Abstract
Lentiviruses are known for their narrow cell- and species-tropisms, which are determined by cellular proteins whose absence or presence either support viral replication (dependency factors, cofactors) or inhibit viral replication (restriction factors). Similar to Human immunodeficiency virus type 1 (HIV-1), the cat lentivirus Feline immunodeficiency virus (FIV) is sensitive to recently discovered cellular restriction factors from non-host species that are able to stop viruses from replicating. Of particular importance are the cellular proteins APOBEC3, TRIM5α and tetherin/BST-2. In general, lentiviruses counteract or escape their species’ own variant of the restriction factor, but are targeted by the orthologous proteins of distantly related species. Most of the knowledge regarding lentiviral restriction factors has been obtained in the HIV-1 system; however, much less is known about their effects on other lentiviruses. We describe here the molecular mechanisms that explain how FIV maintains its replication in feline cells, but is largely prevented from cross-species infections by cellular restriction factors.
Keywords: FIV, antiviral, restriction factor, APOBEC3, TRIM5, tetherin
1. Introduction
In many species of Felidae, including the domestic cat, individuals may be infected with a unique strain of feline immunodeficiency virus (FIV), and a related virus is also found in African hyenas (for a review, see [1,2]). Viruses isolated from various felid species show monophyletic proviral sequences, which supports the idea that FIVs are not frequently transmitted between different felid species [2–4]. FIV was introduced into the existing felid lineages at several different times during evolution. A co-adaptation between FIV and Felidae is assumed to have occurred for at least 10,000 years, and possibly as much as a million years for some species [2,3]. In contrast, FIV infection of the domestic cat appears to be of more recent origin [2]. In addition, singular events of FIV felid-to-felid cross-species transmissions and repeated FIV transmissions from bobcats to pumas have been observed (for an overview, see [1,5,6]).
In mammals, including felids, the innate immune system provides a first line of defense against pathogens. Induction of type I interferon regulates the expression of several interferon-stimulated genes (ISGs) whose protein products have direct antiviral properties. A group of proteins with potent antiviral properties, known collectively as “restriction factors,” are constitutively expressed in cells, but also induced by type I interferon. These proteins are able to limit replication by targeting specific steps in the viral life cycle [7]. However, lentiviruses have developed counteracting proteins to survive and replicate in their host mammal. While orthologous cellular proteins, which differ specifically in the interaction sites of the counteracting viral proteins, limit inter-species transfer, a high genetic variability in the restriction factors might also limit the spread and extent of infections and disease within the natural host population (intra-species transfer) [8].
FIV infection of felids shows substantial variation in disease outcome: in domestic cats, FIV can induce a strong immune pathology with a T cell depletion and immunodeficiency, resulting in opportunistic infections as well as weight loss and neurological disease (for review see [9,10]); however, in non-domestic felids infections with FIV appear to be apathogenic in most individuals [1]. For example, in FIV-infected lions and pumas, T cell depletion without disease has been described [11,12]. Interestingly, different subtypes of lion FIV may have different patterns of pathogenicity and transmissibility among African lions [13]. Some of these variations are likely influenced by differences in viral adaptation to the restriction factors of their hosts.
Much of today’s knowledge of anti-lentiviral restriction factors is derived from the intensive characterization of the interaction of human immunodeficiency virus type 1 (HIV-1) with different host cells. However, recent investigations have also focused on how FIV escapes its host species’ own restriction factors. Many of the accessory gene products of HIV-1 counteract cellular restriction factors. For example, the Vif protein inhibits APOBEC3 cytidine deaminases (see Section 2), while the Vpu protein protects HIV-1 from tetherin (see Section 4). Vpr inhibits a cellular factor that is still unidentified but, interestingly, Vpx, which is closely related to Vpr (found, for example in Human immunodeficiency virus type 2, HIV-2) is a potent inhibitor of SAMHD1 (see Section 5). In contrast to HIV-1, FIV has only a vif gene and genes such as vpr, vpx and vpu are not present. Instead, a gene for the less well-characterized multifunctional protein OrfA (Orf-A, Orf-2) is uniquely found in FIV (see Section 6). A further restriction factor, TRIM5α, is not counteracted by accessory proteins. While all primate lentiviruses evolved in the presence of TRIM5α, the feline lentiviruses did not face this protein in their natural hosts because felids express a truncated, inactive variant of the TRIM5 protein (see Section 3) [14].
The strain-specific evolution of FIVs is likely driven by genetic differences in cellular dependency and restriction factors, in addition to selection by the immune system. We can assume that, besides differences in these factors, the FIV cross-species transfer from a felid to a felid/non-felid is also impaired by other limitations. Since FIV infection in domestic cats induces a fatal immunodeficiency that is very similar to AIDS in humans [9,10], FIV infection of cats not only provides a unique model to investigate the evolutionary role of restriction factors on cross-species transmission, but also virus evolution and its impact on AIDS induction.
2. FIV and APOBEC3
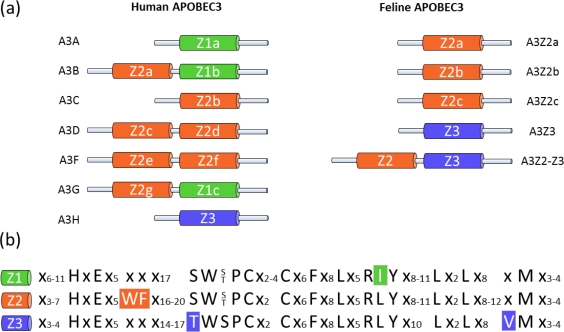
The mammalian APOBEC3 (A3; apolipoprotein B mRNA editing enzyme catalytic polypeptide 3) protein family was discovered through the study of the HIV-1 Vif. HIV-1 virions that are produced in permissive cells are able to infect permissive and non-permissive cells, whereas Δvif virons produced in non-permissive cells are unable to infect target cells [15,16]. These findings suggested the existence of a Vif-sensitive antiviral factor that was later identified as human APOBEC3G (Figure 1a) [17–19]. This interferon-inducible cellular factor is part of the AID/APOBEC gene family, which share a characteristic zinc (Z)-coordinating catalytic motif (His-X-Glu-X23–28-Pro-Cys-X2–4-Cys) [20]. The A3 proteins can be classified according to the presence of a Z1, Z2 or Z3 motif (Figure 1b) [21–23]. A multiplicity of evolutionary events driven by host adaptation to viruses—including preservations, deletions, duplications, subfunctionalizations and neofunctionalizations—have led to a number of different forms of A3 genes within mammals [21,23]. Humans express seven A3 proteins (APOBEC3A, -B, -C, -D, -F, -G and -H, Figure 1a) with either one or two zinc-coordinating domains that can inhibit various retroviruses, endogenous retroelements and DNA viruses [24–28].
Figure 1.
Human and feline APOBEC3 (A3) proteins. (a) Schematic representation of human and feline A3 cytidine deaminases. All A3 proteins share at least one zinc (Z)-coordinating catalytic motif. The color code indicates the amino acid specificity of the different deaminase domains (Z1, Z2 and Z3). Humans express seven A3 proteins, A3A–A3H; cats express four A3 proteins, A3Z2a–A3Z2-Z3. (b) Amino acid sequences of indicated domains. Group-specific distinctions of Z-domains are highlighted.
In the domestic cat, there are three copies of a Z2 gene and a single copy of a Z3 gene (Figure 1a) and are likely present in other feline species as well [23,29,30]. Besides these single-Z-domain proteins, Felidae express three very similar two-domain A3 proteins (Figure 1a, shown as a single A3Z2-Z3 protein for simplicity) by a complex process of read-through transcription and alternative splicing [23,29,30]. A feline A3 gene encoding a Z1 domain protein does not exist. In cats, the A3 genes are polymorphic and are under positive selection, indicating that they are relevant in the “arms race” between host and retrovirus [23].
The enzymatic activity of virion-encapsidated A3 results in the hydrolytic deamination of cytosine bases in the single-stranded viral (−) DNA that is synthesized during reverse transcription, leading to viral genome degradation, or to G-to-A hypermutations on the (+) DNA strand (Figure 2) [31–36]. However, A3 proteins also have deaminase-independent antiviral activities, and deaminase-deficient A3 mutants are still able to reduce accumulation of reverse transcription products [37–41]. The Vif protein of HIV-1 forms an interaction between A3 and a ubiquitin E3 ligase complex consisting of elongin B and C, cullin5, and ring box-1 [42]; this interaction results in the A3 polyubiquitination that leads to proteasome-mediated degradation of the A3 protein [42–46]. In addition, Vif of HIV-1 inhibits A3 proteins by other non-degrading mechanisms (for a review, see [24]). Like HIV-1, FIV also encodes a Vif protein [23,29,30,47]. FIVs with an inactivated vif gene do not productively infect feline blood cells or show replication in cats [48–50].
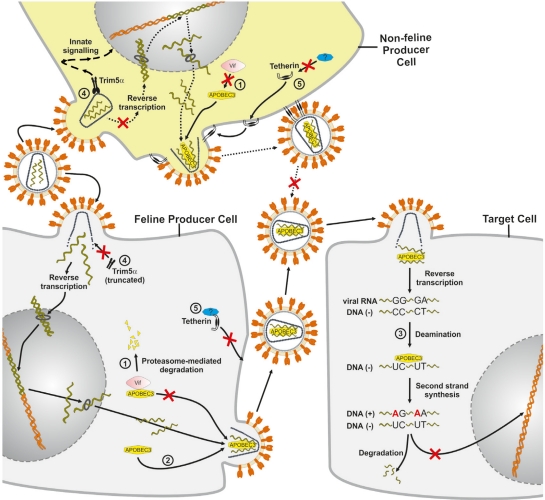
Figure 2.
Impact of the cellular restriction factors APOBEC3 (A3), TRIM5α and tetherin on FIV replication. The FIV replication cycle starts by infection of either feline or non-feline cells and the resulting FIV particles then infect a target cell that shows no TRIM5α restriction. FIV Vif targets the feline A3 protein for proteasomal degradation but it is inactive against A3 proteins from non-felid species ①. If Vif is not expressed or does not bind to A3, A3 is packaged into FIV virions budding from the producer cells ②. During the next round of infection in target cells, encapsidated A3 proteins act as inhibitors of virus replication. Single-stranded viral DNA serves as a substrate for A3-induced cytidine deamination, causing hypermutations of the viral DNA, during which deoxycytidines are converted to deoxyuridines. Uracil-containing minus-strand DNA can be targeted by uracil DNA glycosylase, which could lead to endonucleolytic cleavage ③. The number of integrated, highly mutated proviruses is low. In non-feline cells, TRIM5α restricts FIV soon after post-entry, likely by acceleration of the viral uncoating process. TRIM5α may also promote innate immune signaling. The truncated feline TRIM5α is incapable of restricting retroviruses ④. Feline tetherin is not able to restrict direct cell-to-cell spread of FIV, but can restrict the release of cell-free particles. A FIV antagonist (indicated by a question mark) of feline tetherin has not yet been detected. FIV is also inhibited by non-felid tetherin proteins ⑤. Many details of this model are based on experimental results of HIV-1 in primate cells.
Recent studies show that FIV Vif counteracts the feline A3 antiviral activity [23,29,30,47]. vif-deficient FIV is moderately inhibited by feline A3Z3 and is strongly suppressed by feline A3Z2-Z3, whereas wild-type virus infectivity is not influenced by feline A3 proteins [23,30]. Both FIV and FIVΔvif are very sensitive to three of the seven human A3s (A3F, A3G and A3H) [23,30,47]. The antiviral activity of feline A3s against FIVΔvif correlated with the detection of cytidine deaminated, edited viral genomes [23]. Whether feline A3s also have non-editing antiviral activities has not yet been investigated. Further, it is unknown why feline A3Z2 proteins do not inhibit FIVΔvif or FIV. However, the feline A3Z2 proteins are active cytidine deaminases and they restrict Bet-deficient Feline foamy virus. This feline retrovirus uses its accessory protein, Bet, to counteract the feline A3Z2 proteins via a degradation-independent pathway [23,51]. In contrast, expression of FIV Vif induces the degradation of feline A3s and thus prevents the encapsidation of A3s into FIV particles [29,30,47]. The degradation of feline A3 proteins by FIV Vif occurs efficiently in human cells indicating that this Vif activity does not depend on species-specific host factors [29,30,47]. It has not yet been shown but it is likely that FIV Vif recruits elongin B/C, cullin5, and ring box-1 in human cells as in feline cells to induce polyubiquitination of feline A3s.
Vif proteins can be counteractive, non-active or semi-active against different A3 proteins. Surprisingly, the domestic cat FIV Vif protein can efficiently inhibit A3 restriction factors of three tested Felidae (puma, lion, lynx) and shows a reduced activity to A3s from the tiger [30]. These results indicate that most of the diverse felid A3s of big cats are probably not major determinants that prevent cross-species transmission of FIV from the domestic cat to these closely related animal species. In contrast, FIV Vif is completely inactive against A3 proteins from primates and other non-felid species [23,30,47]. A characterization of the molecular interaction of domestic cat FIV Vif with A3s of different felids may reveal the binding domains of Vif and A3; it may also explain the broad activity of domestic cat FIV Vif.
3. FIV and TRIM5
HIV replication in simian cells is blocked during uncoating at the early post-entry stage—a finding that was used to identify the restriction factor TRIM5α [52], which is constitutively expressed, but interferon treatment can further increase its levels [53]. TRIM5α is a tripartite motif protein with a RING, B-box 2 and coiled-coil (CC) domain (RBCC), as well as a carboxy-terminal B30.2 (SPRY) domain (Figure 3a). The B30.2 domain of TRIM5α binds to the viral capsid of incoming viral particles, while the RBCC domains mediate the localization to cytoplasmic bodies and are important for TRIM5α self-association [54–61]. TRIM5α inhibits HIV-1 and other retroviruses in a species-specific manner. Thus retroviruses replicate in the presence of TRIM5α proteins of their host species and are inhibited by orthologous TRIM5α proteins. Species-adapted retroviruses evolved viral capsids that do not bind TRIM5α proteins expressed in their host species [62]. Simian TRIM5α likely acts at several levels: it restricts HIV-1 by accelerating the viral uncoating process, which leads to an inhibition of the reverse transcription (Figure 2) [63–65], and TRIM5α also leads to an integration block that appears after the reverse transcription block is relieved by inhibition of the proteasome [66]. Insights into the multifunctional nature of TRIM5α were obtained in a recent study by Pertel et al. that suggests that TRIM5α is a pattern-recognition receptor for HIV-1 (Figure 2) [67]. TRIM5α promotes innate immune signaling of the mitogen-activated protein kinase and NF-κB signaling pathways by its association with the retroviral capsid lattice and the E2 ubiquitin-conjugating enzyme complex UBC13-UEV1A that activates TAK1 kinase [67]. Furthermore, the authors demonstrated that TRIM5α has a specific effect on the expression of NF-κB- and AP-1-responsive inflammatory chemokines and cytokines [67]. The knockdown of TRIM5α in myeloid cells attenuates lipopolysaccharide (LPS)-induced immune signaling and also rescues viral infections from the LPS-induced antiviral gene expression [67]. Thus, TRIM5α plays a role in LPS-triggered immune activation through the Toll-like receptor 4 pathway.
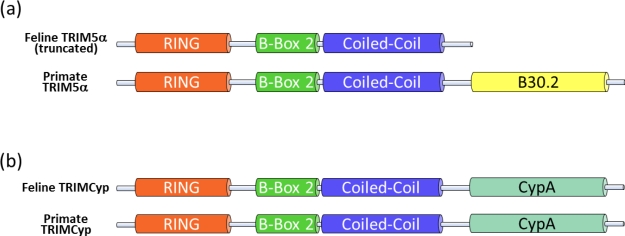
Figure 3.
Domain structure of TRIM5α and TRIMCyp proteins. (a) TRIM5α is defined by four domains: RING finger, B-box 2, coiled-coil and B30.2. The feline TRIM5α lacks the B30.2 capsid-binding domain because of a premature stop codon in the mRNA transcript. (b) In some monkeys, TRIMCyp is expressed, in which the B30.2 domain of TRIM5α is replaced with a cyclophilin A (CypA) domain. Expression of feline TRIMCyp was not observed. Dietrich et al. produced a synthetic feline TRIMCyp by fusion of the truncated TRIM5α and feline cyclophilin A [75].
Human and rhesus macaque TRIM5α can inhibit the infection of FIV if expressed in feline cells [54,68]. In single-round infection assays, it appears that the antiviral activity of the rhesus protein is stronger than that of human TRIM5α. Feline cells expressing human or rhesus TRIM5α also potently block spreading replication of FIV without the appearance of revertants [68]. Most human cell lines, with the exception of HEK 293T, are not permissive for transduction by FIV vectors, especially T cell lines that show a strong restriction [68].
Owl monkeys and macaques express a protein called TRIMCyp due to a cDNA of the cyclophilin A (CypA) gene that retrotransposed into the 3′ region of the TRIM5 gene [69–73]. In owl monkeys, the CypA cDNA is inserted into intron seven of the TRIM5 gene [71]. A distinct evolutionary origin of TRIMCyp is found in three macaque species (M. fascicularis, M. mulatta, and M. nemestrina) where the CypA cDNA retrotransposed in the 3′ untranslated region of the TRIM5 gene and macaque TRIMCyp is expressed by exon skipping from exon six to CypA [69,70,72,73]. Owl monkey as well macaque TRIMCyps consist of the RBCC domains of TRIM5 fused with a carboxy-terminal CypA moiety (Figure 3b) [69–74]. CypA binds the surface of the capsid proteins of HIV-1 and FIV that form the viral core [75–78]. In FIV, the interaction of CypA can be prevented by a P90A mutation in the loop between helix 4 and 5 of the capsid [75,78]. TRIMCyp of Owl monkeys and macaques, or an artificial human TRIMCyp, restricts infection with FIV [54,72,73,78–82]. Similar to TRIM5α, treating rhesus TRIMCyp-expressing feline cells with a proteasome inhibitor relieves the block in the reverse transcription of FIV, but does not restore the capacity of FIV to transduce these cells [73].
McEwan et al. discovered that Feliformia express a truncated TRIM5 gene, which explains why feline cells do not show a TRIM5-typical restriction to retroviruses (Figure 2) [14]. The feline mRNA of TRIM5 contains a premature stop codon expressing a RBCC protein without the B30.2 domain (Figure 3a). In cats, the missing B30.2 domain is not replaced by CypA as seen in some monkeys [14]. The truncated feline TRIM5 appears to be without any antiretroviral activity as overexpression of the feline TRIM5 does not prevent infection with murine leukemia virus, HIV-1 or a simian immunodeficiency virus (SIV) of macaques (SIVmac) [14]. However, it is possible that the truncated feline TRIM5 protein is similar to the human TRIM5α involved in LPS-mediated signaling [67], potentially explaining why this gene is retained in Feliformia. Interestingly, a synthetic fusion of the feline TRIM5 to the feline CypA (Figure 3b) generated a potent inhibitor of FIV and HIV-1 [75,83]. These results show that the RBCC domains of feline TRIM5 retain their intrinsic antiviral function.
4. FIV and Tetherin
The restriction factor tetherin (also known as CD317 or BST-2 or HM1.24), was identified by analyzing the cell type-specific particle release block of HIV-1 deficient for the viral protein Vpu [84,85]. Tetherin is an interferon-induced protein that “tethers” HIV-1Δvpu particles at the cell surface (Figure 2). The Vpu protein of HIV-1 interferes with the cell surface expression of human tetherin in part by inducing its degradation [86,87]. Primate lentiviruses that lack a vpu gene use either their Nef or Env proteins to counteract tetherin in the host cells (for review see [88–90]). In general, the lentiviral proteins that counteract are only active against tetherin proteins of their own host species. In addition to retroviruses, tetherin proteins are also inhibitory to herpesviruses, arenaviruses, filoviruses, rhabdoviruses, orthomyxoviruses and paramyxoviruses [91–98].
Tetherin is an unusual type II integral membrane protein (Figure 4) with an N-terminal transmembrane domain, an extracellular CC domain and a C-terminal glycosylphospatidylinositol (GPI) lipid anchor at its C-terminus [99]. Recent data indicate that the GPI anchor motif functions as a second transmembrane motif [100]. In humans, tetherin is expressed on several specialized cell types such as hepatocytes, monocytes, epithelial cells, terminally differentiated B cells and bone marrow stromal cells. It is upregulated in many cell types upon treatment with interferon [101]. The feline tetherin protein was recently cloned and functionally characterized [83,102]. Similar to human and mouse tetherin, the expression of feline tetherin is inducible by interferons but a detailed tissue expression pattern has not yet been determined [83,102]. In a transient co-expression system, feline tetherin is a potent inhibitor for the release of FIV and HIV-1 particles [83]. The release of FIV virus-like particles and wild-type particles is also strongly inhibited by the human tetherin [83,91]. Apparently, under such experimental conditions, FIV has no viral determinant that inhibits the antiviral activity of feline tetherin. In contrast to transient expression of tetherin, stably expressing feline tetherin in feline CrFK cells did not restrict virus spread of FIV (human tetherin was not tested) [83]. Similarly, in the HIV-1 system, the cell-to-cell transfer of vpu-deficient HIV-1 in T cells was not inhibited by human tetherin [103], although, contrasting results have also been reported [104]. This might indicate that Vpu of HIV-1 is not strongly required for viral spread in vivo. Support of this model comes from the observation, that in contrast to Vpu of HIV-1 pandemic group M, the Vpus of non-pandemic AIDS inducing HIV-1 groups O and N show no, or very low activity, against human tetherin [105]. These findings potentially explain why HIV-1 group O and N viruses propagate less efficiently in the human population than the pandemic HIV-1 group M virus. Together, these data indicate that FIV, in contrast to HIV-1, is transmitted mainly as a cell-associated virus and not as a cell-free virus.
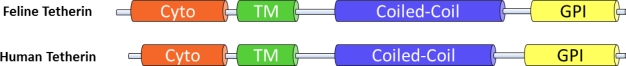
Figure 4.
Schematic structure of feline and human tetherin. Tetherins from both species share their principal elements: Cytoplasmatic (Cyto)-, transmembrane (TM)-, coiled-coil- and glycophosphatidyl-inositol (GPI)-anchor domains.
5. FIV and SAMHD1
Human cells of the myeloid lineage such as blood monocytes or monocyte-derived dendritic cells (mDCs), are highly refractory to infection by HIV-1 due to a post-entry restriction [106–111]. Some primate lentiviruses, such as SIV endemic to sooty mangabeys (SIVsm), SIVmac and HIV-2, do not show this replication restriction (for a review, see [112]). Human and feline mDCs also show a resistance against the productive infection with FIV [113–115]. In contrast to HIV-1, lentiviruses such as SIVsm/SIVmac/HIV-2 express an accessory protein called Vpx that is specifically encapsidated into viral particles [116,117]. Loss of Vpx expression in SIVmac and HIV-2 has no effect on the viral replication in established cell lines, but Vpx mutants show a delayed replication in cultures of PBMCs (peripheral blood mononuclear cells) and a replication block in macrophages [118–120]. In vivo, SIVsm (isolate PBj) needs Vpx expression for efficient dissemination and the acute, unique pathogenesis of SIVsmPBj [121]. In infected monkeys, SIVmac Vpx mutants show a lower viremia, a delayed CD4 cell decline and a later AIDS induction compared to wild type viruses [122]. Thus, a Vpx-function for AIDS induction by SIVmac is not required and it is unclear why only some lentiviruses encode Vpx and infect mDCs.
In myeloid cells, Vpx mutants of HIV-2 and SIVmac show a post-entry block at reverse transcription or uncoating [115,123–125]. The Vpx protein forms a cullin 4-based E3 ubiquitin ligase complex (for a review, see [112]) indicating that, similar to Vif, Vpx acts as a substrate receptor to induce the degradation of a cellular protein. Recently, the human protein SAMHD1 was identified to be causative for the post-entry restriction of HIV-1 in myeloid cells [126,127]. Viral particle-associated Vpx protein induces a proteasome-dependent degradation of SAMHD1 in the target cell very early after entry [126,127]. The SAMHD1 protein has two domains that are widely found in all genomes: an N-terminal SAM domain followed by a HD domain (Figure 5). SAM (sterile α motif) domains have diverse functions, such as binding to kinases, other proteins or RNA [128]. The HD domain with histidine and aspartic acid residues for metal coordination defines a superfamily of metal-dependent phosphohydrolases, which includes many proteins that are involved in nucleic acid metabolism such as dGTPases, nucleotidyltransferases and helicases [129]. Mutations in the SAMHD1 gene lead to Aicardi-Goutieres syndrome, which includes cerebral atrophy, leukoencephalopathy, hepatosplenomegaly, and increased production of α-interferon [130]. The antiviral mechanism of SAMHD1 is not yet identified but, based on the activities of proteins that share homology with SAMHD1, it is likely that SAMHD1 acts either as a nuclease that destroys the viral genetic material of incoming viruses, or functions as a sensor (or sensor-associated protein) that induces antiviral proteins. It would be interesting to determine whether the feline gene for SAMHD1 (found on cat chromosome A3) encodes an antiviral protein, and whether FIV expresses a viral counteracting factor against feline SAMHD1 that functions similarly to Vpx.
Figure 5.
Schematic representation of the human SAMHD1 protein indicating the SAM and the HD domain. A putative feline SAMHD1-encoding gene is found on cat chromosome 13.
6. Major Challenges in Research on FIV Restriction Factors
The HIV-1 origin in humans is one of best studied lentiviral cross-species transmissions [131]. Because of the very high genome sequence identity between the two Hominidae, Pan troglodytes and Homo sapiens, it is not surprising that SIV of chimpanzees (SIVcpz) quickly adapted and developed a pandemic distribution once introduced in the human population. However, the evolution of HIV-2, derived from a SIV endemic to sooty mangabeys (SIVsm) demonstrates that lentiviruses can also rapidly cross between species in different families, in that case from Cercopithecidae to Hominidae [132,133]. FIV cross-species transfers are described so far only in Felidae, and mostly as singular events, with the exception of repeated transmissions of FIVs from bobcats to pumas [1,5,6]. It is unknown what the impact of restriction factors versus the relevance of other factors are in these cross-species transmissions [2]. There is a need for more field and laboratory studies that quantitate the potential of molecularly defined feline lentiviruses to establish cross-felid infections. It would be interesting to learn whether these cross-species transmitted viruses replicate in vivo to similar levels, with similar tissue distributions as the species-adapted FIVs, and whether they are rapidly controlled by the innate and/or adaptive immune system of the cat. Even for regular FIV infections in the domestic cat, information on the importance of cellular restriction factors is limited. Modern methods to knock-down the expression of genes or genetically modify FIV target cells with novel restriction factors (to model human HIV-1 gene therapy) will be useful in gaining further insights into the evolutionary potential of viral adaptation or its intrinsic constraints, the stability of viral genes and genomes, and the impact of restriction factors on the health of infected animals. A recent breakthrough is the generation of rhesus TRIMCyp transgenic cats [82]. PBMCs of theses transgenic animals expressed variable amounts of rhesus TRIMCyp and showed a partial resistance to FIV replication [82].
Besides the impact of known restriction factors on FIV replication and their use in therapeutic models, FIV has not been utilized in a systematic search for antiviral proteins and dependency factors. The recent identification of novel restriction factors that are counteracted by accessory proteins of HIV-1 raises the question as to whether FIV might also be used to identify novel cellular restriction factors. In this regard, it might be interesting to note that FIV encodes the protein OrfA, whose function is still enigmatic [134,135]. OrfA does not counteract of feline tetherin [83], but is important for replication in vivo and in primary T cells, and its exact role in FIV biology is not yet established. Different studies describe various functions of OrfA including: transactivating viral transcription, regulating the infectivity of FIV particles, inducing G2 cell cycle arrest, and downregulating the FIV receptor CD134 [136–139].
The induction of interferon following FIV infection of feline PBMCs and cats suggests that pattern-recognition receptors in hematopoietic cells detect the FIV infection [140]. How FIV or other lentiviruses are sensed, whether FIV has evolved partial escape mechanisms of this detection, and which feline ISGs show anti-FIV activity need much more investigation. A systematic analysis of all ISGs with FIV and FIV deleted in specific genes could identify factors that are antiviral and are counteracted by FIV’s proteins. In human cells, many interaction partners of A3G, TRIM5α, tetherin and SAMHD1, and of HIV-1 accessory proteins, have been described or will be published soon (e.g., [42,67,141–143]). Thus, systems biology approaches for FIV and feline cells will be important to learn about the conserved and differing pathways used by HIV-1 and FIV. It may also allow us to better utilize the FIV cat model to discover novel treatment and healing options for HIV-1-infected patients.
Acknowledgments
We thank Eric Logue for critically reading the manuscript. We thank Dieter Häussinger for constant support. C.M. is supported by the Heinz Ansmann Foundation for AIDS research.
Footnotes
Conflict of Interest
The authors declare no conflict of interest.
References and Notes
- 1.VandeWoude S, Apetrei C. Going wild: Lessons from naturally occurring T-lymphotropic lentiviruses. Clin Microbiol Rev. 2006;19:728–762. doi: 10.1128/CMR.00009-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pecon-Slattery J, Troyer JL, Johnson WE, O'Brien SJ. Evolution of feline immunodeficiency virus in Felidae: Implications for human health and wildlife ecology. Vet Immunol Immunopathol. 2008;123:32–44. doi: 10.1016/j.vetimm.2008.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Troyer JL, Pecon-Slattery J, Roelke ME, Johnson W, VandeWoude S, Vazquez-Salat N, Brown M, Frank L, Woodroffe R, Winterbach C, et al. Seroprevalence and genomic divergence of circulating strains of feline immunodeficiency virus among Felidae and Hyaenidae species. J Virol. 2005;79:8282–8294. doi: 10.1128/JVI.79.13.8282-8294.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Antunes A, Troyer JL, Roelke ME, Pecon-Slattery J, Packer C, Winterbach C, Winterbach H, Hemson G, Frank L, Stander P, et al. The evolutionary dynamics of the lion Panthera leo revealed by host and viral population genomics. PLoS Genet. 2008;4:e1000251. doi: 10.1371/journal.pgen.1000251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Troyer JL, VandeWoude S, Pecon-Slattery J, McIntosh C, Franklin S, Antunes A, Johnson W, O'Brien SJ. FIV cross-species transmission: An evolutionary prospective. Vet Immunol Immunopathol. 2008;123:159–166. doi: 10.1016/j.vetimm.2008.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.VandeWoude S, Troyer J, Poss M. Restrictions to cross-species transmission of lentiviral infection gleaned from studies of FIV. Vet Immunol Immunopathol. 2010;134:25–32. doi: 10.1016/j.vetimm.2009.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wolf D, Goff SP. Host restriction factors blocking retroviral replication. Annu Rev Genet. 2008;42:143–163. doi: 10.1146/annurev.genet.42.110807.091704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Meyerson NR, Sawyer SL. Two-stepping through time: Mammals and viruses. Trends Microbiol. 2011;19:286–294. doi: 10.1016/j.tim.2011.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bendinelli M, Pistello M, Lombardi S, Poli A, Garzelli C, Matteucci D, Ceccherini-Nelli L, Malvaldi G, Tozzini F. Feline immunodeficiency virus: An interesting model for AIDS studies and an important cat pathogen. Clin Microbiol Rev. 1995;8:87–112. doi: 10.1128/cmr.8.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hartmann K. Clinical aspects of feline immunodeficiency and feline leukemia virus infection. Vet Immunol Immunopathol. 2011;143:190–201. doi: 10.1016/j.vetimm.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roelke ME, Pecon-Slattery J, Taylor S, Citino S, Brown E, Packer C, VandeWoude S, O'Brien SJ. T-lymphocyte profiles in FIV-infected wild lions and pumas reveal CD4 depletion. J Wildl Dis. 2006;42:234–248. doi: 10.7589/0090-3558-42.2.234. [DOI] [PubMed] [Google Scholar]
- 12.Bull ME, Kennedy-Stoskopf S, Levine JF, Loomis M, Gebhard DG, Tompkins WA. Evaluation of T lymphocytes in captive african lions (Panthera leo) infected with feline immunodeficiency virus. Am J Vet Res. 2003;64:1293–1300. doi: 10.2460/ajvr.2003.64.1293. [DOI] [PubMed] [Google Scholar]
- 13.Troyer JL, Roelke ME, Jespersen JM, Baggett N, Buckley-Beason V, Macnulty D, Craft M, Packer C, Pecon-Slattery J, O'Brien SJ. FIV diversity: FIV(Ple) subtype composition may influence disease outcome in African lions. Vet Immunol Immunopathol. 2011;143:338–346. doi: 10.1016/j.vetimm.2011.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McEwan WA, Schaller T, Ylinen LM, Hosie MJ, Towers GJ, Willett BJ. Truncation of TRIM5 in the Feliformia explains the absence of retroviral restriction in cells of the domestic cat. J Virol. 2009;83:8270–8275. doi: 10.1128/JVI.00670-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gabuzda DH, Lawrence K, Langhoff E, Terwilliger E, Dorfman T, Haseltine WA, Sodroski J. Role of vif in replication of human immunodeficiency virus type 1 in CD4+ T lymphocytes. J Virol. 1992;66:6489–6495. doi: 10.1128/jvi.66.11.6489-6495.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sakai H, Shibata R, Sakuragi J, Sakuragi S, Kawamura M, Adachi A. Cell-dependent requirement of human immunodeficiency virus type 1 Vif protein for maturation of virus particles. J Virol. 1993;67:1663–1666. doi: 10.1128/jvi.67.3.1663-1666.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Madani N, Kabat D. An endogenous inhibitor of human immunodeficiency virus in human lymphocytes is overcome by the viral Vif protein. J Virol. 1998;72:10251–10255. doi: 10.1128/jvi.72.12.10251-10255.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sheehy AM, Gaddis NC, Choi JD, Malim MH. Isolation of a human gene that inhibits HIV-1 infection and is suppressed by the viral Vif protein. Nature. 2002;418:646–650. doi: 10.1038/nature00939. [DOI] [PubMed] [Google Scholar]
- 19.Simon JH, Gaddis NC, Fouchier RA, Malim MH. Evidence for a newly discovered cellular anti-HIV-1 phenotype. Nat Med. 1998;4:1397–1400. doi: 10.1038/3987. [DOI] [PubMed] [Google Scholar]
- 20.Jarmuz A, Chester A, Bayliss J, Gisbourne J, Dunham I, Scott J, Navaratnam N. An anthropoid-specific locus of orphan C to U RNA-editing enzymes on chromosome 22. Genomics. 2002;79:285–296. doi: 10.1006/geno.2002.6718. [DOI] [PubMed] [Google Scholar]
- 21.LaRue RS, Jonsson SR, Silverstein KA, Lajoie M, Bertrand D, El-Mabrouk N, Hotzel I, Andresdottir V, Smith TP, Harris RS. The artiodactyl APOBEC3 innate immune repertoire shows evidence for a multi-functional domain organization that existed in the ancestor of placental mammals. BMC Mol Biol. 2008;9:104. doi: 10.1186/1471-2199-9-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.LaRue RS, Andresdottir V, Blanchard Y, Conticello SG, Derse D, Emerman M, Greene WC, Jonsson SR, Landau NR, Löchelt M, et al. Guidelines for naming nonprimate APOBEC3 genes and proteins. J Virol. 2009;83:494–497. doi: 10.1128/JVI.01976-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Münk C, Beck T, Zielonka J, Hotz-Wagenblatt A, Chareza S, Battenberg M, Thielebein J, Cichutek K, Bravo IG, O'Brien SJ, et al. Functions, structure, and read-through alternative splicing of feline APOBEC3 genes. Genome Biol. 2008;9:R48. doi: 10.1186/gb-2008-9-3-r48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chiu YL, Greene WC. The APOBEC3 cytidine deaminases: An innate defensive network opposing exogenous retroviruses and endogenous retroelements. Annu Rev Immunol. 2008;26:317–353. doi: 10.1146/annurev.immunol.26.021607.090350. [DOI] [PubMed] [Google Scholar]
- 25.Narvaiza I, Linfesty DC, Greener BN, Hakata Y, Pintel DJ, Logue E, Landau NR, Weitzman MD. Deaminase-independent inhibition of parvoviruses by the APOBEC3A cytidine deaminase. PLoS Pathog. 2009;5:e1000439. doi: 10.1371/journal.ppat.1000439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Suspene R, Aynaud MM, Koch S, Pasdeloup D, Labetoulle M, Gaertner B, Vartanian JP, Meyerhans A, Wain-Hobson S. Genetic editing of herpes simplex virus 1 and Epstein-Barr herpesvirus genomes by human APOBEC3 cytidine deaminases in culture and in vivo. J Virol. 2011;85:7594–7602. doi: 10.1128/JVI.00290-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vartanian JP, Guetard D, Henry M, Wain-Hobson S. Evidence for editing of human papillomavirus DNA by APOBEC3 in benign and precancerous lesions. Science. 2008;320:230–233. doi: 10.1126/science.1153201. [DOI] [PubMed] [Google Scholar]
- 28.Muckenfuss H, Hamdorf M, Held U, Perkovic M, Löwer J, Cichutek K, Flory E, Schumann GG, Münk C. APOBEC3 proteins inhibit human LINE-1 retrotransposition. J Biol Chem. 2006;281:22161–22172. doi: 10.1074/jbc.M601716200. [DOI] [PubMed] [Google Scholar]
- 29.Stern MA, Hu C, Saenz DT, Fadel HJ, Sims O, Peretz M, Poeschla EM. Productive replication of Vif-chimeric HIV-1 in feline cells. J Virol. 2010;84:7378–7395. doi: 10.1128/JVI.00584-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zielonka J, Marino D, Hofmann H, Yuhki N, Löchelt M, Münk C. Vif of feline immunodeficiency virus from domestic cats protects against APOBEC3 restriction factors from many felids. J Virol. 2010;84:7312–7324. doi: 10.1128/JVI.00209-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bishop KN, Holmes RK, Sheehy AM, Davidson NO, Cho SJ, Malim MH. Cytidine deamination of retroviral DNA by diverse APOBEC proteins. Curr Biol. 2004;14:1392–1396. doi: 10.1016/j.cub.2004.06.057. [DOI] [PubMed] [Google Scholar]
- 32.Harris RS, Bishop KN, Sheehy AM, Craig HM, Petersen-Mahrt SK, Watt IN, Neuberger MS, Malim MH. DNA deamination mediates innate immunity to retroviral infection. Cell. 2003;113:803–809. doi: 10.1016/s0092-8674(03)00423-9. [DOI] [PubMed] [Google Scholar]
- 33.Lecossier D, Bouchonnet F, Clavel F, Hance AJ. Hypermutation of HIV-1 DNA in the absence of the Vif protein. Science. 2003;300:1112. doi: 10.1126/science.1083338. [DOI] [PubMed] [Google Scholar]
- 34.Mangeat B, Turelli P, Caron G, Friedli M, Perrin L, Trono D. Broad antiretroviral defence by human APOBEC3G through lethal editing of nascent reverse transcripts. Nature. 2003;424:99–103. doi: 10.1038/nature01709. [DOI] [PubMed] [Google Scholar]
- 35.Mariani R, Chen D, Schröfelbauer B, Navarro F, König R, Bollman B, Münk C, Nymark-McMahon H, Landau NR. Species-specific exclusion of APOBEC3G from HIV-1 virions by Vif. Cell. 2003;114:21–31. doi: 10.1016/s0092-8674(03)00515-4. [DOI] [PubMed] [Google Scholar]
- 36.Zhang H, Yang B, Pomerantz RJ, Zhang C, Arunachalam SC, Gao L. The cytidine deaminase CEM15 induces hypermutation in newly synthesized HIV-1 DNA. Nature. 2003;424:94–98. doi: 10.1038/nature01707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mbisa JL, Barr R, Thomas JA, Vandegraaff N, Dorweiler IJ, Svarovskaia ES, Brown WL, Mansky LM, Gorelick RJ, Harris RS, et al. Human immunodeficiency virus type 1 cDNAs produced in the presence of APOBEC3G exhibit defects in plus-strand DNA transfer and integration. J Virol. 2007;81:7099–7110. doi: 10.1128/JVI.00272-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Newman EN, Holmes RK, Craig HM, Klein KC, Lingappa JR, Malim MH, Sheehy AM. Antiviral function of APOBEC3G can be dissociated from cytidine deaminase activity. Curr Biol. 2005;15:166–170. doi: 10.1016/j.cub.2004.12.068. [DOI] [PubMed] [Google Scholar]
- 39.Iwatani Y, Chan DS, Wang F, Maynard KS, Sugiura W, Gronenborn AM, Rouzina I, Williams MC, Musier-Forsyth K, Levin JG. Deaminase-independent inhibition of HIV-1 reverse transcription by APOBEC3G. Nucleic Acids Res. 2007;35:7096–7108. doi: 10.1093/nar/gkm750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bishop KN, Holmes RK, Malim MH. Antiviral potency of APOBEC proteins does not correlate with cytidine deamination. J Virol. 2006;80:8450–8458. doi: 10.1128/JVI.00839-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Holmes RK, Koning FA, Bishop KN, Malim MH. APOBEC3F can inhibit the accumulation of HIV-1 reverse transcription products in the absence of hypermutation. Comparisons with APOBEC3G. J Biol Chem. 2007;282:2587–2595. doi: 10.1074/jbc.M607298200. [DOI] [PubMed] [Google Scholar]
- 42.Yu X, Yu Y, Liu B, Luo K, Kong W, Mao P, Yu XF. Induction of APOBEC3G ubiquitination and degradation by an HIV-1 Vif-Cul5-SCF complex. Science. 2003;302:1056–1060. doi: 10.1126/science.1089591. [DOI] [PubMed] [Google Scholar]
- 43.Conticello SG, Harris RS, Neuberger MS. The Vif protein of HIV triggers degradation of the human antiretroviral DNA deaminase APOBEC3G. Curr Biol. 2003;13:2009–2013. doi: 10.1016/j.cub.2003.10.034. [DOI] [PubMed] [Google Scholar]
- 44.Marin M, Rose KM, Kozak SL, Kabat D. HIV-1 Vif protein binds the editing enzyme APOBEC3G and induces its degradation. Nat Med. 2003;9:1398–1403. doi: 10.1038/nm946. [DOI] [PubMed] [Google Scholar]
- 45.Mehle A, Strack B, Ancuta P, Zhang C, McPike M, Gabuzda D. Vif overcomes the innate antiviral activity of APOBEC3G by promoting its degradation in the ubiquitin-proteasome pathway. J Biol Chem. 2004;279:7792–7798. doi: 10.1074/jbc.M313093200. [DOI] [PubMed] [Google Scholar]
- 46.Sheehy AM, Gaddis NC, Malim MH. The antiretroviral enzyme APOBEC3G is degraded by the proteasome in response to HIV-1 Vif. Nat Med. 2003;9:1404–1407. doi: 10.1038/nm945. [DOI] [PubMed] [Google Scholar]
- 47.LaRue RS, Lengyel J, Jonsson SR, Andresdottir V, Harris RS. Lentiviral Vif degrades the APOBEC3Z3/APOBEC3H protein of its mammalian host and is capable of cross-species activity. J Virol. 2010;84:8193–8201. doi: 10.1128/JVI.00685-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lockridge KM, Himathongkham S, Sawai ET, Chienand M, Sparger EE. The feline immunodeficiency virus vif gene is required for productive infection of feline peripheral blood mononuclear cells and monocyte-derived macrophages. Virology. 1999;261:25–30. doi: 10.1006/viro.1999.9831. [DOI] [PubMed] [Google Scholar]
- 49.Shen X, Leutenegger CM, Stefano CK, Pedersen NC, Sparger EE. A feline immunodeficiency virus vif-deletion mutant remains attenuated upon infection of newborn kittens. J Gen Virol. 2007;88:2793–2799. doi: 10.1099/vir.0.83268-0. [DOI] [PubMed] [Google Scholar]
- 50.Inoshima Y, Miyazawa T, Mikami T. The roles of vif and ORF-A genes and AP-1 binding site in in vivo replication of feline immunodeficiency virus. Arch Virol. 1998;143:789–795. doi: 10.1007/s007050050330. [DOI] [PubMed] [Google Scholar]
- 51.Löchelt M, Romen F, Bastone P, Muckenfuss H, Kirchner N, Kim YB, Truyen U, Rosler U, Battenberg M, Saib A, et al. The antiretroviral activity of APOBEC3 is inhibited by the foamy virus accessory Bet protein. Proc Natl Acad Sci U S A. 2005;102:7982–7987. doi: 10.1073/pnas.0501445102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stremlau M, Owens CM, Perron MJ, Kiessling M, Autissier P, Sodroski J. The cytoplasmic body component TRIM5alpha restricts HIV-1 infection in Old World monkeys. Nature. 2004;427:848–853. doi: 10.1038/nature02343. [DOI] [PubMed] [Google Scholar]
- 53.Carthagena L, Bergamaschi A, Luna JM, David A, Uchil PD, Margottin-Goguet F, Mothes W, Hazan U, Transy C, Pancino G, et al. Human TRIM gene expression in response to interferons. PLoS One. 2009;4:e4894. doi: 10.1371/journal.pone.0004894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Diaz-Griffero F, Kar A, Lee M, Stremlau M, Poeschla E, Sodroski J. Comparative requirements for the restriction of retrovirus infection by TRIM5alpha and TRIMCyp. Virology. 2007;369:400–410. doi: 10.1016/j.virol.2007.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Diaz-Griffero F, Qin XR, Hayashi F, Kigawa T, Finzi A, Sarnak Z, Lienlaf M, Yokoyama S, Sodroski J. A B-box 2 surface patch important for TRIM5alpha self-association, capsid binding avidity, and retrovirus restriction. J Virol. 2009;83:10737–10751. doi: 10.1128/JVI.01307-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Javanbakht H, Diaz-Griffero F, Stremlau M, Si Z, Sodroski J. The contribution of RING and B-box 2 domains to retroviral restriction mediated by monkey TRIM5alpha. J Biol Chem. 2005;280:26933–26940. doi: 10.1074/jbc.M502145200. [DOI] [PubMed] [Google Scholar]
- 57.Li X, Sodroski J. The TRIM5alpha B-box 2 domain promotes cooperative binding to the retroviral capsid by mediating higher-order self-association. J Virol. 2008;82:11495–11502. doi: 10.1128/JVI.01548-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ohkura S, Yap MW, Sheldon T, Stoye JP. All three variable regions of the TRIM5alpha B30.2 domain can contribute to the specificity of retrovirus restriction. J Virol. 2006;80:8554–8565. doi: 10.1128/JVI.00688-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Stremlau M, Perron M, Welikala S, Sodroski J. Species-specific variation in the B30.2(SPRY) domain of TRIM5alpha determines the potency of human immunodeficiency virus restriction. J Virol. 2005;79:3139–3145. doi: 10.1128/JVI.79.5.3139-3145.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Javanbakht H, Yuan W, Yeung DF, Song B, Diaz-Griffero F, Li Y, Li X, Stremlau M, Sodroski J. Characterization of TRIM5alpha trimerization and its contribution to human immunodeficiency virus capsid binding. Virology. 2006;353:234–246. doi: 10.1016/j.virol.2006.05.017. [DOI] [PubMed] [Google Scholar]
- 61.Yap MW, Nisole S, Stoye JP. A single amino acid change in the SPRY domain of human Trim5alpha leads to HIV-1 restriction. Curr Biol. 2005;15:73–78. doi: 10.1016/j.cub.2004.12.042. [DOI] [PubMed] [Google Scholar]
- 62.Sastri J, Campbell EM. Recent insights into the mechanism and consequences of TRIM5alpha retroviral restriction. AIDS Res Hum Retroviruses. 2011;27:231–238. doi: 10.1089/aid.2010.0367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Perron MJ, Stremlau M, Lee M, Javanbakht H, Song B, Sodroski J. The human TRIM5alpha restriction factor mediates accelerated uncoating of the N-tropic murine leukemia virus capsid. J Virol. 2007;81:2138–2148. doi: 10.1128/JVI.02318-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Stremlau M, Perron M, Lee M, Li Y, Song B, Javanbakht H, Diaz-Griffero F, Anderson DJ, Sundquist WI, Sodroski J. Specific recognition and accelerated uncoating of retroviral capsids by the TRIM5alpha restriction factor. Proc Natl Acad Sci U S A. 2006;103:5514–5519. doi: 10.1073/pnas.0509996103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Münk C, Brandt SM, Lucero G, Landau NR. A dominant block to HIV-1 replication at reverse transcription in simian cells. Proc Natl Acad Sci U S A. 2002;99:13843–13848. doi: 10.1073/pnas.212400099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wu X, Anderson JL, Campbell EM, Joseph AM, Hope TJ. Proteasome inhibitors uncouple rhesus TRIM5alpha restriction of HIV-1 reverse transcription and infection. Proc Natl Acad Sci U S A. 2006;103:7465–7470. doi: 10.1073/pnas.0510483103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pertel T, Hausmann S, Morger D, Zuger S, Guerra J, Lascano J, Reinhard C, Santoni FA, Uchil PD, Chatel L, et al. TRIM5 is an innate immune sensor for the retrovirus capsid lattice. Nature. 2011;472:361–365. doi: 10.1038/nature09976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Saenz DT, Teo W, Olsen JC, Poeschla EM. Restriction of feline immunodeficiency virus by Ref1, Lv1, and primate TRIM5alpha proteins. J Virol. 2005;79:15175–15188. doi: 10.1128/JVI.79.24.15175-15188.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Brennan G, Kozyrev Y, Hu SL. TRIMCyp expression in Old World primates Macaca nemestrina and Macaca fascicularis. Proc Natl Acad Sci U S A. 2008;105:3569–3574. doi: 10.1073/pnas.0709511105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Newman RM, Hall L, Kirmaier A, Pozzi LA, Pery E, Farzan M, O'Neil SP, Johnson W. Evolution of a TRIM5-CypA splice isoform in old world monkeys. PLoS Pathog. 2008;4:e1000003. doi: 10.1371/journal.ppat.1000003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sayah DM, Sokolskaja E, Berthoux L, Luban J. Cyclophilin A retrotransposition into TRIM5 explains owl monkey resistance to HIV-1. Nature. 2004;430:569–573. doi: 10.1038/nature02777. [DOI] [PubMed] [Google Scholar]
- 72.Virgen CA, Kratovac Z, Bieniasz PD, Hatziioannou T. Independent genesis of chimeric TRIM5-cyclophilin proteins in two primate species. Proc Natl Acad Sci U S A. 2008;105:3563–3568. doi: 10.1073/pnas.0709258105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wilson SJ, Webb BL, Ylinen LM, Verschoor E, Heeney JL, Towers GJ. Independent evolution of an antiviral TRIMCyp in rhesus macaques. Proc Natl Acad Sci U S A. 2008;105:3557–3562. doi: 10.1073/pnas.0709003105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nisole S, Lynch C, Stoye JP, Yap MW. A Trim5-cyclophilin A fusion protein found in owl monkey kidney cells can restrict HIV-1. Proc Natl Acad Sci U S A. 2004;101:13324–13328. doi: 10.1073/pnas.0404640101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dietrich I, Macintyre A, McMonagle E, Price AJ, James LC, McEwan WA, Hosie MJ, Willett BJ. Potent lentiviral restriction by a synthetic feline TRIM5 cyclophilin A fusion. J Virol. 2010;84:8980–8985. doi: 10.1128/JVI.00858-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gamble TR, Vajdos FF, Yoo S, Worthylake DK, Houseweart M, Sundquist WI, Hill CP. Crystal structure of human cyclophilin A bound to the amino-terminal domain of HIV-1 capsid. Cell. 1996;87:1285–1294. doi: 10.1016/s0092-8674(00)81823-1. [DOI] [PubMed] [Google Scholar]
- 77.Luban J, Bossolt KL, Franke EK, Kalpana GV, Goff SP. Human immunodeficiency virus type 1 Gag protein binds to cyclophilins A and B. Cell. 1993;73:1067–1078. doi: 10.1016/0092-8674(93)90637-6. [DOI] [PubMed] [Google Scholar]
- 78.Lin TY, Emerman M. Cyclophilin A interacts with diverse lentiviral capsids. Retrovirology. 2006;3:70. doi: 10.1186/1742-4690-3-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Diaz-Griffero F, Vandegraaff N, Li Y, McGee-Estrada K, Stremlau M, Welikala S, Si Z, Engelman A, Sodroski J. Requirements for capsid-binding and an effector function in TRIMCyp-mediated restriction of HIV-1. Virology. 2006;351:404–419. doi: 10.1016/j.virol.2006.03.023. [DOI] [PubMed] [Google Scholar]
- 80.Neagu MR, Ziegler P, Pertel T, Strambio-De-Castillia C, Grutter C, Martinetti G, Mazzucchelli L, Grutter M, Manz MG, Luban J. Potent inhibition of HIV-1 by TRIM5-cyclophilin fusion proteins engineered from human components. J Clin Invest. 2009;119:3035–3047. doi: 10.1172/JCI39354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Dietrich EA, Brennan G, Ferguson B, Wiseman RW, O'Connor D, Hu SL. Variable prevalence and functional diversity of the antiretroviral restriction factor TRIMCyp in Macaca fascicularis. J Virol. 2011;85:9956–9963. doi: 10.1128/JVI.00097-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wongsrikeao P, Saenz D, Rinkoski T, Otoi T, Poeschla E. Antiviral restriction factor transgenesis in the domestic cat. Nat Methods. 2011;8:853–859. doi: 10.1038/nmeth.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Dietrich I, McMonagle EL, Petit SJ, Vijayakrishnan S, Logan N, Chan CN, Towers GJ, Hosie MJ, Willett BJ. Feline tetherin efficiently restricts release of feline immunodeficiency virus but not spreading of infection. J Virol. 2011;85:5840–5852. doi: 10.1128/JVI.00071-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Neil SJ, Zang T, Bieniasz PD. Tetherin inhibits retrovirus release and is antagonized by HIV-1 Vpu. Nature. 2008;451:425–430. doi: 10.1038/nature06553. [DOI] [PubMed] [Google Scholar]
- 85.Van DN, Goff D, Katsura C, Jorgenson RL, Mitchell R, Johnson MC, Stephens EB, Guatelli J. The interferon-induced protein BST-2 restricts HIV-1 release and is downregulated from the cell surface by the viral Vpu protein. Cell Host Microbe. 2008;3:245–252. doi: 10.1016/j.chom.2008.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mitchell RS, Katsura C, Skasko MA, Fitzpatrick K, Lau D, Ruiz A, Stephens EB, Margottin-Goguet F, Benarous R, Guatelli JC. Vpu antagonizes BST-2-mediated restriction of HIV-1 release via beta-TrCP and endo-lysosomal trafficking. PLoS Pathog. 2009;5:e1000450. doi: 10.1371/journal.ppat.1000450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Douglas JL, Viswanathan K, McCarroll MN, Gustin JK, Fruh K, Moses AV. Vpu directs the degradation of the human immunodeficiency virus restriction factor BST-2/Tetherin via a {beta}TrCP-dependent mechanism. J Virol. 2009;83:7931–7947. doi: 10.1128/JVI.00242-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Douglas JL, Gustin JK, Viswanathan K, Mansouri M, Moses AV, Fruh K. The great escape: Viral strategies to counter BST-2/tetherin. PLoS Pathog. 2010;6:e1000913. doi: 10.1371/journal.ppat.1000913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Evans DT, Serra-Moreno R, Singh RK, Guatelli JC. BST-2/tetherin: A new component of the innate immune response to enveloped viruses. Trends Microbiol. 2010;18:388–396. doi: 10.1016/j.tim.2010.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kuhl BD, Cheng V, Wainberg MA, Liang C. Tetherin and its viral antagonists. J Neuroimmune Pharmacol. 2011;6:188–201. doi: 10.1007/s11481-010-9256-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Jouvenet N, Neil SJ, Zhadina M, Zang T, Kratovac Z, Lee Y, McNatt M, Hatziioannou T, Bieniasz PD. Broad-spectrum inhibition of retroviral and filoviral particle release by tetherin. J Virol. 2009;83:1837–1844. doi: 10.1128/JVI.02211-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kaletsky RL, Francica JR, Agrawal-Gamse C, Bates P. Tetherin-mediated restriction of filovirus budding is antagonized by the Ebola glycoprotein. Proc Natl Acad Sci U S A. 2009;106:2886–2891. doi: 10.1073/pnas.0811014106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mansouri M, Viswanathan K, Douglas JL, Hines J, Gustin J, Moses AV, Fruh K. Molecular mechanism of BST2/tetherin downregulation by K5/MIR2 of Kaposi's sarcoma-associated herpesvirus. J Virol. 2009;83:9672–9681. doi: 10.1128/JVI.00597-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sakuma T, Noda T, Urata S, Kawaoka Y, Yasuda J. Inhibition of Lassa and Marburg virus production by tetherin. J Virol. 2009;83:2382–2385. doi: 10.1128/JVI.01607-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Radoshitzky SR, Dong L, Chi X, Clester JC, Retterer C, Spurgers K, Kuhn JH, Sandwick S, Ruthel G, Kota K, et al. Infectious Lassa virus, but not filoviruses, is restricted by BST-2/tetherin. J Virol. 2010;84:10569–10580. doi: 10.1128/JVI.00103-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Weidner JM, Jiang D, Pan XB, Chang J, Block TM, Guo JT. Interferon-induced cell membrane proteins, IFITM3 and tetherin, inhibit vesicular stomatitis virus infection via distinct mechanisms. J Virol. 2010;84:12646–12657. doi: 10.1128/JVI.01328-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Yondola MA, Fernandes F, Belicha-Villanueva A, Uccelini M, Gao Q, Carter C, Palese P. Budding capability of the influenza virus neuraminidase can be modulated by tetherin. J Virol. 2011;85:2480–2491. doi: 10.1128/JVI.02188-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Watanabe R, Leser GP, Lamb RA. Influenza virus is not restricted by tetherin whereas influenza VLP production is restricted by tetherin. Virology. 2011;417:50–56. doi: 10.1016/j.virol.2011.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kupzig S, Korolchuk V, Rollason R, Sugden A, Wilde A, Banting G. Bst-2/HM1.24 is a raft-associated apical membrane protein with an unusual topology. Traffic. 2003;4:694–709. doi: 10.1034/j.1600-0854.2003.00129.x. [DOI] [PubMed] [Google Scholar]
- 100.Andrew AJ, Kao S, Strebel K. The C-terminal hydrophobic region in human BST-2/tetherin functions as a second transmembrane motif. J Biol Chem. 2011 doi: 10.1074/jbc.M111.287011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Erikson E, Adam T, Schmidt S, Lehmann-Koch J, Over B, Goffinet C, Harter C, Bekeredjian-Ding I, Sertel S, Lasitschka F, et al. In vivo expression profile of the antiviral restriction factor and tumor-targeting antigen CD317/BST-2/HM1.24/tetherin in humans. Proc Natl Acad Sci U S A. 2011:108, 13688–13693. doi: 10.1073/pnas.1101684108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Fukuma A, Abe M, Morikawa Y, Miyazawa T, Yasuda J. Cloning and characterization of the antiviral activity of feline Tetherin/BST-2. PLoS One. 2011;6:e18247. doi: 10.1371/journal.pone.0018247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Jolly C, Booth NJ, Neil SJ. Cell-cell spread of human immunodeficiency virus type 1 overcomes tetherin/BST-2-mediated restriction in T cells. J Virol. 2010;84:12185–12199. doi: 10.1128/JVI.01447-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Casartelli N, Sourisseau M, Feldmann J, Guivel-Benhassine F, Mallet A, Marcelin AG, Guatelli J, Schwartz O. Tetherin restricts productive HIV-1 cell-to-cell transmission. PLoS Pathog. 2010;6:e1000955. doi: 10.1371/journal.ppat.1000955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sauter D, Schindler M, Specht A, Landford WN, Munch J, Kim KA, Votteler J, Schubert U, Bibollet-Ruche F, Keele BF, et al. Tetherin-driven adaptation of Vpu and Nef function and the evolution of pandemic and nonpandemic HIV-1 strains. Cell Host Microbe. 2009;6:409–421. doi: 10.1016/j.chom.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Collman R, Hassan NF, Walker R, Godfrey B, Cutilli J, Hastings JC, Friedman H, Douglas SD, Nathanson N. Infection of monocyte-derived macrophages with human immunodeficiency virus type 1 (HIV-1). Monocyte-tropic and lymphocyte-tropic strains of HIV-1 show distinctive patterns of replication in a panel of cell types. J Exp Med. 1989;170:1149–1163. doi: 10.1084/jem.170.4.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Mühlebach MD, Wolfrum N, Schule S, Tschulena U, Sanzenbacher R, Flory E, Cichutek K, Schweizer M. Stable transduction of primary human monocytes by simian lentiviral vector PBj. Mol Ther. 2005;12:1206–1216. doi: 10.1016/j.ymthe.2005.06.483. [DOI] [PubMed] [Google Scholar]
- 108.Naif HM, Li S, Alali M, Sloane A, Wu L, Kelly M, Lynch G, Lloyd A, Cunningham AL. CCR5 expression correlates with susceptibility of maturing monocytes to human immunodeficiency virus type 1 infection. J Virol. 1998;72:830–836. doi: 10.1128/jvi.72.1.830-836.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Neil S, Martin F, Ikeda Y, Collins M. Postentry restriction to human immunodeficiency virus-based vector transduction in human monocytes. J Virol. 2001;75:5448–5456. doi: 10.1128/JVI.75.12.5448-5456.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Rich EA, Chen IS, Zack JA, Leonard ML, O'Brien WA. Increased susceptibility of differentiated mononuclear phagocytes to productive infection with human immunodeficiency virus-1 (HIV-1) J Clin Invest. 1992;89:176–183. doi: 10.1172/JCI115559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Sonza S, Maerz A, Deacon N, Meanger J, Mills J, Crowe S. Human immunodeficiency virus type 1 replication is blocked prior to reverse transcription and integration in freshly isolated peripheral blood monocytes. J Virol. 1996;70:3863–3869. doi: 10.1128/jvi.70.6.3863-3869.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ayinde D, Maudet C, Transy C, Margottin-Goguet F. Limelight on two HIV/SIV accessory proteins in macrophage infection: Is Vpx overshadowing Vpr. Retrovirology. 2010;7:35. doi: 10.1186/1742-4690-7-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Sprague WS, Robbiani M, Avery PR, O'Halloran KP, Hoover EA. Feline immunodeficiency virus dendritic cell infection and transfer. J Gen Virol. 2008;89:709–715. doi: 10.1099/vir.0.83068-0. [DOI] [PubMed] [Google Scholar]
- 114.van der Meer FJ, Schuurman NM, Egberink HF. Feline immunodeficiency virus infection is enhanced by feline bone marrow-derived dendritic cells. J Gen Virol. 2007;88:251–258. doi: 10.1099/vir.0.82450-0. [DOI] [PubMed] [Google Scholar]
- 115.Goujon C, Riviere L, Jarrosson-Wuilleme L, Bernaud J, Rigal D, Darlix JL, Cimarelli A. SIVSM/HIV-2 Vpx proteins promote retroviral escape from a proteasome-dependent restriction pathway present in human dendritic cells. Retrovirology. 2007;4:2. doi: 10.1186/1742-4690-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Henderson LE, Sowder RC, Copeland TD, Benveniste RE, Oroszlan S. Isolation and characterization of a novel protein (X-ORF product) from SIV and HIV-2. Science. 1988;241:199–201. doi: 10.1126/science.3388031. [DOI] [PubMed] [Google Scholar]
- 117.Horton R, Spearman P, Ratner L. HIV-2 viral protein X association with the GAG p27 capsid protein. Virology. 1994;199:453–457. doi: 10.1006/viro.1994.1144. [DOI] [PubMed] [Google Scholar]
- 118.Guyader M, Emerman M, Montagnier L, Peden K. VPX mutants of HIV-2 are infectious in established cell lines but display a severe defect in peripheral blood lymphocytes. EMBO J. 1989;8:1169–1175. doi: 10.1002/j.1460-2075.1989.tb03488.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Yu XF, Yu QC, Essex M, Lee TH. The vpx gene of simian immunodeficiency virus facilitates efficient viral replication in fresh lymphocytes and macrophage. J Virol. 1991;65:5088–5091. doi: 10.1128/jvi.65.9.5088-5091.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Gibbs JS, Regier DA, Desrosiers RC. Construction and in vitro properties of SIVmac mutants with deletions in “nonessential” genes. AIDS Res Hum Retroviruses. 1994;10:607–616. doi: 10.1089/aid.1994.10.607. [DOI] [PubMed] [Google Scholar]
- 121.Hirsch VM, Sharkey ME, Brown CR, Brichacek B, Goldstein S, Wakefield J, Byrum R, Elkins WR, Hahn BH, Lifson JD, et al. Vpx is required for dissemination and pathogenesis of SIV(SM) PBj: Evidence of macrophage-dependent viral amplification. Nat Med. 1998;4:1401–1408. doi: 10.1038/3992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Gibbs JS, Lackner AA, Lang SM, Simon MA, Sehgal PK, Daniel MD, Desrosiers RC. Progression to AIDS in the absence of a gene for vpr or vpx. J Virol. 1995;69:2378–2383. doi: 10.1128/jvi.69.4.2378-2383.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Fujita M, Otsuka M, Miyoshi M, Khamsri B, Nomaguchi M, Adachi A. Vpx is critical for reverse transcription of the human immunodeficiency virus type 2 genome in macrophages. J Virol. 2008;82:7752–7756. doi: 10.1128/JVI.01003-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Gramberg T, Sunseri N, Landau NR. Evidence for an activation domain at the amino terminus of simian immunodeficiency virus Vpx. J Virol. 2010;84:1387–1396. doi: 10.1128/JVI.01437-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Srivastava S, Swanson SK, Manel N, Florens L, Washburn MP, Skowronski J. Lentiviral Vpx accessory factor targets VprBP/DCAF1 substrate adaptor for cullin 4 E3 ubiquitin ligase to enable macrophage infection. PLoS Pathog. 2008;4:e1000059. doi: 10.1371/journal.ppat.1000059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Hrecka K, Hao C, Gierszewska M, Swanson SK, Kesik-Brodacka M, Srivastava S, Florens L, Washburn MP, Skowronski J. Vpx relieves inhibition of HIV-1 infection of macrophages mediated by the SAMHD1 protein. Nature. 2011;474:658–661. doi: 10.1038/nature10195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Laguette N, Sobhian B, Casartelli N, Ringeard M, Chable-Bessia C, Segeral E, Yatim A, Emiliani S, Schwartz O, Benkirane M. SAMHD1 is the dendritic- and myeloid-cell-specific HIV-1 restriction factor counteracted by Vpx. Nature. 2011;474:654–657. doi: 10.1038/nature10117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Qiao F, Bowie JU. The many faces of SAM. Sci STKE. 2005;2005:re7. doi: 10.1126/stke.2862005re7. [DOI] [PubMed] [Google Scholar]
- 129.Aravind L, Koonin EV. The HD domain defines a new superfamily of metal-dependent phosphohydrolases. Trends Biochem Sci. 1998;23:469–472. doi: 10.1016/s0968-0004(98)01293-6. [DOI] [PubMed] [Google Scholar]
- 130.Rice GI, Bond J, Asipu A, Brunette RL, Manfield IW, Carr IM, Fuller JC, Jackson RM, Lamb T, Briggs TA, et al. Mutations involved in Aicardi-Goutieres syndrome implicate SAMHD1 as regulator of the innate immune response. Nat Genet. 2009;41:829–832. doi: 10.1038/ng.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Gao F, Bailes E, Robertson DL, Chen Y, Rodenburg CM, Michael SF, Cummins LB, Arthur LO, Peeters M, Shaw GM, et al. Origin of HIV-1 in the chimpanzee Pan troglodytes troglodytes. Nature. 1999;397:436–441. doi: 10.1038/17130. [DOI] [PubMed] [Google Scholar]
- 132.Hirsch VM, Olmsted RA, Murphey-Corb M, Purcell RH, Johnson PR. An African primate lentivirus (SIVsm) closely related to HIV-2. Nature. 1989;339:389–392. doi: 10.1038/339389a0. [DOI] [PubMed] [Google Scholar]
- 133.Lemey P, Pybus OG, Wang B, Saksena NK, Salemi M, Vandamme AM. Tracing the origin and history of the HIV-2 epidemic. Proc Natl Acad Sci U S A. 2003;100:6588–6592. doi: 10.1073/pnas.0936469100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Tomonaga K, Miyazawa T, Sakuragi J, Mori T, Adachi A, Mikami T. The feline immunodeficiency virus ORF-A gene facilitates efficient viral replication in established T-cell lines and peripheral blood lymphocytes. J Virol. 1993;67:5889–5895. doi: 10.1128/jvi.67.10.5889-5895.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Pistello M, Moscardini M, Mazzetti P, Bonci F, Zaccaro L, Isola P, Freer G, Specter S, Matteucci D, Bendinelli M. Development of feline immunodeficiency virus ORF-A (tat) mutants: In vitro and in vivo characterization. Virology. 2002;298:84–95. doi: 10.1006/viro.2002.1442. [DOI] [PubMed] [Google Scholar]
- 136.Chatterji U, de PA, Elder JH. Feline immunodeficiency virus OrfA is distinct from other lentivirus transactivators. J Virol. 2002;76:9624–9634. doi: 10.1128/JVI.76.19.9624-9634.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Gemeniano MC, Sawai ET, Leutenegger CM, Sparger EE. Feline immunodeficiency virus ORF-Ais required for virus particle formation and virus infectivity. J Virol. 2003;77:8819–8830. doi: 10.1128/JVI.77.16.8819-8830.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Gemeniano MC, Sawai ET, Sparger EE. Feline immunodeficiency virus Orf-A localizes to the nucleus and induces cell cycle arrest. Virology. 2004;325:167–174. doi: 10.1016/j.virol.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 139.Hong Y, Fink E, Hu QY, Kiosses WB, Elder JH. OrfA downregulates feline immunodeficiency virus primary receptor CD134 on the host cell surface and is important in viral infection. J Virol. 2010;84:7225–7232. doi: 10.1128/JVI.00434-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Robert-Tissot C, Ruegger VL, Cattori V, Meli ML, Riond B, Gomes-Keller MA, Vogtlin A, Wittig B, Juhls C, Hofmann-Lehmann R, et al. The innate antiviral immune system of the cat: Molecular tools for the measurement of its state of activation. Vet Immunol Immunopathol. 2011;143:269–281. doi: 10.1016/j.vetimm.2011.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Schrofelbauer B, Hakata Y, Landau NR. HIV-1 Vpr function is mediated by interaction with the damage-specific DNA-binding protein DDB1. Proc Natl Acad Sci U S A. 2007;104:4130–4135. doi: 10.1073/pnas.0610167104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Gallois-Montbrun S, Kramer B, Swanson CM, Byers H, Lynham S, Ward M, Malim MH. Antiviral protein APOBEC3G localizes to ribonucleoprotein complexes found in P bodies and stress granules. J Virol. 2007;81:2165–2178. doi: 10.1128/JVI.02287-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Mitchell RS, Katsura C, Skasko MA, Fitzpatrick K, Lau D, Ruiz A, Stephens EB, Margottin-Goguet F, Benarous R, Guatelli JC. Vpu antagonizes BST-2-mediated restriction of HIV-1 release via beta-TrCP and endo-lysosomal trafficking. PLoS Pathog. 2009;5:e1000450. doi: 10.1371/journal.ppat.1000450. [DOI] [PMC free article] [PubMed] [Google Scholar]