed] [Google Scholar]
- Carbon M, Ma Y, Barnes A, Dhawan V, Chaly T, Ghilardi MF, Eidelberg D. Caudate nucleus: influence of dopaminergic input on sequence learning and brain activation in Parkinsonism. Neuroimage. 2004;21:1497–507. doi: 10.1016/j.neuroimage.2003.12.014. [DOI] [PubMed] [Google Scholar]
- Carbon M, Eidelberg D. Functional imaging of sequence learning in Parkinson’s disease. J Neurol Sci. 2006;248:72–7. doi: 10.1016/j.jns.2006.05.005. [DOI] [PubMed] [Google Scholar]
- Carli M, Evenden JL, Robbins TW. Depletion of unilateral striatal dopamine impairs initiation of contralateral actions and not sensory attention. Nature. 1985;313:679–82. doi: 10.1038/313679a0. [DOI] [PubMed] [Google Scholar]
- Cavaco S, Anderson SW, Correia M, Magalhaes M, Pereira C, Tuna A, Taipa R, Pinto P, Pinto C, Cruz R, Lima AB, Castro-Caldas A, da Silva AM, Damasio H. Task-specific contribution of the human striatum to perceptual-motor skill learning. J Clin Exp Neuropsychol. 2010:1–12. doi: 10.1080/13803395.2010.493144. [DOI] [PubMed] [Google Scholar]
- Centonze D, Picconi B, Gubellini P, Bernardi G, Calabresi P. Dopaminergic control of synaptic plasticity in the dorsal striatum. Eur J Neurosci. 2001;13:1071–7. doi: 10.1046/j.0953-816x.2001.01485.x. [DOI] [PubMed] [Google Scholar]
- Centonze D, Usiello A, Costa C, Picconi B, Erbs E, Bernardi G, Borrelli E, Calabresi P. Chronic haloperidol promotes corticostriatal long-term potentiation by targeting dopamine D2L receptors. J Neurosci. 2004;24:8214–22. doi: 10.1523/JNEUROSCI.1274-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cepeda C, Hurst RS, Altemus KL, Flores-Hernandez J, Calvert CR, Jokel ES, Grandy DK, Low MJ, Rubinstein M, Ariano MA, Levine MS. Facilitated glutamatergic transmission in the striatum of D2 dopamine receptor-deficient mice. J Neurophysiol. 2001;85:659–70. doi: 10.1152/jn.2001.85.2.659. [DOI] [PubMed] [Google Scholar]
- Chaudhuri KR, Healy DG, Schapira AH. Non-motor symptoms of Parkinson’s disease: diagnosis and management. Lancet Neurol. 2006;5:235–45. doi: 10.1016/S1474-4422(06)70373-8. [DOI] [PubMed] [Google Scholar]
- Chaudhuri KR, Schapira AH. Non-motor symptoms of Parkinson’s disease: dopaminergic pathophysiology and treatment. Lancet Neurol. 2009;8:464–74. doi: 10.1016/S1474-4422(09)70068-7. [DOI] [PubMed] [Google Scholar]
- Chesselet MF, Delfs JM. Basal ganglia and movement disorders: an update. Trends Neurosci. 1996;19:417–22. doi: 10.1016/0166-2236(96)10052-7. [DOI] [PubMed] [Google Scholar]
- Chevalier G, Deniau JM. Disinhibition as a basic process in the expression of striatal functions. Trends Neurosci. 1990;13:277–80. doi: 10.1016/0166-2236(90)90109-n. [DOI] [PubMed] [Google Scholar]
- Choi WY, Balsam PD, Horvitz JC. Extended habit training reduces dopamine mediation of appetitive response expression. J Neurosci. 2005;25:6729–33. doi: 10.1523/JNEUROSCI.1498-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen H, Pourcher E. Intact encoding, impaired consolidation in procedural learning in Parkinson’s disease. Exp Brain Res. 2007;179:703–8. doi: 10.1007/s00221-006-0827-6. [DOI] [PubMed] [Google Scholar]
- Cools R, Clark L, Robbins TW. Differential responses in human striatum and prefrontal cortex to changes in object and rule relevance. J Neurosci. 2004;24:1129–35. doi: 10.1523/JNEUROSCI.4312-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cools R. Dopaminergic modulation of cognitive function-implications for L-DOPA treatment in Parkinson’s disease. Neurosci Biobehav Rev. 2006;30:1–23. doi: 10.1016/j.neubiorev.2005.03.024. [DOI] [PubMed] [Google Scholar]
- Cools R, Altamirano L, D’Esposito M. Reversal learning in Parkinson’s disease depends on medication status and outcome valence. Neuropsychologia. 2006a;44:1663–73. doi: 10.1016/j.neuropsychologia.2006.03.030. [DOI] [PubMed] [Google Scholar]
- Cools R, Ivry RB, D’Esposito M. The human striatum is necessary for responding to changes in stimulus relevance. J Cogn Neurosci. 2006b;18:1973–83. doi: 10.1162/jocn.2006.18.12.1973. [DOI] [PubMed] [Google Scholar]
- Cools R, Miyakawa A, Sheridan M, D’Esposito M. Enhanced frontal function in Parkinson’s disease. Brain. 2010;133:225–33. doi: 10.1093/brain/awp301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa RM, Cohen D, Nicolelis MA. Differential corticostriatal plasticity during fast and slow motor skill learning in mice. Curr Biol. 2004;14:1124–34. doi: 10.1016/j.cub.2004.06.053. [DOI] [PubMed] [Google Scholar]
- Costa RM, Lin SC, Sotnikova TD, Cyr M, Gainetdinov RR, Caron MG, Nicolelis MA. Rapid alterations in corticostriatal ensemble coordination during acute dopamine-dependent motor dysfunction. Neuron. 2006;52:359–69. doi: 10.1016/j.neuron.2006.07.030. [DOI] [PubMed] [Google Scholar]
- Costa RM. Plastic corticostriatal circuits for action learning: what’s dopamine got to do with it? Ann N Y Acad Sci. 2007;1104:172–91. doi: 10.1196/annals.1390.015. [DOI] [PubMed] [Google Scholar]
- Cotzias GC, Papavasiliou PS, Gellene R. Modification of Parkinsonism--chronic treatment with L-dopa. N Engl J Med. 1969;280:337–45. doi: 10.1056/NEJM196902132800701. [DOI] [PubMed] [Google Scholar]
- Dauer W, Przedborski S. Parkinson’s disease: mechanisms and models. Neuron. 2003;39:889–909. doi: 10.1016/s0896-6273(03)00568-3. [DOI] [PubMed] [Google Scholar]
- Dawson TM, Ko HS, Dawson VL. Genetic animal models of Parkinson’s disease. Neuron. 2010;66:646–61. doi: 10.1016/j.neuron.2010.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day M, Wokosin D, Plotkin JL, Tian X, Surmeier DJ. Differential excitability and modulation of striatal medium spiny neuron dendrites. J Neurosci. 2008;28:11603–14. doi: 10.1523/JNEUROSCI.1840-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wit S, Barker RA, Dickinson AD, Cools R. Habitual versus goal-directed action control in Parkinson disease. J Cogn Neurosci. 2011;23:1218–29. doi: 10.1162/jocn.2010.21514. [DOI] [PubMed] [Google Scholar]
- Deane KH, Ellis-Hill C, Jones D, Whurr R, Ben-Shlomo Y, Playford ED, Clarke CE. Systematic review of paramedical therapies for Parkinson’s disease. Mov Disord. 2002;17:984–91. doi: 10.1002/mds.10197. [DOI] [PubMed] [Google Scholar]
- DeLong M, Wichmann T. Update on models of basal ganglia function and dysfunction. Parkinsonism Relat Disord. 2009;15(Suppl 3):S237–40. doi: 10.1016/S1353-8020(09)70822-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLong MR. Primate models of movement disorders of basal ganglia origin. Trends Neurosci. 1990;13:281–5. doi: 10.1016/0166-2236(90)90110-v. [DOI] [PubMed] [Google Scholar]
- DeLong MR, Wichmann T. Circuits and circuit disorders of the basal ganglia. Arch Neurol. 2007;64:20–4. doi: 10.1001/archneur.64.1.20. [DOI] [PubMed] [Google Scholar]
- Divac I, Rosvold HE, Szwarcbart MK. Behavioral effects of selective ablation of the caudate nucleus. J Comp Physiol Psychol. 1967;63:184–90. doi: 10.1037/h0024348. [DOI] [PubMed] [Google Scholar]
- Doller HJ, Connor JD. Changes in neostriatal dopamine concentrations in response to levodopa infusions. J Neurochem. 1980;34:1264–9. doi: 10.1111/j.1471-4159.1980.tb09969.x. [DOI] [PubMed] [Google Scholar]
- Doron O, Goelman G. Evidence for asymmetric intra substantia nigra functional connectivity-application to basal ganglia processing. Neuroimage. 2010;49:2940–6. doi: 10.1016/j.neuroimage.2009.11.032. [DOI] [PubMed] [Google Scholar]
- Dorsey ER, Constantinescu R, Thompson JP, Biglan KM, Holloway RG, Kieburtz K, Marshall FJ, Ravina BM, Schifitto G, Siderowf A, Tanner CM. Projected number of people with Parkinson disease in the most populous nations, 2005 through 2030. Neurology. 2007;68:384–6. doi: 10.1212/01.wnl.0000247740.47667.03. [DOI] [PubMed] [Google Scholar]
- Doyon J, Benali H. Reorganization and plasticity in the adult brain during learning of motor skills. Curr Opin Neurobiol. 2005;15:161–7. doi: 10.1016/j.conb.2005.03.004. [DOI] [PubMed] [Google Scholar]
- Doyon J. Motor sequence learning and movement disorders. Curr Opin Neurol. 2008;21:478–83. doi: 10.1097/WCO.0b013e328304b6a3. [DOI] [PubMed] [Google Scholar]
- Doyon J, Bellec P, Amsel R, Penhune V, Monchi O, Carrier J, Lehericy S, Benali H. Contributions of the basal ganglia and functionally related brain structures to motor learning. Behav Brain Res. 2009;199:61–75. doi: 10.1016/j.bbr.2008.11.012. [DOI] [PubMed] [Google Scholar]
- Draganski B, Kherif F, Kloppel S, Cook PA, Alexander DC, Parker GJ, Deichmann R, Ashburner J, Frackowiak RS. Evidence for segregated and integrative connectivity patterns in the human Basal Ganglia. J Neurosci. 2008;28:7143–52. doi: 10.1523/JNEUROSCI.1486-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunnett SB, Robbins TW. The functional role of mesotelencephalic dopamine systems. Biol Rev Camb Philos Soc. 1992;67:491–518. doi: 10.1111/j.1469-185x.1992.tb01191.x. [DOI] [PubMed] [Google Scholar]
- Espay AJ. Management of motor complications in Parkinson disease: current and emerging therapies. Neurol Clin. 2010;28:913–25. doi: 10.1016/j.ncl.2010.03.014. [DOI] [PubMed] [Google Scholar]
- Fahn S, Oakes D, Shoulson I, Kieburtz K, Rudolph A, Lang A, Olanow CW, Tanner C, Marek K. Levodopa and the progression of Parkinson’s disease. N Engl J Med. 2004;351:2498–508. doi: 10.1056/NEJMoa033447. [DOI] [PubMed] [Google Scholar]
- Faure A, Haberland U, Conde F, El Massioui N. Lesion to the nigrostriatal dopamine system disrupts stimulus-response habit formation. J Neurosci. 2005;25:2771–80. doi: 10.1523/JNEUROSCI.3894-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filion M, Tremblay L. Abnormal spontaneous activity of globus pallidus neurons in monkeys with MPTP-induced parkinsonism. Brain Res. 1991;547:142–51. [PubMed] [Google Scholar]
- Fleming SM, Fernagut PO, Chesselet MF. Genetic mouse models of parkinsonism: strengths and limitations. NeuroRx. 2005;2:495–503. doi: 10.1602/neurorx.2.3.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming SM, Chesselet MF. Behavioral phenotypes and pharmacology in genetic mouse models of Parkinsonism. Behav Pharmacol. 2006;17:383–91. doi: 10.1097/00008877-200609000-00004. [DOI] [PubMed] [Google Scholar]
- Flores-Hernandez J, Galarraga E, Bargas J. Dopamine selects glutamatergic inputs to neostriatal neurons. Synapse. 1997;25:185–95. doi: 10.1002/(SICI)1098-2396(199702)25:2<185::AID-SYN9>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Floyer-Lea A, Matthews PM. Distinguishable brain activation networks for short- and long-term motor skill learning. J Neurophysiol. 2005;94:512–8. doi: 10.1152/jn.00717.2004. [DOI] [PubMed] [Google Scholar]
- Foerde K, Knowlton BJ, Poldrack RA. Modulation of competing memory systems by distraction. Proc Natl Acad Sci U S A. 2006;103:11778–83. doi: 10.1073/pnas.0602659103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fornaguera J, Carey RJ, Dai H, Huston JP, Schwarting RK. Differentiation of motor inactivation from movement asymmetry effects in an animal model of hemi-parkinsonism. Neuroreport. 1994;6:173–6. doi: 10.1097/00001756-199412300-00044. [DOI] [PubMed] [Google Scholar]
- Frank MJ. Dynamic dopamine modulation in the basal ganglia: a neurocomputational account of cognitive deficits in medicated and nonmedicated Parkinsonism. J Cogn Neurosci. 2005;17:51–72. doi: 10.1162/0898929052880093. [DOI] [PubMed] [Google Scholar]
- Gelb DJ, Oliver E, Gilman S. Diagnostic criteria for Parkinson disease. Arch Neurol. 1999;56:33–9. doi: 10.1001/archneur.56.1.33. [DOI] [PubMed] [Google Scholar]
- Gerfen CR, Engber TM, Mahan LC, Susel Z, Chase TN, Monsma FJ, Jr, Sibley DR. D1 and D2 dopamine receptor-regulated gene expression of striatonigral and striatopallidal neurons. Science. 1990;250:1429–32. doi: 10.1126/science.2147780. [DOI] [PubMed] [Google Scholar]
- Ghilardi MF, Eidelberg D, Silvestri G, Ghez C. The differential effect of PD and normal aging on early explicit sequence learning. Neurology. 2003;60:1313–9. doi: 10.1212/01.wnl.0000059545.69089.ee. [DOI] [PubMed] [Google Scholar]
- Ghilardi MF, Moisello C, Silvestri G, Ghez C, Krakauer JW. Learning of a sequential motor skill comprises explicit and implicit components that consolidate differently. J Neurophysiol. 2009;101:2218–29. doi: 10.1152/jn.01138.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg MS, Pisani A, Haburcak M, Vortherms TA, Kitada T, Costa C, Tong Y, Martella G, Tscherter A, Martins A, Bernardi G, Roth BL, Pothos EN, Calabresi P, Shen J. Nigrostriatal dopaminergic deficits and hypokinesia caused by inactivation of the familial Parkinsonism-linked gene DJ-1. Neuron. 2005;45:489–96. doi: 10.1016/j.neuron.2005.01.041. [DOI] [PubMed] [Google Scholar]
- Gottesman, Gould TD. The endophenotype concept in psychiatry: etymology and strategic intentions. Am J Psychiatry. 2003;160:636–45. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- Gould TD, Gottesman II. Psychiatric endophenotypes and the development of valid animal models. Genes Brain Behav. 2006;5:113–9. doi: 10.1111/j.1601-183X.2005.00186.x. [DOI] [PubMed] [Google Scholar]
- Graybiel AM, Hirsch EC, Agid Y. The nigrostriatal system in Parkinson’s disease. Adv Neurol. 1990;53:17–29. [PubMed] [Google Scholar]
- Graybiel AM. Building action repertoires: memory and learning functions of the basal ganglia. Curr Opin Neurobiol. 1995;5:733–41. doi: 10.1016/0959-4388(95)80100-6. [DOI] [PubMed] [Google Scholar]
- Graybiel AM. The basal ganglia and chunking of action repertoires. Neurobiol Learn Mem. 1998;70:119–36. doi: 10.1006/nlme.1998.3843. [DOI] [PubMed] [Google Scholar]
- Graybiel AM. The basal ganglia: learning new tricks and loving it. Curr Opin Neurobiol. 2005;15:638–44. doi: 10.1016/j.conb.2005.10.006. [DOI] [PubMed] [Google Scholar]
- Graybiel AM. Habits, rituals, and the evaluative brain. Annu Rev Neurosci. 2008;31:359–87. doi: 10.1146/annurev.neuro.29.051605.112851. [DOI] [PubMed] [Google Scholar]
- Haber SN. The primate basal ganglia: parallel and integrative networks. J Chem Neuroanat. 2003;26:317–30. doi: 10.1016/j.jchemneu.2003.10.003. [DOI] [PubMed] [Google Scholar]
- Hammond C, Bergman H, Brown P. Pathological synchronization in Parkinson’s disease: networks, models and treatments. Trends Neurosci. 2007;30:357–64. doi: 10.1016/j.tins.2007.05.004. [DOI] [PubMed] [Google Scholar]
- Harrington DL, Haaland KY, Yeo RA, Marder E. Procedural memory in Parkinson’s disease: impaired motor but not visuoperceptual learning. J Clin Exp Neuropsychol. 1990;12:323–39. doi: 10.1080/01688639008400978. [DOI] [PubMed] [Google Scholar]
- Haruno M, Kawato M. Different neural correlates of reward expectation and reward expectation error in the putamen and caudate nucleus during stimulus-action-reward association learning. J Neurophysiol. 2006;95:948–59. doi: 10.1152/jn.00382.2005. [DOI] [PubMed] [Google Scholar]
- Hauser RA, Holford NH. Quantitative description of loss of clinical benefit following withdrawal of levodopa-carbidopa and bromocriptine in early Parkinson’s disease. Mov Disord. 2002;17:961–8. doi: 10.1002/mds.10226. [DOI] [PubMed] [Google Scholar]
- Helmich RC, de Lange FP, Bloem BR, Toni I. Cerebral compensation during motor imagery in Parkinson’s disease. Neuropsychologia. 2007;45:2201–15. doi: 10.1016/j.neuropsychologia.2007.02.024. [DOI] [PubMed] [Google Scholar]
- Helmich RC, Derikx LC, Bakker M, Scheeringa R, Bloem BR, Toni I. Spatial remapping of cortico-striatal connectivity in Parkinson’s disease. Cereb Cortex. 2010;20:1175–86. doi: 10.1093/cercor/bhp178. [DOI] [PubMed] [Google Scholar]
- Hernandez-Lopez S, Bargas J, Surmeier DJ, Reyes A, Galarraga E. D1 receptor activation enhances evoked discharge in neostriatal medium spiny neurons by modulating an L-type Ca2+ conductance. J Neurosci. 1997;17:3334–42. doi: 10.1523/JNEUROSCI.17-09-03334.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez-Lopez S, Tkatch T, Perez-Garci E, Galarraga E, Bargas J, Hamm H, Surmeier DJ. D2 dopamine receptors in striatal medium spiny neurons reduce L-type Ca2+ currents and excitability via a novel PLC[beta]1-IP3-calcineurin-signaling cascade. J Neurosci. 2000;20:8987–95. doi: 10.1523/JNEUROSCI.20-24-08987.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hikosaka O, Nakahara H, Rand MK, Sakai K, Lu X, Nakamura K, Miyachi S, Doya K. Parallel neural networks for learning sequential procedures. Trends Neurosci. 1999;22:464–71. doi: 10.1016/s0166-2236(99)01439-3. [DOI] [PubMed] [Google Scholar]
- Hikosaka O, Isoda M. Switching from automatic to controlled behavior: cortico-basal ganglia mechanisms. Trends Cogn Sci. 2010;14:154–61. doi: 10.1016/j.tics.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirtz D, Thurman DJ, Gwinn-Hardy K, Mohamed M, Chaudhuri AR, Zalutsky R. How common are the “common” neurologic disorders? Neurology. 2007;68:326–37. doi: 10.1212/01.wnl.0000252807.38124.a3. [DOI] [PubMed] [Google Scholar]
- Hoover JE, Strick PL. Multiple output channels in the basal ganglia. Science. 1993;259:819–21. doi: 10.1126/science.7679223. [DOI] [PubMed] [Google Scholar]
- Hornykiewicz O. Chemical neuroanatomy of the basal ganglia--normal and in Parkinson’s disease. J Chem Neuroanat. 2001;22:3–12. doi: 10.1016/s0891-0618(01)00100-4. [DOI] [PubMed] [Google Scholar]
- Humphries MD, Prescott TJ. The ventral basal ganglia, a selection mechanism at the crossroads of space, strategy, and reward. Prog Neurobiol. 2010;90:385–417. doi: 10.1016/j.pneurobio.2009.11.003. [DOI] [PubMed] [Google Scholar]
- Hwang DY, Ardayfio P, Kang UJ, Semina EV, Kim KS. Selective loss of dopaminergic neurons in the substantia nigra of Pitx3-deficient aphakia mice. Brain Res Mol Brain Res. 2003;114:123–31. doi: 10.1016/s0169-328x(03)00162-1. [DOI] [PubMed] [Google Scholar]
- Israel Z, Bergman H. Pathophysiology of the basal ganglia and movement disorders: from animal models to human clinical applications. Neurosci Biobehav Rev. 2008;32:367–77. doi: 10.1016/j.neubiorev.2007.08.005. [DOI] [PubMed] [Google Scholar]
- Iversen SD. Behavioural effects of manipulation of basal ganglia neurotransmitters. Ciba Found Symp. 1984;107:183–200. doi: 10.1002/9780470720882.ch10. [DOI] [PubMed] [Google Scholar]
- Jackson GM, Jackson SR, Harrison J, Henderson L, Kennard C. Serial reaction time learning and Parkinson’s disease: evidence for a procedural learning deficit. Neuropsychologia. 1995;33:577–93. doi: 10.1016/0028-3932(95)00010-z. [DOI] [PubMed] [Google Scholar]
- Jankovic J. Parkinson’s disease: clinical features and diagnosis. J Neurol Neurosurg Psychiatry. 2008;79:368–76. doi: 10.1136/jnnp.2007.131045. [DOI] [PubMed] [Google Scholar]
- Jenner P. Functional models of Parkinson’s disease: a valuable tool in the development of novel therapies. Ann Neurol. 2008;64(Suppl 2):S16–29. doi: 10.1002/ana.21489. [DOI] [PubMed] [Google Scholar]
- Jin X, Costa RM. Start/stop signals emerge in nigrostriatal circuits during sequence learning. Nature. 2010;466:457–62. doi: 10.1038/nature09263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joel D, Weiner I. The organization of the basal ganglia-thalamocortical circuits: open interconnected rather than closed segregated. Neuroscience. 1994;63:363–79. doi: 10.1016/0306-4522(94)90536-3. [DOI] [PubMed] [Google Scholar]
- Jog MS, Kubota Y, Connolly CI, Hillegaart V, Graybiel AM. Building neural representations of habits. Science. 1999;286:1745–9. doi: 10.1126/science.286.5445.1745. [DOI] [PubMed] [Google Scholar]
- Jordan N, Sagar HJ. The role of the striatum in motor learning: dissociations between isometric motor control processes in Parkinson’s disease. Int J Neurosci. 1994;77:153–65. doi: 10.3109/00207459408986027. [DOI] [PubMed] [Google Scholar]
- Kermadi I, Jurquet Y, Arzi M, Joseph JP. Neural activity in the caudate nucleus of monkeys during spatial sequencing. Exp Brain Res. 1993;94:352–6. doi: 10.1007/BF00230305. [DOI] [PubMed] [Google Scholar]
- Kerr JN, Wickens JR. Dopamine D-1/D-5 receptor activation is required for long-term potentiation in the rat neostriatum in vitro. J Neurophysiol. 2001;85:117–24. doi: 10.1152/jn.2001.85.1.117. [DOI] [PubMed] [Google Scholar]
- Keus SH, Munneke M, Nijkrake MJ, Kwakkel G, Bloem BR. Physical therapy in Parkinson’s disease: evolution and future challenges. Mov Disord. 2009;24:1–14. doi: 10.1002/mds.22141. [DOI] [PubMed] [Google Scholar]
- Kheirbek MA, Beeler JA, Ishikawa Y, Zhuang X. A cAMP pathway underlying reward prediction in associative learning. J Neurosci. 2008;28:11401–8. doi: 10.1523/JNEUROSCI.4115-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kheirbek MA, Britt JP, Beeler JA, Ishikawa Y, McGehee DS, Zhuang X. Adenylyl cyclase type 5 contributes to corticostriatal plasticity and striatum-dependent learning. J Neurosci. 2009;29:12115–24. doi: 10.1523/JNEUROSCI.3343-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimchi EY, Torregrossa MM, Taylor JR, Laubach M. Neuronal correlates of instrumental learning in the dorsal striatum. J Neurophysiol. 2009;102:475–89. doi: 10.1152/jn.00262.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kish SJ, Shannak K, Hornykiewicz O. Uneven pattern of dopamine loss in the striatum of patients with idiopathic Parkinson’s disease. Pathophysiologic and clinical implications. N Engl J Med. 1988;318:876–80. doi: 10.1056/NEJM198804073181402. [DOI] [PubMed] [Google Scholar]
- Kitada T, Pisani A, Porter DR, Yamaguchi H, Tscherter A, Martella G, Bonsi P, Zhang C, Pothos EN, Shen J. Impaired dopamine release and synaptic plasticity in the striatum of PINK1-deficient mice. Proc Natl Acad Sci U S A. 2007;104:11441–6. doi: 10.1073/pnas.0702717104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitada T, Pisani A, Karouani M, Haburcak M, Martella G, Tscherter A, Platania P, Wu B, Pothos EN, Shen J. Impaired dopamine release and synaptic plasticity in the striatum of parkin−/− mice. J Neurochem. 2009;110:613–21. doi: 10.1111/j.1471-4159.2009.06152.x. [DOI] [PubMed] [Google Scholar]
- Knowlton BJ, Mangels JA, Squire LR. A neostriatal habit learning system in humans. Science. 1996;273:1399–402. doi: 10.1126/science.273.5280.1399. [DOI] [PubMed] [Google Scholar]
- Konczak J, Corcos DM, Horak F, Poizner H, Shapiro M, Tuite P, Volkmann J, Maschke M. Proprioception and motor control in Parkinson’s disease. J Mot Behav. 2009;41:543–52. doi: 10.3200/35-09-002. [DOI] [PubMed] [Google Scholar]
- Konorski J. Integrative Activity of the Brain. University of Chicago Press; Chicago: 1967. [Google Scholar]
- Krebs HI, Hogan N, Hening W, Adamovich SV, Poizner H. Procedural motor learning in Parkinson’s disease. Exp Brain Res. 2001;141:425–37. doi: 10.1007/s002210100871. [DOI] [PubMed] [Google Scholar]
- Kreitzer AC, Malenka RC. Endocannabinoid-mediated rescue of striatal LTD and motor deficits in Parkinson’s disease models. Nature. 2007;445:643–7. doi: 10.1038/nature05506. [DOI] [PubMed] [Google Scholar]
- Kreitzer AC, Malenka RC. Striatal plasticity and basal ganglia circuit function. Neuron. 2008;60:543–54. doi: 10.1016/j.neuron.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang AE, Lozano AM. Parkinson’s disease. First of two parts. N Engl J Med. 1998;339:1044–53. doi: 10.1056/NEJM199810083391506. [DOI] [PubMed] [Google Scholar]
- Lehericy S, Ducros M, Krainik A, Francois C, Van de Moortele PF, Ugurbil K, Kim DS. 3-D diffusion tensor axonal tracking shows distinct SMA and pre-SMA projections to the human striatum. Cereb Cortex. 2004;14:1302–9. doi: 10.1093/cercor/bhh091. [DOI] [PubMed] [Google Scholar]
- Lehericy S, Benali H, Van de Moortele PF, Pelegrini-Issac M, Waechter T, Ugurbil K, Doyon J. Distinct basal ganglia territories are engaged in early and advanced motor sequence learning. Proc Natl Acad Sci U S A. 2005;102:12566–71. doi: 10.1073/pnas.0502762102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerner TN, Kreitzer AC. Neuromodulatory control of striatal plasticity and behavior. Curr Opin Neurobiol. 2011;21:322–7. doi: 10.1016/j.conb.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin BE, Katzen HL. Early cognitive changes and nondementing behavioral abnormalities in Parkinson’s disease. Adv Neurol. 2005;96:84–94. [PubMed] [Google Scholar]
- Lidsky TI, Manetto C, Schneider JS. A consideration of sensory factors involved in motor functions of the basal ganglia. Brain Res. 1985;356:133–46. doi: 10.1016/0165-0173(85)90010-4. [DOI] [PubMed] [Google Scholar]
- Lovinger DM, Partridge JG, Tang KC. Plastic control of striatal glutamatergic transmission by ensemble actions of several neurotransmitters and targets for drugs of abuse. Ann N Y Acad Sci. 2003;1003:226–40. doi: 10.1196/annals.1300.014. [DOI] [PubMed] [Google Scholar]
- Lovinger DM. Neurotransmitter roles in synaptic modulation, plasticity and learning in the dorsal striatum. Neuropharmacology. 2010 doi: 10.1016/j.neuropharm.2010.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maetzler W, Liepelt I, Berg D. Progression of Parkinson’s disease in the clinical phase: potential markers. Lancet Neurol. 2009;8:1158–71. doi: 10.1016/S1474-4422(09)70291-1. [DOI] [PubMed] [Google Scholar]
- Mahon S, Deniau JM, Charpier S. Corticostriatal plasticity: life after the depression. Trends Neurosci. 2004;27:460–7. doi: 10.1016/j.tins.2004.06.010. [DOI] [PubMed] [Google Scholar]
- Mallet N, Ballion B, Le Moine C, Gonon F. Cortical inputs and GABA interneurons imbalance projection neurons in the striatum of parkinsonian rats. J Neurosci. 2006;26:3875–84. doi: 10.1523/JNEUROSCI.4439-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manetto C, Lidsky TI. Caudate neuronal activity in cats during head turning: selectivity for sensory-triggered movements. Brain Res Bull. 1986;16:425–8. doi: 10.1016/0361-9230(86)90067-5. [DOI] [PubMed] [Google Scholar]
- Marinelli L, Crupi D, Di Rocco A, Bove M, Eidelberg D, Abbruzzese G, Ghilardi MF. Learning and consolidation of visuo-motor adaptation in Parkinson’s disease. Parkinsonism Relat Disord. 2009;15:6–11. doi: 10.1016/j.parkreldis.2008.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsden CD, Parkes JD. Success and problems of long-term levodopa therapy in Parkinson’s disease. Lancet. 1977;1:345–9. doi: 10.1016/s0140-6736(77)91146-1. [DOI] [PubMed] [Google Scholar]
- Marsden CD. The mysterious motor function of the basal gnaglia: the Robert Wartenberg Lecture. Neurology. 1982;32:514–539. doi: 10.1212/wnl.32.5.514. [DOI] [PubMed] [Google Scholar]
- McGeorge AJ, Faull RL. The organization of the projection from the cerebral cortex to the striatum in the rat. Neuroscience. 1989;29:503–37. doi: 10.1016/0306-4522(89)90128-0. [DOI] [PubMed] [Google Scholar]
- Mentis MJ, Dhawan V, Feigin A, Delalot D, Zgaljardic D, Edwards C, Eidelberg D. Early stage Parkinson’s disease patients and normal volunteers: comparative mechanisms of sequence learning. Hum Brain Mapp. 2003a;20:246–58. doi: 10.1002/hbm.10142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mentis MJ, Dhawan V, Nakamura T, Ghilardi MF, Feigin A, Edwards C, Ghez C, Eidelberg D. Enhancement of brain activation during trial-and-error sequence learning in early PD. Neurology. 2003b;60:612–9. doi: 10.1212/01.wnl.0000044154.92143.dc. [DOI] [PubMed] [Google Scholar]
- Messier J, Adamovich S, Jack D, Hening W, Sage J, Poizner H. Visuomotor learning in immersive 3D virtual reality in Parkinson’s disease and in aging. Exp Brain Res. 2007;179:457–74. doi: 10.1007/s00221-006-0802-2. [DOI] [PubMed] [Google Scholar]
- Metman LV, Locatelli ER, Bravi D, Mouradian MM, Chase TN. Apomorphine responses in Parkinson’s disease and the pathogenesis of motor complications. Neurology. 1997;48:369–372. doi: 10.1212/wnl.48.2.369. [DOI] [PubMed] [Google Scholar]
- Middleton FA, Strick PL. Basal ganglia and cerebellar loops: motor and cognitive circuits. Brain Res Brain Res Rev. 2000;31:236–50. doi: 10.1016/s0165-0173(99)00040-5. [DOI] [PubMed] [Google Scholar]
- Mink JW. The basal ganglia: focused selection and inhibition of competing motor programs. Prog Neurobiol. 1996;50:381–425. doi: 10.1016/s0301-0082(96)00042-1. [DOI] [PubMed] [Google Scholar]
- Miyachi S, Hikosaka O, Miyashita K, Karadi Z, Rand MK. Differential roles of monkey striatum in learning of sequential hand movement. Exp Brain Res. 1997;115:1–5. doi: 10.1007/pl00005669. [DOI] [PubMed] [Google Scholar]
- Miyachi S, Hikosaka O, Lu X. Differential activation of monkey striatal neurons in the early and late stages of procedural learning. Exp Brain Res. 2002;146:122–6. doi: 10.1007/s00221-002-1213-7. [DOI] [PubMed] [Google Scholar]
- Muenter MD, Tyce GM. L-dopa therapy of Parkinson’s disease: plasma L-dopa concentration, therapeutic response, and side effects. Mayo Clin Proc. 1971;46:231–9. [PubMed] [Google Scholar]
- Muslimovic D, Post B, Speelman JD, Schmand B. Motor procedural learning in Parkinson’s disease. Brain. 2007;130:2887–97. doi: 10.1093/brain/awm211. [DOI] [PubMed] [Google Scholar]
- Nakamura T, Ghilardi MF, Mentis M, Dhawan V, Fukuda M, Hacking A, Moeller JR, Ghez C, Eidelberg D. Functional networks in motor sequence learning: abnormal topographies in Parkinson’s disease. Hum Brain Mapp. 2001;12:42–60. doi: 10.1002/1097-0193(200101)12:1<42::AID-HBM40>3.0.CO;2-D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicola SM, Surmeier J, Malenka RC. Dopaminergic modulation of neuronal excitability in the striatum and nucleus accumbens. Annu Rev Neurosci. 2000;23:185–215. doi: 10.1146/annurev.neuro.23.1.185. [DOI] [PubMed] [Google Scholar]
- Nicola SM. The nucleus accumbens as part of a basal ganglia action selection circuit. Psychopharmacology (Berl) 2007;191:521–50. doi: 10.1007/s00213-006-0510-4. [DOI] [PubMed] [Google Scholar]
- Nieuwboer A, Rochester L, Muncks L, Swinnen SP. Motor learning in Parkinson’s disease: limitations and potential for rehabilitation. Parkinsonism Relat Disord. 2009;15(Suppl 3):S53–8. doi: 10.1016/S1353-8020(09)70781-3. [DOI] [PubMed] [Google Scholar]
- Nunes I, Tovmasian LT, Silva RM, Burke RE, Goff SP. Pitx3 is required for development of substantia nigra dopaminergic neurons. Proc Natl Acad Sci U S A. 2003;100:4245–50. doi: 10.1073/pnas.0230529100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nutt JG, Carter JH, Woodward WR. Long-duration response to levodopa. Neurology. 1995;45:1613–6. doi: 10.1212/wnl.45.8.1613. [DOI] [PubMed] [Google Scholar]
- Nutt JG, Holford NH. The response to levodopa in Parkinson’s disease: imposing pharmacological law and order. Ann Neurol. 1996;39:561–73. doi: 10.1002/ana.410390504. [DOI] [PubMed] [Google Scholar]
- Nutt JG, Carter JH, Van Houten L, Woodward WR. Short- and long-duration responses to levodopa during the first year of levodopa therapy. Ann Neurol. 1997;42:349–55. doi: 10.1002/ana.410420311. [DOI] [PubMed] [Google Scholar]
- Nutt JG, Carter JH. Apomorphine can sustain the long-duration response to L-DOPA in fluctuating PD. Neurology. 2000;54:247–50. doi: 10.1212/wnl.54.1.247. [DOI] [PubMed] [Google Scholar]
- Nutt JG, Rufener SL, Carter JH, Anderson VC, Pahwa R, Hammerstad JP, Burchiel KJ. Interactions between deep brain stimulation and levodopa in Parkinson’s disease. Neurology. 2001;57:1835–42. doi: 10.1212/wnl.57.10.1835. [DOI] [PubMed] [Google Scholar]
- Nutt JG. Long-term L-DOPA therapy: challenges to our understanding and for the care of people with Parkinson’s disease. Exp Neurol. 2003;184:9–13. doi: 10.1016/s0014-4886(03)00304-2. [DOI] [PubMed] [Google Scholar]
- Nutt JG. Pharmacokinetics and pharmacodynamics of levodopa. Mov Disord. 2008;23(Suppl 3):S580–4. doi: 10.1002/mds.22037. [DOI] [PubMed] [Google Scholar]
- Obeso JA, Rodriguez-Oroz MC, Rodriguez M, Macias R, Alvarez L, Guridi J, Vitek J, DeLong MR. Pathophysiologic basis of surgery for Parkinson’s disease. Neurology. 2000;55:S7–12. [PubMed] [Google Scholar]
- Ogura T, Ogata M, Akita H, Jitsuki S, Akiba L, Noda K, Hoka S, Saji M. Impaired acquisition of skilled behavior in rotarod task by moderate depletion of striatal dopamine in a pre-symptomatic stage model of Parkinson’s disease. Neurosci Res. 2005;51:299–308. doi: 10.1016/j.neures.2004.12.006. [DOI] [PubMed] [Google Scholar]
- Owen AM, James M, Leigh PN, Summers BA, Marsden CD, Quinn NP, Lange KW, Robbins TW. Fronto-striatal cognitive deficits at different stages of Parkinson’s disease. Brain. 1992;115(Pt 6):1727–51. doi: 10.1093/brain/115.6.1727. [DOI] [PubMed] [Google Scholar]
- Packard MG, White NM. Dissociation of hippocampus and caudate nucleus memory systems by posttraining intracerebral injection of dopamine agonists. Behav Neurosci. 1991;105:295–306. doi: 10.1037//0735-7044.105.2.295. [DOI] [PubMed] [Google Scholar]
- Packard MG, Knowlton BJ. Learning and memory functions of the Basal Ganglia. Annu Rev Neurosci. 2002;25:563–93. doi: 10.1146/annurev.neuro.25.112701.142937. [DOI] [PubMed] [Google Scholar]
- Parent A. Extrinsic connections of the basal ganglia. Trends Neurosci. 1990;13:254–8. doi: 10.1016/0166-2236(90)90105-j. [DOI] [PubMed] [Google Scholar]
- Parent A, Hazrati LN. Functional anatomy of the basal ganglia. I. The cortico-basal ganglia-thalamo-cortical loop. Brain Res Brain Res Rev. 1995;20:91–127. doi: 10.1016/0165-0173(94)00007-c. [DOI] [PubMed] [Google Scholar]
- Penney JB, Jr, Young AB. Striatal inhomogeneities and basal ganglia function. Mov Disord. 1986;1:3–15. doi: 10.1002/mds.870010102. [DOI] [PubMed] [Google Scholar]
- Poewe W, Antonini A, Zijlmans JC, Burkhard PR, Vingerhoets F. Levodopa in the treatment of Parkinson’s disease: an old drug still going strong. Clin Interv Aging. 2010;5:229–38. doi: 10.2147/cia.s6456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poldrack RA, Clark J, Pare-Blagoev EJ, Shohamy D, Creso Moyano J, Myers C, Gluck MA. Interactive memory systems in the human brain. Nature. 2001;414:546–50. doi: 10.1038/35107080. [DOI] [PubMed] [Google Scholar]
- Poldrack RA, Sabb FW, Foerde K, Tom SM, Asarnow RF, Bookheimer SY, Knowlton BJ. The neural correlates of motor skill automaticity. J Neurosci. 2005;25:5356–64. doi: 10.1523/JNEUROSCI.3880-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puttemans V, Wenderoth N, Swinnen SP. Changes in brain activation during the acquisition of a multifrequency bimanual coordination task: from the cognitive stage to advanced levels of automaticity. J Neurosci. 2005;25:4270–8. doi: 10.1523/JNEUROSCI.3866-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quattrone A, Zappia M, Aguglia U, Branca D, Colao R, Montesanti R, Nicoletti G, Palmieri A, Parlato G, Rizzo M. The subacute levodopa test for evaluating long-duration response in Parkinson’s disease. Ann Neurol. 1995;38:389–95. doi: 10.1002/ana.410380308. [DOI] [PubMed] [Google Scholar]
- Racette BA, Good L, Antenor JA, McGee-Minnich L, Moerlein SM, Videen TO, Perlmutter JS. [18F]FDOPA PET as an endophenotype for Parkinson’s Disease linkage studies. Am J Med Genet B Neuropsychiatr Genet. 2006;141B:245–9. doi: 10.1002/ajmg.b.30293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramanathan S, Hanley JJ, Deniau JM, Bolam JP. Synaptic convergence of motor and somatosensory cortical afferents onto GABAergic interneurons in the rat striatum. J Neurosci. 2002;22:8158–69. doi: 10.1523/JNEUROSCI.22-18-08158.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redgrave P, Rodriguez M, Smith Y, Rodriguez-Oroz MC, Lehericy S, Bergman H, Agid Y, DeLong MR, Obeso JA. Goal-directed and habitual control in the basal ganglia: implications for Parkinson’s disease. Nat Rev Neurosci. 2010;11:760–72. doi: 10.1038/nrn2915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds JN, Hyland BI, Wickens JR. A cellular mechanism of reward-related learning. Nature. 2001;413:67–70. doi: 10.1038/35092560. [DOI] [PubMed] [Google Scholar]
- Reynolds JN, Wickens JR. Dopamine-dependent plasticity of corticostriatal synapses. Neural Netw. 2002;15:507–21. doi: 10.1016/s0893-6080(02)00045-x. [DOI] [PubMed] [Google Scholar]
- Rivlin-Etzion M, Elias S, Heimer G, Bergman H. Computational physiology of the basal ganglia in Parkinson’s disease. Prog Brain Res. 2010;183:259–73. doi: 10.1016/S0079-6123(10)83013-4. [DOI] [PubMed] [Google Scholar]
- Sakai K, Kitaguchi K, Hikosaka O. Chunking during human visuomotor sequence learning. Exp Brain Res. 2003;152:229–42. doi: 10.1007/s00221-003-1548-8. [DOI] [PubMed] [Google Scholar]
- Salamone JD. Functions of mesolimbic dopamine: changing concepts and shifting paradigms. Psychopharmacology (Berl) 2007;191:389. doi: 10.1007/s00213-006-0623-9. [DOI] [PubMed] [Google Scholar]
- Schapira AH, Emre M, Jenner P, Poewe W. Levodopa in the treatment of Parkinson’s disease. Eur J Neurol. 2009;16:982–9. doi: 10.1111/j.1468-1331.2009.02697.x. [DOI] [PubMed] [Google Scholar]
- Schwarz M, Sontag KH, Wand P. Sensory-motor processing in substantia nigra pars reticulata in conscious cats. J Physiol. 1984;347:129–47. doi: 10.1113/jphysiol.1984.sp015057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedelis M, Schwarting RK, Huston JP. Behavioral phenotyping of the MPTP mouse model of Parkinson’s disease. Behav Brain Res. 2001;125:109–25. doi: 10.1016/s0166-4328(01)00309-6. [DOI] [PubMed] [Google Scholar]
- Seidler RD, Noll DC, Chintalapati P. Bilateral basal ganglia activation associated with sensorimotor adaptation. Exp Brain Res. 2006;175:544–55. doi: 10.1007/s00221-006-0571-y. [DOI] [PubMed] [Google Scholar]
- Seidler RD, Tuite P, Ashe J. Selective impairments in implicit learning in Parkinson’s disease. Brain Res. 2007;1137:104–10. doi: 10.1016/j.brainres.2006.12.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Servan-Schreiber D, Printz H, Cohen JD. A network model of catecholamine effects: gain, signal-to-noise ratio, and behavior. Science. 1990;249:892–5. doi: 10.1126/science.2392679. [DOI] [PubMed] [Google Scholar]
- Sethi KD. The impact of levodopa on quality of life in patients with Parkinson disease. Neurologist. 2010;16:76–83. doi: 10.1097/NRL.0b013e3181be6d15. [DOI] [PubMed] [Google Scholar]
- Shen W, Flajolet M, Greengard P, Surmeier DJ. Dichotomous dopaminergic control of striatal synaptic plasticity. Science. 2008;321:848–51. doi: 10.1126/science.1160575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegert RJ, Taylor KD, Weatherall M, Abernethy DA. Is implicit sequence learning impaired in Parkinson’s disease? A meta-analysis. Neuropsychology. 2006;20:490–5. doi: 10.1037/0894-4105.20.4.490. [DOI] [PubMed] [Google Scholar]
- Smith JG, McDowall J. The implicit sequence learning deficit in patients with Parkinson’s disease: a matter of impaired sequence integration? Neuropsychologia. 2006;44:275–88. doi: 10.1016/j.neuropsychologia.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Smits SM, Mathon DS, Burbach JP, Ramakers GM, Smidt MP. Molecular and cellular alterations in the Pitx3-deficient midbrain dopaminergic system. Mol Cell Neurosci. 2005;30:352–63. doi: 10.1016/j.mcn.2005.07.018. [DOI] [PubMed] [Google Scholar]
- Stefanova ED, Kostic VS, Ziropadja L, Markovic M, Ocic GG. Visuomotor skill learning on serial reaction time task in patients with early Parkinson’s disease. Mov Disord. 2000;15:1095–103. doi: 10.1002/1531-8257(200011)15:6<1095::aid-mds1006>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Surmeier DJ, Ding J, Day M, Wang Z, Shen W. D1 and D2 dopamine-receptor modulation of striatal glutamatergic signaling in striatal medium spiny neurons. Trends Neurosci. 2007;30:228–35. doi: 10.1016/j.tins.2007.03.008. [DOI] [PubMed] [Google Scholar]
- Tang C, Pawlak AP, Prokopenko V, West MO. Changes in activity of the striatum during formation of a motor habit. Eur J Neurosci. 2007;25:1212–27. doi: 10.1111/j.1460-9568.2007.05353.x. [DOI] [PubMed] [Google Scholar]
- Taylor TN, Greene JG, Miller GW. Behavioral phenotyping of mouse models of Parkinson’s disease. Behav Brain Res. 2010;211:1–10. doi: 10.1016/j.bbr.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tedroff J, Pedersen M, Aquilonius SM, Hartvig P, Jacobsson G, Langstrom B. Levodopa-induced changes in synaptic dopamine in patients with Parkinson’s disease as measured by [11C]raclopride displacement and PET. Neurology. 1996;46:1430–6. doi: 10.1212/wnl.46.5.1430. [DOI] [PubMed] [Google Scholar]
- Thivierge JP, Rivest F, Monchi O. Spiking neurons, dopamine, and plasticity: timing is everything, but concentration also matters. Synapse. 2007;61:375–90. doi: 10.1002/syn.20378. [DOI] [PubMed] [Google Scholar]
- Ungerstedt U. 6-Hydroxy-dopamine induced degeneration of central monoamine neurons. Eur J Pharmacol. 1968;5:107–10. doi: 10.1016/0014-2999(68)90164-7. [DOI] [PubMed] [Google Scholar]
- van den Munckhof P, Luk KC, Ste-Marie L, Montgomery J, Blanchet PJ, Sadikot AF, Drouin J. Pitx3 is required for motor activity and for survival of a subset of midbrain dopaminergic neurons. Development. 2003;130:2535–42. doi: 10.1242/dev.00464. [DOI] [PubMed] [Google Scholar]
- van der Vegt JP, van Nuenen BF, Bloem BR, Klein C, Siebner HR. Imaging the impact of genes on Parkinson’s disease. Neuroscience. 2009;164:191–204. doi: 10.1016/j.neuroscience.2009.01.055. [DOI] [PubMed] [Google Scholar]
- van Nuenen BF, Weiss MM, Bloem BR, Reetz K, van Eimeren T, Lohmann K, Hagenah J, Pramstaller PP, Binkofski F, Klein C, Siebner HR. Heterozygous carriers of a Parkin or PINK1 mutation share a common functional endophenotype. Neurology. 2009;72:1041–7. doi: 10.1212/01.wnl.0000338699.56379.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varnum DS, Stevens LC. Aphakia, a new mutation in the mouse. J Hered. 1968;59:147–50. doi: 10.1093/oxfordjournals.jhered.a107667. [DOI] [PubMed] [Google Scholar]
- Verschueren SM, Swinnen SP, Dom R, De Weerdt W. Interlimb coordination in patients with Parkinson’s disease: motor learning deficits and the importance of augmented information feedback. Exp Brain Res. 1997;113:497–508. doi: 10.1007/pl00005602. [DOI] [PubMed] [Google Scholar]
- Wickens JR, Begg AJ, Arbuthnott GW. Dopamine reverses the depression of rat corticostriatal synapses which normally follows high-frequency stimulation of cortex in vitro. Neuroscience. 1996;70:1–5. doi: 10.1016/0306-4522(95)00436-m. [DOI] [PubMed] [Google Scholar]
- Wider C, Russmann H, Villemure JG, Robert B, Bogousslavsky J, Burkhard PR, Vingerhoets FJ. Long-duration response to levodopa in patients with advanced Parkinson disease treated with subthalamic deep brain stimulation. Arch Neurol. 2006;63:951–5. doi: 10.1001/archneur.63.7.951. [DOI] [PubMed] [Google Scholar]
- Wiecki TV, Riedinger K, von Ameln-Mayerhofer A, Schmidt WJ, Frank MJ. A neurocomputational account of catalepsy sensitization induced by D2 receptor blockade in rats: context dependency, extinction, and renewal. Psychopharmacology (Berl) 2009;204:265–77. doi: 10.1007/s00213-008-1457-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiecki TV, Frank MJ. Neurocomputational models of motor and cognitive deficits in Parkinson’s disease. Prog Brain Res. 2010;183:275–97. doi: 10.1016/S0079-6123(10)83014-6. [DOI] [PubMed] [Google Scholar]
- Wiesendanger E, Clarke S, Kraftsik R, Tardif E. Topography of cortico-striatal connections in man: anatomical evidence for parallel organization. Eur J Neurosci. 2004;20:1915–22. doi: 10.1111/j.1460-9568.2004.03640.x. [DOI] [PubMed] [Google Scholar]
- Wilkinson L, Jahanshahi M. The striatum and probabilistic implicit sequence learning. Brain Res. 2007;1137:117–30. doi: 10.1016/j.brainres.2006.12.051. [DOI] [PubMed] [Google Scholar]
- Wilkinson L, Khan Z, Jahanshahi M. The role of the basal ganglia and its cortical connections in sequence learning: evidence from implicit and explicit sequence learning in Parkinson’s disease. Neuropsychologia. 2009;47:2564–73. doi: 10.1016/j.neuropsychologia.2009.05.003. [DOI] [PubMed] [Google Scholar]
- Willingham DB, Salidis J, Gabrieli JD. Direct comparison of neural systems mediating conscious and unconscious skill learning. J Neurophysiol. 2002;88:1451–60. doi: 10.1152/jn.2002.88.3.1451. [DOI] [PubMed] [Google Scholar]
- Wilson CJ. Basal Ganglia. In: Shepard GM, editor. The synaptic organization of the brain. Oxford University Press; New York: 1998. pp. 329–375. [Google Scholar]
- Wu N, Cepeda C, Zhuang X, Levine MS. Altered corticostriatal neurotransmission and modulation in dopamine transporter knock-down mice. J Neurophysiol. 2007;98:423–32. doi: 10.1152/jn.00971.2006. [DOI] [PubMed] [Google Scholar]
- Wu T, Hallett M. A functional MRI study of automatic movements in patients with Parkinson’s disease. Brain. 2005;128:2250–9. doi: 10.1093/brain/awh569. [DOI] [PubMed] [Google Scholar]
- Yin HH, Knowlton BJ, Balleine BW. Lesions of dorsolateral striatum preserve outcome expectancy but disrupt habit formation in instrumental learning. Eur J Neurosci. 2004;19:181–9. doi: 10.1111/j.1460-9568.2004.03095.x. [DOI] [PubMed] [Google Scholar]
- Yin HH, Knowlton BJ. The role of the basal ganglia in habit formation. Nat Rev Neurosci. 2006;7:464–76. doi: 10.1038/nrn1919. [DOI] [PubMed] [Google Scholar]
- Yin HH, Lovinger DM. Frequency-specific and D2 receptor-mediated inhibition of glutamate release by retrograde endocannabinoid signaling. Proc Natl Acad Sci U S A. 2006;103:8251–6. doi: 10.1073/pnas.0510797103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin HH, Mulcare SP, Hilario MR, Clouse E, Holloway T, Davis MI, Hansson AC, Lovinger DM, Costa RM. Dynamic reorganization of striatal circuits during the acquisition and consolidation of a skill. Nat Neurosci. 2009;12:333–41. doi: 10.1038/nn.2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaidel A, Arkadir D, Israel Z, Bergman H. Akineto-rigid vs. tremor syndromes in Parkinsonism. Curr Opin Neurol. 2009;22:387–93. doi: 10.1097/WCO.0b013e32832d9d67. [DOI] [PubMed] [Google Scholar]
- Zaidel A, Bergman H, Ritov Y, Md ZI. Levodopa and subthalamic deep brain stimulation responses are not congruent. Mov Disord. 2010;25:2379–86. doi: 10.1002/mds.23294. [DOI] [PubMed] [Google Scholar]
- Zappia M, Colao R, Montesanti R, Rizzo M, Aguglia U, Gambardella A, Oliveri RL, Quattrone A. Long-duration response to levodopa influences the pharmacodynamics of short-duration response in Parkinson’s disease. Ann Neurol. 1997;42:245–8. doi: 10.1002/ana.410420217. [DOI] [PubMed] [Google Scholar]
- Zappia M, Oliveri RL, Montesanti R, Rizzo M, Bosco D, Plastino M, Crescibene L, Bastone L, Aguglia U, Gambardella A, Quattrone A. Loss of long-duration response to levodopa over time in PD: implications for wearing-off. Neurology. 1999;52:763–7. doi: 10.1212/wnl.52.4.763. [DOI] [PubMed] [Google Scholar]
- Zappia M, Oliveri RL, Bosco D, Nicoletti G, Branca D, Caracciolo M, Napoli ID, Gambardella A, Quattrone A. The long-duration response to L-dopa in the treatment of early PD. Neurology. 2000;54:1910–5. doi: 10.1212/wnl.54.10.1910. [DOI] [PubMed] [Google Scholar]
- Zgaljardic DJ, Borod JC, Foldi NS, Mattis P. A review of the cognitive and behavioral sequelae of Parkinson’s disease: relationship to frontostriatal circuitry. Cogn Behav Neurol. 2003;16:193–210. doi: 10.1097/00146965-200312000-00001. [DOI] [PubMed] [Google Scholar]
- Zgaljardic DJ, Foldi NS, Borod JC. Cognitive and behavioral dysfunction in Parkinson’s disease: neurochemical and clinicopathological contributions. J Neural Transm. 2004;111:1287–301. doi: 10.1007/s00702-004-0178-z. [DOI] [PubMed] [Google Scholar]