Abstract
Objectives/Hypothesis
Sinonasal respiratory epithelial mucociliary clearance (MCC) is dependent on the transepithelial transport of ions such as Cl−. The objectives of the present study were to investigate the role of oxygen restriction in 1) Cl− transport across primary sinonasal epithelial monolayers, 2) expression of the apical Cl− channels CFTR and TMEM16A, and 3) the pathogenesis of chronic rhinosinusitis (CRS).
Study Design
In vitro investigation.
Methods
Murine nasal septal epithelial (MNSE, wild type) and human sinonasal epithelial (HSNE) cultures were incubated under hypoxic conditions (1% O2, 5% CO2). Cultures were mounted in Ussing chambers for ion transport measurements. CFTR and TMEM16A expression were measured using quantitative RT-PCR.
Results
The change in short-circuit current (ΔISC (µA/cm2) attributable to CFTR (forskolin-stimulated) was significantly decreased due to a 12 hour hypoxia exposure in both MNSE (13.55+/− 0.46 vs. 19.23+/−0.18) and HSNE (19.55+/−0.56 vs. 25.49+/−1.48 (control); p<0.05. TMEM16A (UTP-stimulated transport) was inhibited by 48 hours of hypoxic exposure in MNSE (15.92+/−2.87 vs. 51.44+/−3.71(control) p<0.05] and by 12 hours of hypoxic exposure in HSNE (16.75+/−0.68 vs. 24.15+/−1.35 (control). Quantitative RT-PCR (reported as relative mRNA levels+/−S.D.) demonstrated significant reductions in both CFTR and TMEM16A mRNA expression in MNSE and HSNE due to airway epithelial hypoxia.
Conclusions
Sinonasal epithelial CFTR and TMEM16A-mediated Cl− transport and mRNA expression were robustly decreased in an oxygen restricted environment. The findings in the present study indicate persistent hypoxia may lead to acquired defects in sinonasal Cl− transport in a fashion likely to confer mucociliary dysfunction in CRS.
Level of Evidence
1b
Keywords: Transepithelial Ion Transport, Hypoxia, CFTR, TMEM16A, Calcium-activated Chloride Channel, Chronic Sinusitis, Chloride Secretion, Murine Nasal Culture, Human Sinus Epithelium, Mucociliary Clearance
INTRODUCTION
Healthy sinonasal respiratory epithelium is dependent upon the removal of inhaled pathogens by mucociliary clearance (MCC) – a primary innate defense utilized by the airways. Normal MCC is the result of proper ciliary beating and hydration of airway surface liquid (ASL). The vectorial transport of ions such as Cl−1 influences both biological and physical characteristics of the ASL, including macromolecular composition. The cystic fibrosis transmembrane conductance regulator (CFTR) is the primary apical ion channel responsible for Cl− transport within respiratory epithelial cells.2 Calcium-activated chloride channels (CaCCs) serve as secondary Cl− transport pathways, although these may have a more prominent role in the murine airway. The identity of the CaCC was actively sought for nearly 20 years and recently identified as TMEM16A.3,4 CFTR and TMEM16A, in combination with epithelial sodium (Na+) channels (ENaCs), contribute to the delicate balance of epithelial ion transport critical for ASL depth, composition, and functional MCC.
In cystic fibrosis (CF), CFTR mutations decrease transepithelial anion (Cl−) transport (with relative Na+ hyperabsorption) resulting in ASL dehydration, chronic stasis of inspissated mucus, bacterial infection, and widespread chronic disease of the nose and paranasal sinuses.5 Accumulated secretions and impacted mucus lead to poor oxygen (O2) delivery, resulting in near anaerobic tissue conditions that accelerate progression of disease.6 Similarly, hypoxia has been suggested to play a significant role in the pathophysiology of chronic rhinosinusitis (CRS) among non-CF individuals.7–9 Blockage of sinus ostia may lead to hypoxia, resulting in decreased MCC, increased inflammatory factors, and bacterial colonization.9 However, very little is known regarding the mechanism by which hypoxia contributes to the underlying pathogenesis of CRS, particularly among individuals who do not harbor mutations in CFTR.
Various environmental insults, such as cigarette smoke exposure, high altitude/hypoxemia, inflammation, and infectious agents can lead to acquired defects in CFTR by repressing wild type CFTR processing, endocytic recycling, or function.10–15 Whether hypoxia impairs transepithelial ion transport in sinus and nasal epithelium has not been previously evaluated. The purpose of the current study was to investigate the effects of O2 restriction on transepithelial ion transport in murine nasal septal epithelium (MNSE) and human sinonasal epithelium (HSNE) and better elucidate the role of hypoxia and its mechanistic relationship to the pathophysiology of CRS.
MATERIALS AND METHODS
Institutional Review Board and Institutional Animal Care and Use Committee approval were obtained prior to the initiation of this study.
Tissue Culture
The technique of culturing MNSE and HSNE at an air-liquid interface has been described previously by our laboratory.11,16–22 In brief, tissue was harvested and grown on Costar 6.5-mm-diameter permeable filter supports (Corning Life Sciences, Lowell, MA) submerged in culture medium. After achieving confluence on culture day 4, media were removed from the surface of the monolayers and cells fed via the basal chamber. MNSE cultures were genetically identical and derived from the C57 strain. Differentiation and ciliogenesis occurred in all cultures within 10 to 14 days. All experiments were performed only when cell monolayers were fully differentiated with widespread ciliogenesis and transepithelial resistances (Rt) > 500 Ω/cm2. To evaluate the role of oxygen restriction, monolayers were incubated in 1% O2, 5% CO2 (the remainder consisting of nitrogen) or a physiologic environment (21% O2, 5% CO2) in a sealed modular incubator chamber at 37°C. For all experiments, n ≥ 3 was used for each condition.
Electrophysiology
Solutions and Chemicals
The bath solution contained (mM): 120 NaCl, 25 NaHCO3, 3.3 KH2PO4, 0.8 K2HPO4, 1.2 MgCl2, 1.2 CaCl2, and 10 glucose. The pH of this solution is 7.3 – 7.4 when gassed with a mixture of 94% N2, 1% O2, 5% CO2 at 37°C. Chemicals were acquired from Sigma (St. Louis, MO). Each solution was formulated as 1000x stock and used at 1x in the Ussing chamber. All experiments were performed with low Cl− (6 mM) in the mucosal bath. Pharmacologic preparations were the following: amiloride (100µM); forskolin (20 µM); INH-172 (10 µM) and uridine triphosphate (UTP) (150 µM). Amiloride blocks epithelial sodium (Na+) channels, and supports the notion that variation in short-circuit current (ΔISC) from subsequent manipulation is due to effects on Cl− channel activity. Forskolin stimulates the cystic fibrosis transmembrane conductance regulator (CFTR) via a cAMP-mediated mechanism. INH-172 is a selective inhibitor of CFTR and aids in determining the degree of contribution of CFTR to the ISC under experimental conditions. UTP stimulates the CaCC, TMEM16A, by triggering P2Y receptor-mediated increases in intracellular Ca2+. ISC attributable to TMEM16A activation was measured in both MNSE (wild type) and HSNE.
Short circuit (Isc) Measurements
Transwell inserts (Costar) were mounted in vertical Ussing chambers and continuously short-circuited after fluid resistance compensation using automatic voltage clamps (VCC 600, Physiologic Instruments). Inserts were mounted in bath solution warmed to 37°C, and the solution continuously gas-lifted with a 94% N2/1% O2/5% CO2 mixture. The ISC was measured at 1 sample per second. By convention, a positive deflection in ISC is defined as the net movement of anion in the serosal to mucosal direction. Statistical analysis was performed using two-tailed paired t test, unpaired t test, or analysis of variance as appropriate.
Gene Expression
RNA isolation
Total RNA was isolated with RNeasy mini kit (Qiagen) according to the manufacturer's instructions. To prevent possible DNA contamination, samples were pretreated with RNase-free DNase (Qiagen) and column purified.
Primers and probes
Sequences used for murine CFTR, human CFTR and 18S rRNA were purchased from Assays on Demand (ABI); with assay ID for murine CFTR, Mm00445197_m1; and human CFTR, Hs00357011_m1. Sequences used for TMEM16A were purchased from APPLIED BIOSYSTEMS; with assay ID for murine TMEM16A, Mm01138366-m1; and human TMEM16A Hs01008057-m1.
RT-PCR
In order to detect TMEM16A gene expression in HSNE and MNSE, cDNA was synthesized from 1 µg total RNA using the First-Strand kit (Amersham Biosciences, Piscataway, N.J., USA) with oligo-(dT) priming, and 10% of the cDNA reaction used as template for PCR. The thermocycler conditions were as follows: Stage 1: 48°C for 30 min; Stage 2: 95°C for 10 min; Stage 3: 95°C for 15 sec; Stage 4: 60°C for 1 min; 40 cycles. PCR products were separated on 4–12% polyacrylamide TBE gels (Invitrogen) and visualized by ethidium bromide staining.
Quantitative RT-PCR
Once TMEM16A gene expression was confirmed in MNSE and HSNE, a one-step Applied Biosystems PCR protocol was used to quantify CFTR and TMEM16A mRNA transcripts on ABI Prism 7500 sequence detection system on six serial dilutions of RNA isolates according to the manufacturer’s instructions (Applied Biosystems, Foster City, CA). TaqMan OneStep PCR Master Mix Reagents Kit (ABI) was used for reverse transcription and PCR. The thermocycler conditions were as follows: Stage 1: 48°C for 30 min; Stage 2: 95°C for 10 min; Stage 3: 95°C for 15 sec; Stage 4: 60°C for 1 min; 40 cycles.. All CFTR and TMEM16A values were normalized to 18S rRNA (from the same sample) according to the Applied Biosystems relative quantification method as described in ABI manual to establish specific changes in CFTR and TMEM16A mRNA levels as opposed to more general effects on widespread suppression of cellular transcription. All experiments were performed in triplicate.
Protein Analyses
Western Blot Analysis
Western blotting was used to determine the presence of TMEM16A protein in MNSE. Total protein concentration of homogenized cultured cells was measured using Bio-Rad Benchmark Plus Multiplate Spectrophotometer (Bio-Rad, Hercules, CA) and 20 µg of total protein were loaded on a 12% Tris-HCl–sodium dodecyl sulfate–polyacrylamide gel and run for 1 hour at 120V. Protein was electrotransferred to a nitrocellulose membrane and then blocked with 5% nonfat dry milk and Tris-buffered saline (composition 25mM Tris, 150mM NaCl, 2mM KCl, pH 7.4) with 0.1% Tween 20. The membrane was then incubated overnight at 4°C with a rabbit polyclonal primary antibody to TMEM16A (Abcam) at a dilution of 1:100 followed by anti-mouse horseradish peroxidase–coupled secondary antibody (Bio-Rad) at a dilution of 1:10,000. After three washings, bands were detected using Enhanced Chemiluminescence Plus Western blotting detection reagents (Amersham Pharmacia Biotech, Piscataway, NJ).
Immunofluorescence
Localization by immunofluorescence of TMEM16A was performed on human cultured monolayers in transwell inserts to confirm presence on the apical membrane. Mouse anti-human type IV β-tubulin monoclonal antibodies (Invitrogen) were utilized to assist with apical localization by staining cilia. Negative controls were performed in parallel without a primary antibody incubation step. Nonspecific staining was blocked with 5% goat serum and 1% bovine serum albumin (BSA). The cells were permeabilized with 0.3% Triton® X-100 and incubated in primary antibody (type IV β-tubulin, 1:500; TMEM16A, 1:100) overnight at 4°C. After three washes with PBS, the transwell insert was incubated in fluorescein isothiocyanate (FITC)-coupled goat anti-mouse immunoglobulin G (IgG; 1:500) and rhodamine-coupled goat anti-rabbit IgG (1:500) at room temperature for 90 min. The membranes were washed three times in 1× PBS and then cut from the plastic support mold. They were mounted with Gel Mount™ aqueous mounting medium (Sigma-Aldrich) on a glass slide. The slides were then imaged on a Zeiss LSM510META confocal microscope.
RESULTS
ISC Measurements in Hypoxic MNSE
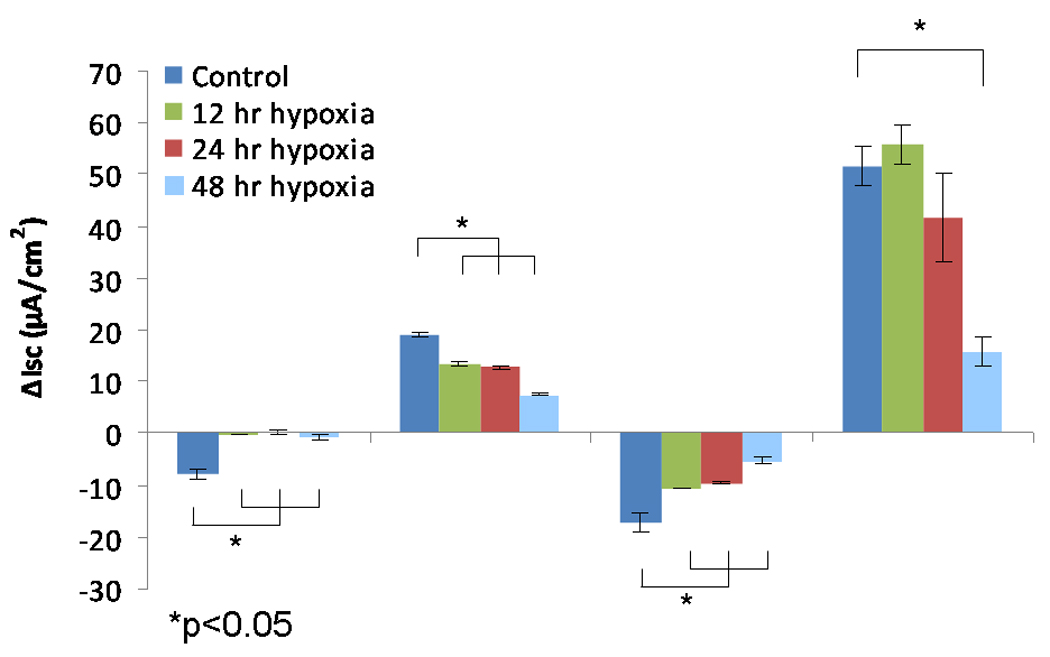
The change in short-circuit current (ΔISC (µA/cm2) attributable to CFTR (forskolin-stimulated transport) was significantly decreased at 12, 24, and 48 hours in MNSE (Figure 1A, 1B) incubated in an oxygen-restricted environment [13.55+/− 0.46 (12 hours); 12.75+/−0.07 (24 hours); 7.41+/−0.18 (48 hours) vs. 19.23+/−0.18 (control); p<0.001)]. Inhibition with the specific CFTR inhibitor, INH-172, confirmed a time-dependent decrease in CFTR-mediated ISC [−10.79+/− 0.10 (12 hours); −9.57+/−0.28 (24 hours); −5.33+/−0.65 (48 hours) vs. −17.19+/−1.80 (control); p<0.001)]. The ΔISC attributable to TMEM16A as measured by UTP was more stable and not significantly abrogated until 48 hours under hypoxic conditions [55.94+/−3.81 (12 hours); 41.68+/−8.40 (24 hours); 15.92+/−2.87 (48 hours) vs. 51.44+/−3.71 (control); p<0.001)]. Of note, ISC attributable to Na+ channel transport (ENaC) as measured by amiloride blockade was nearly absent by 12 hours [−0.16+/−0.01 vs. −7.76+/−0.80; p<0.001].
Figure 1.
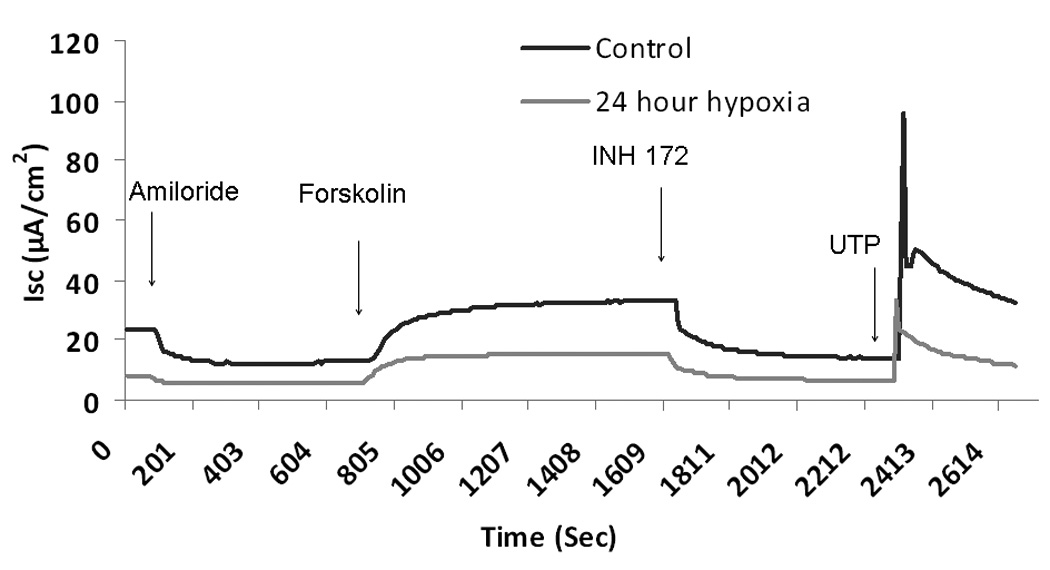
A. Representative ISC tracing of wild type murine nasal septal epithelial (MNSE) cultures. Wild type MNSE cells grown on transwell permeable supports and incubated in 1% O2 or physiologic O2 (control, 21%) were mounted in modified Ussing chambers under short-circuit conditions and sequentially exposed to amiloride (100µM); forskolin (20 µM); INH-172 (10 µM) and uridine triphosphate (UTP) (150 µM). By convention, a positive deflection in the tracing represents the movement of anion in the serosal to mucosal direction. Note the decrease in ISC in hypoxic MNSE (lower tracing) in all conditions compared to control (upper tracing).
B. Change in short-circuit current (ΔISC) in murine nasal septal epithelial cultures after 12, 24, and 48 hours under hypoxic conditions. Forskolin-stimulated ΔISC and CFTR blockade by INH-72 were significantly decreased compared to control (n ≥ 6 per condition). UTP-stimulated ΔISC was significantly inhibited at 48 hours.
ISC Measurements in Hypoxic HSNE
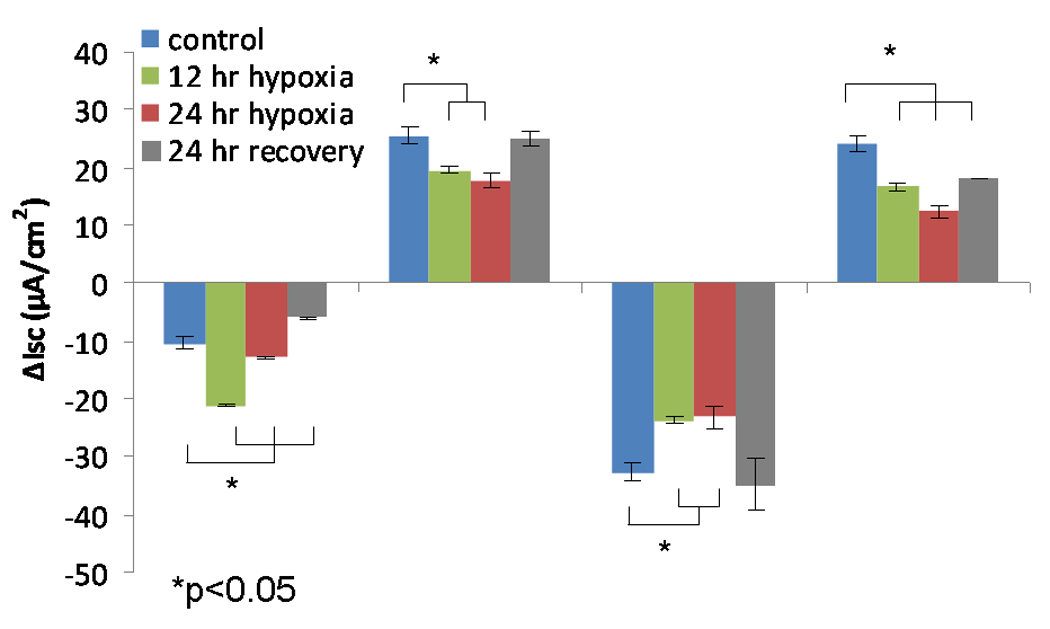
Similar to MNSE, forskolin-stimulated ΔISC in HSNE (Figure 2A, 2B) was exquisitely sensitive to hypoxic stress, and demonstrated significant reductions in CFTR-mediated Cl− transport [19.55+/−0.56 (12 hours); 17.67+/−1.13 (24 hours) vs. 25.49+/−1.48 (control); p<0.001)]. Decreased ΔISC following INH-172 pharmacologic blockade verified a decreased contribution of CFTR to the ISC [−23.67+/−0.05 (12 hours); −23.21+/−1.86 (24 hours) vs. −32.66+/−1.15 (control); p<0.001)]. Conversely, CaCC-mediated ΔISC (UTP-stimulated) was more susceptible to oxygen restriction when compared to MNSE and demonstrated early inhibition at 12 hours [16.75+/−0.68 (12 hours); 12.44+/−0.98 (24 hours) vs. 24.15+/−1.35 (control); p<0.001)]. Sodium absorption in HSNE (amiloride blockade) appeared to be resistant to hypoxia and, in fact, was significantly increased at 12 hours [−21.13+/−0.27 (12 hours) vs. −10.45+/−1.05 (24 hours); p <0.001] and returned to baseline by 24 hours (−12.89+/−0.37). In addition, HSNE demonstrated significant recovery of Cl− transport following 24 hours in physiologic O2 (21%) [forskolin-stimulated transport, 25.12+/−1.24 (p<0.05) and UTP-stimulated, 18.09+/−0.02 (p<0.01)]. However, Na+ absorption was significantly inhibited following recovery (−6.05+/−0.33).
Figure 2.
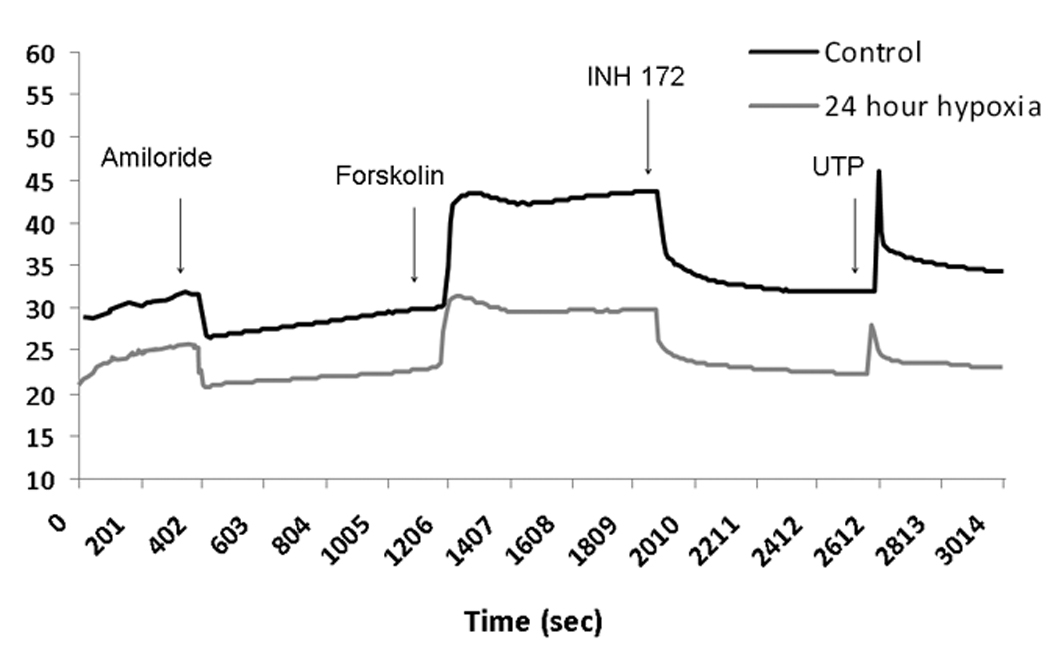
A: Representative ISC tracing of human sinonasal epithelial (HSNE) cultures. HSNE cells grown on transwell permeable supports and incubated in 1% O2 or physiologic O2 (control, 21%) were mounted in modified Ussing chambers under short-circuit conditions and sequentially exposed to amiloride (100µM); forskolin (20 µM); INH-172 (10 µM) and uridine triphosphate (UTP) (150 µM). Note the relative increase in ΔISC attributable to sodium blockade with amiloride (negative deflection) and decrease in forskolin-stimulated ΔISC at 24 hours under hypoxic conditions.
B. Change in short-circuit current (ΔISC) in human sinonasal epithelial cultures after 12 and 24 hours under hypoxic (1% O2) conditions and following 24 hour recovery period under physiologic conditions (21% O2). Forskolin-stimulated ΔISC was sensitive to hypoxic stress, and demonstrated significant reduction in CFTR-mediated Cl− transport (n ≥ 6 per condition). Decreased ΔISC from INH-172 blockade verified the contribution of CFTR to the ISC. Conversely, CaCC-mediated ΔISC (UTP-stimulated) was more susceptible to oxygen restriction compared to MNSE and demonstrated early inhibition at 12 hours. Sodium absorption (amiloride blockade) was significantly increased at 12 hours and returned back to baseline by 24 hours. HSNE demonstrated significant recovery of Cl− transport following 24 hours in a physiologic O2 (21%) environment.
Gene Expression
TMEM16A RT-PCR
RT-PCR products of the expected sizes (murine, 299 base pairs and human, 158 base pairs) were amplified using TMEM16A-specific primer pairs in both MNSE and HSNE and are consistent with the recently identified CaCC in sinus and nasal respiratory epithelium (Figure 3).
Figure 3. RT-PCR demonstrating presence of TMEM16A mRNA in murine nasal septal and human sinonasal epithelial cultures.
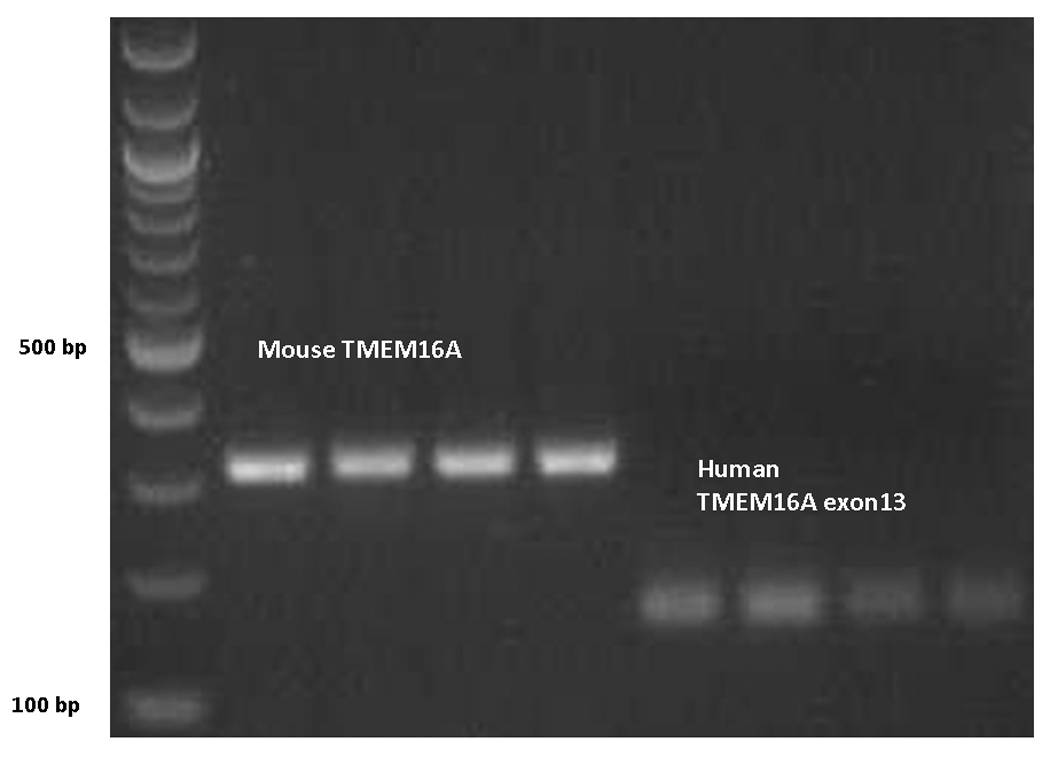
PCR products of the expected sizes were amplified using TMEM16A-specific primer pairs in both species and are consistent with the expression of the calcium-activated chloride channel in sinus and nasal respiratory epithelium.
Quantitative RT-PCR
In order to examine the effects of hypoxia on CFTR and TMEM16A gene expression, quantitative RT-PCR (reported as relative mRNA levels +/− S.D.) was performed on MNSE and HSNE incubated in 1% O2 for 48 hrs. CFTR (55.2+/−16.1 vs 102.8+/−10.3), p <0.05) and TMEM16A (54.6+/−12.1 vs. 134.3+/−26.1; p<0.05) mRNA were markedly decreased in MNSE following incubation in this oxygen restricted environment (Figure 4A). Furthermore, gene expression of CFTR (76.5+/−21.8 vs 251.3+/−33.9, p <0.05) and TMEM16A (101.1+/−35.7 vs. 362.3+/−30.9; p<0.01) were even more sensitive to hypoxic conditions in HSNE (Figure 4B).
Figure 4. Effect of 48 hours hypoxia on TMEM16A and CFTR gene expression.
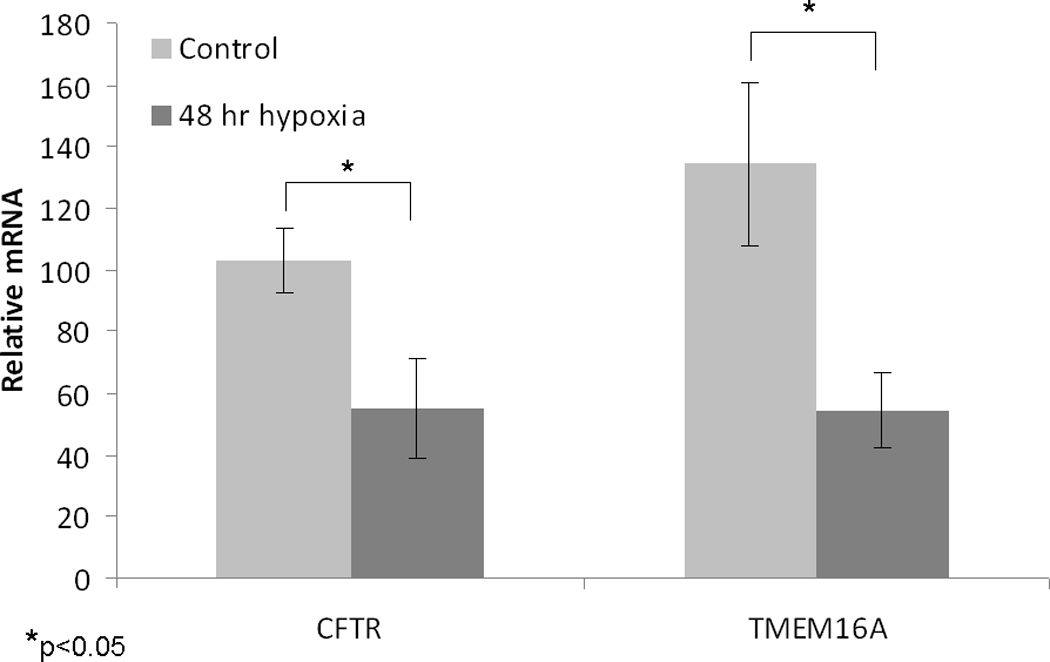
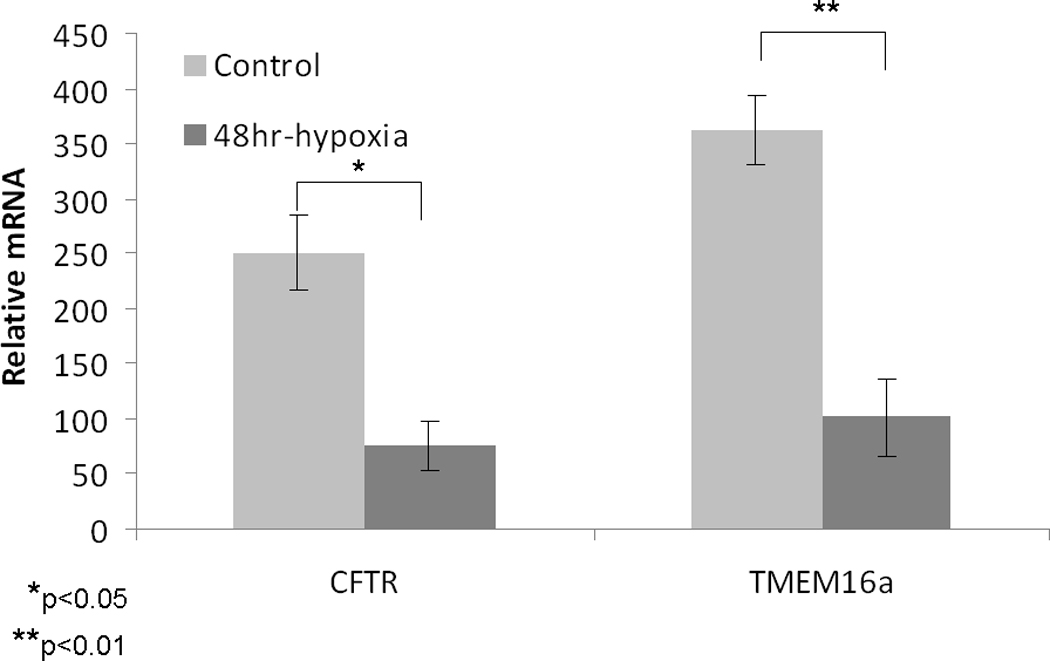
Quantitative RT-PCR (reported as relative mRNA levels +/− S.D.) was performed on MNSE (A) and HSNE (B) incubated in 1% O2 for 48 hours (n ≥ 6 per condition). CFTR and TMEM16A mRNA were robustly decreased following incubation in this oxygen restricted environment.
Protein Analyses
Western blotting confirmed that the TMEM16A channel protein was present in MNSE (Figure 5A). Colocalization of TMEM16A and type IV tubulin was also performed in HSNE to determine whether the channel is present on the apical membrane. Apical localization of TMEM16A was verified in HSNE at the base of the cilia (Figure 5B).
Figure 5. A (top) and B (bottom): Confirmation and localization of the TMEM16A channel protein.
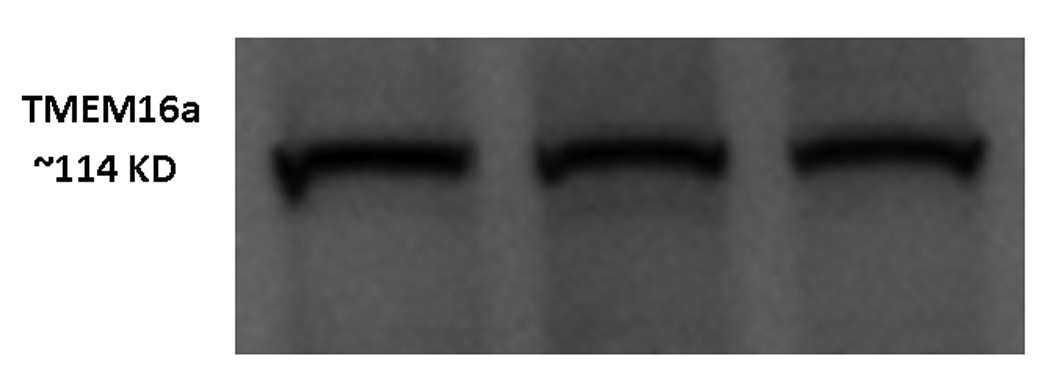
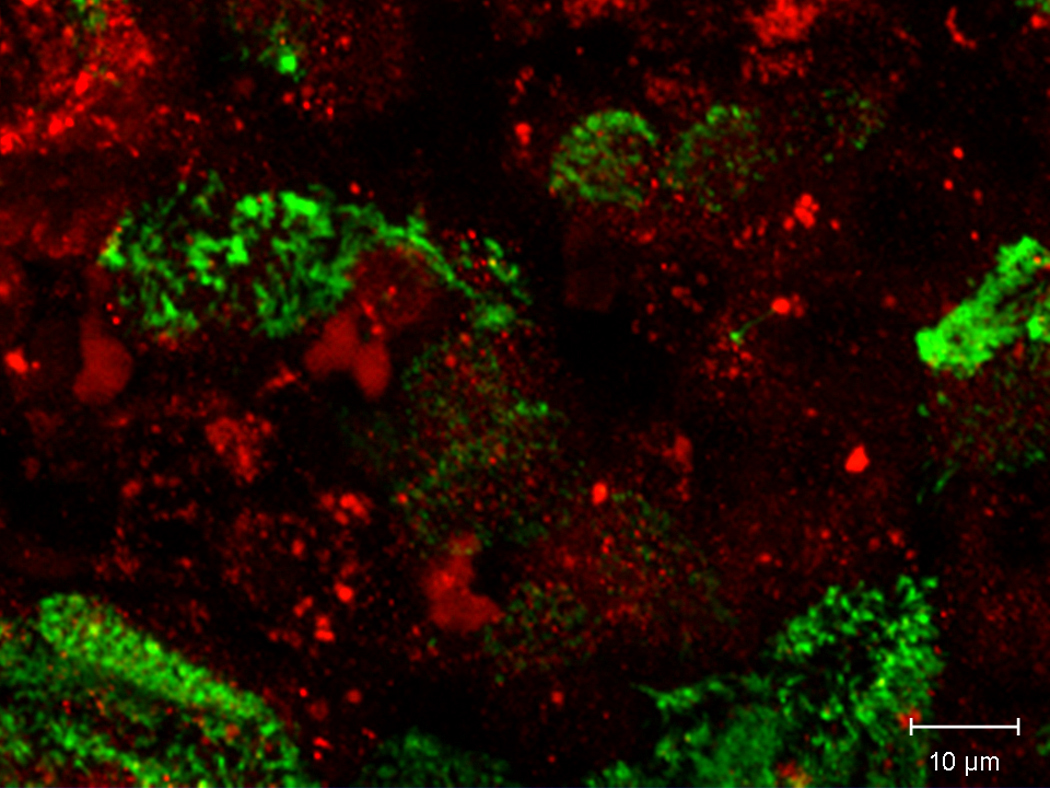
Western blotting confirmed that the TMEM16A channel was present in MNSE identifying the protein with a molecular weight of approximately 114 kDa (A). Co-localization of TMEM16A (red) and type IV Beta-tubulin (cilia – green) was also performed in HSNE to determine whether the channel is present on the apical membrane. Apical localization of TMEM16A was verified in HSNE at the base of the cilia (B).
Discussion
The current investigation examined effects of hypoxia on the major apical ion channels responsible for ASL homeostasis and MCC in sinus and nasal epithelium. Primary cell cultures derived from the murine nasal septum and human sinonasal tissues closely resemble the in vivo situation and are reliable models for testing the significance of environmental perturbations on sinus and nasal physiology.11,17–19,21,23 Reduced CFTR-mediated Cl− transport secondary to hypoxia has been demonstrated in multiple epithelial cell types15,24, but has not been previously investigated in the murine or human upper airway. Furthermore, the effects of hypoxia on TMEM16A, to our knowledge, have not been reported previously.
The data presented in the current study indicate that a low oxygen environment profoundly affects normal ion transport physiology in both MNSE and HSNE. Although apical secretion of anions (e.g. Cl−) was significantly reduced, there were also differences between species that may influence the pathophysiology of rhinosinusitis. While stimulation of transepithelial Cl− transport would be expected to hydrate the ASL, increased Na+ absorption (as demonstrated in CF) reduces water content leading to decreased clearance of dehydrated and, eventually, impacted mucus. Notably, blockade of ENaC channels with amiloride resulted in significant reductions in the Na+ transport contribution to the ISC in MNSE. Thus, both Na+ absorption and Cl− secretion are reduced in the murine model, an indication that the overall influence of hypoxia on the ASL could be reduced or compensated by opposing effects on epithelial ion transport. In addition, CaCC-mediated ΔISC was somewhat resistant to the effects of hypoxia in MNSE. CaCC’s provide a more substantial contribution to the overall transport of Cl− ions in murine epithelium versus human airways, and may reflect the absence of pulmonary disease in transgenic CF mice.25 While forskolin-mediated ISC consistently dropped with O2 restriction, the UTP-stimulated ISC was relatively stable until 48 hours of hypoxic stress. Overall, the ion transport phenotype demonstrated in MNSE appears to be relatively resistant to hypoxic stress (at least initially) and may have a less pronounced effect on ASL hydration due to a simultaneous decrease in Na+ absorption and residual CaCC activity.
Like MNSE, HSNE exhibit a marked reduction in forskolin-stimulated ISC over 24 hours. In contrast to the murine situation, however, Na+ absorption increased during the first 12 hours (as measured with amiloride blockade of ENaC) and a concomitant decrease in CaCC-mediated ISC was also observed. The finding that HSNE develops a globally decreased transepithelial Cl− secretion and increased Na+ absorption in response to hypoxic conditions indicates that in human sinonasal mucosa, all three of the major pathways necessary to maintain normal ASL hydration (Cl- and HCO3- secretion through CFTR and CaCC; Na absorption through ENaC) are affected by tissue hypoxia. Thus, the ion transport properties are consistent with CF airway pathophysiology (decreased Cl− secretion and enhanced Na+ absorption) resulting in dehydration of the ASL and, ultimately, disrupted mucociliary transport.
TMEM16A was only recently characterized as the CaCC present in airway epithelium.3,4 While the identification of TMEM16A in sinus and nasal epithelium had not been previously reported, the present study confirmed both expression, localization, and function of this channel protein in murine and human sinonasal tissues. Importantly, future investigations may now focus on this channel as a target for therapeutic intervention.
Although the mechanisms of hypoxia-induced inhibition of CFTR and TMEM16A remain incompletely characterized, the findings in the present study indicate that these effects may be mediated, at least in part, by suppression of mRNA expression. Decreased CFTR mRNA expression in hypoxic environments has been demonstrated in several tissues26, but hypoxic suppression of TMEM16A mRNA expression has not been shown previously. Although a decrease in steady-state mRNA is likely to contribute to a reduction of cell surface CFTR and TMEM16A within 48 hours, other mechanisms such as effects on protein folding, endocytic recycling, and ion channel gating could occur within a shorter time frame and remain areas of active investigation. Factors such as inhibition of basolateral Na/K+ ATPase (supplies driving force for basolateral Cl−) are not primarily responsible for these effects because forskolin-stimulated ISC was still significantly reduced in permeability studies (Data not shown).
Conclusion
The present studies suggest that hypoxia alters active transepithelial ion transport in both MNSE and HSNE. While transepithelial Cl− secretion is decreased in both of these primary epithelial culture models, our findings indicate the MNSE are more resistant to alterations in ASL due to a concomitant decrease in Na+ absorption and increased stability of TMEM16A. In contrast, the decreased anion transport and increased Na+ absorption demonstrated in HSNE will confer ASL dehydration and elicit persistent mucociliary dysfunction in CRS. These findings have important implications concerning the role of hypoxia in the pathogenesis of CRS and indicate usefulness of Cl− secretagogues as a new therapeutic strategy in CRS.
Acknowledgments
Research Support: This research was funded by the American Rhinologic Society New Investigator Award (2009), Flight Attendant’s Medical Research Institute Young Clinical Scientist Award (072218), and NIH/NHLBI (1K08HL107142-01) to B.A.W.; and NIH/NIDDK (5P30DK072482-03) to E.J.S.
Footnotes
Conflict of Interest: Bradford Woodworth M.D. is a consultant for Arthrocare ENT and Gyrus ENT. Dr. Sorscher and Dr. Woodworth are inventors on a patent submitted regarding the possible activity of chloride secretagogues for therapy of sinus disease (Provisional Patent Application Under 35 U.S.C. ξ111(b) and 37 C.F.R ξ.53 (c) in the United States Patent and Trademark Office.
References
- 1.Trout L, King M, Feng W, Inglis SK, Ballard ST. Inhibition of airway liquid secretion and its effect on the physical properties of airway mucus. Am J Physiol. 1998;274:L258–L263. doi: 10.1152/ajplung.1998.274.2.L258. [DOI] [PubMed] [Google Scholar]
- 2.Anderson MP, Berger HA, Rich DP, Gregory RJ, Smith AE, Welsh MJ. Nucleoside triphosphates are required to open the CFTR chloride channel. Cell. 1991;67:775–784. doi: 10.1016/0092-8674(91)90072-7. [DOI] [PubMed] [Google Scholar]
- 3.Caputo A, Caci E, Ferrera L, et al. TMEM16A, a membrane protein associated with calcium-dependent chloride channel activity. Science. 2008;322:590–594. doi: 10.1126/science.1163518. [DOI] [PubMed] [Google Scholar]
- 4.Rock JR, O'Neal WK, Gabriel SE, et al. Transmembrane protein 16A (TMEM16A) is a Ca2+-regulated Cl-secretory channel in mouse airways. J Biol Chem. 2009;284:14875–14880. doi: 10.1074/jbc.C109.000869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moller W, Haussinger K, Ziegler-Heitbrock L, Heyder J. Mucociliary and long-term particle clearance in airways of patients with immotile cilia. Respir Res. 2006;7:10. doi: 10.1186/1465-9921-7-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Worlitzsch D, Tarran R, Ulrich M, et al. Effects of reduced mucus oxygen concentration in airway Pseudomonas infections of cystic fibrosis patients. J Clin Invest. 2002;109:317–325. doi: 10.1172/JCI13870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baroody FM. Mucociliary transport in chronic rhinosinusitis. Clin Allergy Immunol. 2007;20:103–119. [PubMed] [Google Scholar]
- 8.Pahl A, Szelenyi S, Brune K. Hypoxia induced chemokine expression in nasal epithelial cells: development of an in vitro model for chronic rhinosinusitis. ALTEX. 2006;23:59–63. [PubMed] [Google Scholar]
- 9.Steinke JW, Woodard CR, Borish L. Role of hypoxia in inflammatory upper airway disease. Curr Opin Allergy Clin Immunol. 2008;8:16–20. doi: 10.1097/ACI.0b013e3282f3f488. [DOI] [PubMed] [Google Scholar]
- 10.Kreindler JL, Jackson AD, Kemp PA, Bridges RJ, Danahay H. Inhibition of chloride secretion in human bronchial epithelial cells by cigarette smoke extract. Am J Physiol Lung Cell Mol Physiol. 2005;288:L894–L902. doi: 10.1152/ajplung.00376.2004. [DOI] [PubMed] [Google Scholar]
- 11.Cohen NA, Zhang S, Sharp DB, et al. Cigarette smoke condensate inhibits transepithelial chloride transport and ciliary beat frequency. Laryngoscope. 2009 doi: 10.1002/lary.20223. [DOI] [PubMed] [Google Scholar]
- 12.MacEachran DP, Stanton BA, O'Toole GA. Cif is negatively regulated by the TetR family repressor CifR. Infect Immun. 2008;76:3197–3206. doi: 10.1128/IAI.00305-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.MacEachran DP, Ye S, Bomberger JM, et al. The Pseudomonas aeruginosa secreted protein PA2934 decreases apical membrane expression of the cystic fibrosis transmembrane conductance regulator. Infect Immun. 2007;75:3902–3912. doi: 10.1128/IAI.00338-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bebok Z, Tousson A, Schwiebert LM, Venglarik CJ. Improved oxygenation promotes CFTR maturation and trafficking in MDCK monolayers. Am J Physiol Cell Physiol. 2001;280:C135–C145. doi: 10.1152/ajpcell.2001.280.1.C135. [DOI] [PubMed] [Google Scholar]
- 15.Bebok Z, Varga K, Hicks JK, et al. Reactive oxygen nitrogen species decrease cystic fibrosis transmembrane conductance regulator expression and cAMP-mediated Cl-secretion in airway epithelia. J Biol Chem. 2002;277:43041–43049. doi: 10.1074/jbc.M203154200. [DOI] [PubMed] [Google Scholar]
- 16.Virgin F, Zhang S, Schuster D, et al. The bioflavonoid compound, sinupret, stimulates transepithelial chloride transport in vitro and in vivo. Laryngoscope. 2010;120:1051–1056. doi: 10.1002/lary.20871. [DOI] [PubMed] [Google Scholar]
- 17.Virgin FW, Azbell C, Schuster D, et al. Exposure to cigarette smoke condensate reduces calcium activated chloride channel transport in primary sinonasal epithelial cultures. Laryngoscope. 2010;120:1465–1469. doi: 10.1002/lary.20930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang S, Fortenberry JA, Cohen NA, Sorscher EJ, Woodworth BA. Comparison of vectorial ion transport in primary murine airway and human sinonasal air-liquid interface cultures, models for studies of cystic fibrosis, and other airway diseases. Am J Rhinol Allergy. 2009;23:149–152. doi: 10.2500/ajra.2009.23.3285. [DOI] [PubMed] [Google Scholar]
- 19.Woodworth BA, Tamashiro E, Bhargave G, Cohen NA, Palmer JN. An in vitro model of Pseudomonas aeruginosa biofilms on viable airway epithelial cell monolayers. Am J Rhinol. 2008;22:234–238. doi: 10.2500/ajr.2008.22.3178. [DOI] [PubMed] [Google Scholar]
- 20.Bhargave G, Woodworth BA, Xiong G, Wolfe SG, Antunes MB, Cohen NA. Transient receptor potential vanilloid type 4 channel expression in chronic rhinosinusitis. Am J Rhinol. 2008;22:7–12. doi: 10.2500/ajr.2008.22.3125. [DOI] [PubMed] [Google Scholar]
- 21.Woodworth BA, Antunes MB, Bhargave G, et al. Murine nasal septa for respiratory epithelial air-liquid interface cultures. Biotechniques. 2007;43:195–204. doi: 10.2144/000112531. [DOI] [PubMed] [Google Scholar]
- 22.Azbell C, Zhang S, Skinner D, Fortenberry J, Sorscher EJ, Woodworth BA. Hesperidin stimulates cystic fibrosis transmembrane conductance regulator-mediated chloride secretion and ciliary beat frequency in sinonasal epithelium. Otolaryngol Head Neck Surg. 2010;143:397–404. doi: 10.1016/j.otohns.2010.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Woodworth BA, Antunes MB, Bhargave G, Palmer JN, Cohen NA. Murine tracheal and nasal septal epithelium for air-liquid interface cultures: A comparative study. Am J Rhinol. 2007;21(5):533–537. doi: 10.2500/ajr.2007.21.3068. [DOI] [PubMed] [Google Scholar]
- 24.Zheng W, Kuhlicke J, Jackel K, et al. Hypoxia inducible factor-1 (HIF-1)-mediated repression of cystic fibrosis transmembrane conductance regulator (CFTR) in the intestinal epithelium. FASEB J. 2009;23:204–213. doi: 10.1096/fj.08-110221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grubb BR, Boucher RC. Pathophysiology of gene-targeted mouse models for cystic fibrosis. Physiol Rev. 1999;79:S193–S214. doi: 10.1152/physrev.1999.79.1.S193. [DOI] [PubMed] [Google Scholar]
- 26.Guimbellot JS, Fortenberry JA, Siegal GP, et al. Role of oxygen availability in CFTR expression and function. Am J Respir Cell Mol Biol. 2008;39:514–521. doi: 10.1165/rcmb.2007-0452OC. [DOI] [PMC free article] [PubMed] [Google Scholar]


