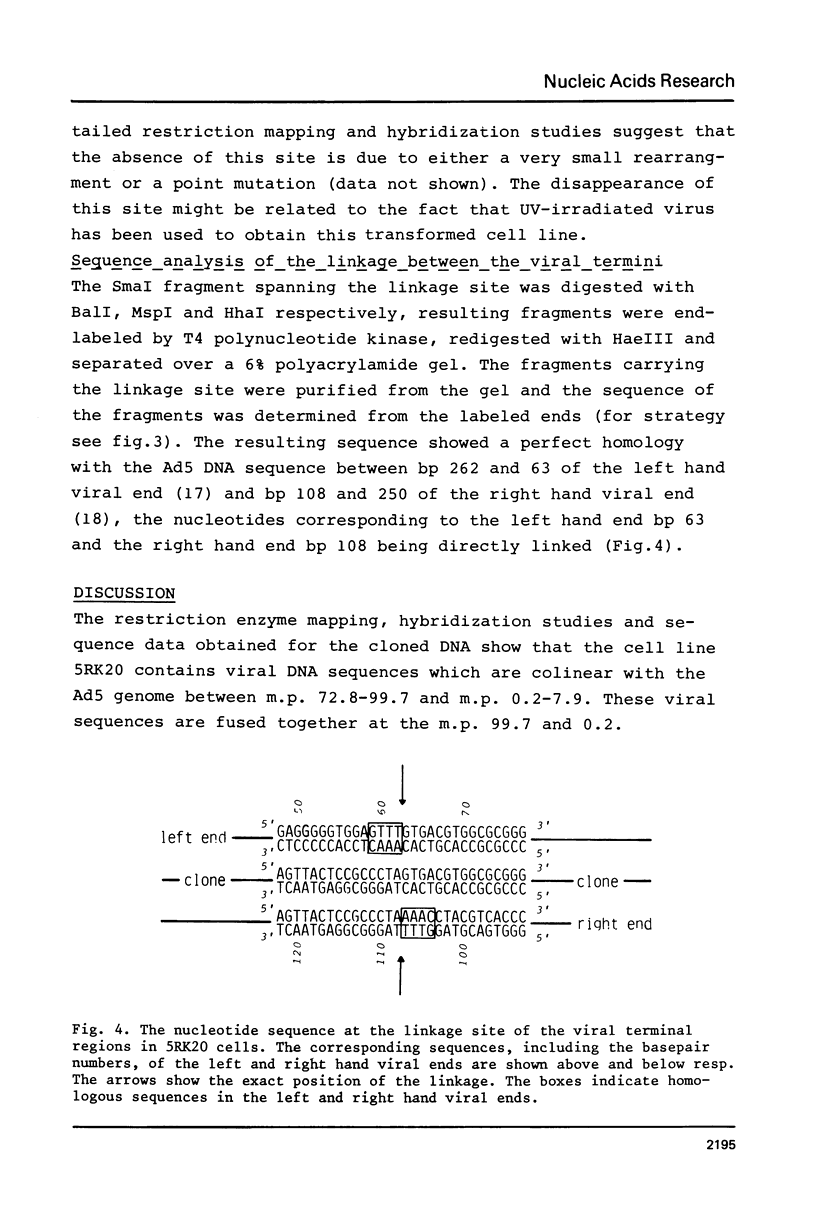
Abstract
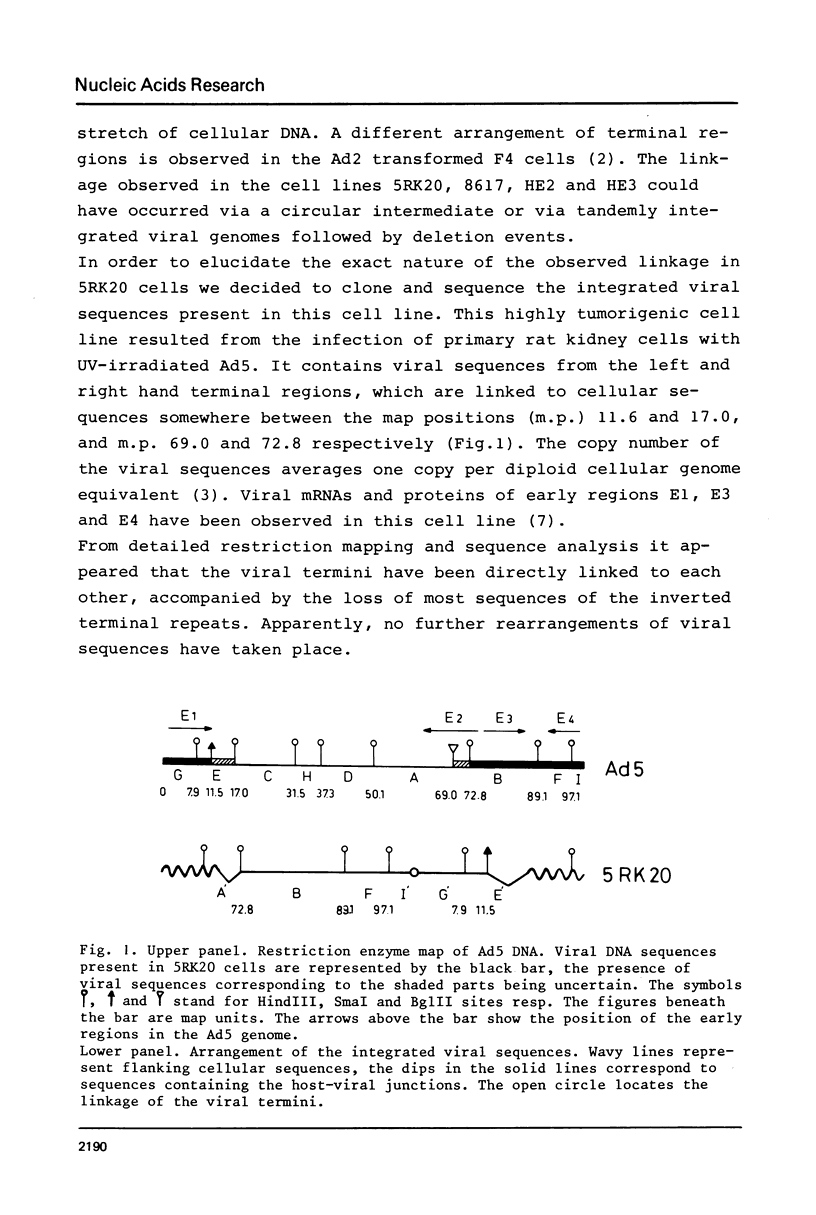
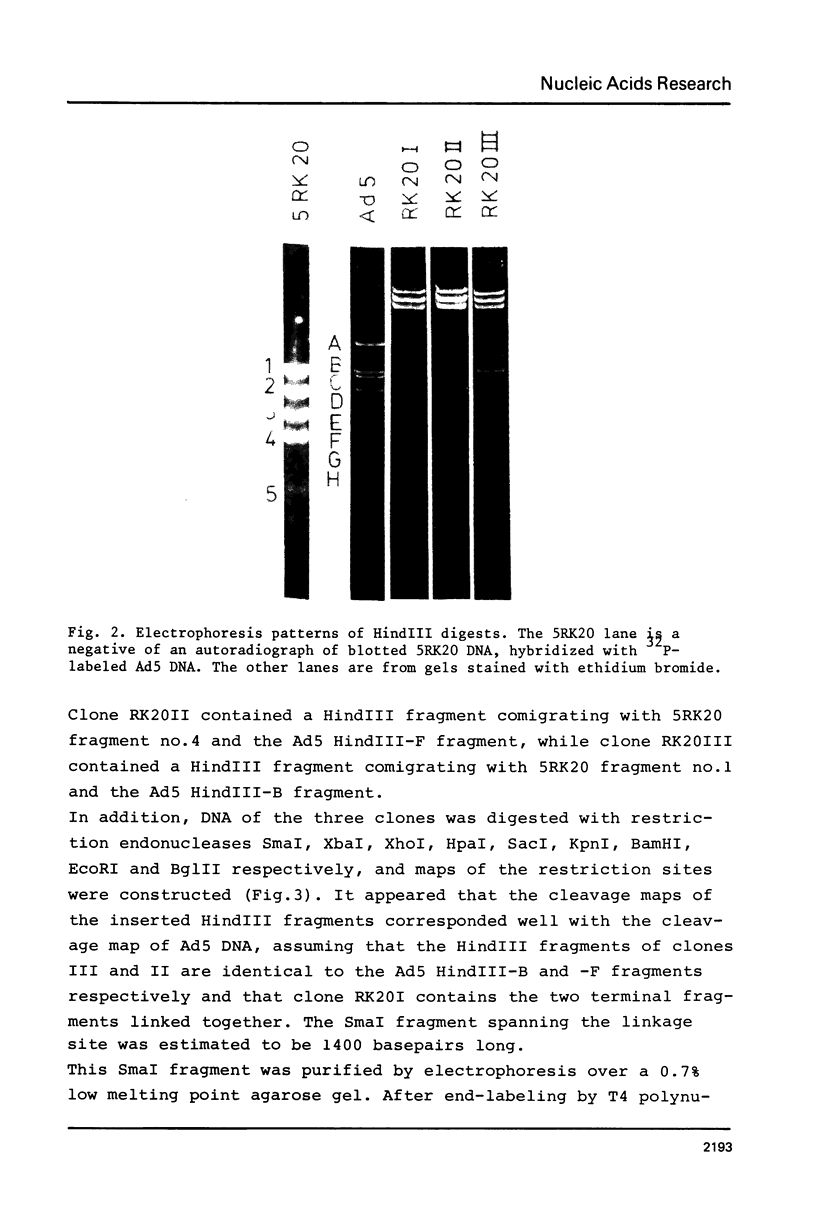
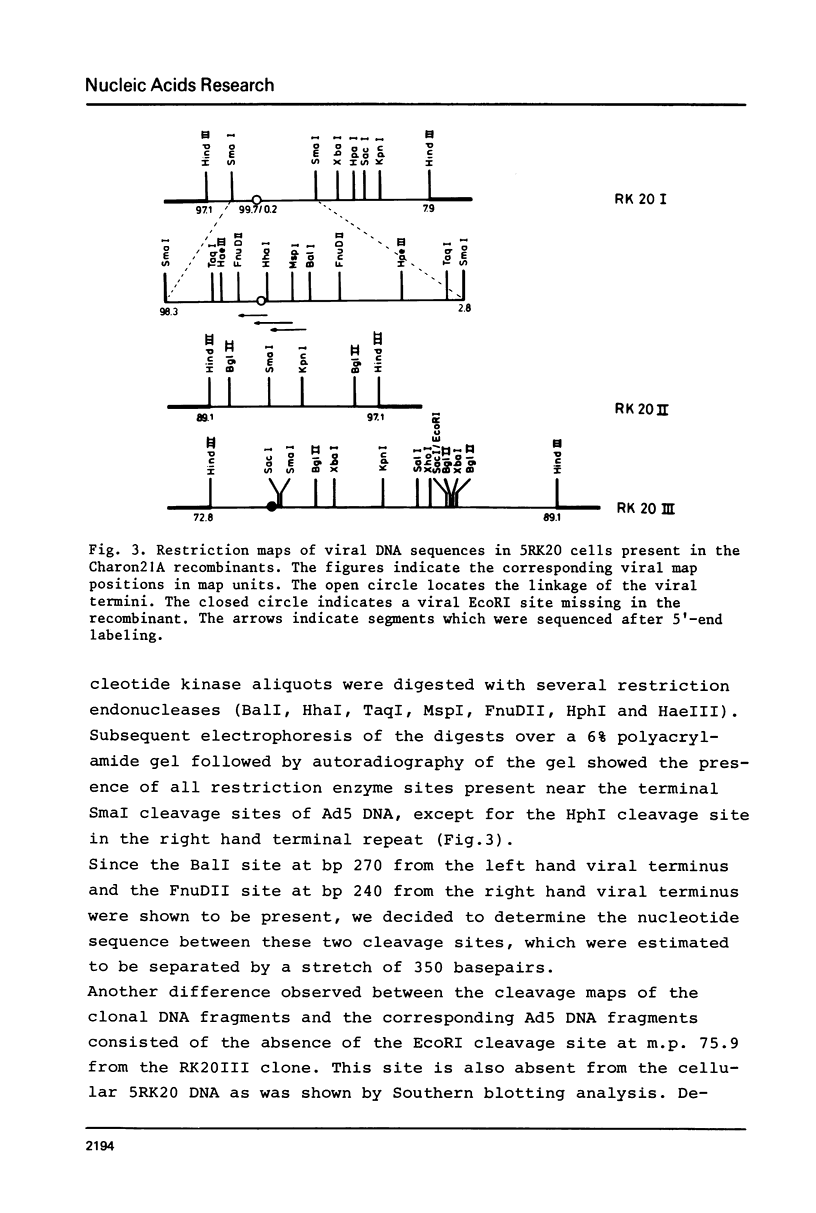
A peculiar phenomenon is observed in several adenovirus type 2 or 5 (Ad2 or Ad5) transformed cell lines: the right hand and left hand terminal regions of the viral genome present in the viral DNA insertions of these cell lines are found to be linked together. A large part of the viral DNA insertion present in the Ad5 transformed rat cell line 5RK20 has been cloned in the lambda vector Charon21A, including the segment containing the linked terminal regions. Sequence analysis of the linkage region showed a perfect homology with the Ad5 DNA sequence and a direct linkage of basepair (bp) 63 of the left hand end of the viral genome to bp 108 of the right hand end. No cellular or rearranged viral sequences were present. Our findings suggest that the joining of viral sequences into the cellular genome.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arrand J. R., Roberts R. J. The nucleotide sequences at the termini of adenovirus-2 DNA. J Mol Biol. 1979 Mar 15;128(4):577–594. doi: 10.1016/0022-2836(79)90294-8. [DOI] [PubMed] [Google Scholar]
- Benton W. D., Davis R. W. Screening lambdagt recombinant clones by hybridization to single plaques in situ. Science. 1977 Apr 8;196(4286):180–182. doi: 10.1126/science.322279. [DOI] [PubMed] [Google Scholar]
- Challberg M. D., Desiderio S. V., Kelly T. J., Jr Adenovirus DNA replication in vitro: characterization of a protein covalently linked to nascent DNA strands. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5105–5109. doi: 10.1073/pnas.77.9.5105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green M., Wold W. S., Büttner W. Integration and transcription of group C human adenovirus sequences in the DNA of five lines of transformed rat cells. J Mol Biol. 1981 Sep 25;151(3):337–366. doi: 10.1016/0022-2836(81)90001-2. [DOI] [PubMed] [Google Scholar]
- Hohn B. In vitro packaging of lambda and cosmid DNA. Methods Enzymol. 1979;68:299–309. doi: 10.1016/0076-6879(79)68021-7. [DOI] [PubMed] [Google Scholar]
- Jeffreys A. J., Flavell R. A. A physical map of the DNA regions flanking the rabbit beta-globin gene. Cell. 1977 Oct;12(2):429–439. doi: 10.1016/0092-8674(77)90119-2. [DOI] [PubMed] [Google Scholar]
- Keegstra W., Van Wielink P. S., Sussenbach J. S. The visualization of a circular DNA-protein complex from adenovirions. Virology. 1977 Jan;76(1):444–447. doi: 10.1016/0042-6822(77)90320-8. [DOI] [PubMed] [Google Scholar]
- Kruijer W., van Schaik F. M., Sussenbach J. S. Nucleotide sequence analysis of a region of adenovirus 5 DNA encoding a hitherto unidentified gene. Nucleic Acids Res. 1980 Dec 20;8(24):6033–6042. doi: 10.1093/nar/8.24.6033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniatis T., Hardison R. C., Lacy E., Lauer J., O'Connell C., Quon D., Sim G. K., Efstratiadis A. The isolation of structural genes from libraries of eucaryotic DNA. Cell. 1978 Oct;15(2):687–701. doi: 10.1016/0092-8674(78)90036-3. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Sambrook J., Greene R., Stringer J., Mitchison T., Hu S. L., Botchan M. Analysis of the sites of integration of viral DNA sequences in rat cells transformed by adenovirus 2 or SV40. Cold Spring Harb Symp Quant Biol. 1980;44(Pt 1):569–584. doi: 10.1101/sqb.1980.044.01.059. [DOI] [PubMed] [Google Scholar]
- Steenbergh P. H., Sussenbach J. S. The nucleotide sequence of the right-hand terminus of adenovirus type 5 DNA: implications for the mechanism of DNA replication. Gene. 1979 Aug;6(4):307–318. doi: 10.1016/0378-1119(79)90071-4. [DOI] [PubMed] [Google Scholar]
- Stenlund A., Perricaudet M., Tiollais P., Pettersson U. Construction of restriction enzyme fragment libraries containing DNA from human adenovirus types 2 and 5. Gene. 1980 Jun;10(1):47–52. doi: 10.1016/0378-1119(80)90142-0. [DOI] [PubMed] [Google Scholar]
- Vardimon L., Doerfler W. Patterns of integration of viral DNA in adenovirus type 2-transformed hamster cells. J Mol Biol. 1981 Apr 5;147(2):227–246. doi: 10.1016/0022-2836(81)90439-3. [DOI] [PubMed] [Google Scholar]
- Visser L., Wassenaar A. T., van Maarschalkerweerd M. W., Rozijn T. H. Arrangement of integrated viral DNA sequences in cells transformed by adenovirus types 2 and 5. J Virol. 1981 Sep;39(3):684–693. doi: 10.1128/jvi.39.3.684-693.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieslander L. A simple method to recover intact high molecular weight RNA and DNA after electrophoretic separation in low gelling temperature agarose gels. Anal Biochem. 1979 Oct 1;98(2):305–309. doi: 10.1016/0003-2697(79)90145-3. [DOI] [PubMed] [Google Scholar]
- van Ormondt H., Maat J., van Beveren C. P. The nucleotide sequence of the transforming early region E1 of adenovirus type 5 DNA. Gene. 1980 Nov;11(3-4):299–309. doi: 10.1016/0378-1119(80)90070-0. [DOI] [PubMed] [Google Scholar]