Abstract
A continuously increasing body of knowledge shows that the brain is an extremely complex neural network and single neurons possess their own complicated interactive signaling pathways. Such complexity of the nervous system makes it increasingly difficult to investigate the functions of specific neural components such as genes, proteins, transcription factors, neurons and nuclei in the brain. Technically, it has been even more of a significant challenge to identify the molecular and cellular adaptations that are both sufficient and necessary to underlie behavioral functions in health and disease states. Defining such neural adaptations is a critical step to identify the potential therapeutic targets within the complex neural network that are beneficial to treat psychiatric disorders. Recently, the newly development and extensive application of in vivo viral-mediated gene transfer (virogenetics) and optical manipulation of specific neurons or selective neural circuits in freely-moving animals (optogenetics) make it feasible, through loss- and gain-of-function approaches, to reliably define sufficient and necessary neuroadaptations in the behavioral models of psychiatric disorders, including drug addiction, depression, anxiety and bipolar disorders. In this article, we focus on recent studies that successfully employ these advanced virogenetic and optogenetic techniques as a powerful tool to identify potential targets in the brain, and to provide highly useful information in the development of novel therapeutic strategies for psychiatric disorders.
Keywords: Neural target, Target defining, Viral-mediated gene transfer, Virogenetics, Optogenetics, Depression, Anxiety, Drug addiction, Bipolar disorder, Neurophysiology
1. Introduction
Prolonged environmental stimulation such as chronic stress, a major cause of major depressive disorder, can induce responsive or adaptive changes in a vast amount of genes and cellular functions of the brain (Herman et al., 2008; Hyman and Malenka, 2001; Krishnan et al., 2007; Nestler, 2001; Tsankova et al., 2007). One major challenge in neuropsychiatry is to define the molecular and cellular adaptations, among the huge array of induced changes, that are both sufficient and necessary to underlie the environmental stimulation-induced behavioral abnormalities (disease conditions). To identify these neuroadaptations, a suitable research strategy is essential. Here we introduce a “systematic research strategy”: the research starts with behavioral phenotypes observed in rodent models of psychiatric disorders, and is followed by the studies of neural circuitry, cellular and even molecular (channels and receptors) adaptations in the brain; and importantly, to further define these neural adaptations as therapeutic targets, virogenetics and optogenetics are used to precisely and reliably imitate these molecular or cellular adaptations, and behavioral outcomes of these imitations are retested in freely behaving animals (Fig. 1). Virogenetics is a viral-mediated gene transfer technique, in which modified DNA is packed into a viral vector, and the vector act as a vehicle to deliver the DNA into cell nucleus and produce a new protein using the delivered gene (Fig. 2); optogenetics is a technique of delivering light-sensitive channels such as channelrhodopsin-2 (ChR2), halorhodopsin (NpHR) or other proteins into neurons and regulating their activity (Fig. 3). In this research strategy model, in vivo virogenetics is used to virally transfer genes in alive animals through stereotaxic surgery (Fig. 4); and optogenetic techniques is employed to optically control cell type- or circuit-specific neurons in freely-moving animals (Fig. 5). These advanced techniques have a unique ability to probe specific molecules, neurons and circuit pathways with space and temporal precision in behaving animals. Therefore, virogenetics and optogenetics are increasingly and extensively used in the neuroscience field and play a crucial role in defining the molecular and cellular adaptations that underlie behavioral functions. The rapidly accumulating functional knowledge of specific brain components provided by these novel techniques has never been achieved with conventional pharmacology (for a technical comparison, see Table 1). Recent studies have highlighted the importance of virogenetic and optogenetic approaches in the systematic research strategy for identifying potential therapeutic targets within the complex brain. In this review, we first explain the importance of focusing on neuronal electrical activity, and then, describe and discuss the potential therapeutic targets that are related to neuronal activity and identified through the use of virogenetic and optogenetic approaches in different models of psychiatric disorders.
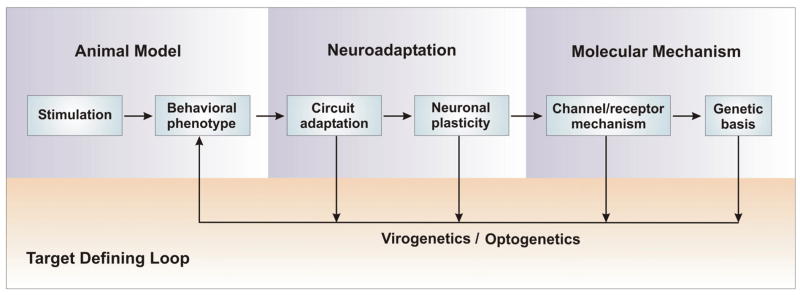
Fig. 1. Systematic research strategy for defining potential therapeutic targets.
Strong environmental stimulation such as chronic stress and addictive drugs can induce behavioral dysfunctions, which can be mediated by neuroadaptations at the levels of neural circuitry and neuronal plasticity. The underlying molecular mechanisms such as ionic, receptor and genetic basis for the neuroadaptations are potential therapeutic targets. An up-down-up research strategy model can be used to define these potential targets from a vast amount of prolonged stimulation-induced adaptive changes. A key component to this strategy is the Target Defining Loop, which links various neuroadaptations at different levels back to behavioral endpoints by utilizing in vivo viral-mediated gene transfer (virogenetics) and optical manipulations of specific neurons or neural circuits in freely moving animals (optogenetics). Through the gain- or loss-of-function, the sufficient and necessary molecular and cellular adaptations for the behavioral abnormalities are defined as potential therapeutic targets.
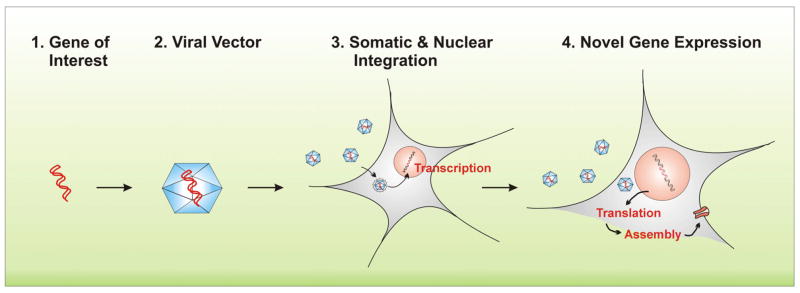
Fig. 2. Viral-mediated gene transfer.
1. Cloning and isolating gene of interest. 2. Viral transfection with the ideal viral vector based on genome size, infection/tropism, host genome interaction, transgene expression and packaging capacity. Viral vector options include adenovirus, adeno-associated virus, retrovirus, herpes simplex viruses, vaccinia virus and lentivirus vehicles. Each differs on how well they transfer genes to the cells they recognize and are able to infect, and whether they alter the cell’s DNA permanently or temporarily. 3. Binding of virus with cell surface receptors. Cell-bound virions are internalized via clathrin-dependent endocytotic pathway and imported to the nucleus, where transcription and replication of the vRNA/DNA molecules occur. 4. Translation of viral mRNA molecules takes place in the cytoplasm. Produced proteins and complexes are finally assembled at the plasma membrane.
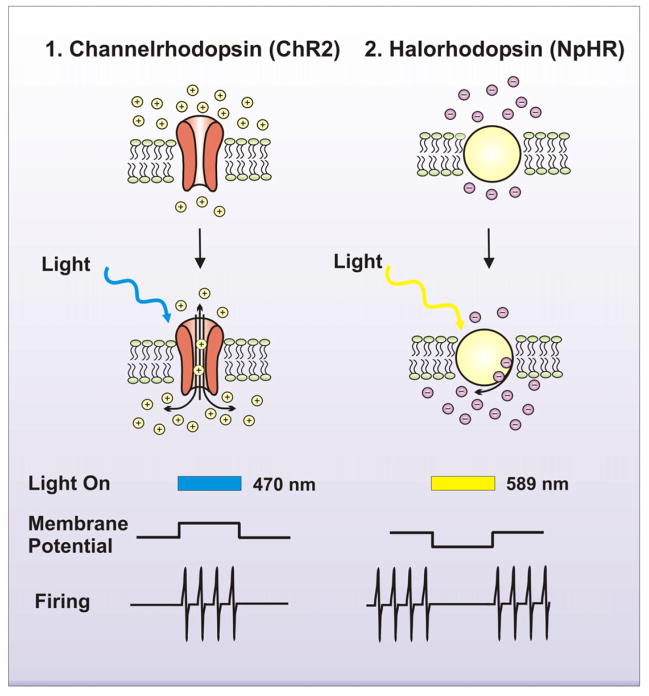
Fig. 3. Light sensitive channels and their functions.
Fast light activated channels and enzymes allow for temporally precise manipulation of electrical and biochemical events and are used as tools to investigate the function of multiple neural systems. Specifically, 1. Channelrhodopsin (ChR2), a light activated proton channel (as well as ChR1, VChR1, and SFOs), excites neurons to investigate gain of function by depolarizing the membrane and eliciting firing activity. 2. Halorhodopsin, a chloride ion pump (NpHR, eNpHR2.0 and eNpHR3.0), inhibits neurons to investigate loss of function by hyperpolarizing the membrane and inhibiting firing activity. Alternatively, specific G-protein coupled receptors can be fused with opsins to manipulate cAMP, GTPases and IP3 optically.
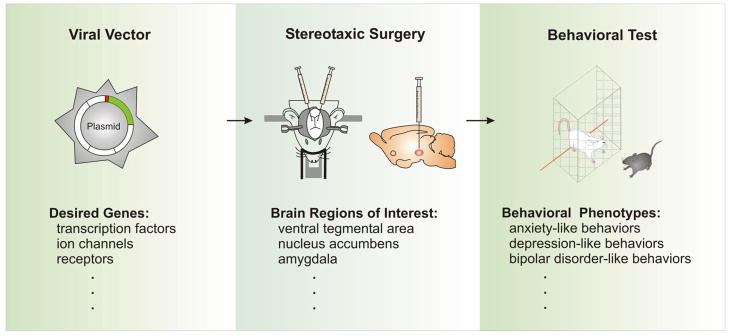
Fig. 4. In vivo viral-mediated gene transfer – virogenetics.
A number of viruses such as adeno-associated virus (AAV) and herpes simplex virus (HSV) are used to carry genes of interest, including transcription factors, ion channels and receptors, and successfully express them in the brain in vivo. In this sense, the virus is acting as a “gene delivery vehicle” (viral vector), by which desired genes are delivered into the neurons of specific brain regions through standard laboratory stereotaxic surgery. Thus, the gene expression is locally regulated in the brain region or nucleus of interest, and ready for specific behavioral tests to investigate the contribution of these local genes to the tested behaviors. By expressing wildtype genes, constitutively active genes, or dominant negative genes in the restricted brain region, the gain- and loss-of-function is realized with this virogenetic approach, which is a key tool to mine the genes that are not only induced by stimuli in a behavioral model, but primarily contribute to the behavioral phenotypes seen in this model. For more information about virogenetics, see reviews (Carlezon et al., 2000b; Neve et al., 2005).
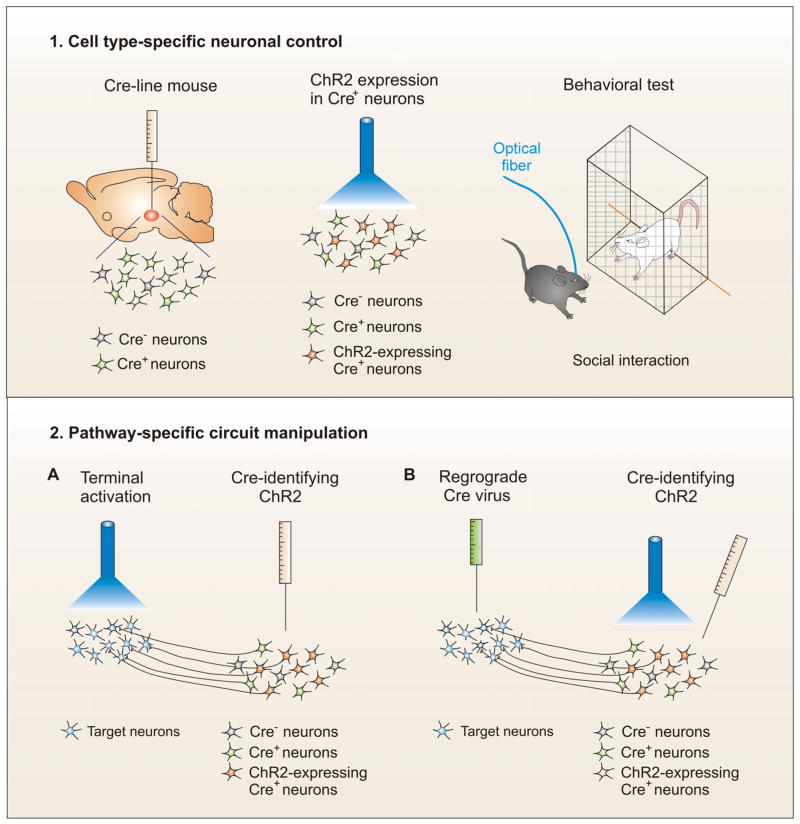
Fig. 5. In vivo optical manipulations of specific neurons or neural circuits – optogenetics.
1. Cell type-specific neuronal control. The targeted neurons are engineered to express Cre in Cre line mice. Therefore, there are Cre positive (Cre+) and Cre negative (Cre-) neurons in the brain region of interest. When Cre-identifying (conditional) ChR2 viral vectors are injected into the brain region, they selectively infect Cre-containing neurons. Behavioral test can be carried out during the blue light activation of infected neurons in freely-moving animals. For instance, conditional AAV-ChR2 is locally injected into the ventral tegmental area (VTA) of tyrosine hydroxylase (TH)-Cre mice so that ChR2 is only expressed in VTA dopamine neurons, but not GABA interneurons in this brain region. Then, the optical fiber reaches to the VTA through a guide cannula that fixes to scull. Through the optical fiber, blue light is delivered to the VTA and selectively activates the dopamine neurons during behavioral tests such as a social interaction measurement. 2. Pathway specific circuit manipulation. There are two ways to realize the circuit-specific control. (A) Conditional ChR2 is injected to one brain nucleus and then light activation of infected neuronal terminals in its target area. For example, conditional AAV-ChR2 is injected into the VTA of TH-Cre mice and an optical fiber is implanted into the nucleus accumbens (NAc), a target brain region of the VTA dopamine neurons. The VTA-NAc pathway can be selectively activated through the light stimulation of ChR2-expressing dopamine neuron’s terminals in the NAc when the behaving animals are tested. (B) Retrograde Cre virus is injected to a target area so that Cre is retrograded to the cell body from the target area, and then conditional AAV-ChR2 is delivered into the cell body brain region to infect Cre-containing neurons. Therefore, the light activation of cell body region selectively stimulates the specific circuit pathway (Gradinaru et al., 2010). For more detailed information, see reviews (Deisseroth et al., 2006; Gradinaru et al., 2007; Zhang et al., 2007)
Table 1.
A comparison of conventional pharmacology, virogenetics and optogenetics.
| Technique | Advantages | Disadvantages |
|---|---|---|
| Conventional Pharmacology |
|
|
| Virogenetics |
|
|
| Optogenetics |
|
|
There is an accumulating body of evidence supporting the idea that some psychiatric disorders such as drug addiction, depression and bipolar disorder or different addictive drugs interact with overlapping brain mechanisms, and even share the same molecular pathways or neural circuits (Nestler, 2005; Roybal et al., 2007; Saal et al., 2003). Drug addiction is characterized by an experience-driven strong desire for the use of alcohol or other drugs despite severe problems related to use of the substance. One of the core symptoms of major depressive disorder is anhedonia, a phenomenon in which patients are unable to experience pleasure from activities usually found enjoyable. It is not surprising that these two conditions are associated to the brain reward neural circuit. Moreover, recent studies demonstrate that bipolar disorder, which is typified by depressive episodes and manic episodes, is also linked to the brain reward circuit. Similarly, dysfunctions of medial prefrontal cortex are sometimes found in both depressed and schizophrenia patients or in rodent models of these disorders. The shared dysfunction of the same brain region or mechanism may explain why these patients have a high risk of co-morbidity, a fact that makes the understanding of these disorders more difficult.
Psychiatric disorders are also a huge burden on worldwide society (Eaton et al., 2008; Insel and Scolnick, 2006). As the most common mental health problem, major depressive disorder alone afflicts 5–6% of the United States population, ranking 4th in terms of illness burden; and, according to the WHO (World Health Organization), these numbers are continuously increasing. While antidepressants are one of most commonly prescribed medications in the United States, less than half of depressed patients achieve full remission and many are not responsive, with currently available monoamine-based antidepressants, most of which were discovered by serendipity over 50 years ago (Berton and Nestler, 2006; Nestler et al., 2002b). Surprisingly, despite tremendous efforts in the psychiatric field, there have been only one new class of antidepressant medications developed for depression treatments and none for schizophrenia over the past 50 years (NAMHCW-Report, 2010). This is in stark contrast to other diseases, such as heart disease, where 13 mechanistically novel drug classes were developed during the same period (NAMHCW-Report, 2010). Thus, there is a clearly urgent need to develop mechanistically novel therapeutic drugs based on the understanding of the fundamental nature of these disorders. Recent years, utilizing virogenetic and optogenetic techniques, studies have dramatically improved our understanding of the molecular and cellular mechanisms of psychiatric disorders, and an increasing number of potential therapeutic targets have been reliably defined in the brain. In this article, we review these studies, in which the sophisticated virogenetic and optogenetic techniques are effectively used as a mining tool to identify the molecular and cellular adaptations that have effects on both neuronal activity and behavioral functions. These studies have provided novel, conceptually innovative therapeutic targets for the treatment of psychiatric disorders.
2. Neuronal activity-associated mechanisms as potential therapeutic targets
The biological brain and artificial electronic systems, such as a computer, both use surprisingly simple codes to encode complicated higher functions. A computer system, like the highly intelligent IBM chess computer Deep Blue, is composed of very basic elements; namely, simple functional units such as resistors (strength of connection), transistors (electric switch), diodes (signal rectification) and capacitors (signal filter), which construct higher functional units for the system. The system’s ultimate function is supported by these higher functional units such as a logic gate (implementing logical operations, e.g., logic “and” and “or”), arithmetic unit (performing addition, subtraction, and multiplication etc) and control unit (performing the duty to direct its operations). Surprisingly, a computer system’s ability to perform complex functions, such as the artificial intelligence of Deep Blue, is simply encoded by two electric states – low voltage state (logic 0) and high voltage state (logic 1) or Boolean logic conditions.
In the biological brain, a typical neuron has dendritic branches and a long axon, which is structurally different from other types of cells in the biological system. This highly differentiated morphology of neurons are precisely formed for collecting and transmitting signals, and are ideally shaped to make connections and communicate with each other. There are about 100 billion neurons in the human brain and each neuron receives on average 7,000 synaptic inputs from other neurons, which forms an extremely complicated neural network and is the physical basis for the brain’s primitive functions such as sensation (e.g., vision, hearing) and motor function (e.g., strength, coordination), and higher brain functions such as memory, language skills, and perception of time and space. Compared to a computer system, the biological brain is much more complicated. However, the brain functions of such complicated neural networks seem to be encoded by traveling action potentials (spikes), which are generated by neurons with a restrict rule called “none” or “all”, similar to logic 0 and logic 1 in a computer system, respectively. This means that neuronal spikes are one of key elements for the brain to encode its functions.
A neuronal spike is an integrated signal generated by the cell body of a neuron, and the integrative function of a neuron is determined by the extrinsic synaptic inputs received from other neurons and the neuron’s intrinsic excitability (the neuron’s ability to fire spikes in response to synaptic inputs). In contrast to stable, artificial electric circuits, the brain’s neural network is a dynamic, plastic system: the strength of synaptic connections and the excitability of a neuron are all subject to adaptive change by an individual’s activity or experience in both physiological and disease conditions. The neural factors that can adaptively regulate the integrative function of a neuron include synaptic plasticity (e.g., long-term synaptic potentiation, long-term synaptic depression, and homeostatic synaptic scaling) and neuronal excitability (e.g., homeostatic intrinsic plasticity). These neuroadaptations determine the firing frequency of spikes and the firing patterns of a neuron, which has been demonstrated to encode various brain functions (see below).
As mentioned above, strong environmental stimulations such as prolonged life stress and drugs of abuse can induce a vast amount of changes in the genes, proteins, transcription factors, channels and receptors. However, it is not necessary that all the induced changes regulate the neuronal activity. In this review, the potential therapeutic targets are those neuroadaptations that (1) are associated with the neuronal activity, and (2) are both sufficient and necessary to underlie the behavioral or brain functions. Due to the complexity of the brain, it has been beyond our technical capabilities to easily identify the specific molecular and cellular adaptations that are both sufficient and necessary to underlie psychiatric malfunctions. With the development and extensive application of virogenetic and optogenetic techniques, an increasing number of potential therapeutic targets were found in the brain through loss- and gain-of-function approaches.
3. Synaptic plasticity
We have known since the nineteenth century that neuroadaptations can occur at synaptic levels in response to synaptic stimulation (Bliss and Gardner-Medwin, 1973; Bliss and Lomo, 1973; Neves et al., 2008). High frequency stimulation for a few seconds or low frequency stimulation for minutes can respectively induce long-term potentiation (LTP) and long-term depression (LTD) in the strength of synaptic connection. This type of short-time stimulation-induced LTP and LTD has been primarily used as a model of learning and memory (Collingridge et al., 2010; Neves et al., 2008). Interestingly, a similar type of synaptic plasticity has been found to be induced by drugs of abuse – relatively long-term stimulations (Kauer and Malenka, 2007; Liu et al., 2005; Saal et al., 2003). In vivo administration of different addictive drugs, including cocaine, amphetamine, morphine, nicotine and ethanol, induced an increase of AMPA (a-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid)/NMDA (N-menthyl-D-aspartic acid) ratio in excitatory synaptic inputs onto ventral tegmental area (VTA) dopamine neurons in the brain reward circuit (Saal et al., 2003; Ungless et al., 2001). Moreover, stress, a well-known factor that can induce drug or alcohol relapse, has similar effects on AMPA/NMDA ratio on these synaptic connections. Emerging evidence has showed that the adaptations in AMPA/NMDA ratio are highly associated with LTP (Bellone and Luscher, 2006; Hawasli et al., 2007; Marie et al., 2005). Using viral-mediated gene transfer (virogenetic approach), it was demonstrated that in vivo expression of constitutively active calcium/calmodulin-dependent protein kinase IV (CaMK IV) and cAMP response element binding protein (CREB) in the hippocampus induced both LTP and synaptic adaptation in AMPA/NMDA ratio by enhancing NMDA receptor-mediated responses (Marie et al., 2005). The same signal pathway may also play a role in mediating anxiety-like behavior (Schneider et al., 2007).
Consistent with these studies, viral-mediated activation of the active form of CREB was found to increase the surface expression of NMDA receptors in nucleus accumbens (NAc) medium spiny neurons. Functionally, this increase of surface level NMDA receptors maintains these neurons in an upstate for a longer duration and produces additional spikes in each upstate, an active functional state for this type of highly hyperpolarized neuron (Huang et al., 2008). Moreover, overexpression of constitutively active CREB upregulates the level of NR2B, a NMDA receptor subunit, in the NAc, which links to the increase of silent synapses, a phenomenon that is induced by cocaine (Brown et al., 2011; Huang et al., 2009). Additionally, CREB in the NAc exerts its effects on synaptic transmission not only through the regulation of NMDA receptor-mediated synaptic transmission, but also through the regulation of intrinsic excitability of these neurons (see below Neuronal excitability).
In another study, utilizing the social defeat stress model of depression and anxiety, viral-mediated induction of transcription factor ΔFosB in the NAc was found to be both necessary and sufficient for resilience to social defeat stress, an ability of some mice to escape the deleterious effects of chronic stress (Vialou et al., 2010). Interestingly, these effects of ΔFosB are produced through the induction of GluR2 AMPA glutamate receptor, an important receptor subunit that regulates the rectification of AMPA receptor-mediated excitatory postsynaptic currents (EPSCs). More recently, a study shows that the AMPA receptor rectification is increased in VTA dopamine neurons when GluR1 is overexpressed in brain slice cultures (Choi et al., 2011), a system that has been successfully used to virally infect neurons (Cao et al., 2010b; Choi et al., 2011; Coque et al., 2011; Han et al., 2006; Huang et al., 2008; Iniguez et al., 2010; Krishnan et al., 2007). Importantly, in vivo overexpression of GluR1 in the VTA potentiates locomotor responses to cocaine and increases the motivation for cocaine in cocaine self-administrating animals (Choi et al., 2011). Interestingly, virally expressed GluR1 and GluR2 in the shell component of NAc showed opposing effects on the regulation of motivation evidenced by measurements of intracranial self-stimulation (Todtenkopf et al., 2006). Consistent with these findings, chronic morphine selectively increases expression of GluR1 in the VTA, and virally mimicking this change in GluR1 expression enhances morphine reward (Carlezon et al., 1997). Surprisingly, morphine is both rewarding and aversive when GluR1 is virally expressed in the rostral and caudal VTA, respectively (Carlezon et al., 2000a). Virally elevated expression of 5-HT1B receptors in NAc shell increases cocaine-induced locomotor sensitization and rewarding functions (Neumaier et al., 2002). Similar overexpression of 5-HT1B receptors in the NAc, pairing with repeated mild stress, increases sensitivity to psychostimulant amphetamine (Ferguson et al., 2009), implicating the role of 5-HT1B receptors in the interaction between stress and psychostimulants. Consistent with these observations, viral overexpression of 5-HT1B receptors in the NAc increases alcohol consumption (Hoplight et al., 2006). NAc 5-HT1B receptors play an important role in depression, aggressive behavior and drug abuse, and neurophysiologically, these receptors may mediate their effects by regulating presynaptic function of NAc medium spiny neurons (Neumaier et al., 2002; Sari, 2004).
Studies of viral-mediated Homer gene transfer demonstrate that this family of post synaptic density proteins is also involved in mediating drug- and alcohol-induced plasticity (Klugmann and Szumlinski, 2008; Knackstedt et al., 2010). Physiologically, glutamate receptors are associatively regulated with Homer2 in the NAc (Szumlinski et al., 2007; Szumlinski et al., 2005). Homer2 knockout mice do not exhibit glutamate sensitization in the NAc, which is rescued by Homer2b expression in the NAc. Interestingly, NR2B stays close to the upregulation of Homer2 during ethanol withdrawal, although it does not last as long as Homer2 (Szumlinski et al., 2008).
Together, these studies mentioned above suggest that the synaptic transmissions onto hippocampal neurons, NAc medium spiny neurons and VTA dopamine neurons play important roles in mediating the behavioral responses to drugs of abuse and stress, in which glutamate receptors, serotonin receptors, Homer, transcription factors CREB and ΔFosB are important mediators. Thus, serotonin, NMDA and AMPA receptors, CREB, ΔFosB and their associated signaling pathways are potential targets for the treatment of drug addiction and stress-induced relapse, although the exact interactions between these receptors and proteins remain to be elucidated.
An increasing body of evidence shows that synaptic adaptations also present as structural plasticity such as adaptive changes in spine density and cell size of neurons (Russo et al., 2007; Russo et al., 2010; Sklair-Tavron et al., 1996). Changes in dendritic spine density can induce alterations in synaptic transmission, which can be directly measured by studying inhibitory postsynaptic currents (IPSCs) or EPSCs. Repeated exposure to cocaine induces a significant long-lasting increase in dendritic spine density in the NAc (Martin et al., 2011; Robinson and Kolb, 1999), a phenomenon that is mediated by myocyte enhancer factor 2 (MEF2) transcription factor (Pulipparacharuvil et al., 2008). Moreover, utilizing control adeno-associated virus (AAV)-short hair RNA (shRNA) against MEF2A/2D, control AAV-MEF2-VP16 and AAV-MEF2-VP16, a study demonstrated that the transcription factor MEF2 is sufficient and necessary to underlie both dentritic spine density plasticity and cocaine-induced behavioral plasticity, including locomotor sensitization and cocaine conditioned place preference (CPP). Furthermore, MEF2 is known as one of cyclin-dependent kinase 5 (Cdk5) substrates, a signal pathway that is also regulated by repeated exposure of cocaine (Bibb et al., 2001). Both MEF2 and Cdk5 play important roles in the regulation of synaptic transmission in hippocampal neurons. A conditional knockout of Cdk5 enhances LTP and AMPA/NMDA ratio in the hippocampal Schaffer collaterals-CA1 pathway (Hawasli et al., 2007). Specifically, the viral-mediated reduction of MEF2 increased the number of synapses and miniature EPSC frequency (Flavell et al., 2006), whereas the activation of MEF2 induces synapse elimination and consistently decreases miniature EPSC frequency (Pfeiffer et al., 2010).
Similar structural plasticity is seen in the models of depression and anxiety. In the prefrontal cortex, the number and function of new spine synapses are found to be increased by NMDA receptor antagonist ketamine, a rapidly acting antidepressant when the dosage is significantly below that used as a stimulant (Li et al., 2010). The mammalian target of rapamycin (mTOR) is involved in mediating these adaptive changes, and blockade of mTOR signaling abolishes ketamine-induced synaptogenesis and depression-like behaviors. In the social defeat stress model, chronic defeat significantly increases the stubby spine density of NAc medium spiny neurons, and consequently increases the frequency of miniature EPSCs in these neurons (Christoffel et al., 2011). Furthermore, these structural effects of social defeat were found to be mediated by IkB kinase through viral regulation of this kinase, which was shown to be sufficient and necessary to underlie both chronic defeat-induced spine plasticity and social avoidance, a profound depression-like behavior in this model. Interestingly, IkB kinase also mediates cocaine-induced structural and behavioral plasticity in these NAc medium spiny neurons of the brain reward circuit (Russo et al., 2009). It is notable, as mentioned above in Section 2, that depression and drug addiction share the same neural circuit, and even the same signaling pathway. These studies implicate that the structural plasticity is one of the important factors that can adaptively alter synaptic transmission by regulating the synapse number. As compared to LTP and LTD, relatively less is known about the molecular mechanisms of structural plasticity. Further studies in this field, utilizing advanced viral and optical regulation, may improve the understanding of how this form of adaptation exerts its effects on synaptic transmission and on behavioral functions, and provide valuable targets for disease treatments.
4. Neuronal excitability
The ability of a neuron to fire spikes (neuronal excitability) is intrinsically determined by the neuron’s molecular and structural composition. Thus, different types of neurons exhibit intrinsically distinct excitability in the brain. For instance, norepinephrinergic neurons in the locus coeruleus (LC) and dopaminergic neurons in the VTA have relatively depolarized resting membrane potential (approximately −55 mV) and exhibit spontaneous firing during functional states (Cao et al., 2010b; Han et al., 2006; van den Pol et al., 2002), whereas GABAergic medium spiny neurons in the NAc have a very negative resting membrane potential (about −80 mV) and need to be driven to an upstate to fire spikes as mentioned above (Dong et al., 2006; Huang et al., 2008). An increasing body of evidence reveals that neuronal excitability is a key mechanism that underlies certain forms of long-lasting, experience-dependent plasticity (Rabinak et al., 2008; Saar and Barkai, 2009; Zhang and Linden, 2003). Evidence also shows that intrinsic neuronal excitability is linked to disease conditions such as Alzheimer disease and drug addiction (Santos et al., 2010; Wolf, 2010), indicating that the molecular and ionic mechanisms of neuronal excitability are potential therapeutic targets.
Studies demonstrate that the adaptive changes in intrinsic neuronal excitability occur in the spontaneously firing neurons such as VTA and LC neurons and the neurons that have negative resting membrane potential, such as NAc medium spiny neurons (Cao et al., 2010a; Cao et al., 2010b; Coque et al., 2011; Han et al., 2006; Krishnan et al., 2007; Lobo et al., 2010; Renthal et al., 2009; Wallace et al., 2009). Indeed, repeated exposure to cocaine induces a decrease in the excitability of NAc neurons (Dong et al., 2006). Viral-mediated overexpression of dominant negative CREB in these neurons mimics the effect of cocaine, while overexpression of constitutively active CREB increases the excitability (Dong et al., 2006). Further work demonstrates that the expression of active CREB prolongs the upstate of these neurons and increases the spike number in each upstate, while dominant negative CREB expression conversely decreases the spike number (Huang et al., 2008). Importantly, decreasing NAc neuronal excitability via the in vivo overexpression of a voltage-gated potassium channel Kir2.1, which mimics dominant negative CREB effects, facilitates cocaine-induced locomotor sensitization (Dong et al., 2006). These results demonstrate that the intrinsic excitability of NAc neurons plays a critical role in mediating cocaine actions via the CREB signaling pathway.
Consistent with these studies, brain-derived neurotrophic factor (BDNF), one of downstream substrates of CREB, is found to play interesting roles in mediating the stress responses and cocaine actions in NAc neurons (Berton and Nestler, 2006; Krishnan et al., 2007; Lobo et al., 2010). In the social defeat model, chronic defeat increases the level of BDNF in the NAc. Utilizing floxed BDNF mice and AAV-Cre, local knockdown of BDNF gene in the VTA blocks the BDNF increase in the NAc and reverses the social avoidance behavior demonstrated following social defeat (Berton et al., 2006). These results demonstrate that the increased BDNF in the NAc is released from the terminals of VTA dopamine neurons, and plays an essential role in mediating social avoidance behavior (Berton et al., 2006). Highly consistent with these findings, a further study shows that a similar increase in the level of BDNF of the NAc occurs in susceptible mice, but not in the resilient subgroup (Krishnan et al., 2007). These data suggest that the local BDNF in NAc is involved in determining the vulnerability to social defeat. Deficits found in activity-dependent BDNF release in Met/Met mice, a common single-nucleotide polymorphism in the BDNF gene, (Chen et al., 2004) promote a resilient phenotype (Krishnan et al., 2007). In addition, by combined use of Cre viral vector and floxed BDNF and TrkB mice, the same BDNF/TrkB signal mechanisms in the VTA-NAc pathway is evidenced to play a key role in mediating cocaine self-administration and condition place preference behavior (Graham et al., 2007; Graham et al., 2009). The detailed ionic mechanisms that underlie these effects of BDNF/TrkB signal pathway remain to be elucidated.
Interestingly, BDNF has opposing effects in different types of NAc neurons (Lobo et al., 2010). Utilizing AAV-Cre and floxed TrkB mice, selective deletion of TrkB, a BDNF receptor, in dopamine receptor 2-containing (D2+) neurons increases the excitability of these neurons and suppresses cocaine reward (evidenced by decreased cocaine CPP). In contrast, an opposite effect was induced by the selective loss of TrkB in D1+ neurons. Importantly, utilizing optogenetic approaches, selective in vivo light activation of ChR2 in D2+ neurons, mimicking the loss of TrkB, decreases cocaine reward, while selective light activation of D1+ neurons in the NAc induces an opposite effect. In another study, the enkephalin- and dynorphin-containing medium spiny neurons in the NAc are selectively targeted through virally engineered G-protein coupled muscarinic M4 receptors under control of either enkephalin or dynorphin promoter (Ferguson et al., 2011). Pharmacological activation of these engineered receptors decreases the excitability of both types of neurons. Decreasing the activity of enkephalin neurons facilitates amphetamine-induced behavioral sensitization, while the activation of dynorphin neurons impairs its persistence. These studies demonstrate that different cell types of NAc neurons play distinct, even opposing roles in the development of drug dependence, and highlight that without the use of advanced virogenetic and optogenetic approaches, it has not been possible for investigators to explore the roles of cell type-specific or circuit specific signaling pathways in mediating behavioral functions in freely-moving animals. Recently, cell type-specific (circuit-specific) manipulation has been successfully used in amygdala circuitry and reveals that different circuit pathways have clearly specific functions in drug seeking behavior, anxiety and conditioned fear (Ciocchi et al., 2011; Stuber et al., 2011; Tye et al., 2011).
Another family of CREB target genes, sirtuins, which are NAD+-dependent enzymes that regulate cellular functions through deacetylation of various proteins, are regulated in the NAc by in vivo cocaine administration (Renthal et al., 2009). In detail, SIRT1 and SIRT2 catalytic activity in the NAc are increased by chronic cocaine treatment. Importantly, these enzymes exhibit an ability to regulate the excitability of NAc neurons in brain slice preparations: a pharmacological activator of sirtuins, resveratrol, increases the excitability of NAc neurons, whereas an inhibitor, sirtinol, induces a decrease in their excitability. Thus, it would be of therapeutic interest to test if the local infusion of these inhibitors and activators of sirtuins or viral regulation of these enzymes have effects on cocaine sensitization and cocaine CPP. Together, these studies suggest that the genetic or pharmacologic regulations of CREB, BDNF, SIRT1, SIRT2 and their related signaling pathways may have therapeutic effects on cocaine addiction and stress-induced disorders such as depression. Very recent studies found that a non-peptide antagonist of BDNF, receptor tropomyosin-related kinase B (TrkB), which intervenes in the ligand-receptor interaction and reduces anxiety- and depression-related behaviors in mouse models (Cazorla et al., 2011; Harrison, 2011).
Emerging evidence reveals that ion channels, such as K+ channels and Ih (hyperpolarization-activated cation channels), play an important role as intrinsic factors in the regulation of neuronal excitability. Recent study shows that Ba2+-sensitive K+ channel function is significantly increased in the NAc by chronic social isolation stress, a model of depression and anxiety. And this increase in K+ channel function leads to a down-regulation of the intrinsic excitability of NAc medium spiny neurons (Wallace et al., 2009). Mimicking this effect by viral-mediated expression of Kir2.1 in the NAc shell causes an anxiety-like phenotype. Further intriguing evidence comes from the firing regulation of VTA dopamine neurons in the social defeat model. Chronic social defeat increases Ih channels, a driving force that intrinsically up-regulates the firing rate of these neurons in susceptible mice, whereas this channel is up-regulated to a greater extent in resilient mice. Thus, the firing rate of VTA dopamine neurons in resilient mice ought to fire higher than that in susceptible mice, but the bigger Ih driving force is blocked by the increased K+ channel function selectively in resilient mice, which normalizes the firing rate in resilient mice (Friedman et al., 2011; Krishnan et al., 2007). Moreover, microarray studies show that four different types of K+ channel subunits, including Kcnf1, Kcnh3, Kcnk4 and kcnq3, are increased selectively in resilient mice (Krishnan et al., 2007). These studies suggest that K+ channels gate the neuronal excitability as an intrinsic factor, even when the ionic mechanisms are primed to up-regulate the activity of neurons.
One intriguing question is whether other types of K+ channels serve similar functions to regulate neuronal activity. It is believed that GIRK channels may conceptually function as a gatekeeper of neuronal excitability (Balana et al., 2011). GIRK channels are also a new target of ethanol actions in the brain, an exciting finding in the field of alcohol addiction studies (Aryal et al., 2009; Kobayashi et al., 1999; Lewohl et al., 1999). Currently, there is accumulating evidence showing that this type of K+ channel is involved in mediating the regulation of neuronal excitability (see review,(Luscher and Slesinger, 2010)).
Further evidence that implicates the roles of neuronal excitability in the regulation of emotional processing is from a genetic model of bipolar disorder. In this model, the Clock mutation mice exhibit mania-like behaviors, one of the two opposing episodes seen in bipolar disorder patients (Coque et al., 2011; Roybal et al., 2007). These mice show a significantly higher firing rate of VTA dopamine neurons compared to wildtype mice, an effect that is completely normalized by lithium – a commonly used mood stabilizer for bipolar disorder patients. Consistently, knockdown of Clock expression with AAV-Clock shRNA increases the excitability of VTA dopamine neurons obtained from naïve mice (Mukherjee et al., 2010), whereas viral-mediated expression of Kir2.1 effectively decreases the firing rate of VTA neurons in the Clock mutation mice, and also decreases “risk taking” behaviors as measured in dark light and elevated plus maze tests (Coque et al., 2011; Roybal et al., 2007).
Together, these studies strongly support the notion that the intrinsic excitability of neurons is an important neural mechanism that underlies the neuroadaptations involved in psychiatric disorders. Further investigations on the detailed molecular and ionic mechanisms have enormous potential to provide valuable therapeutic targets for the treatment of these diseases. Very recent studies reveal that K+ and Ih channels as the intrinsic regulators of VTA dopamine neuronal activity and therefore as promising targets for depression treatment (Friedman et al., 2011; Krishnan et al., 2007) (see below).
5. Homeostatic plasticity
In artificial electronic systems, feedback circuits are commonly used to realize certain functions of signal processing or to stabilize the circuitry functions. A decade ago, investigators in the field of synaptic plasticity started to understand the similar compensatory feedback mechanisms in the brain circuitry (LeMasson et al., 1993; Turrigiano et al., 1994). Recent findings reveal that the neurons in primary or slice cultures exhibit an ability to develop compensatory homeostatic adaptations in intrinsic excitability or strength of synaptic connections in response to excessive inhibition and excitation (LeMasson et al., 1993; Turrigiano et al., 1994; Turrigiano, 2008; Zhang and Linden, 2003). Homeostatic intrinsic plasticity is a neuronal feedback mechanism by which neurons compensate for a strong stimulus by re-stabilizing the activity of neurons within a physiologically normal range, while homeostatic synaptic scaling is a circuitry mechanism by which neurons re-balance the inhibitory and excitatory functions of the neural network. For instance, blockade of the firing of cortical pyramidal neurons in primary cell cultures by TTX (tetrodotoxin) or by AMPA receptor blocker CNQX (6-cyano-7-nitroquinoxaline-2,3-dione) induces an increase in the amplitude of miniature EPSCs. Conversely, an increase in the firing by blockade of GABA (γ-aminobutyric acid)-mediated inhibition with bicuculline significantly decreases miniature EPSC amplitude (Turrigiano et al., 1998). Since this new form of homeostatic plasticity was reported (LeMasson et al., 1993; Turrigiano et al., 1994), investigators have begun to understand the molecular mechanisms of homeostatic plasticity, but relatively less is known about the functional roles of homeostatic plasticity at the behavioral level (Turrigiano, 2011; Turrigiano, 2008).
Impressive progress in this research area is from monocular deprivation studies, a classic paradigm for experience-dependent cortical plasticity (Desai et al., 2002; Maffei et al., 2004). Evidence shows that neurons receiving synaptic inputs from the deprived eye exhibit an increased sensitivity to visual stimulation, which is functionally mediated by a compensatory increase in synaptic strength and/or intrinsic excitability (Turrigiano, 2008; Turrigiano and Nelson, 2004). Recently, utilizing optogenetic tools, homeostatic adaptations in glutamate receptors are successfully induced in organotypic rodent hippocampal slices (Goold and Nicoll, 2010). Cell-autonomous increases in excitation of CA1 pyramidal neurons induces a compensatory postsynaptic depression of AMPA and NMDA receptor functions via a pathway involving CaM kinase kinase and CaM kinase 4. This is exciting news for the future of the homeostatic plasticity field because with the combined use of optogenetic techniques and behavioral models of learning and memory there is the potential to reveal the detailed functions of homeostatic plasticity in behaving animals.
It is well known that prolonged neuronal perturbations such as chronic stress and repeated exposure to drugs of abuse induce long-lasting or even life time long changes in the brain (Hyman et al., 2006; Luscher and Malenka, 2011; Nestler, 2001; Nestler et al., 2002a). Recent research has revealed the role of homeostatic plasticity in these conditions. Chronic administration of morphine or morphine pellets develop morphine dependence in animal models, which is evidenced by the development of deleterious physical signs and symptoms of morphine withdrawal (Han et al., 2006; Lane-Ladd et al., 1997; Nestler and Aghajanian, 1997). Accumulated evidence has showed that the norepinephrinergic neurons of LC are involved in mediating the physical withdrawal. Acute in vivo morphine administration decreases the firing rate of LC neurons (Wang and Aghajanian, 1990). During chronic morphine exposure, LC firing rates return to normal levels (tolerance) and, on antagonist precipitation of withdrawal, increase dramatically above normal levels (Aghajanian, 1978; Rasmussen et al., 1990). This chronic morphine-induced homeostatic plasticity is induced by both synaptic homeostatic plasticity and adaptive changes in the intrinsic excitability of LC neurons (Cao et al., 2010b; Han et al., 2006; Ivanov and Aston-Jones, 2001; Nestler and Aghajanian, 1997; Rasmussen, 1995). Chronic morphine significantly increases the level of CREB in the LC and viral-mediated expression of the active form of CREB increases the firing rate of LC neurons, whereas overexpression of dominant negative CREB has an opposite effect on the firing rate. Importantly, local infusion of CREB into the LC aggravates certain signs of withdrawal, while these withdrawal behaviors show significant attenuation by the expression of dominant negative CREB (Han et al., 2006). These results provide direct evidence that the LC neuronal homeostatic plasticity plays an important role in mediating the development of morphine dependence and withdrawal behaviors, indicating that these neuroadaptative changes may be valuable targets for therapeutic treatment.
Similar intrinsic homeostatic plasticity is found in VTA dopamine neurons. As mentioned above, in a social defeat stress model, chronic defeat increases the firing rate and bursting properties of VTA dopamine neurons in susceptible mice, but not in the resilient subgroup. Further research demonstrates that Ih is significantly up-regulated in susceptible mice, suggesting that this channel is a pathogenic ion mechanism that underlies the susceptible phenotype. Surprisingly, this pathogenic mechanism persists in resilient mice in conjunction with the selective increase in K+ channel function, which drives the pathophysiological hyperactivity back to normal levels (Friedman et al., 2011). These results strongly support the notion that the homeostatic, compensational plasticity in K+ channels is the critical mechanism of resilience to social defeat. Thus, the balance between Ih and K+ channels is a key determinant for susceptibility versus resilience to social defeat. Importantly, decreased firing through viral-mediated expression of Kir2.1 in the VTA in vivo promotes a resilient phenotype, whereas increased firing oppositely promotes susceptibility (Krishnan et al., 2007). Accordingly, in continuing studies, a K+ channel activator is used to mimic the compensational, active ionic mechanism of naturally occurring resilience, and found to normalize the social defeat-induced avoidance behavior (Friedman et al., 2011). This research, based on the understanding of detailed stress responses in the brain, provides novel targets, which are conceptually distinct from traditional monoamine-based antidepressants.
6. Adaptive changes in the firing rate and firing pattern
In a computer system, certain-length binary digits encode an instruction, which presents a processing step such as addition. For instance, a binary code of addition is sent to a control unit, which fetches the binary code and directs the addition operation of arithmetic unit. Comparatively, how do neuronal spikes encode brain functions? Does a certain number of spikes from different parallel neurons or a certain number of spikes from the same neuron encode neural functions? Studies have showed that the nervous system uses multiple ways, including the frequency of neuronal spikes (firing rate), the firing patterns (tonic or phasic firing), spike timing-dependent plasticity and spike synchronization of neuronal population, to encode a multitude of brain functions (Caporale and Dan, 2008; Ermentrout et al., 2008; Grace et al., 2007; Lestienne, 2001). Recent optogenetic studies show that highly synchronized activation of specific neurons in certain brain nuclei or specific pathways reliably regulates behavioral functions. For instance, it was demonstrated that the light stimulation of ChR2 in the motor cortex induces instant locomotor behavior: light on, mouse quickly moves around (unilateral stimulation), while light off, mouse moves only randomly (see the video at Karl Deisseroth Laboratory website: http://www.stanford.edu/group/dlab/optogenetics/hardware.html). In addition, optogenetic research also demonstrates that the firing patterns of neurons also carry functional signals (see below).
The amygdala, an important brain region for emotional processing, is involved in the development and expression of Pavlovian conditioned fear responses (LeDoux, 2000; Maren and Quirk, 2004). Recent research has defined the detailed inhibitory microcircuits for the fear conditioning through combined use of in vivo eletrophysiological, optogenetic and pharmacological approaches (Ciocchi et al., 2010; Haubensak et al., 2010). A further study demonstrates that the neuronal projection from the basolateral amygdala (Lewis et al., 2011) to the central nucleus of the amygdala (CeA) is a critical circuit pathway that controls acute anxiety (Tye et al., 2011). High frequency (20 Hz) optical stimulation of ChR2 expressed at the CeA terminals of BLA neurons induces an acute, reversible anxiolytic effect, whereas inhibition of the same projection pathway by light activation of halorhodopsin (eNpHR3.0) increases anxiety-like behaviors.
It has been known for a long time that the VTA dopamine neurons in the brain reward circuit exhibit two different in vivo firing patterns: low-frequency tonic and high-frequency phasic firing (Grace et al., 2007). The phasic firing of these neurons releases more dopamine in their target area and encode reward signals. Therefore, the phasic firing is functionally more important, a notion that is further supported by a recent study. In this study, the high-frequency light stimulation of ChR2, specifically in VTA dopamine neurons, is used to mimic the in vivo phasic firing, and is delivered when the mice approach a conditioning chamber during a conditioned place preference test. The phasic activation of ChR2 in VTA dopamine neurons results in mice exhibiting a place preference by spending more time in the conditioning chamber, an effect that could not be induced by the tonic activation of ChR2 in the same group of neurons (Tsai et al., 2009). This research provides the first direct evidence that the phasic firing pattern of VTA neurons encodes conditioned place preference behavior.
Another interesting finding obtained from the social defeat mouse model, demonstrates that in vivo phasic firing of VTA dopamine neurons is significantly increased in susceptible mice, but not in the resilient subgroup. As mentioned above, resilient mice are a subgroup of mice that undergo the social defeat paradigm, but exhibit no depression-like behavioral abnormalities such as social avoidance and anhedonia (lower sucrose preference) (Cao et al., 2010a; Krishnan et al., 2007; Vialou et al., 2010; Wilkinson et al., 2009). Moreover, phasic optogenetic activation of VTA DA neurons induces avoidance behavior in resilient mice, as well as in mice that undergo a sub-threshold social defeat paradigm, a procedure that is used to measure sensitivity to further stress (Chaudhury et al., 2011). These data provide direct evidence that the phasic firing of VTA dopamine neurons in the brain reward circuit encodes a signal of susceptibility to social defeat stress. Intensive investigation of the ionic mechanisms that underlie the phasic firing have the potential to reveal novel drug targets for the treatment of major depressive disorder. As mentioned above, one possible target is the Ih channel, not only because it is a featured pacemaker channel in VTA dopamine neurons, but because this channel is known to play an important role in the transition between tonic and phasic firing patterns (Arencibia-Albite et al., 2007; Inyushin et al., 2010; Neuhoff et al., 2002). As expected, local infusion of Ih inhibitors ZD7288 and DK-AH269 shows antidepressant effects: Ih inhibitors completely reverse depression-like avoidance behaviors (Cao et al., 2010a). Importantly, systematic administration of DK-AH269 has similar antidepressant effects in susceptible mice (Friedman et al., 2011). Surprisingly, Ih inhibitors showed antidepressant effect within a few hours, and the antidepressant effects induced by a single-dose local infusion into the VTA or systematic administration of DK-AH269 lasted at least two weeks, which is very different from traditional antidepressant medications that take weeks to reach clinical efficacy (Friedman et al., 2011). Consistent with these exciting findings, the deletion of Ih channel auxiliary subunit TRIP8b impairs Ih function in the hippocampus, and interestingly, shows an antidepressant effect in mice (Lewis et al., 2011).
In the same stress model, the phosphorylation of extracellular signal-regulated kinase-2 (ERK2) in the VTA is found to be increased by chronic unpredictable stress. Overexpression of dominant negative ERK2 decreases the firing rate of VTA dopamine neurons and promotes resilience to social defeat stress, whereas expressing ERK2 promotes susceptibility (Iniguez et al., 2010). In addition, the phosphorylation of AKT in the VTA is decreased in susceptible mice. Decreasing the AKT activity through a dominant negative AKT promotes the susceptibility to social defeat, whereas increasing the AKT activity by constitutively active AKT reverses the susceptible mice phenotype (Krishnan et al., 2008). The deleterious effects of AKT on the social behavior may be mediated by increasing the firing rate of VTA dopamine neurons since the firing rate of these neurons is increased by an inhibitor of AKT signaling. Together, these investigations suggest that the firing activity of VTA dopamine neurons in the reward system is important in encoding the brain’s response to stress and may provide an ideal neuroadaptation to target for beneficial therapeutic treatments.
7. Conclusion
Virogenetics and optogenetics are newly developed and highly effective techniques to intensively understand the in vivo functional roles of specific molecular components in the brain and specific types of neurons in a complex neural network. These advanced techniques are to date, the most powerful tools that can be used to precisely and reliably imitate the molecular and cellular neuroadaptations seen in rodent models of psychiatric disorders, and to further and directly link these adaptive changes in the brain to behavioral dysfunctions in freely moving animals. After over 50 years of studies of psychiatric medications found by serendipity, these new molecular mechanisms provide highly useful information for the development of biological mechanism-based therapeutics. And importantly, the circuit understanding of psychiatric disorders provides precise information for the development of more targeted and effective treatments for severe forms of psychiatric disorders with deep brain stimulation. Given the emerging view of addiction and depression as a “neural circuit disorder”, which in part arouse from the rapid treatment efficacy of deep brain stimulation, the goal of understanding these psychiatric disorders on the circuit level has become even more therapeutically important. As still developing techniques, virogenetics and optogenetics have their limitation (see Table 1 for their advantages and disadvantages) in research aspects, and will require significant advancement before they are reliably used to assist in treatment for severe forms of currently treatment-resistant psychiatric patients.
Through the effective use of virogenetic and optogenetic approaches as a powerful tool, the studies mentioned above have made strides to isolate the molecular and cellular adaptations that are sufficient and necessary to underlie various behavioral abnormities in rodent models of psychiatric disorders. One consistent finding across different types of neurons in the NAc and LC is that transcription factor CREB increases the firing rate of LC neurons and the excitability of NAc medium spiny neurons, a net effect that enhances the neuronal activity. Similar effects have been reported in the neurons of hippocampus and amygdala (Benito and Barco, 2010). As emphasized in this review, neuronal activity is a crucial component that encodes neural functions and reliably links to behavioral outputs. Importantly, the findings about BDNF, TrkB, CaMKII, ERK, Cdk5, and Sirtuins are not separated single items; rather they are closely related each other in several signal pathways such as TrkB-CaMKII-CREB, TrkB-ERK-CREB, and TrkB-AKT-CREB pathways. There may also have local feedback regulations because BDNF is a downstream product of CREB. Therefore, CREB and CREB-related signaling pathways, including BDNF, ΔFosB, Cdk5 and Sirtuins, are potentially valuable targets to regulate the neuronal activity and further to treat behavioral dysfunctions in these psychiatric conditions. In addition, structural plasticity is a fast growing research area that may provide opportunities to understand the underlying molecular mechanisms of mood disorders, and reveal useful targets for the development of mechanistically new antidepressants such as NMDA receptor antagonist ketamine, a rapidly acting antidepressant. A glutamate theory of depression has also attracted increasing attention and provides promising potential to identify new therapies for major depression. Glutamate receptor-mediated synaptic and structural plasticity can contribute directly to the neuronal activity and exert its effects on behavioral functions as mentioned above in cocaine-related studies. Another chance to identify mechanistically novel classes of antidepressant medications is to effectively regulate the firing rate, especially phasic firing pattern, of VTA dopamine neurons in the brain reward circuitry. The impressive rapid and long-lasting antidepressant effects of Ih inhibitors open a new avenue to develop therapeutic medications that are conceptually different from traditional monoamine-based antidepressants. Finally, the accumulating body of consistent evidence supports the important role of K+ channels in gating neuronal excitability. K+ channel-based antidepressant medications would be conceptually innovative therapy for stress-induced mood disorders. Together, utilizing virogenetic and optogenetic approaches, these studies reveal highly specific neuronal and molecular neuroadaptations underlying different psychiatric disorders that ultimately may lead to a number of potential new therapeutic targets.
Highlights.
A systematic research strategy with a target defining loop is introduced.
Virogenetic and optogenetic approaches are used as an effective defining tool.
Focusing on the neuronal activity during the target defining is highly important.
The identified potential therapeutic targets and related studies are reviewed.
Acknowledgments
Preparation of this review was supported by grants from the National Institute of Mental Health (MH092306) and the International Mental Health Research Organization (IMHRO).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aghajanian GK. Tolerance of locus coeruleus neurones to morphine and suppression of withdrawal response by clonidine. Nature. 1978;276:186–188. doi: 10.1038/276186a0. [DOI] [PubMed] [Google Scholar]
- Arencibia-Albite F, Paladini C, Williams JT, Jimenez-Rivera CA. Noradrenergic modulation of the hyperpolarization-activated cation current (Ih) in dopamine neurons of the ventral tegmental area. Neuroscience. 2007;149:303–314. doi: 10.1016/j.neuroscience.2007.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aryal P, Dvir H, Choe S, Slesinger PA. A discrete alcohol pocket involved in GIRK channel activation. Nat Neurosci. 2009;12:988–995. doi: 10.1038/nn.2358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balana B, Maslennikov I, Kwiatkowski W, Stern KM, Bahima L, Choe S, Slesinger PA. Mechanism underlying selective regulation of G protein-gated inwardly rectifying potassium channels by the psychostimulant-sensitive sorting nexin 27. Proceedings of the National Academy of Sciences. 2011;108:5831–5836. doi: 10.1073/pnas.1018645108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellone C, Luscher C. Cocaine triggered AMPA receptor redistribution is reversed in vivo by mGluR-dependent long-term depression. Nat Neurosci. 2006;9:636–641. doi: 10.1038/nn1682. [DOI] [PubMed] [Google Scholar]
- Benito E, Barco A. CREB’s control of intrinsic and synaptic plasticity: implications for CREB-dependent memory models. Trends in Neurosciences. 2010;33:230–240. doi: 10.1016/j.tins.2010.02.001. [DOI] [PubMed] [Google Scholar]
- Berton O, McClung CA, Dileone RJ, Krishnan V, Renthal W, Russo SJ, Graham D, Tsankova NM, Bolanos CA, Rios M, Monteggia LM, Self DW, Nestler EJ. Essential role of BDNF in the mesolimbic dopamine pathway in social defeat stress. Science. 2006;311:864–868. doi: 10.1126/science.1120972. [DOI] [PubMed] [Google Scholar]
- Berton O, Nestler EJ. New approaches to antidepressant drug discovery: beyond monoamines. Nat Rev Neurosci. 2006;7:137–151. doi: 10.1038/nrn1846. [DOI] [PubMed] [Google Scholar]
- Bibb JA, Chen J, Taylor JR, Svenningsson P, Nishi A, Snyder GL, Yan Z, Sagawa ZK, Ouimet CC, Nairn AC, Nestler EJ, Greengard P. Effects of chronic exposure to cocaine are regulated by the neuronal protein Cdk5. Nature. 2001;410:376–380. doi: 10.1038/35066591. [DOI] [PubMed] [Google Scholar]
- Bliss TV, Gardner-Medwin AR. Long-lasting potentiation of synaptic transmission in the dentate area of the unanaestetized rabbit following stimulation of the perforant path. J Physiol. 1973;232:357–374. doi: 10.1113/jphysiol.1973.sp010274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliss TV, Lomo T. Long-lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path. J Physiol. 1973;232:331–356. doi: 10.1113/jphysiol.1973.sp010273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown TE, Lee BR, Mu P, Ferguson D, Dietz D, Ohnishi YN, Lin Y, Suska A, Ishikawa M, Huang YH, Shen H, Kalivas PW, Sorg BA, Zukin RS, Nestler EJ, Dong Y, Schluter OM. A Silent Synapse-Based Mechanism for Cocaine-Induced Locomotor Sensitization. The Journal of Neuroscience. 2011;31:8163–8174. doi: 10.1523/JNEUROSCI.0016-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao JL, Covington HE, 3rd, Friedman AK, Wilkinson MB, Walsh JJ, Cooper DC, Nestler EJ, Han MH. Mesolimbic dopamine neurons in the brain reward circuit mediate susceptibility to social defeat and antidepressant action. J Neurosci. 2010a;30:16453–16458. doi: 10.1523/JNEUROSCI.3177-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao JL, Vialou VF, Lobo MK, Robison AJ, Neve RL, Cooper DC, Nestler EJ, Han MH. Essential role of the cAMP-cAMP response-element binding protein pathway in opiateinduced homeostatic adaptations of locus coeruleus neurons. Proc Natl Acad Sci U S A. 2010b;107:17011–17016. doi: 10.1073/pnas.1010077107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caporale N, Dan Y. Spike timing-dependent plasticity: a Hebbian learning rule. Annu Rev Neurosci. 2008;31:25–46. doi: 10.1146/annurev.neuro.31.060407.125639. [DOI] [PubMed] [Google Scholar]
- Carlezon WA, Boundy VA, Haile CN, Lane SB, Kalb RG, Neve RL, Nestler EJ. Sensitization to Morphine Induced by Viral-Mediated Gene Transfer. Science. 1997;277:812–815. doi: 10.1126/science.277.5327.812. [DOI] [PubMed] [Google Scholar]
- Carlezon WA, Haile CN, Coppersmith R, Hayashi Y, Malinow R, Neve RL, Nestler EJ. Distinct Sites of Opiate Reward and Aversion within the Midbrain Identified Using a Herpes Simplex Virus Vector Expressing GluR1. The Journal of Neuroscience. 2000a;20:RC62. doi: 10.1523/JNEUROSCI.20-05-j0002.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlezon WA, Jr, Nestler EJ, Neve RL. Herpes simplex virus-mediated gene transfer as a tool for neuropsychiatric research. Crit Rev Neurobiol. 2000b;14:47–67. doi: 10.1080/08913810008443546. [DOI] [PubMed] [Google Scholar]
- Cazorla M, Premont J, Mann A, Girard N, Kellendonk C, Rognan D. Identification of a low-molecular weight TrkB antagonist with anxiolytic and antidepressant activity in mice. J Clin Invest. 2011;121:1846–1857. doi: 10.1172/JCI43992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhury D, Juarez B, Tsai H, Lobo M, Walsh J, Friedman A, Mouzon E, Mogri M, Deisseroth K, Nestler E, Han M. Optogenetic manipulation of dopaminergic neurons in the brain reward circuit modulates susceptibility to social defeat stress [Abstract] Society for Neuroscie 2011 [Google Scholar]
- Chen ZY, Patel PD, Sant G, Meng CX, Teng KK, Hempstead BL, Lee FS. Variant brain-derived neurotrophic factor (BDNF) (Met66) alters the intracellular trafficking and activity-dependent secretion of wild-type BDNF in neurosecretory cells and cortical neurons. J Neurosci. 2004;24:4401–4411. doi: 10.1523/JNEUROSCI.0348-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi KH, Edwards S, Graham DL, Larson EB, Whisler KN, Simmons D, Friedman AK, Walsh JJ, Rahman Z, Monteggia LM, Eisch AJ, Neve RL, Nestler EJ, Han MH, Self DW. Reinforcement-related regulation of AMPA glutamate receptor subunits in the ventral tegmental area enhances motivation for cocaine. J Neurosci. 2011;31:7927–7937. doi: 10.1523/JNEUROSCI.6014-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christoffel DJ, Golden SA, Dumitriu D, Robison AJ, Janssen WG, Ahn HF, Krishnan V, Reyes CM, Han MH, Ables JL, Eisch AJ, Dietz DM, Ferguson D, Neve RL, Greengard P, Kim Y, Morrison JH, Russo SJ. IkappaB kinase regulates social defeat stress-induced synaptic and behavioral plasticity. J Neurosci. 2011;31:314–321. doi: 10.1523/JNEUROSCI.4763-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciocchi S, Herry C, Grenier F, Wolff SBE, Letzkus JJ, Vlachos I, Ehrlich I, Sprengel R, Deisseroth K, Stadler MB, Muller C, Luthi A. Encoding of conditioned fear in central amygdala inhibitory circuits. Nature. 2010;468:277–282. doi: 10.1038/nature09559. [DOI] [PubMed] [Google Scholar]
- Collingridge GL, Peineau S, Howland JG, Wang YT. Long-term depression in the CNS. Nat Rev Neurosci. 2010;11:459–473. doi: 10.1038/nrn2867. [DOI] [PubMed] [Google Scholar]
- Coque L, Mukherjee S, Cao JL, Spencer S, Marvin M, Falcon E, Sidor MM, Birnbaum SG, Graham A, Neve RL, Gordon E, Ozburn AR, Goldberg MS, Han MH, Cooper DC, McClung CA. Specific Role of VTA Dopamine Neuronal Firing Rates and Morphology in the Reversal of Anxiety-Related, but not Depression-Related Behavior in the Clock[Delta]19 Mouse Model of Mania. Neuropsychopharmacology. 2011;36:1478–1488. doi: 10.1038/npp.2011.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deisseroth K, Feng G, Majewska AK, Miesenbock G, Ting A, Schnitzer MJ. Next-generation optical technologies for illuminating genetically targeted brain circuits. J Neurosci. 2006;26:10380–10386. doi: 10.1523/JNEUROSCI.3863-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai NS, Cudmore RH, Nelson SB, Turrigiano GG. Critical periods for experience-dependent synaptic scaling in visual cortex. Nat Neurosci. 2002;5:783–789. doi: 10.1038/nn878. [DOI] [PubMed] [Google Scholar]
- Dong Y, Green T, Saal D, Marie H, Neve R, Nestler EJ, Malenka RC. CREB modulates excitability of nucleus accumbens neurons. Nat Neurosci. 2006;9:475–477. doi: 10.1038/nn1661. [DOI] [PubMed] [Google Scholar]
- Eaton WW, Martins SS, Nestadt G, Bienvenu OJ, Clarke D, Alexandre P. The burden of mental disorders. Epidemiol Rev. 2008;30:1–14. doi: 10.1093/epirev/mxn011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ermentrout GB, Galan RF, Urban NN. Reliability, synchrony and noise. Trends Neurosci. 2008;31:428–434. doi: 10.1016/j.tins.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson SM, Eskenazi D, Ishikawa M, Wanat MJ, Phillips PE, Dong Y, Roth BL, Neumaier JF. Transient neuronal inhibition reveals opposing roles of indirect and direct pathways in sensitization. Nat Neurosci. 2011;14:22–24. doi: 10.1038/nn.2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson SM, Sandygren NA, Neumaier JF. Pairing mild stress with increased serotonin-1B receptor expression in the nucleus accumbens increases susceptibility to amphetamine. European Journal of Neuroscience. 2009;30:1576–1584. doi: 10.1111/j.1460-9568.2009.06933.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flavell SW, Cowan CW, Kim TK, Greer PL, Lin Y, Paradis S, Griffith EC, Hu LS, Chen C, Greenberg ME. Activity-Dependent Regulation of MEF2 Transcription Factors Suppresses Excitatory Synapse Number. Science. 2006;311:1008–1012. doi: 10.1126/science.1122511. [DOI] [PubMed] [Google Scholar]
- Friedman AK, Walsh JJ, Covington HE, III, Juarez B, Dietz DM, Li X, Pan N, Chaudhury D, Vialou VF, Yue Z, Ribadeneira M, Wong E, Han MH. Ih and K+ channels as mechanistically novel drug targets for depression treatment [Abstract] Society for Neuroscie 2011 [Google Scholar]
- Goold CP, Nicoll RA. Single-Cell Optogenetic Excitation Drives Homeostatic Synaptic Depression. Neuron. 2010;68:512–528. doi: 10.1016/j.neuron.2010.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace AA, Floresco SB, Goto Y, Lodge DJ. Regulation of firing of dopaminergic neurons and control of goal-directed behaviors. Trends in Neurosciences. 2007;30:220–227. doi: 10.1016/j.tins.2007.03.003. [DOI] [PubMed] [Google Scholar]
- Gradinaru V, Thompson KR, Zhang F, Mogri M, Kay K, Schneider MB, Deisseroth K. Targeting and readout strategies for fast optical neural control in vitro and in vivo. J Neurosci. 2007;27:14231–14238. doi: 10.1523/JNEUROSCI.3578-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gradinaru V, Zhang F, Ramakrishnan C, Mattis J, Prakash R, Diester I, Goshen I, Thompson KR, Deisseroth K. Molecular and Cellular Approaches for Diversifying and Extending Optogenetics. Cell. 2010;141:154–165. doi: 10.1016/j.cell.2010.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham DL, Edwards S, Bachtell RK, DiLeone RJ, Rios M, Self DW. Dynamic BDNF activity in nucleus accumbens with cocaine use increases self-administration and relapse. Nat Neurosci. 2007;10:1029–1037. doi: 10.1038/nn1929. [DOI] [PubMed] [Google Scholar]
- Graham DL, Krishnan V, Larson EB, Graham A, Edwards S, Bachtell RK, Simmons D, Gent LM, Berton O, Bolanos CA, DiLeone RJ, Parada LF, Nestler EJ, Self DW. Tropomyosin-Related Kinase B in the Mesolimbic Dopamine System: Region-Specific Effects on Cocaine Reward. Biological Psychiatry. 2009;65:696–701. doi: 10.1016/j.biopsych.2008.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han MH, Bolanos CA, Green TA, Olson VG, Neve RL, Liu RJ, Aghajanian GK, Nestler EJ. Role of cAMP response element-binding protein in the rat locus ceruleus: regulation of neuronal activity and opiate withdrawal behaviors. J Neurosci. 2006;26:4624–4629. doi: 10.1523/JNEUROSCI.4701-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison C. Mood disorders: Small-molecule neurotrophin antagonist reduces anxiety. Nat Rev Drug Discov. 2011;10:415. doi: 10.1038/nrd3470. [DOI] [PubMed] [Google Scholar]
- Haubensak W, Kunwar PS, Cai H, Ciocchi S, Wall NR, Ponnusamy R, Biag J, Dong HW, Deisseroth K, Callaway EM, Fanselow MS, Luthi A, Anderson DJ. Genetic dissection of an amygdala microcircuit that gates conditioned fear. Nature. 2010;468:270–276. doi: 10.1038/nature09553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawasli AH, Benavides DR, Nguyen C, Kansy JW, Hayashi K, Chambon P, Greengard P, Powell CM, Cooper DC, Bibb JA. Cyclin-dependent kinase 5 governs learning and synaptic plasticity via control of NMDAR degradation. Nat Neurosci. 2007;10:880–886. doi: 10.1038/nn1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman JP, Flak J, Jankord R. Chronic stress plasticity in the hypothalamic paraventricular nucleus. Prog Brain Res. 2008;170:353–364. doi: 10.1016/S0079-6123(08)00429-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoplight BJ, Sandygren NA, Neumaier JF. Increased expression of 5-HT1B receptors in rat nucleus accumbens via virally mediated gene transfer increases voluntary alcohol consumption. Alcohol. 2006;38:73–79. doi: 10.1016/j.alcohol.2006.04.003. [DOI] [PubMed] [Google Scholar]
- Huang YH, Lin Y, Brown TE, Han MH, Saal DB, Neve RL, Zukin RS, Sorg BA, Nestler EJ, Malenka RC, Dong Y. CREB modulates the functional output of nucleus accumbens neurons: a critical role of N-methyl-D-aspartate glutamate receptor (NMDAR) receptors. J Biol Chem. 2008;283:2751–2760. doi: 10.1074/jbc.M706578200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YH, Lin Y, Mu P, Lee BR, Brown TE, Wayman G, Marie H, Liu W, Yan Z, Sorg BA, Schluter OM, Zukin RS, Dong Y. In vivo cocaine experience generates silent synapses. Neuron. 2009;63:40–47. doi: 10.1016/j.neuron.2009.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman SE, Malenka RC. Addiction and the brain: the neurobiology of compulsion and its persistence. Nat Rev Neurosci. 2001;2:695–703. doi: 10.1038/35094560. [DOI] [PubMed] [Google Scholar]
- Hyman SE, Malenka RC, Nestler EJ. Neural mechanisms of addiction: the role of reward-related learning and memory. Annu Rev Neurosci. 2006;29:565–598. doi: 10.1146/annurev.neuro.29.051605.113009. [DOI] [PubMed] [Google Scholar]
- Iniguez SD, Vialou V, Warren BL, Cao JL, Alcantara LF, Davis LC, Manojlovic Z, Neve RL, Russo SJ, Han MH, Nestler EJ, Bolanos-Guzman CA. Extracellular signal-regulated kinase-2 within the ventral tegmental area regulates responses to stress. J Neurosci. 2010;30:7652–7663. doi: 10.1523/JNEUROSCI.0951-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel TR, Scolnick EM. Cure therapeutics and strategic prevention: raising the bar for mental health research. Mol Psychiatry. 2006;11:11–17. doi: 10.1038/sj.mp.4001777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inyushin MU, Arencibia-Albite F, Vazquez-Torres R, Velez-Hernandez ME, Jimenez-Rivera CA. Alpha-2 noradrenergic receptor activation inhibits the hyperpolarization-activated cation current (Ih) in neurons of the ventral tegmental area. Neuroscience. 2010;167:287–297. doi: 10.1016/j.neuroscience.2010.01.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov A, Aston-Jones G. Local opiate withdrawal in locus coeruleus neurons in vitro. J Neurophysiol. 2001;85:2388–2397. doi: 10.1152/jn.2001.85.6.2388. [DOI] [PubMed] [Google Scholar]
- Kauer JA, Malenka RC. Synaptic plasticity and addiction. Nat Rev Neurosci. 2007;8:844–858. doi: 10.1038/nrn2234. [DOI] [PubMed] [Google Scholar]
- Klugmann M, Szumlinski KK. Targeting Homer genes using adeno-associated viral vector: lessons learned from behavioural and neurochemical studies. Behavioural Pharmacology. 2008;19:485–500. doi: 10.1097/FBP.0b013e32830c369f. 410.1097/FBP.1090b1013e32830c32369f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knackstedt LA, Moussawi K, Lalumiere R, Schwendt M, Klugmann M, Kalivas PW. Extinction Training after Cocaine Self-Administration Induces Glutamatergic Plasticity to Inhibit Cocaine Seeking. The Journal of Neuroscience. 2010;30:7984–7992. doi: 10.1523/JNEUROSCI.1244-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi T, Ikeda K, Kojima H, Niki H, Yano R, Yoshioka T, Kumanishi T. Ethanol opens G-protein-activated inwardly rectifying K+ channels. Nat Neurosci. 1999;2:1091–1097. doi: 10.1038/16019. [DOI] [PubMed] [Google Scholar]
- Krishnan V, Han MH, Graham DL, Berton O, Renthal W, Russo SJ, Laplant Q, Graham A, Lutter M, Lagace DC, Ghose S, Reister R, Tannous P, Green TA, Neve RL, Chakravarty S, Kumar A, Eisch AJ, Self DW, Lee FS, Tamminga CA, Cooper DC, Gershenfeld HK, Nestler EJ. Molecular adaptations underlying susceptibility and resistance to social defeat in brain reward regions. Cell. 2007;131:391–404. doi: 10.1016/j.cell.2007.09.018. [DOI] [PubMed] [Google Scholar]
- Krishnan V, Han MH, Mazei-Robison M, Iniguez SD, Ables JL, Vialou V, Berton O, Ghose S, Covington HE, 3rd, Wiley MD, Henderson RP, Neve RL, Eisch AJ, Tamminga CA, Russo SJ, Bolanos CA, Nestler EJ. AKT signaling within the ventral tegmental area regulates cellular and behavioral responses to stressful stimuli. Biol Psychiatry. 2008;64:691–700. doi: 10.1016/j.biopsych.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane-Ladd SB, Pineda J, Boundy VA, Pfeuffer T, Krupinski J, Aghajanian GK, Nestler EJ. CREB (cAMP response element-binding protein) in the locus coeruleus: biochemical, physiological, and behavioral evidence for a role in opiate dependence. J Neurosci. 1997;17:7890–7901. doi: 10.1523/JNEUROSCI.17-20-07890.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux JE. Emotion Circuits in the Brain. Annual Review of Neuroscience. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- LeMasson G, Marder E, Abbott LF. Activity-dependent regulation of conductances in model neurons. Science. 1993;259:1915–1917. doi: 10.1126/science.8456317. [DOI] [PubMed] [Google Scholar]
- Lestienne R. Spike timing, synchronization and information processing on the sensory side of the central nervous system. Prog Neurobiol. 2001;65:545–591. doi: 10.1016/s0301-0082(01)00019-3. [DOI] [PubMed] [Google Scholar]
- Lewis AS, Vaidya SP, Blaiss CA, Liu Z, Stoub TR, Brager DH, Chen X, Bender RA, Estep CM, Popov AB, Kang CE, Van Veldhoven PP, Bayliss DA, Nicholson DA, Powell CM, Johnston D, Chetkovich DM. Deletion of the Hyperpolarization-Activated Cyclic Nucleotide-Gated Channel Auxiliary Subunit TRIP8b Impairs Hippocampal Ih Localization and Function and Promotes Antidepressant Behavior in Mice. J Neurosci. 2011;31:7424–7440. doi: 10.1523/JNEUROSCI.0936-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewohl JM, Wilson WR, Mayfield RD, Brozowski SJ, Morrisett RA, Harris RA. G-protein-coupled inwardly rectifying potassium channels are targets of alcohol action. Nat Neurosci. 1999;2:1084–1090. doi: 10.1038/16012. [DOI] [PubMed] [Google Scholar]
- Li N, Lee B, Liu RJ, Banasr M, Dwyer JM, Iwata M, Li XY, Aghajanian G, Duman RS. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science. 2010;329:959–964. doi: 10.1126/science.1190287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu QS, Pu L, Poo MM. Repeated cocaine exposure in vivo facilitates LTP induction in midbrain dopamine neurons. Nature. 2005;437:1027–1031. doi: 10.1038/nature04050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobo MK, Covington HE, 3rd, Chaudhury D, Friedman AK, Sun H, Damez-Werno D, Dietz DM, Zaman S, Koo JW, Kennedy PJ, Mouzon E, Mogri M, Neve RL, Deisseroth K, Han MH, Nestler EJ. Cell type-specific loss of BDNF signaling mimics optogenetic control of cocaine reward. Science. 2010;330:385–390. doi: 10.1126/science.1188472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luscher C, Malenka RC. Drug-evoked synaptic plasticity in addiction: from molecular changes to circuit remodeling. Neuron. 2011;69:650–663. doi: 10.1016/j.neuron.2011.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luscher C, Slesinger PA. Emerging roles for G protein-gated inwardly rectifying potassium (GIRK) channels in health and disease. Nat Rev Neurosci. 2010;11:301–315. doi: 10.1038/nrn2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maffei A, Nelson SB, Turrigiano GG. Selective reconfiguration of layer 4 visual cortical circuitry by visual deprivation. Nat Neurosci. 2004;7:1353–1359. doi: 10.1038/nn1351. [DOI] [PubMed] [Google Scholar]
- Maren S, Quirk GJ. Neuronal signalling of fear memory. Nat Rev Neurosci. 2004;5:844–852. doi: 10.1038/nrn1535. [DOI] [PubMed] [Google Scholar]
- Marie H, Morishita W, Yu X, Calakos N, Malenka RC. Generation of silent synapses by acute in vivo expression of CaMKIV and CREB. Neuron. 2005;45:741–752. doi: 10.1016/j.neuron.2005.01.039. [DOI] [PubMed] [Google Scholar]
- Martin BJ, Naughton BJ, Thirtamara-Rajamani K, Yoon DJ, Han DD, Devries AC, Gu HH. Dopamine transporter inhibition is necessary for cocaine-induced increases in dendritic spine density in the nucleus accumbens. Synapse. 2011;65:490–496. doi: 10.1002/syn.20865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee S, Coque L, Cao JL, Kumar J, Chakravarty S, Asaithamby A, Graham A, Gordon E, Enwright JF, 3rd, DiLeone RJ, Birnbaum SG, Cooper DC, McClung CA. Knockdown of Clock in the ventral tegmental area through RNA interference results in a mixed state of mania and depression-like behavior. Biol Psychiatry. 2010;68:503–511. doi: 10.1016/j.biopsych.2010.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NAMHCW-Report. Report of the National Advisory Mental Health Council’s Workgroup. 2010. From Discovery to Cure. [Google Scholar]
- Nestler EJ. Molecular basis of long-term plasticity underlying addiction. Nat Rev Neurosci. 2001;2:119–128. doi: 10.1038/35053570. [DOI] [PubMed] [Google Scholar]
- Nestler EJ. Is there a common molecular pathway for addiction? Nat Neurosci. 2005;8:1445–1449. doi: 10.1038/nn1578. [DOI] [PubMed] [Google Scholar]
- Nestler EJ, Aghajanian GK. Molecular and cellular basis of addiction. Science. 1997;278:58–63. doi: 10.1126/science.278.5335.58. [DOI] [PubMed] [Google Scholar]
- Nestler EJ, Barrot M, DiLeone RJ, Eisch AJ, Gold SJ, Monteggia LM. Neurobiology of Depression. Neuron. 2002a;34:13–25. doi: 10.1016/s0896-6273(02)00653-0. [DOI] [PubMed] [Google Scholar]
- Nestler EJ, Gould E, Manji H, Buncan M, Duman RS, Greshenfeld HK, Hen R, Koester S, Lederhendler I, Meaney M, Robbins T, Winsky L, Zalcman S. Preclinical models: status of basic research in depression. Biol Psychiatry. 2002b;52:503–528. doi: 10.1016/s0006-3223(02)01405-1. [DOI] [PubMed] [Google Scholar]
- Neuhoff H, Neu A, Liss B, Roeper J. I(h) channels contribute to the different functional properties of identified dopaminergic subpopulations in the midbrain. J Neurosci. 2002;22:1290–1302. doi: 10.1523/JNEUROSCI.22-04-01290.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumaier JF, Vincow ES, Arvanitogiannis A, Wise RA, Carlezon WA. Elevated Expression of 5-HT1B Receptors in Nucleus Accumbens Efferents Sensitizes Animals to Cocaine. The Journal of Neuroscience. 2002;22:10856–10863. doi: 10.1523/JNEUROSCI.22-24-10856.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neve RL, Neve KA, Nestler EJ, Carlezon WA., Jr Use of herpes virus amplicon vectors to study brain disorders. Biotechniques. 2005;39:381–391. doi: 10.2144/05393PS01. [DOI] [PubMed] [Google Scholar]
- Neves G, Cooke SF, Bliss TV. Synaptic plasticity, memory and the hippocampus: a neural network approach to causality. Nat Rev Neurosci. 2008;9:65–75. doi: 10.1038/nrn2303. [DOI] [PubMed] [Google Scholar]
- Pfeiffer BE, Zang T, Wilkerson JR, Taniguchi M, Maksimova MA, Smith LN, Cowan CW, Huber KM. Fragile X mental retardation protein is required for synapse elimination by the activity-dependent transcription factor MEF2. Neuron. 2010;66:191–197. doi: 10.1016/j.neuron.2010.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulipparacharuvil S, Renthal W, Hale CF, Taniguchi M, Xiao G, Kumar A, Russo SJ, Sikder D, Dewey CM, Davis MM, Greengard P, Nairn AC, Nestler EJ, Cowan CW. Cocaine regulates MEF2 to control synaptic and behavioral plasticity. Neuron. 2008;59:621–633. doi: 10.1016/j.neuron.2008.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinak CA, Zimmerman JM, Chang CH, Orsini CA. Bidirectional changes in the intrinsic excitability of infralimbic neurons reflect a possible regulatory role in the acquisition and extinction of Pavlovian conditioned fear. J Neurosci. 2008;28:7245–7247. doi: 10.1523/JNEUROSCI.2130-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen K. The role of the locus coeruleus and N-methyl-D-aspartic acid (NMDA) and AMPA receptors in opiate withdrawal. Neuropsychopharmacology. 1995;13:295–300. doi: 10.1016/0893-133X(95)00082-O. [DOI] [PubMed] [Google Scholar]
- Rasmussen K, Beitner-Johnson DB, Krystal JH, Aghajanian GK, Nestler EJ. Opiate withdrawal and the rat locus coeruleus: behavioral, electrophysiological, and biochemical correlates. J Neurosci. 1990;10:2308–2317. doi: 10.1523/JNEUROSCI.10-07-02308.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renthal W, Kumar A, Xiao G, Wilkinson M, Covington HE, 3rd, Maze I, Sikder D, Robison AJ, LaPlant Q, Dietz DM, Russo SJ, Vialou V, Chakravarty S, Kodadek TJ, Stack A, Kabbaj M, Nestler EJ. Genome-wide analysis of chromatin regulation by cocaine reveals a role for sirtuins. Neuron. 2009;62:335–348. doi: 10.1016/j.neuron.2009.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE, Kolb B. Morphine alters the structure of neurons in the nucleus accumbens and neocortex of rats. Synapse. 1999;33:160–162. doi: 10.1002/(SICI)1098-2396(199908)33:2<160::AID-SYN6>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Roybal K, Theobold D, Graham A, DiNieri JA, Russo SJ, Krishnan V, Chakravarty S, Peevey J, Oehrlein N, Birnbaum S, Vitaterna MH, Orsulak P, Takahashi JS, Nestler EJ, Carlezon WA, Jr, McClung CA. Mania-like behavior induced by disruption of CLOCK. Proc Natl Acad Sci U S A. 2007;104:6406–6411. doi: 10.1073/pnas.0609625104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo SJ, Bolanos CA, Theobald DE, DeCarolis NA, Renthal W, Kumar A, Winstanley CA, Renthal NE, Wiley MD, Self DW, Russell DS, Neve RL, Eisch AJ, Nestler EJ. IRS2-Akt pathway in midbrain dopamine neurons regulates behavioral and cellular responses to opiates. Nat Neurosci. 2007;10:93–99. doi: 10.1038/nn1812. [DOI] [PubMed] [Google Scholar]
- Russo SJ, Dietz DM, Dumitriu D, Morrison JH, Malenka RC, Nestler EJ. The addicted synapse: mechanisms of synaptic and structural plasticity in nucleus accumbens. Trends Neurosci. 2010;33:267–276. doi: 10.1016/j.tins.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo SJ, Wilkinson MB, Mazei-Robison MS, Dietz DM, Maze I, Krishnan V, Renthal W, Graham A, Birnbaum SG, Green TA, Robison B, Lesselyong A, Perrotti LI, Bolanos CA, Kumar A, Clark MS, Neumaier JF, Neve RL, Bhakar AL, Barker PA, Nestler EJ. Nuclear factor kappa B signaling regulates neuronal morphology and cocaine reward. J Neurosci. 2009;29:3529–3537. doi: 10.1523/JNEUROSCI.6173-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saal D, Dong Y, Bonci A, Malenka RC. Drugs of abuse and stress trigger a common synaptic adaptation in dopamine neurons. Neuron. 2003;37:577–582. doi: 10.1016/s0896-6273(03)00021-7. [DOI] [PubMed] [Google Scholar]
- Saar D, Barkai E. Long-lasting maintenance of learning-induced enhanced neuronal excitability: mechanisms and functional significance. Mol Neurobiol. 2009;39:171–177. doi: 10.1007/s12035-009-8060-5. [DOI] [PubMed] [Google Scholar]
- Santos SF, Pierrot N, Octave JN. Network excitability dysfunction in Alzheimer’s disease: insights from in vitro and in vivo models. Rev Neurosci. 2010;21:153–171. doi: 10.1515/revneuro.2010.21.3.153. [DOI] [PubMed] [Google Scholar]
- Sari Y. Serotonin1B receptors: from protein to physiological function and behavior. Neurosci Biobehav Rev. 2004;28:565–582. doi: 10.1016/j.neubiorev.2004.08.008. [DOI] [PubMed] [Google Scholar]
- Schneider M, Spanagel R, Zhang SJ, Bading H, Klugmann M. Adeno-associated virus (AAV)-mediated suppression of Ca2+/calmodulin kinase IV activity in the nucleus accumbens modulates emotional behaviour in mice. BMC Neurosci. 2007;8:105. doi: 10.1186/1471-2202-8-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sklair-Tavron L, Shi WX, Lane SB, Harris HW, Bunney BS, Nestler EJ. Chronic morphine induces visible changes in the morphology of mesolimbic dopamine neurons. Proc Natl Acad Sci U S A. 1996;93:11202–11207. doi: 10.1073/pnas.93.20.11202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuber GD, Sparta DR, Stamatakis AM, van Leeuwen WA, Hardjoprajitno JE, Cho S, Tye KM, Kempadoo KA, Zhang F, Deisseroth K, Bonci A. Excitatory transmission from the amygdala to nucleus accumbens facilitates reward seeking. Nature. 2011;475:377–380. doi: 10.1038/nature10194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szumlinski KK, Ary AW, Lominac KD, Klugmann M, Kippin TE. Accumbens Homer2 overexpression facilitates alcohol-induced neuroplasticity in C57BL/6J mice. Neuropsychopharmacology. 2008;33:1365–1378. doi: 10.1038/sj.npp.1301473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szumlinski KK, Lominac KD, Oleson EB, Walker JK, Mason A, Dehoff MH, Klugman M, Cagle S, Welt K, During M, Worley PF, Middaugh LD, Kalivas PW. Homer2 Is Necessary for EtOH-Induced Neuroplasticity. The Journal of Neuroscience. 2005;25:7054–7061. doi: 10.1523/JNEUROSCI.1529-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todtenkopf MS, Parsegian A, Naydenov A, Neve RL, Konradi C, Carlezon WA. Brain Reward Regulated by AMPA Receptor Subunits in Nucleus Accumbens Shell. The Journal of Neuroscience. 2006;26:11665–11669. doi: 10.1523/JNEUROSCI.3070-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai HC, Zhang F, Adamantidis A, Stuber GD, Bonci A, de Lecea L, Deisseroth K. Phasic firing in dopaminergic neurons is sufficient for behavioral conditioning. Science. 2009;324:1080–1084. doi: 10.1126/science.1168878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsankova N, Renthal W, Kumar A, Nestler EJ. Epigenetic regulation in psychiatric disorders. Nat Rev Neurosci. 2007;8:355–367. doi: 10.1038/nrn2132. [DOI] [PubMed] [Google Scholar]
- Turrigiano G. Too Many Cooks? Intrinsic and Synaptic Homeostatic Mechanisms in Cortical Circuit Refinement. Annu Rev Neurosci. 2011 doi: 10.1146/annurev-neuro-060909-153238. [DOI] [PubMed] [Google Scholar]
- Turrigiano G, Abbott LF, Marder E. Activity-dependent changes in the intrinsic properties of cultured neurons. Science. 1994;264:974–977. doi: 10.1126/science.8178157. [DOI] [PubMed] [Google Scholar]
- Turrigiano GG. The self-tuning neuron: synaptic scaling of excitatory synapses. Cell. 2008;135:422–435. doi: 10.1016/j.cell.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turrigiano GG, Leslie KR, Desai NS, Rutherford LC, Nelson SB. Activity-dependent scaling of quantal amplitude in neocortical neurons. Nature. 1998;391:892–896. doi: 10.1038/36103. [DOI] [PubMed] [Google Scholar]
- Turrigiano GG, Nelson SB. Homeostatic plasticity in the developing nervous system. Nat Rev Neurosci. 2004;5:97–107. doi: 10.1038/nrn1327. [DOI] [PubMed] [Google Scholar]
- Tye KM, Prakash R, Kim SY, Fenno LE, Grosenick L, Zarabi H, Thompson KR, Gradinaru V, Ramakrishnan C, Deisseroth K. Amygdala circuitry mediating reversible and bidirectional control of anxiety. Nature. 2011;471:358–362. doi: 10.1038/nature09820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungless MA, Whistler JL, Malenka RC, Bonci A. Single cocaine exposure in vivo induces long-term potentiation in dopamine neurons. Nature. 2001;411:583–587. doi: 10.1038/35079077. [DOI] [PubMed] [Google Scholar]
- van den Pol AN, Ghosh PK, Liu RJ, Li Y, Aghajanian GK, Gao XB. Hypocretin (orexin) enhances neuron activity and cell synchrony in developing mouse GFP-expressing locus coeruleus. J Physiol. 2002;541:169–185. doi: 10.1113/jphysiol.2002.017426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vialou V, Robison AJ, Laplant QC, Covington HE, 3rd, Dietz DM, Ohnishi YN, Mouzon E, Rush AJ, 3rd, Watts EL, Wallace DL, Iniguez SD, Ohnishi YH, Steiner MA, Warren BL, Krishnan V, Bolanos CA, Neve RL, Ghose S, Berton O, Tamminga CA, Nestler EJ. DeltaFosB in brain reward circuits mediates resilience to stress and antidepressant responses. Nat Neurosci. 2010;13:745–752. doi: 10.1038/nn.2551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace DL, Han MH, Graham DL, Green TA, Vialou V, Iniguez SD, Cao JL, Kirk A, Chakravarty S, Kumar A, Krishnan V, Neve RL, Cooper DC, Bolanos CA, Barrot M, McClung CA, Nestler EJ. CREB regulation of nucleus accumbens excitability mediates social isolation-induced behavioral deficits. Nat Neurosci. 2009;12:200–209. doi: 10.1038/nn.2257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YY, Aghajanian GK. Excitation of locus coeruleus neurons by vasoactive intestinal peptide: role of a cAMP and protein kinase A. J Neurosci. 1990;10:3335–3343. doi: 10.1523/JNEUROSCI.10-10-03335.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson MB, Xiao G, Kumar A, LaPlant Q, Renthal W, Sikder D, Kodadek TJ, Nestler EJ. Imipramine treatment and resiliency exhibit similar chromatin regulation in the mouse nucleus accumbens in depression models. J Neurosci. 2009;29:7820–7832. doi: 10.1523/JNEUROSCI.0932-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf ME. The Bermuda Triangle of cocaine-induced neuroadaptations. Trends Neurosci. 2010;33:391–398. doi: 10.1016/j.tins.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F, Aravanis AM, Adamantidis A, de Lecea L, Deisseroth K. Circuit-breakers: optical technologies for probing neural signals and systems. Nat Rev Neurosci. 2007;8:577–581. doi: 10.1038/nrn2192. [DOI] [PubMed] [Google Scholar]
- Zhang W, Linden DJ. The other side of the engram: experience-driven changes in neuronal intrinsic excitability. Nat Rev Neurosci. 2003;4:885–900. doi: 10.1038/nrn1248. [DOI] [PubMed] [Google Scholar]