Abstract
Rationale:
Systemic glucocorticosteroids (steroids) are commonly prescribed for patients with exacerbations of COPD during acute viral infections such as respiratory syncytial virus (RSV). The effects of short-term high-dose steroid treatment on viral load and adaptive immunity to RSV have not been examined in adults.
Objectives:
The objectives of this study were to measure peak viral load and duration of viral shedding, serum and nasal cytokines, RSV-specific antibody response, and lymphocyte subsets in patients admitted to the hospital with RSV infection and to compare patients treated with steroids to patients untreated with steroids.
Methods:
Hospitalized adults who tested positive for RSV by reverse transcription-polymerase chain reaction (RT-PCR) on admission had respiratory samples collected for quantitative RT-PCR and cytokine analysis. Serum and nasal secretions were tested for RSV antibody and lymphocyte subsets were analyzed by flow cytometry at 2 days, 2 weeks, and 1 month.
Main Results:
Thirty-three of 50 (66%) patients hospitalized with RSV received systemic steroids for a mean duration of 11 days. Those who received steroids more frequently wheezed and were less often febrile. There were no serious adverse events related to steroids and no significant differences in peak viral load, duration of RSV shedding, nasal cytokines, or lymphocyte subsets in patients treated with steroids and patients untreated with steroids. Antibody responses to RSV were slightly blunted in the steroid-treated group.
Conclusions:
Short courses of systemic steroids in patients hospitalized with RSV infection did not affect viral load or shedding. Humoral immunity may be mildly diminished, and thus potential benefits of systemic steroids must be balanced against potential risks.
Systemic glucocorticosteroids (steroids) are commonly prescribed in dyspneic patients with wheezing. Studies support the use of steroids for the treatment of exacerbations of COPD and acute asthma, as both conditions are increasingly recognized as inflammatory syndromes.1,2 Short courses of systemic steroids have been shown to improve spirometric and clinical outcomes in patients with COPD exacerbations, although patients with documented viral infections have not been studied.3,4 Precipitants of exacerbations of COPD and asthma include infection, environmental irritants, and allergens. Although corticosteroids reduce inflammation, long-term use has clear detrimental effects on adaptive immunity, glucose control, bone metabolism, and other organ systems.5‐8
Respiratory syncytial virus (RSV) is a common winter virus that infects persons of all ages and is associated with wheezing, especially in young children.9 In children with RSV-related bronchiolitis and wheezing, systemic corticosteroids have been studied and found to provide no significant clinical benefit.10‐12 In adults, the virus has been implicated as a cause of COPD and asthma exacerbations, yet is rarely specifically diagnosed in clinical practice.13‐15 Because wheezing is associated with the infection, systemic corticosteroids are commonly prescribed.16 However, the effects of short-term high-dose steroid treatment on viral load and adaptive immunity to a specific virus during acute infection have never been examined. Therefore, we took advantage of an ongoing study of the pathogenesis of RSV disease in adults to address these issues.
Materials and Methods
Study Design
The study was conducted during three consecutive winters between 2005 and 2008 at Rochester General Hospital in Rochester, New York. Hospitalized patients with diagnoses of upper respiratory infection, bronchitis, pneumonia, COPD, asthma, viral illness, or respiratory failure were evaluated within 48 h of admission. All participants or guardians provided informed consent. The University of Rochester Research Subjects Review Board and the Rochester General Hospital Clinical Investigation Committee approved the study.
Sample Collection
Nasal samples were obtained from all participants by rubbing the nasal turbinates for 5 s with a cotton swab. Reverse transcription polymerase chain reaction (RT-PCR) on nasal samples was performed within 24 h of collection. Study subjects who tested positive for RSV were visited daily. Expectorated sputum and endotracheal aspirated secretions were obtained if possible; however, sputum was not induced. Respiratory samples were collected daily for the first 7 days of illness and every other day thereafter until testing negative by RT-PCR on two consecutive samples. Nasal samples were also collected at days 8 to 14 and at 1 month. Whole blood and serum were collected at the time RSV was first identified, at days 8 to 14, and at 1 month after symptom onset. Peripheral blood mononuclear cells (PBMCs) were isolated from whole blood.
Illness Evaluations
Evaluations consisted of a medical history, chart review, and physical examination. Steroid use prior to admission and the timing and dose of steroid administration during hospitalization were recorded. Subjects underwent daily clinical assessments while hospitalized and were reevaluated at 1 month.
Laboratory Methods
Reverse Transcription-Polymerase Chain Reaction:
RNA was extracted into 12 μL of water from 250 μL of respiratory specimens. Sputum was diluted with an equal volume of distilled water and vortexed before extraction. RSV RNA was detected using real-time multiplex RT-PCR to identify RSV A or B infections.17 A quantitative RT-PCR for RSV A was performed using a published assay and modified for group B RSV using a different reverse primer (5′ TCCTCTATCAGTCCTTGTT).18 A standard curve was run for each assay using 10-fold dilutions of stock RSV A2 and B1 (106 plaque-forming units/mL) and cyclic threshold values for samples converted to PFU per mL equivalents.
Serum IgG and Nasal IgA Enzyme Immunoassay and Serum Neutralization Assay:
The titer of serum IgG and nasal IgA to purified RSV F and G glycoproteins was determined using established methods.19 Nasal titers were standardized to a total protein of 100 μg/mL of nasal secretion. Neutralization titers were determined by a microneutralization assay as previously published.19
Cytokine Assays
Serum:
Levels of IL-6 were measured using a commercially available enzyme immunoassay (Biosource; Camarillo, California). The range of detection for IL-6 is 0.16 to 10 pg/mL.
Nasal Samples:
IL-6, IL-8, and MIP-1α levels were measured using commercial enzyme immunoassay kits (Biosource). Cytokine results were corrected to 100 μg/mL of total protein.
Polychromatic Flow Cytometry:
Fresh PBMCs from whole blood were stained using the following antibody panel to identify T-cell subsets: FITC-CD45RO, PE-CD28, Pacific Blue-CD3, APC-CD16 (BioLegend; San Diego, California); PE-Texas Red-CD8, PE-Cy5.5-CD4 (Invitrogen; Carlsbad, California); PE-Cy7-CD56, Alexa Fluor700-CD14, APC-Cy7-CD19 (BD Biosciences; San Jose, California); and B cell subsets: FITC-IgD, PE-IgG, PE-Cy5-IgM, PE-Cy7-B220, Alexa Fluor700-CD14, APC-Cy7-CD19 (BD Biosciences); Pacific-Blue-CD38 (Sanz Laboratory; Rochester, New York); APC-CD27 (BioLegend); and PE-Cy5.5-CD3 (Invitrogen). Cells were collected on an LSRII instrument (BD Biosciences) configured to detect 11 fluorochromes. One to 2 million events were collected per sample. Analysis was performed using FlowJo software (Treestar, Inc; version 8.8.6). Total PBMC were gated on lymphocytes and monocytes using forward scatter and sideways scatter. To exclude nonspecific staining of B and T cells, CD14 and CD3 or CD14 and CD19 were used, respectively.
Statistical Methods
Differences in categorical and continuous distributions were evaluated by Fisher exact and Student t tests, respectively. Two-tailed Mann-Whitney (nonparametric) was used to compare the nonsteroid- and steroid-treated flow data.
Results
Overall, 111 subjects were identified as RSV-infected by RT-PCR and agreed to participate in the study. Of these, 50 were hospitalized and 61 were treated as outpatients. Thirty-three of 50 inpatients (66%) received at least 1 day of systemic corticosteroids compared with three of 61 (5%) outpatients. The analysis of immunologic and virologic changes associated with systemic corticosteroid use was restricted to the hospitalized cohort to provide more balanced illness severity in the comparison groups. Although a variety of steroid regimens were prescribed, most patients received 4 to 10 mg of dexamethasone or 40 to 60 mg of methylprednisolone every 6 h for 1 to 2 days followed by an oral prednisone taper. The mean duration of steroid therapy was 11.3 ± 7.3 days with a range from 1 to 27 days. Five subjects received 2 or fewer days of corticosteroid treatment. Seven subjects were taking oral corticosteroids prior to admission, and all were given IV corticosteroids or higher doses of oral prednisone treatment while hospitalized.
Predictably, the clinical features of the subjects who received corticosteroids were different from those who were not treated (Table 1). Although most differences did not achieve statistical significance, those given steroids in the hospital were more often men with a history of COPD and were receiving oral steroids and supplemental oxygen prior to admission. The presence of diabetes mellitus and coronary artery disease was slightly more common among those not treated with corticosteroids. Admitting diagnoses were similar between the two groups, although COPD exacerbation was most common among those treated with steroids, and a diagnosis of pneumonia was the leading diagnosis for those not treated. Wheezing at the time of admission was more common in the steroid group, whereas the patients not treated with steroids patients were more often febrile. Although not significantly different, there was a trend toward higher rates of infiltrates on chest radiographs and mean peripheral WBC counts in those not treated with steroids.
Table 1.
—Clinical Characteristics of Subjects With RSV Infection
| Characteristic | Steroid Treatment (n = 33) | No Steroid Treatment (n = 17) | P Value |
| Age, y | 69.8 ± 14.9 | 72.0 ± 14.8 | .62 |
| Male sex, | 23 (70) | 8 (47) | .13 |
| Medical conditions. | |||
| COPD | 19 (58) | 5 (29) | .08 |
| Asthma | 9 (27) | 4 (24) | 1.0 |
| Coronary artery disease | 8 (24) | 8 (47) | .12 |
| Congestive heart failure | 9 (27) | 4 (24) | 1.0 |
| Diabetes | 7 (21) | 8 (47) | .10 |
| Smokersa | 23 (67) | 12 (71) | 1.0 |
| Oral steroids | 7 (21) | 0 | .08 |
| Inhaled steroids | 14 (42) | 4 (24) | .23 |
| Home oxygen | 8 (24) | 2 (12) | .46 |
| Admitting diagnosis | |||
| COPD | 10 (30) | 1 (6) | .07 |
| Asthma | 3 (9) | 3 (18) | .40 |
| Pneumonia | 8 (24) | 7 (41) | .33 |
| CHF | 1 (3) | 2 (12) | .26 |
| Other | 11 (33) | 4 (24) | .53 |
| Symptoms | |||
| Cough | 31 (94) | 17 (100) | .54 |
| Sputum | 27 (82) | 16 (94) | .40 |
| Dyspnea | 32 (97) | 15 (88) | .26 |
| Physical findings | |||
| Temperature | 36.9 ± 0.9 | 37.7 ± 1.0 | .006 |
| Respiratory rate | 22.8 ± 6.6 | 25.7 ± 7.5 | .17 |
| Oxygen saturation | 91.1 ± 8.1 | 93.9 ± 5.1 | .20 |
| Wheezing | 31 (94) | 9 (53) | .001 |
| Laboratory | |||
| Chest radiograph infiltrate, | 5 (15) | 4 (24) | .47 |
| WBC | 9.4 ± 5.4 | 11.9 ± 6.9 | .17 |
Data are shown as No. (%) or mean ± SD. RSV = respiratory syncytial virus.
Past or active smokers.
Overall, the hospital course for patients who received corticosteroids was similar to those who were not treated (Table 2). Rates of respiratory failure, intensive care admission, and in-hospital mortality were not significantly different. Two patients in the steroid-treated group died, and in both cases patients were placed on hospice care because of longstanding severe COPD. No patients developed nosocomial bacterial infections, and there were no overt complications related to steroid treatment, such as psychosis or severe glucose dysregulation. Although the mean last days of reported wheezing or dyspnea were significantly longer in the steroid-treated group compared with those not treated, the length of hospital stay and illness duration were not significantly different.
Table 2.
—Hospital Course of Steroid-Treated and Untreated Subjects With RSV Infection
| Hospital Course | Steroid Treatment N = 33 | No Steroid Treatment N = 17 | P Value |
| Intensive care | 6 (18) | 5 (29) | .48 |
| Respiratory failure | 5 (15) | 2 (12) | 1.0 |
| Mean high serum glucose | 218 ± 113 | 202 ± 126 | .65 |
| Last day of wheezing | 12.2 ± 6.1 | 8.2 ± 4.8 | .02 |
| Last day of dyspnea | 15.6 ± 10.0 | 9.4 ± 5.3 | .02 |
| Oxygenation < 95% on room air at day 28 | 13 (39) | 3 (18) | .20 |
| Length of hospitalization | 6.2 ± 5.6 | 5.9 ± 3.2 | .84 |
| Length of illness | 26.0 ± 10.1 | 22.7 ± 8.6 | .26 |
| Death | 2 (6) | 0 | .54 |
Data are presented as No. (%) or mean ± SD. See Table 1 for expansion of abbreviation.
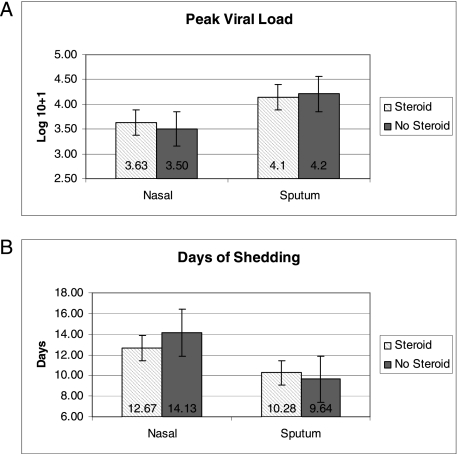
Group A and B viruses were evenly distributed in those treated with steroids vs those who were not treated. Viral shedding was not prolonged, nor was the mean viral load higher in the steroid-treated group (Figs 1A, 1B). Inflammatory mediators were measured in nasal samples at enrollment, day 12, and day 28 and were not consistently different in either group (Table 3). There was a trend toward lower acute serum IL-6 levels in patients treated with steroids compared with those not treated (5.4 ± 8.5 vs 17.2 ± 34.4, P = .08). Of note, blood samples were collected on average 2 days after steroids were begun. By day 28, serum IL-6 levels were similar in both groups.
Figure 1.
A, Comparison of mean peak nasal viral load of patients who received steroids (cross-hatched bar) to patients who were not treated with steroids (solid bar). SE bars are shown and viral load is expressed as a log10 value + 1. B, Comparison of mean duration of shedding of respiratory syncytial virus (RSV) among patients who received steroids (cross-hatched bar) to patients who were not treated with steroids (solid bar). SE bars are shown and values are expressed in days.
Table 3.
—Nasal and Serum Cytokines
| Time of Measure | No. | Steroid Treatment | No. | No Steroid Treatment | P Value |
| Nasal | |||||
| IL-6 | |||||
| Acute | 33 | 7.4 ± 8.3 | 17 | 8.0 ± 6.8 | .80 |
| Day 12 | 33 | 5.9 ± 5.9 | 16 | 3.6 ± 3.4 | .15 |
| Day 28 | 30 | 6.11 ± 4.6 | 16 | 4.9 ± 4.3 | .37 |
| IL-8 | |||||
| Acute | 33 | 288.7 ± 242.4 | 17 | 413.4 ± 264.5 | .10 |
| Day 12 | 33 | 368.6 ± 392.0 | 16 | 324.3 ± 250.1 | .68 |
| Day 28 | 30 | 265.0 ± 240.0 | 16 | 334.1 ± 315.9 | .41 |
| MIP-1α | |||||
| Acute | 33 | 31.4 ± 45.2 | 17 | 34.0 ± 31.1 | .83 |
| Day 12 | 33 | 18.6 ± 27.0 | 15 | 13.2 ± 13.0 | .45 |
| Day 28 | 30 | 5.3 ± 6.8 | 15 | 7.2 ± 9.8 | .46 |
| Serum | |||||
| Acute IL-6 | 31 | 5.4 ± 8.5 | 15 | 17.16 ± 34.4 | .08 |
| 28 d IL-6 | 29 | 7.0 ± 12.3 | 15 | 8.0 ± 20.7 | .84 |
MIP = macrophage inflammatory protein.
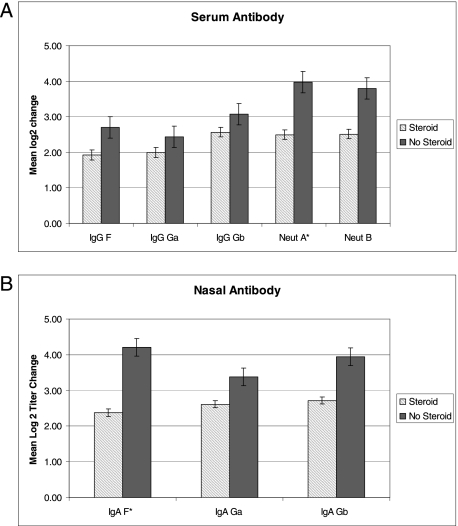
RSV-specific serum and nasal antibody titers measured at the time of enrollment were not significantly different between groups. Serum and nasal antibody responses to RSV infection were slightly blunted in the steroid-treated group (Figs 2A, 2B) with convalescent titers approximately twofold lower compared with the untreated group. However, only serum-neutralizing antibody responses to group A virus (P = .05) and nasal IgA to the fusion protein (P = .01) were significantly lower.
Figure 2.
A, Mean increase in serum antibody titers from acute to day 28 samples from patients who received steroids (cross-hatched bar) and patients who were not treated with steroids (solid bar) are shown. Change in antibody titers are expressed in log2 titers with SE bars shown. Serum IgG to RSV fusion (F) and attachment proteins for A strain (Ga) and B strain (Gb) and microneutralization titers to A strain (Neut A) and B strain (Neut B) are shown. *Indicates significant difference between steroid and nonsteroid group. B, Mean increase in nasal antibody titers from acute to day 28 samples from patients who received steroids (cross-hatched bar) and patients who were not treated with steroids (solid bar) are shown. Change in antibody titers are expressed in log2 titers with SE bars shown. Nasal IgA to RSV fusion (F) and attachment proteins for A strain (Ga) are shown. *Indicates significant difference between steroid and nonsteroid group. See Figure 1 for expansion of abbreviation.
Twenty-nine randomly selected subjects (16 steroid treated and 13 untreated) had T and B lymphocyte subpopulations analyzed at three time points: acute (∼ 36-48 h after initiating steroids), day 12 to 14, and day 28 post illness. Admission absolute lymphocyte counts (ALCs) prior to steroid administration were similar (1.1 ± 0.5 × 103/μL vs 1.2 ± 0.7 × 103/μL) in the steroid-treated and -untreated groups, respectively. Notably, 31% of the steroid-treated group (of whom two were receiving steroids prior to admission) and 46% of the untreated group had an ALC of < 1.0 × 103/μL at presentation to the hospital. For those receiving steroids who had repeat measurements, the mean ALC was 0.8 ± 0.5 × 103/μL at 24 h, 1.2 ± 0.7 × 103/μL between days 2 and 6, and 1.2 ± 0.4 × 103/μL after day 9.
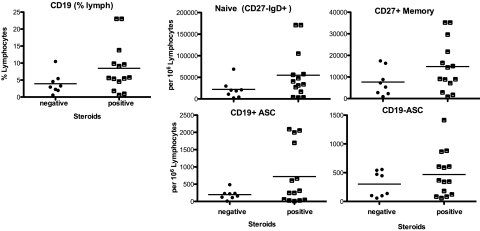
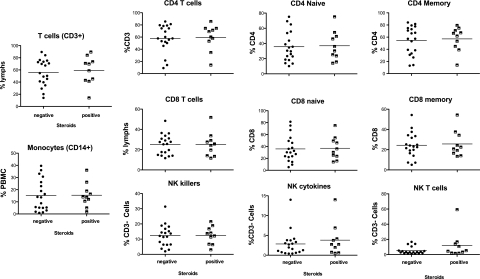
Representative flow cytometry results are shown in Figures 3 and 4 for a random subset of 35 and 23 patients for T- and B-cell panels, respectively, at the mid time point (day 8-14). Analysis of early and late testing demonstrated similar findings in B- and T-cell subsets (data not shown). A representative sample of B- and T-cell subsets is shown in e-Figure 1A and 1B. No significant differences between steroid-treated and steroid-untreated subjects at any of the time points were noted regarding the total percentage of B cells (% CD19+), CD27+ memory B cells, antibody secreting cells (ASC), or plasmablasts (Fig 3). Additionally, there were no significant differences in the fraction of CD3+ T cells, monocytes, CD4 and CD8 T cells (naive and memory), and natural killer cells (subsets of CD56+CD16hi and CD56hi) (Fig 4).
Figure 3.
Flow cytometry for B-cell subpopulations for 23 subjects. Patients not treated with steroids are referred to as negative, and patients treated with steroids as positive. Flow cytometry data at the midpoint (days 8-14) are shown in the graph. The median value of the group is indicated by the bar. ASC = antibody-secreting cell.
Figure 4.
Flow cytometry data gated for monocytes, NK cells, and T-cell subsets for 35 subjects. Patients not treated with steroids are referred to as negative, and patients treated with steroids as positive. Flow cytometry data at the midpoint (days 8-14) are shown in the graph. The median value of the group is indicated by the bar. NK = natural killer.
Discussion
In our study of patients hospitalized with RSV-related respiratory illness, short-term systemic corticosteroids were not associated with significant adverse clinical or virologic outcomes. No episodes of severe hyperglycemia, avascular bone necrosis, or steroid-induced psychosis were observed during the hospitalization or 1-month follow-up period. However, it is important to note that our study was not powered to detect important clinical steroid-related adverse events. Given the importance of cellular immunity for viral clearance and the recognized effects of long-term steroid use, we were surprised that peak RSV viral loads and duration of viral shedding were not increased in the steroid-treated group.20 This finding may reflect the relatively brief course of systemic steroids received by study participants. Although steroids exert an antiinflammatory effect, no uniform trend was detected in serum or nasal cytokine levels in patients receiving steroids.
The complex quantitative and qualitative effects of systemic glucocorticosteroids on the host immune response are well described.21 Nearly every type of immune cell, including B and T lymphocytes, neutrophils, and monocytes, are affected. A substantial reduction in circulating lymphocytes can be demonstrated almost immediately upon injection of hydrocortisone in animal studies, with no further effect of increased dose or duration.22 Lymphopenia is reversible but lasts throughout the period of exposure. Steroids also inhibit T-cell activation, lymphocyte proliferation, and T helper 1/T helper 2 regulation.21 Given the known effects of steroids on the immune system, the lack of significant differences in lymphocyte numbers and subsets in our steroid-treated subjects is puzzling. However, very few data are available on the immune affects of steroids in acutely ill patients, particularly those with viral infections. Lymphopenia is associated with a number of acute viral infections, such as influenza and severe acute respiratory syndrome coronavirus.23,24 It is noteworthy that many of the patients in this study were lymphopenic at the time of presentation, and thus it is possible that RSV infection induces significant immunologic perturbations that may complicate interpretation of a steroid effect.
The primary adverse effect of steroid use appeared to be a modestly diminished serum and secretory antibody response. Although differences were only statistically significant in serum-neutralizing titers against group A virus and nasal antibody to the F protein, it is likely that a larger sample size would have yielded significant differences in other parameters as well. High levels of serum and nasal RSV antibody have been shown to be protective from infection and severe disease in adults, although a specific protective antibody titer is not known.19,25 Therefore, lower postinfection titers may leave steroid-treated patients more susceptible to future RSV exposures. However, the absolute differences in postinfection antibody titers we observed were relatively small, making the clinical implications of these findings uncertain. The diminished antibody response in the steroid group was not explained by a significant reduction in the number of B lymphocytes, ASCs, or CD4+ T-cell lymphocytes. However, it is possible that function or activation was impaired despite no discernible difference in cell numbers or subsets. In addition, we did not measure RSV-specific T-cell responses or ASC, which may have been preferentially affected by steroid administration.
Our study is limited by the small sample size, the lack of randomization, and the restriction to one type of viral infection. Because steroids were more commonly used in patients with COPD, which is an inflammatory condition, the effects of steroids on inflammatory markers and immune function may have been difficult to discern given the unequal distribution of patients with COPD in the analysis groups. Although it is reasonable to presume steroid effects would be similar for other respiratory viruses, RSV has unique immunomodulatory effects, and, therefore, our findings may not be broadly applicable.26‐28
In conclusion, a short course of systemic corticosteroids in patients hospitalized with acute RSV infection was not associated with major deleterious side effects. Since specific respiratory viral infections, with the exception of influenza, are rarely diagnosed in adults, and steroids are commonly prescribed for acute exacerbations of COPD, these data are reassuring. A modestly diminished humoral response may predispose patients to future RSV infections, but this risk must be balanced with the immediate antiinflammatory effects and potential clinical benefits.
Supplementary Material
Acknowledgments
Author contributions: Dr Lee: contributed to acquisition of data, data analysis, and preparation of the manuscript.
Dr Walsh: contributed to conception, design of study, acquisition of data, and revision of the manuscript.
Dr Falsey: contributed to conception, design of study, acquisition of data, and preparation of the manuscript.
Financial/nonfinancial disclosures: The authors have reported to CHEST the following conflicts of interest: Dr Falsey has received research grants from Sanofi Pasteur and GlaxoSmithKline, and has served on advisory boards for Medimmune and Sanofi Pasteur. Dr Walsh has received research grants from Sanofi Pasteur and has served on advisory boards for Alnylam. Dr Lee has reported to CHEST that no potential conflicts of interest exist with any companies/organizations whose products or services may be discussed in this article.
Role of sponsors: The sponsor had no role in the design of the study, the collection and analysis of the data, or in the preparation of the manuscript.
Other contributions: We thank Patricia Hennessey, RN, and Mary Criddle, RN, for patient evaluation and specimen collection; Mary Anne Formica, MS; Jamie Biear, MPH; Gloria Andolina, BS; Jessica Halliley, BS; Aja Kalkanoglu, BS; and Megan Lumb, BS, for technical assistance; Hongyu Miao, PhD, for his biostatistical consultation; and Sarah Korones, BA, for chart review.
Additional information: The e-Figure can be found in the Online Supplement at http://chestjournal.chestpubs.org/content/140/5/1155/DC1.
Abbreviations
- ALC
absolute lymphocyte count
- ASC
antibody secreting cell
- PBMC
peripheral blood mononuclear cell
- RSV
respiratory syncytial virus
- RT-PCR
reverse transcription polymerase chain reaction
Footnotes
Funding/Support: This study was funded by the National Institutes of Health-National Institute of Allergy and Infectious Disease [Grant 2UO1 AI045969-05A1].
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians (http://www.chestpubs.org/site/misc/reprints.xhtml).
References
- 1.Pauwels RA, Buist AS, Calverley PM, Jenkins CR, Hurd SS. GOLD Scientific Committee Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. NHLBI/WHO Global Initiative for Chronic Obstructive Lung Disease (GOLD) Workshop summary. Am J Respir Crit Care Med. 2001;163(5):1256–1276. doi: 10.1164/ajrccm.163.5.2101039. [DOI] [PubMed] [Google Scholar]
- 2.Rodrigo GJ, Rodrigo C, Hall JB. Acute asthma in adults: a review. Chest. 2004;125(3):1081–1102. doi: 10.1378/chest.125.3.1081. [DOI] [PubMed] [Google Scholar]
- 3.Niewoehner DE, Erbland ML, Deupree RH, et al. Department of Veterans Affairs Cooperative Study Group Effect of systemic glucocorticoids on exacerbations of chronic obstructive pulmonary disease. N Engl J Med. 1999;340(25):1941–1947. doi: 10.1056/NEJM199906243402502. [DOI] [PubMed] [Google Scholar]
- 4.Singh JM, Palda VA, Stanbrook MB, Chapman KR. Corticosteroid therapy for patients with acute exacerbations of chronic obstructive pulmonary disease: a systematic review. Arch Intern Med. 2002;162(22):2527–2536. doi: 10.1001/archinte.162.22.2527. [DOI] [PubMed] [Google Scholar]
- 5.Anderton JM, Helm R. Multiple joint osteonecrosis following short-term steroid therapy. Case report. J Bone Joint Surg Am. 1982;64(1):139–141. [PubMed] [Google Scholar]
- 6.Niewoehner CB, Niewoehner DE. Steroid-induced osteoporosis. Are your asthmatic patients at risk? Postgrad Med. 1999;105(3):79–83. doi: 10.3810/pgm.1999.03.598. [DOI] [PubMed] [Google Scholar]
- 7.Niewoehner DE. Systemic corticosteroids for chronic obstructive pulmonary disease: benefits and risks. Monaldi Arch Chest Dis. 1999;54(5):422–426. [PubMed] [Google Scholar]
- 8.Smyllie HC, Connolly CK. Incidence of serious complications of corticosteroid therapy in respiratory disease. A retrospective survey of patients in the Brompton hospital. Thorax. 1968;23(6):571–581. doi: 10.1136/thx.23.6.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hall CB. Respiratory syncytial virus and parainfluenza virus. N Engl J Med. 2001;344(25):1917–1928. doi: 10.1056/NEJM200106213442507. [DOI] [PubMed] [Google Scholar]
- 10.Garrison MM, Christakis DA, Harvey E, Cummings P, Davis RL. Systemic corticosteroids in infant bronchiolitis: a meta-analysis. Pediatrics. 2000;105(4):E44. doi: 10.1542/peds.105.4.e44. [DOI] [PubMed] [Google Scholar]
- 11.De Boeck K, Van der Aa N, Van Lierde S, Corbeel L, Eeckels R. Respiratory syncytial virus bronchiolitis: a double-blind dexamethasone efficacy study. J Pediatr. 1997;131(6):919–921. doi: 10.1016/s0022-3476(97)70044-1. [DOI] [PubMed] [Google Scholar]
- 12.van Woensel JB, Wolfs TF, van Aalderen WM, Brand PL, Kimpen JL. Randomised double blind placebo controlled trial of prednisolone in children admitted to hospital with respiratory syncytial virus bronchiolitis. Thorax. 1997;52(7):634–637. doi: 10.1136/thx.52.7.634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De Serres G, Lampron N, La Forge J, et al. Importance of viral and bacterial infections in chronic obstructive pulmonary disease exacerbations. J Clin Virol. 2009;46(2):129–133. doi: 10.1016/j.jcv.2009.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jacoby DB. Virus-induced asthma attacks. JAMA. 2002;287(6):755–761. doi: 10.1001/jama.287.6.755. [DOI] [PubMed] [Google Scholar]
- 15.Proud D, Chow CW. Role of viral infections in asthma and chronic obstructive pulmonary disease. Am J Respir Cell Mol Biol. 2006;35(5):513–518. doi: 10.1165/rcmb.2006-0199TR. [DOI] [PubMed] [Google Scholar]
- 16.Falsey AR, Hennessey PA, Formica MA, Cox C, Walsh EE. Respiratory syncytial virus infection in elderly and high-risk adults. N Engl J Med. 2005;352(17):1749–1759. doi: 10.1056/NEJMoa043951. [DOI] [PubMed] [Google Scholar]
- 17.Duncan CB, Walsh EE, Peterson DR, Lee FE, Falsey AR. Risk factors for respiratory failure associated with respiratory syncytial virus infection in adults. J Infect Dis. 2009;200(8):1242–1246. doi: 10.1086/605948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Falsey AR, Formica MA, Treanor JJ, Walsh EE. Comparison of quantitative reverse transcription-PCR to viral culture for assessment of respiratory syncytial virus shedding. J Clin Microbiol. 2003;41(9):4160–4165. doi: 10.1128/JCM.41.9.4160-4165.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Walsh EE, Falsey AR. Humoral and mucosal immunity in protection from natural respiratory syncytial virus infection in adults. J Infect Dis. 2004;190(2):373–378. doi: 10.1086/421524. [DOI] [PubMed] [Google Scholar]
- 20.Hall CB, Powell KR, MacDonald NE, et al. Respiratory syncytial viral infection in children with compromised immune function. N Engl J Med. 1986;315(2):77–81. doi: 10.1056/NEJM198607103150201. [DOI] [PubMed] [Google Scholar]
- 21.Lionakis MS, Kontoyiannis DP. Glucocorticoids and invasive fungal infections. Lancet. 2003;362(9398):1828–1838. doi: 10.1016/S0140-6736(03)14904-5. [DOI] [PubMed] [Google Scholar]
- 22.Balow JE, Hurley DL, Fauci AS. Immunosuppressive effects of glucocorticosteroids: differential effects of acute vs chronic administration on cell-mediated immunity. J Immunol. 1975;114(3):1072–1076. [PubMed] [Google Scholar]
- 23.Cunha BA, Pherez FM, Schoch P. Diagnostic importance of relative lymphopenia as a marker of swine influenza (H1N1) in adults. Clin Infect Dis. 2009;49(9):1454–1456. doi: 10.1086/644496. [DOI] [PubMed] [Google Scholar]
- 24.Peiris JS, Chu CM, Cheng VC, et al. HKU/UCH SARS Study Group Clinical progression and viral load in a community outbreak of coronavirus-associated SARS pneumonia: a prospective study. Lancet. 2003;361(9371):1767–1772. doi: 10.1016/S0140-6736(03)13412-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Walsh EE, Peterson DR, Falsey AR. Risk factors for severe respiratory syncytial virus infection in elderly persons. J Infect Dis. 2004;189(2):233–238. doi: 10.1086/380907. [DOI] [PubMed] [Google Scholar]
- 26.Tripp RA, Jones LP, Haynes LM, Zheng H, Murphy PM, Anderson LJ. CX3C chemokine mimicry by respiratory syncytial virus G glycoprotein. Nat Immunol. 2001;2(8):732–738. doi: 10.1038/90675. [DOI] [PubMed] [Google Scholar]
- 27.Chi B, Dickensheets HL, Spann KM, et al. Alpha and lambda interferon together mediate suppression of CD4 T cells induced by respiratory syncytial virus. J Virol. 2006;80(10):5032–5040. doi: 10.1128/JVI.80.10.5032-5040.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bossert B, Marozin S, Conzelmann KK. Nonstructural proteins NS1 and NS2 of bovine respiratory syncytial virus block activation of interferon regulatory factor 3. J Virol. 2003;77(16):8661–8668. doi: 10.1128/JVI.77.16.8661-8668.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.