Abstract
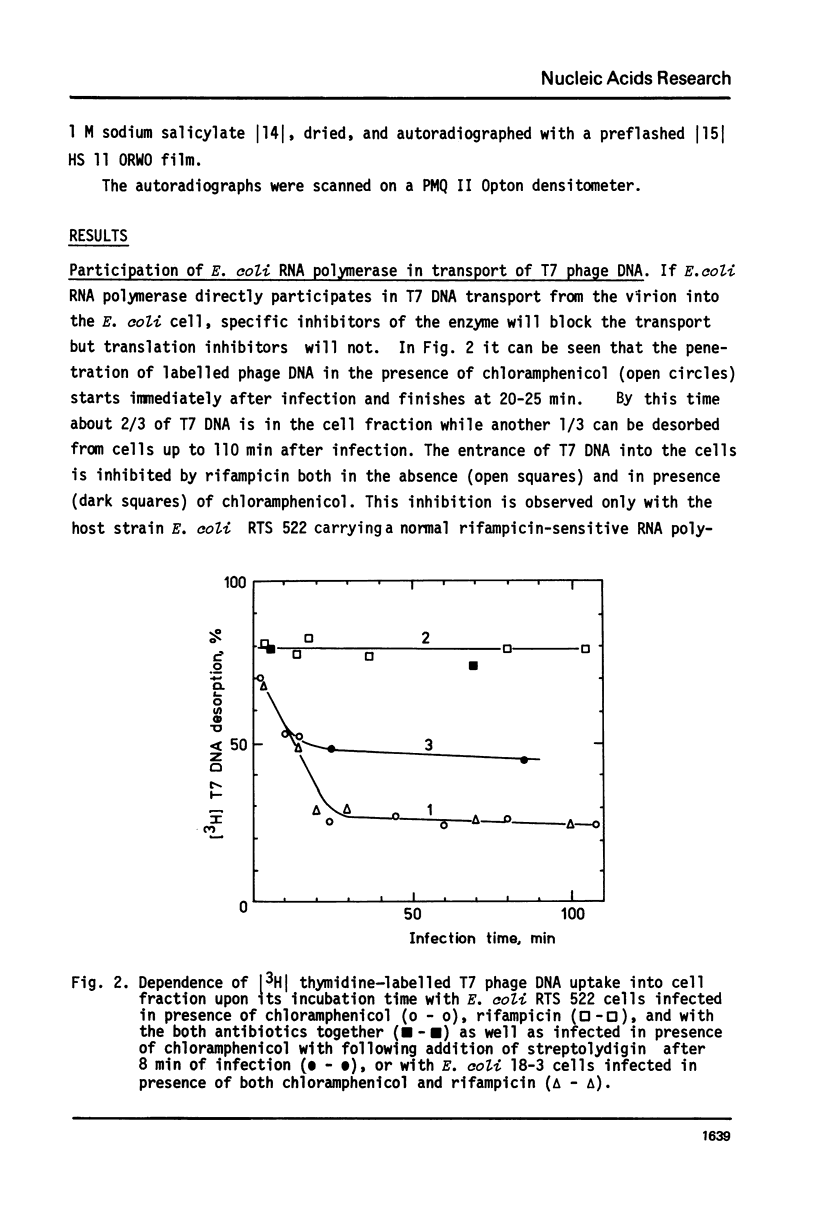
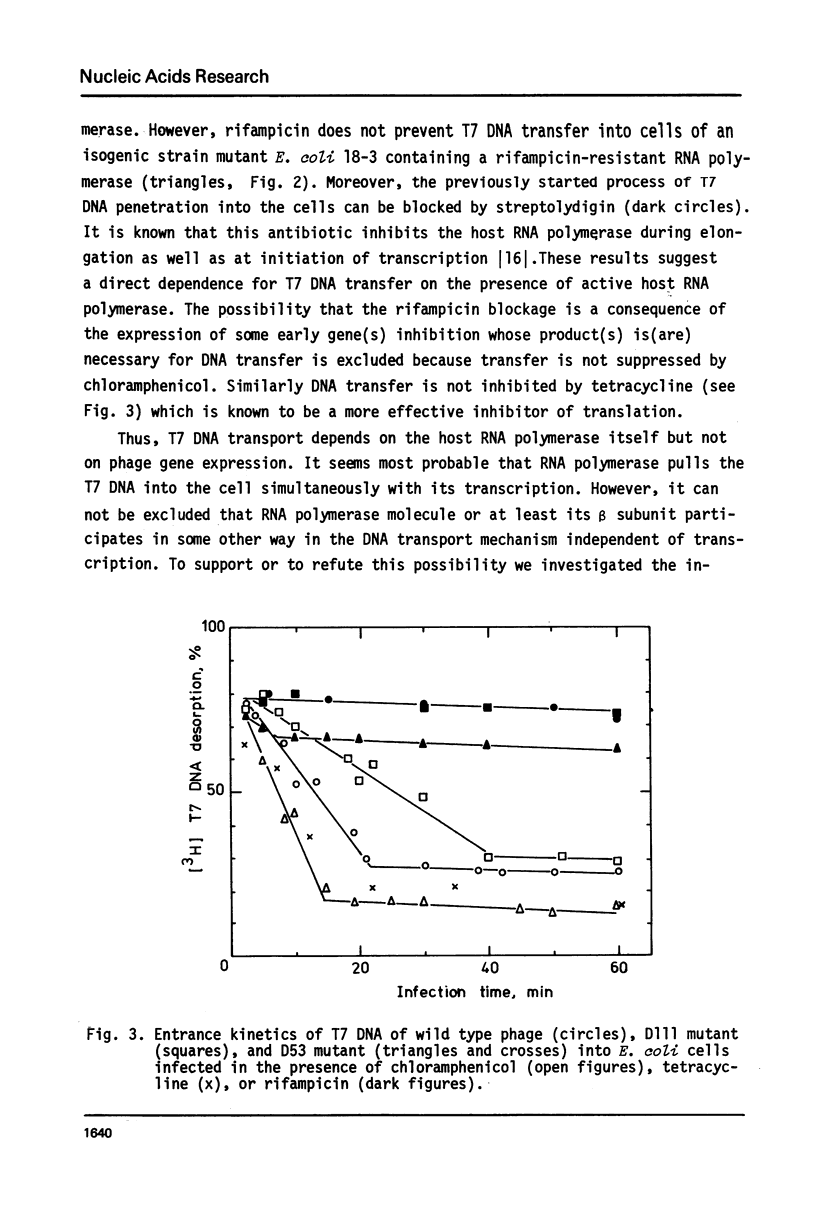
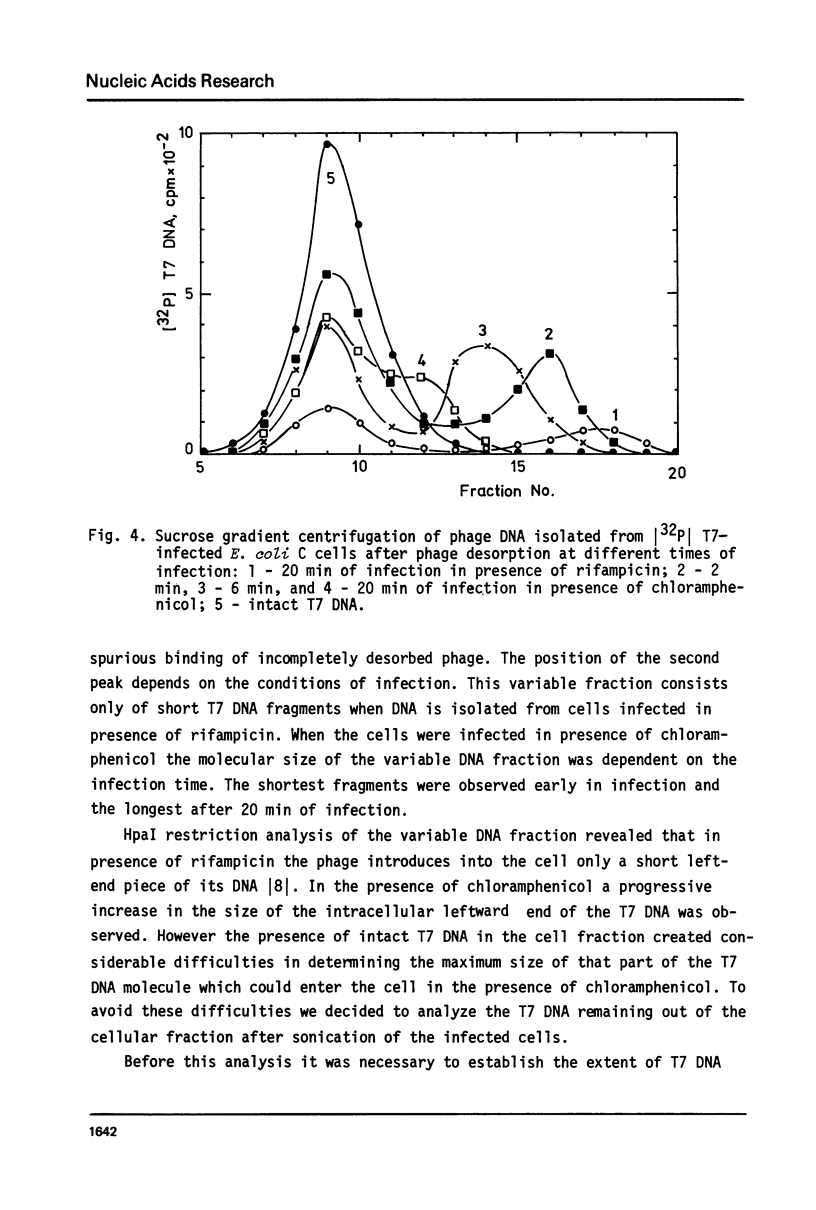
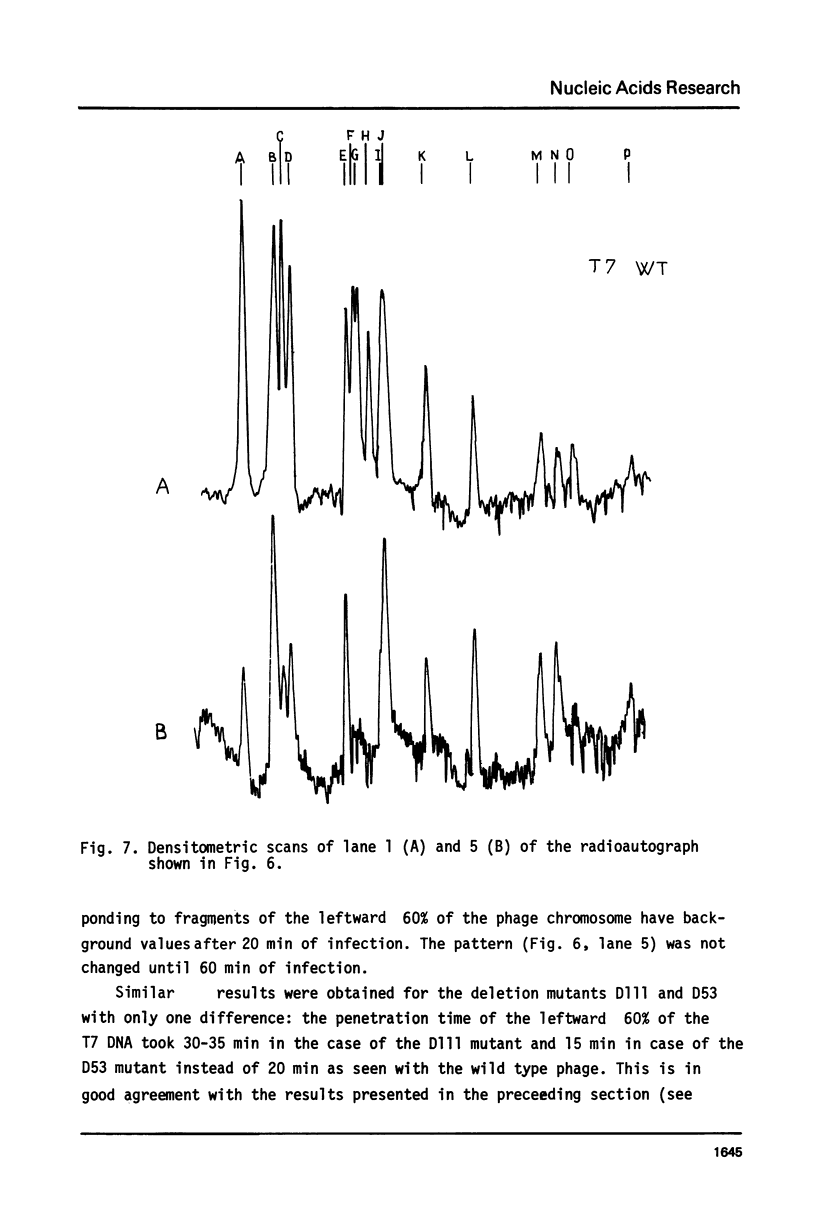
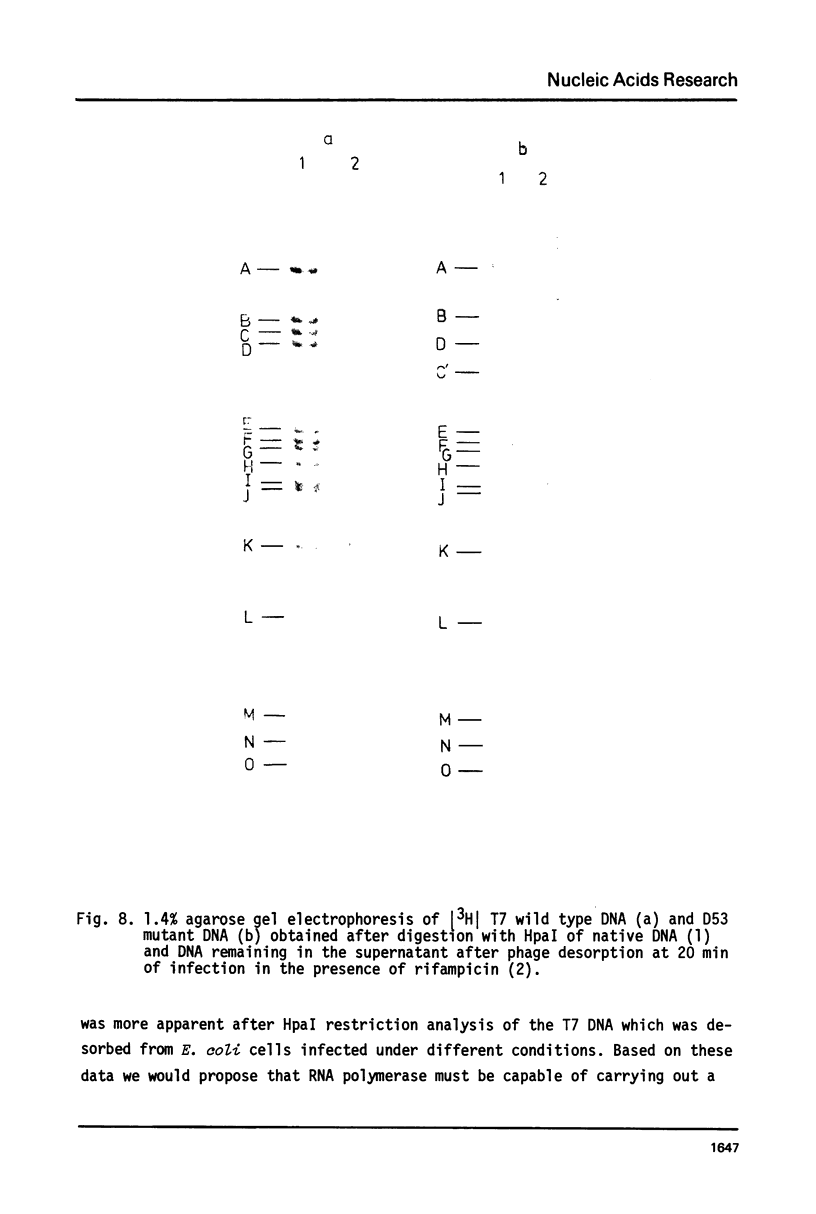
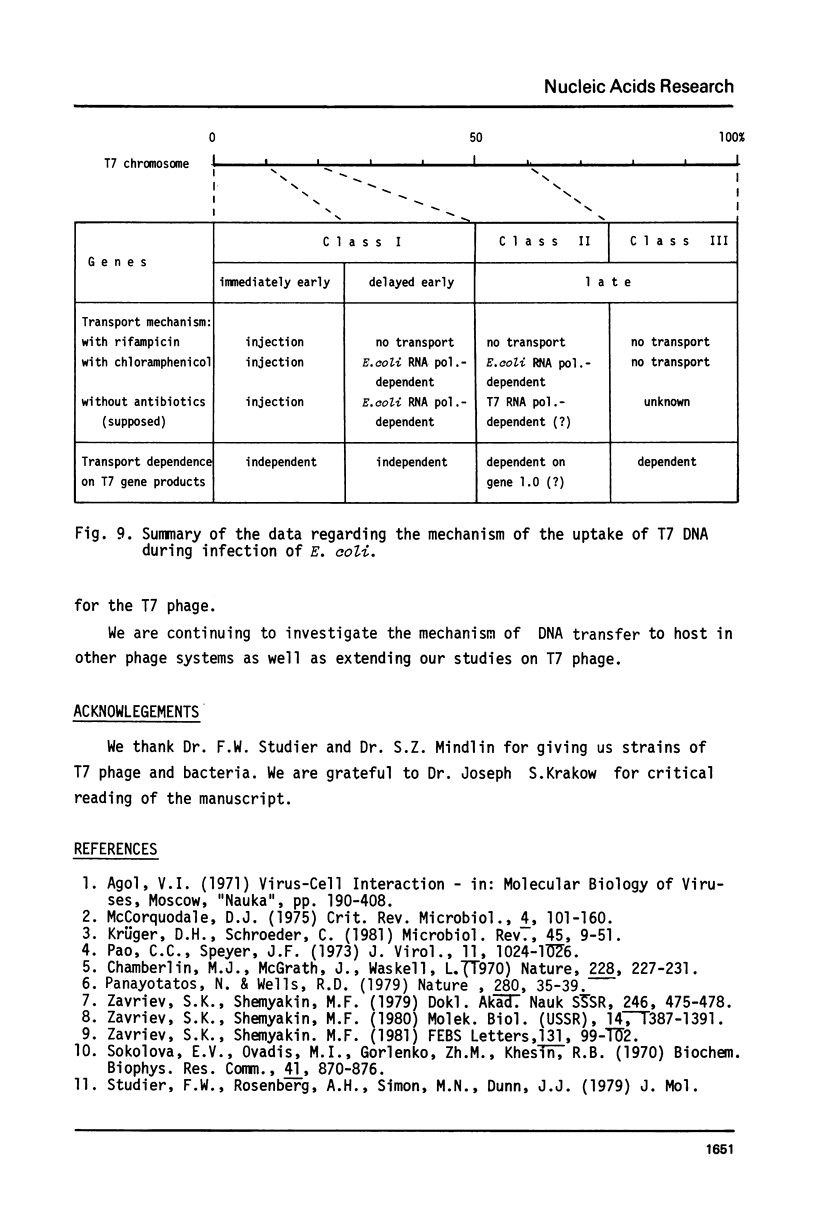
The influence of rifampicin, streptolydigin, tetracycline and chloramphenicol on phage DNA transport from the T7 virion into the E. coli cell was studied. It has been found that the DNA transport proceeds in at least three stages. During the initial stage the phage injects into the host cell the left approximately 10 per cent of its DNA molecule. The entrance of the next 50 per cent of 17 DNA molecule is blocked by inhibitors which block transcription but not translation. Moreover, the entrance time of this part of the T7 DNA increases in the case of the T7 mutant D111 (which contains a deletion of the A2 and A3 promoters) and decreases in the case of the D53 mutant (which contains a deletion in the region of the early gene transcription terminator). It would appear, that the second stage of the phage DNA transport is tightly coupled with its transcription and that a mechanical function is carried out by RNA polymerase. The translation inhibitors completely block the entrance of the remaining 40 per cent of the 17 DNA molecule (class III genes) into the host cell. It would appear that some class I and (or) II gene product(s) are obligatory components of the final stage of 17 DNA transport. Some probable consequences of this virus DNA transport model as well as its agreement with the functional structure of T7 chromosome and with T7 development are discussed.
Full text
PDF











Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chamberlain J. P. Fluorographic detection of radioactivity in polyacrylamide gels with the water-soluble fluor, sodium salicylate. Anal Biochem. 1979 Sep 15;98(1):132–135. doi: 10.1016/0003-2697(79)90716-4. [DOI] [PubMed] [Google Scholar]
- Eskin B., Lautenberger J. A., Linn S. Host-controlled modification and restriction of bacteriophage T7 by escherichia coli B. J Virol. 1973 Jun;11(6):1020–1023. doi: 10.1128/jvi.11.6.1020-1023.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman R. W., Brunovskis I., Summers W. C. A biochemical comparison of the related bacteriophages T7, phiI, phiII, W31, H, and T3. Virology. 1974 Jan;57(1):189–206. doi: 10.1016/0042-6822(74)90120-2. [DOI] [PubMed] [Google Scholar]
- Karska-Wysocki B., Mamet-Bratley M. D., Przewlocki G. DNA injection and genetic recombination of alkylated bacteriophage T7 in the presence of nalidixic acid. J Virol. 1977 Nov;24(2):716–719. doi: 10.1128/jvi.24.2.716-719.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karska-Wysocki B., Thibodeau L., Verly W. G. Inactivation of the T7 coliphage by monofunctional alkylating agents. Action of phage adsorption and injection of its DNA. Biochim Biophys Acta. 1976 Jun 18;435(2):184–191. doi: 10.1016/0005-2787(76)90249-5. [DOI] [PubMed] [Google Scholar]
- Kassavetis G. A., Chamberlin M. J. Mapping of class II promoter sites utilized in vitro by T7-specific RNA polymerase on bacteriophage T7 DNA. J Virol. 1979 Jan;29(1):196–208. doi: 10.1128/jvi.29.1.196-208.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- McAllister W. T., Wu H. L. Regulation of transcription of the late genes of bacteriophage T7. Proc Natl Acad Sci U S A. 1978 Feb;75(2):804–808. doi: 10.1073/pnas.75.2.804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCorquodale D. J. The T-odd bacteriophages. CRC Crit Rev Microbiol. 1975 Dec;4(2):101–159. doi: 10.3109/10408417509111574. [DOI] [PubMed] [Google Scholar]
- McDonell M. W., Simon M. N., Studier F. W. Analysis of restriction fragments of T7 DNA and determination of molecular weights by electrophoresis in neutral and alkaline gels. J Mol Biol. 1977 Feb 15;110(1):119–146. doi: 10.1016/s0022-2836(77)80102-2. [DOI] [PubMed] [Google Scholar]
- Panayotatos N., Wells R. D. Recognition and initiation site for four late promoters of phage T7 is a 22-base pair DNA sequence. Nature. 1979 Jul 5;280(5717):35–39. doi: 10.1038/280035a0. [DOI] [PubMed] [Google Scholar]
- Pao C. C., Speyer J. F. Order of injection of T7 bacteriophage DNA. J Virol. 1973 Jun;11(6):1024–1026. doi: 10.1128/jvi.11.6.1024-1026.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosa M. D. DNA sequence for the T7 RNA polymerase promoter for T7 RNA species II. J Mol Biol. 1981 Mar 25;147(1):199–204. doi: 10.1016/0022-2836(81)90088-7. [DOI] [PubMed] [Google Scholar]
- Rosa M. D. Four T7 RNA polymerase promoters contain an identical 23 bp sequence. Cell. 1979 Apr;16(4):815–825. doi: 10.1016/0092-8674(79)90097-7. [DOI] [PubMed] [Google Scholar]
- Schleif R. Isolation and characterization of streptolydigin resistant RNA polymerase. Nature. 1969 Sep 6;223(5210):1068–1069. doi: 10.1038/2231068a0. [DOI] [PubMed] [Google Scholar]
- Sokolova E. V., Ovadis M. I., Gorlenko Z. M., Khesin R. B. Localization of streptolydigin resistant mutation in E. coli chromosome and effect of streptolydigin on T2 phage development in stl-r and stl-s strains of E. coli. Biochem Biophys Res Commun. 1970 Nov 25;41(4):870–876. doi: 10.1016/0006-291x(70)90164-6. [DOI] [PubMed] [Google Scholar]
- Spoerel N., Herrlich P., Bickle T. A. A novel bacteriophage defence mechanism: the anti-restriction protein. Nature. 1979 Mar 1;278(5699):30–34. doi: 10.1038/278030a0. [DOI] [PubMed] [Google Scholar]
- Studier F. W. Bacteriophage T7. Science. 1972 Apr 28;176(4033):367–376. doi: 10.1126/science.176.4033.367. [DOI] [PubMed] [Google Scholar]
- Studier F. W. Gene 0.3 of bacteriophage T7 acts to overcome the DNA restriction system of the host. J Mol Biol. 1975 May 15;94(2):283–295. doi: 10.1016/0022-2836(75)90083-2. [DOI] [PubMed] [Google Scholar]
- Studier F. W. Relationships among different strains of T7 and among T7-related bacteriophages. Virology. 1979 May;95(1):70–84. doi: 10.1016/0042-6822(79)90402-1. [DOI] [PubMed] [Google Scholar]
- Transfection of restrictionless Escherichia coli by bacteriophage T7 dna: effect of in vitro erosion of DNA by gamma exonuclease. J Mol Biol. 1976 Aug 25;105(4):603–609. doi: 10.1016/0022-2836(76)90238-2. [DOI] [PubMed] [Google Scholar]
- Zavriev S. K., Belintsev B. N., Shemiakin M. F. Initsiatsiia transkriptsii: vliianie ionnoi sily i aktinomitsina D na kinetiku formirovaniia otkrytykh promotornykh kompleksov RNK-polimerazy Escherichia coli s DNK faga T7. Mol Biol (Mosk) 1979 Sep-Oct;13(5):1077–1084. [PubMed] [Google Scholar]
- Zavriev S. K., Shemiakin M. F. Blokirovanie perenosa DNK iz viriona faga T7 v kletku Escherichia coli ingibitorami bakterial'noi RNK-polimerazy. Dokl Akad Nauk SSSR. 1979;246(2):475–478. [PubMed] [Google Scholar]