Abstract
Background
We investigated the clinical and biological significance of p130cas, an important cell signaling molecule, in ovarian carcinoma.
Methods
Expression of p130cas in ovarian tumors, as assessed by immunohistochemistry, was associated with tumor characteristics and patient survival. The effects of p130cas gene silencing with small interfering RNAs incorporated into neutral nanoliposomes (siRNA-DOPC), alone and in combination with docetaxel, on in vivo tumor growth and on tumor cell proliferation (proliferating cell nuclear antigen) and apoptosis (terminal deoxynucleotidyl transferase dUTP nick-end labeling) were examined in mice bearing orthotopic taxane-sensitive (HeyA8 and SKOV3ip1) or taxane-resistant (HeyA8-MDR) ovarian tumors (n = 10 per group). To determine the specific mechanisms by which p130cas gene silencing abrogates tumor growth, we measured cell viability (MTT assay), apoptosis (fluorescence-activated cell sorting), autophagy (immunoblotting, fluorescence, and transmission electron microscopy), and cell signaling (immunoblotting) in vitro. All statistical tests were two-sided.
Results
Of 91 ovarian cancer specimens, 70 (76%) had high p130cas expression; and 21 (24%) had low p130cas expression. High p130cas expression was associated with advanced tumor stage (P < .001) and higher residual disease (>1 cm) following primary cytoreduction surgery (P = .007) and inversely associated with overall survival and progression-free survival (median overall survival: high p130cas expression vs low expression, 2.14 vs 9.1 years, difference = 6.96 years, 95% confidence interval = 1.69 to 9.48 years, P < .001; median progression-free survival: high p130cas expression vs low expression, 1.04 vs 2.13 years, difference = 1.09 years, 95% confidence interval = 0.47 to 2.60 years, P = .01). In mice bearing orthotopically implanted HeyA8 or SKOV3ip1 ovarian tumors, treatment with p130cas siRNA-DOPC in combination with docetaxel chemotherapy resulted in the greatest reduction in tumor growth compared with control siRNA therapy (92%–95% reduction in tumor growth; P < .001 for all). Compared with control siRNA therapy, p130cas siRNA-DOPC reduced SKOV3ip1 cell proliferation (31% reduction, P < .001) and increased apoptosis (143% increase, P < .001) in vivo. Increased tumor cell apoptosis may have persisted despite pan-caspase inhibition by the induction of autophagy and related signaling pathways.
Conclusions
Increased p130cas expression is associated with poor clinical outcome in human ovarian carcinoma, and p130cas gene silencing decreases tumor growth through stimulation of apoptotic and autophagic cell death.
CONTEXT AND CAVEATS
Prior knowledge
The signaling scaffold protein, p130cas, is involved in cellular signaling pathways related to cell migration and transformation. Overexpression of p130cas has been linked to poor prognosis in breast and prostate cancer, but its role in ovarian cancer was unclear.
Study design
p130Cas expression was examined in 91 ovarian tumor specimens. Small interfering RNA (siRNA) was used to silence p130cas expression in mice bearing orthotopically grafted human ovarian tumors. The effect of p130cas siRNA on apoptosis, autophagy, and cell signaling was studied in SKOV3ip1 and HeyA8 ovarian cancer cells in vitro.
Contribution
High p130cas expression was associated with more advanced ovarian cancer stage and poorer prognosis. Liposomes carrying p130 siRNA reduced growth and increased apoptosis in tumor xenografts, especially in combination with docetaxel chemotherapy. In vitro tests suggested that this was likely due to changes in cell signaling that coincided with the induction of autophagy.
Implications
Overexpression of p130 cas is associated with poor ovarian cancer outcome; its inhibition is a potential target for ovarian cancer therapy.
Limitations
All mechanistic and therapeutic tests were performed in cultured human cells and immunodeficient mice with xenografts, respectively. Further testing is necessary to determine whether p130cas is a viable target in humans with cancer.
From the Editors
Ovarian cancer remains the deadliest among all gynecologic malignancies (1). Because the premalignant state is poorly understood and there is no efficient screening strategy (2–5), most ovarian cancer patients present with advanced-stage disease (6). Despite initial response rates of 80% with the current standard therapy (7,8), the probability of a sustained response remains poor; most patients experience tumor recurrence and eventual emergence of multidrug resistance that contribute to poor overall survival rates (9). This clinical reality highlights the need for more efficacious therapies. As we learn more about the molecular mechanisms of ovarian carcinogenesis and progression, several putative targets have been identified (10–13).
The cas (Crk-associated substrate) family of proteins serves as an integral player in many signaling pathways that govern normal and pathological intracellular processes. p130Cas (product of the breast cancer anti-estrogen resistance 1, or BCAR1, gene), the premier member of this group of scaffold proteins, was first described as a heavily tyrosine-phosphorylated component of v-src and v-crk transformed cells (14). Posttranslational phosphorylation of tyrosine residues in the substrate binding domain of p130cas opens binding sites for a variety of effector proteins (15). p130Cas itself has no intrinsic kinase activity but instead functions as a signal assembler, promoting protein-protein interactions. These protein interactions affect numerous downstream signaling pathways that influence cell migration (16–18), shape (16–21), apoptosis (22–24), and cell transformation (25,26). The vital role of p130cas in cellular function is further supported by the early lethality that accompanies cardiovascular anomalies noted in p130cas-null embryos (27) and the inability of p130cas-null fibroblasts to achieve a fully transformed phenotype despite the presence of activated src (28). Although the relevance of p130cas expression to ovarian cancer remains to be delineated, p130cas overexpression has been linked to decreased patient survival and to poor prognostic features in patients with breast and prostate carcinomas (29–33). Collectively, these characteristics uniquely qualify p130cas as a desirable target in cancer therapeutics. Furthermore, targeting the upstream location of p130cas confers a theoretical advantage over existing molecular therapies because it has the potential to affect several distinct downstream signaling pathways simultaneously; if only a single pathway were inhibited, it might induce a compensatory response resulting in no net change in cell growth properties.
Despite the promise of p130cas as a therapeutic target, there are several noteworthy limitations associated with designing drugs capable of affecting the scaffolding function of p130cas (34–37). Such limitations include the absence of an intrinsic kinase activity and a large, unstable, and incompletely characterized three-dimensional structure. Previously, our laboratory developed a novel method for in vivo gene silencing using neutral nanoliposomes containing small interfering RNA (siRNA) (38–40). RNA interference can potentially be used to overcome each of the known limitations in targeting p130cas, making it an attainable cancer therapeutic.
In this study, we examined levels of p130cas expression in tumors from patients with epithelial ovarian cancer and tested whether silencing p130cas with siRNA is effective in reducing tumor growth in a preclinical model of ovarian carcinoma.
Methods
Human Ovarian Cancer Specimens
Following approval from the University of Texas M. D. Anderson Cancer Center Institutional Review Board for the Protection of Human Subjects, 91 paraffin-embedded epithelial ovarian cancer specimens with available clinical outcome data were obtained from the University of Texas M. D. Anderson Cancer Center tumor bank. Diagnosis of epithelial ovarian cancer was first confirmed by a board certified gynecologic pathologist. All patients were diagnosed from 1985 to 2003 and treated with a primary attempt at cytoreductive surgery. Eighty-one percent of patients were treated with paclitaxel- and platinum-based adjuvant chemotherapy. Clinical variables obtained for correlative analyses included age at diagnosis, tumor stage as determined by the International Federation of Gynecology and Obstetrics staging system, tumor grade, presence or absence of ascitic fluid, degree of cytoreduction (optimal vs suboptimal, with optimal surgical cytoreduction defined as all disease nodules at completion of primary cytoreductive surgery being <1 cm), date of tumor recurrence, and patient status relative to disease-specific survival at the time of chart review.
Immunohistochemistry
Immunohistochemical analysis of p130cas and of proliferating cell nuclear antigen (PCNA) was conducted on 4-μm thick, formalin-fixed paraffin-embedded epithelial ovarian cancer specimens from patient tumor samples and from orthotopic xenografts (described below), respectively. Slides were deparaffinized and rehydrated. Antigen retrieval for p130cas was performed using 0.1 M citrate buffer (pH 6.0) under steam for 40 minutes followed by a 30-minute cooling to room temperature. Antigen retrieval for PCNA was performed by heating the slides to 98°C for 10 minutes in 0.1 M citrate buffer (pH 6.0) in a microwave oven (EZ-retriever System; BioGenex, San Ramon, CA).
Following antigen retrieval, endogenous peroxidases were blocked with 3% hydrogen peroxide in phosphate-buffered saline (PBS) for 12 minutes at room temperature followed by nonspecific protein blocking with either 5% normal horse serum in PBS (in the case of p130cas) or 5% normal horse serum and 1% normal goat serum in PBS (in the case of PCNA) for 30 minutes at room temperature. Sections were then incubated with primary antibodies to p130cas (mouse monoclonal anti-human, 1:200 dilution; Neomarkers, Fremont, CA) or PCNA (PC-10 mouse monoclonal IgG, 1:50 dilution; Dako, Carpinteria, CA) in their respective blocking solutions overnight at 4°C. Secondary amplification was performed using either the MACH4 polymer detection system (for p130cas: Biocare Medical, Concord, CA) or the appropriate horseradish peroxidase (HRP)–conjugated secondary antibody. Visualization was achieved with 3,3′-diaminobezidine (DAB; Open Biosystems, Huntsville, AL). Slides were counterstained with Gill No. 2 hematoxylin (Sigma-Aldrich, St Louis, MO) and mounted with Universal Mount (Research Genetics, Huntsville, AL).
Clinical samples were scored for staining with the p130cas antibody by a board certified gynecologic pathologist blinded to the clinical outcome of the patients (MTD). p130cas expression was determined semiquantitatively by assessing the distribution of the positive cells and the staining intensity in the tumor cells. The distribution of positive cells was rated as follows: 0 points, no staining; 1 point, focal or less than 25%; 2 points, 25%–50%, 3 points, 50%–75%; 4 points, 75%–100%. The staining intensity was rated as focal or weak (1 point), moderate (2 points), or heavy (3 points). Points for intensity and distribution were added, and an overall score ranging from 0 to 2 was assigned. An overall score of 0 was assigned for negative expression of p130cas if 5% or fewer cells were stained, regardless of the intensity. An overall score of 1 (1–4 points) designated weak expression of p130cas, and an overall score of 2 (5–7 points) designated p130cas overexpression (41).
Quantification of tumor cell proliferation was done as previously described (40). Briefly, images were taken of five random fields from each slide after PCNA staining (×100 final magnification; Nikon MICROPHOT-FX, 3CCD camera [Sony Corporation of America, Montavale, NJ] and Optimas Image Analysis Software [Bioscan, Edmond, VA]). Five slides (one slide per mouse) were stained for PCNA for all respective treatment groups.
Immunofluorescence Staining
Terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate nick-end labeling (TUNEL) staining was performed on frozen orthotopic tumors that were fixed with 4% paraformaldehyde using the commercially available Promega TUNEL Kit (Promega, Madison, WI) as previously described (42). DNA fragmentation was detected by localized green fluorescence within the nucleus of apoptotic cells. Five slides (one slide per mouse) were stained for TUNEL for all respective treatment groups. Quantification of TUNEL was determined by the number of TUNEL-positive cells in five random fields at ×200 magnification.
Cell Lines and Cultures
The derivation and source of the SKOV3ip1 and HeyA8 cell lines (derived from human ovarian cancer cell lines passaged through mice) has been previously described (39,40). The taxane-resistant cell lines HeyA8-MDR (a kind gift from Dr Isaiah J. Fidler, Department of Cancer Biology, University of Texas M. D. Anderson Cancer Center) and SKOV3TRip2 (derived from intraperitoneal tumors formed after injection of SKOV3TR cells in mice; a kind gift from Dr Michael Seiden, Department of Medicine, Fox Chase Cancer Center, Philadelphia, PA) were also used. All cell lines were maintained and propagated in RPMI-1640 media supplemented with 15% fetal bovine serum (FBS) and 0.1% gentamicin sulfate (Gemini Bioproducts, Calabasas, CA). The media for the HeyA8-MDR and SKOV3TRip2 cells also contained 100 nM docetaxel. All cell lines were purchased from the University of Texas M. D. Anderson Cancer Center Characterized Cell Line Core Facility which provides authenticated cell lines. In addition, cell lines were routinely tested to confirm the absence of Mycoplasma, and all experiments were performed with cell lines at 60%–80% confluence.
p130Cas Gene Silencing by Transfection With siRNA
Human-specific p130cas siRNA was purchased from Sigma-Aldrich and used to silence p130cas expression in ovarian cancer cell lines (target sequence: 5′-CAGCATCACGCAGGGCAA-3′). A non-targeting siRNA (target sequence: 5′-AATTCTCCGAACGTGTCACGT-3′; purchased from Sigma-Aldrich and confirmed to have no sequence homology with any known human or murine mRNA by BLAST analysis [http://www.ncbi.nlm.nih.gov/blast/Blast.cgi]) was used as a control for all in vitro and in vivo experiments. For in vitro transfections, 5 × 105 cells per plate were plated into 10-cm plates. After cells were attached, the medium was replaced with RPMI-1640 supplemented with 0.1% gentamicin sulfate, 8 μg of siRNA (p130cas or control), and 30 μL of Lipofectamine 2000 transfection reagent (Invitrogen, Carlsbad, CA). The cells were incubated until the desired time points were reached. Cells were washed with cold PBS supplemented with 1 mM sodium orthovanadate and incubated with modified radioimmunoprecipitation assay (RIPA) lysis buffer with 1× protease inhibitor (Roche, Mannheim, Germany) and 1 mM sodium orthovanadate for 20 minutes on ice. Cell lysates were collected and centrifuged at 19 000g for 20 minutes at 4°C. Protein concentration of each sample was determined by a bicinconinic acid Protein Assay Reagent kit (Thermo Scientific, Rockford, IL). Twenty micrograms of whole-cell lysate was fractionated by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), transferred to nitrocellulose, blocked with 5% nonfat milk for 1 hour at room temperature, and probed with mouse anti-human p130cas antibody (Neomarkers) at 1:3000 dilution overnight at 4°C. Blots were then incubated with HRP-conjugated anti-mouse secondary antibody (The Jackson Laboratory, Bar Harbor, ME) at 1:2000 dilution and developed with the use of an enhanced chemiluminescence detection kit (ECL; Amersham Pharmacia Biotech, Piscataway, NJ). For protein loading, a monoclonal actin antibody (Sigma-Aldrich) was used at 1:2000 dilution.
Liposome Incorporation of siRNA
For in vivo delivery, siRNAs (p130cas and control) were incorporated into neutral nanoliposomes (1,2-dioleoyl-dn-glycero-3-phosphatidylcholine [DOPC]), lyophilized, and stored at −20°C as previously described (39). Before in vivo delivery, liposomal siRNA was rehydrated with PBS to a final concentration of 5.0 μg per 200 μL.
p130Cas siRNA Therapy in an Orthotopic Ovarian Cancer Model
Female athymic nude mice were purchased from the National Cancer Institute-Frederick Cancer Research and Development Center (Frederick, MD) and housed in specific pathogen-free conditions. Mice were cared for in accordance with the guidelines set forth by the American Association for Accreditation for Laboratory Animal Care and the US Public Health Service Policy on Human Care and Use of Laboratory Animals. All studies were approved and supervised by the MDACC Institutional Animal Care and Use Committee.
Development and characterization of the orthotopic murine model of advanced ovarian cancer used in these experiments has been previous described by our laboratory (39,40). Briefly, human ovarian cancer cells grown in culture were incubated with either EDTA (HeyA8 or HeyA8-MDR) or trypsin in EDTA (SKOV3ip1), centrifuged, washed twice with Hank’s balanced salt solution (Gibco, Carlsbad, CA), and resuspended at a concentration of 1.25 × 106 cells per mL (HeyA8) or 5 × 106 cells per mL (HeyA8-MDR or SKOV3ip1). Each mouse was injected intraperitoneally with 200 μL of the cell suspension. To test the effect of p130cas siRNA-DOPC on tumor growth alone and in combination with docetaxel (Sanofi-aventis, Bridgewater, NJ), 8 days after tumor cell injection, mice (n = 10 mice per treatment group) were randomly assigned to receive treatment by intraperitoneal injection with control siRNA-DOPC (5.0 μg of siRNA [200 μL injection] per mouse, twice weekly), p130cas siRNA-DOPC (5.0 μg of siRNA [200 μL injection] per mouse, twice weekly), control siRNA-DOPC plus docetaxel (50 μg per mouse weekly for HeyA8 and HeyA8-MDR and 35 μg per mouse weekly for SKOV3ip1), or p130cas siRNA-DOPC plus docetaxel. Mice were monitored for signs of adverse events. All mice were necropsied when the control mice became moribund, which was approximately 28 days after HeyA8 cell injection, 32 days after HeyA8-MDR cell injection, and 38 days after SKOV3ip1 cell injection. Before necropsy, mice were killed by cervical dislocation. Mean tumor weights, the number of tumor nodules, the pattern or disease spread, and the presence and amount of ascites and mouse body weight were recorded. Tumors were snap frozen for later protein or RNA analysis and fixed in formalin for paraffin bedding or snap frozen in optimal cutting media (OCT; Miles Inc, Elkhart, IN) for frozen slide preparation.
Tumor Cell Viability Assay
SKOV3ip1 and SKOV3TRip2 cells (2 × 103) were plated in each well of a 96-well plate. Experimental conditions were set in quadruplicate. After cells were attached, the culture medium was replaced with serum-free medium plus 5 pmol siRNA per well (p130cas or control) and 0.25 μL per well of Lipofectamine 2000 (Invitrogen) transfection reagent. The following day, the cells were incubated with decreasing concentrations of docetaxel (ranging from 100 to 0.05 nM), and incubated at 37°C until the untreated cells became 100% confluent (approximately 120 hours). To assess cell viability, 50 μL of 0.15% 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (Sigma-Aldrich) was added to each well and incubated for 2 hours at 37°C. The medium containing 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide was then removed, and 100 μL of dimethyl sulfoxide (DMSO) was added. Absorbance was read at 570 nm (Ceres UV 900C; Bio-Tek Instrument Inc, Winooski, VT) after incubation at room temperature for 5 minutes. Experimental conditions were set in quadruplicate, and viability assays were repeated three times to confirm results.
Tumor Cell Apoptosis Assay
Apoptosis was studied using the Annexin V-phycoerythrin (PE) apoptosis detection kit (BD Biosciences, San Diego, CA). Approximately 5 × 105 SKOV3ip1 or SKOV3TRip2 ovarian cancer cells were plated per 10-cm plate. After cells were attached, media was replaced and cells were incubated with siRNA (p130cas or control) as described above. After incubation at 37°C for 6 hours, the cells were incubated with docetaxel at IC50 concentrations (1 nM for SKOV3ip1 and 100 nM for SKOV3TRip2). Cells were harvested at 48-, 72-, and 96-hour time points following siRNA therapy. At each respective time point, cells were incubated in trypsin–EDTA and collected. For purposes of this experiment, both the floating and attached cells were harvested. Cell pellets were resuspended in 1 mL of 1× binding buffer. One hundred microliters of the cell suspension was incubated with 5 μL of Annexin V-PE and 5 μL of 7-aminoactinomycin D (7-AAD) at room temperature (25°C) in the dark for 15 minutes. Following this incubation, 400 μL of 1× binding buffer was added to each tube, and samples were analyzed by fluorescence-activated cell sorting (FACS). Each time point was repeated three times to confirm results.
Apoptosis was also examined after treatment with a control or p130cas siRNA in the presence of a pan-caspase inhibitor, benzyloxycarbonyl-Val-Ala-Asp (OMe) fluoromethylketone (ZVAD-fmk; R&D Systems, Minneapolis, MN). For these experiments, SKOV3ip1 or SKOV3TRip2 cells (5.0 × 104 cells per well) were plated into six-well plates. After the cells were attached, the medium was replaced with serum-free medium containing either control siRNA alone (100 pmol siRNA and 5 μL Lipofectamine 2000 [Invitrogen]), control siRNA plus 20 μM ZVAD-fmk (R&D Systems), p130cas siRNA alone (100 pmol siRNA and 5 μL Lipofectamine 2000 [Invitrogen]), or p130cas siRNA plus 20 μM ZVAD-fmk (R&D Systems). Cell culture medium was replaced with serum-containing medium alone or serum-containing medium plus 20 μM ZVAD-fmk (R&D Systems) daily until 72 hours. At 72 hours, both floating and attached cells were harvested and analyzed by flow cytometry for Annexin V-PE staining according to the methods described above.
Tumor Cell Migration and Invasion Assays
Motility in the absence of a chemoattractant was determined in membrane invasion culture system chambers containing a polycarbonate filter with 10-μm pores coated with 0.1% gelatin, as previously described (43). Untreated HeyA8 cells (7.5 × 104) or HeyA8 cells that had been transfected 48 hours earlier with either p130cas or control siRNA were seeded in each upper well and were incubated at 37°C for 6 hours in RPMI-1640 medium containing 10% FBS and subsequently processed by fixation with staining. The number of cells that migrated to the lower side of the membrane was determined by examining five random fields (at ×100 magnification) per test condition. Triplicate samples were measured for each condition and results were averaged. Invasion assays were carried out in a similar fashion, using membrane invasion culture system chambers containing a polycarbonate filter with 10-μm pores coated with a collagen-based matrix. For invasion assay, the time frame of incubation was extended to overnight (ie, approximately 20 hours).
p130Cas Signaling Detection by RNA Microarray
Total RNA was extracted from the HeyA8 cell line following transfection with control and p130cas siRNA in vitro using a mirVana RNA Isolation labeling kit (Ambion, Inc., Austin, TX). Five hundred nanograms of total RNA were used for labeling and hybridization, according to the manufacturer’s protocols (Illumina). After the bead chips were scanned with an Illumina BeadArray Reader (Illumina, San Diego, CA), the microarray data were normalized using the quantile normalization method in the Linear Models for Microarray Data (LIMMA) package in the R language environment. The expression level of each gene was transformed into a log2 base before further analysis.
The comprehensive network of human genes was constructed by compiling protein–protein interactions in the Human Protein Reference Database (HPRD), Gene (44), the Biomarker Interaction Network Database (BIND) (45), and IntAct. Signaling interactions were compiled from Biocarta and the Kyoto Encyclopedia of Genes and Genomes [KEGG (46)] as well as through manual curation. Transcription factor–target interactions were obtained from the open regulatory annotation database [ORegAnno; (47)] and TRANSFAC (Biobase, Beverly, MA). Functional interactions between genes were constructed based on the statistical significance of overlap of their Gene Ontology (48) annotations as well as their common assignment to a KEGG metabolic pathway. Scoring of network components (nodes, edges) for their relevance to a given dataset is done by a random walk–based scoring strategy by using the data values of nodes as transition probabilities (P. T. Ram, unpublished data). Briefly, each interaction i–j is assigned a probability value (pij) based on the data values of nodes in the neighborhood:
where wj is the experimental value for node j and Ni is the set of immediate downstream neighbors of node i. If there are no downstream nodes of the node i (|Ni| = 0), pij is set to pij = 1/n for all j, where n is the total number of nodes in the network. Also at each step, we assign a small probability (q = .01) that the random walk will “jump” to any other node in the network. Final relevance scores of nodes are given by their visitation frequencies by the random walk in the end of infinite iterations (ie, value at the stationary distribution of the random walk) (49).
Immunoblot Analysis of Cleaved poly-ADP ribose polymerase (PARP) and Bcl-2 Family Members in Ovarian Cancer Cells
SKOV3ip1 and SKOV3TRip2 cells were plated at concentrations of 5 × 105 cells per 10-cm plate and treated with control or p130cas siRNA as described above. Six hours following siRNA transfection, cells were treated with docetaxel at IC50 concentrations (1 nM for SKOV3ip1 and 100 nM for SKOV3TRip2) and incubated at 37°C for 72 hours. Cell lysates were prepared following either no treatment or treatment with vehicle, p130cas siRNA alone, or in combination with docetaxel chemotherapy in modified RIPA buffer (50 mM Tris–HCl [pH 7.4], 150 mM NaCl, 1% Triton, 0.5% deoxycholate) plus 25 μg/mL leupeptin, 10 μg/mL aprotinin, 2 mM EDTA, and 1 mM sodium orthovanadate. For analysis of cleaved PARP, proteins (50 μg lysate per lane) were resolved by SDS-PAGE on 10% cells and transferred to a nitrocellulose membrane. The membrane was incubated in 5% milk at room temperature for 1 hour to block nonspecific binding and then with an activated PARP antibody to detect PARP cleavage (1:1000 dilution; Cell Signaling, Danvers, MA) overnight at 4°C followed by a HRP-conjugated sheep anti-rabbit IgG antibody (1:2000 dilution; GE Healthcare UK Limited, Buckinghamshire, UK) for 2 hours at room temperature. For analysis of Bcl-2 family members, proteins (50 μg lysate per lane) were resolved by SDS-PAGE on 12% gels and transferred to nitrocellulose membranes. Membranes were incubated in 5% milk for 1 hour at room temperature to block nonspecific binding and then with the respective antibody (1:1000 dilution, Cell Signaling) overnight at 4°C followed by an HRP-conjugated sheep anti-rabbit IgG antibody (1:2000 dilution; GE Healthcare) for 2 hours at room temperature. The membranes were washed, and immunoreactive proteins were detected with the use of an enhanced chemiluminescence Western Blotting Detection Kit (GE Healthcare). Total actin was detected (1:2000 dilution, Sigma-Aldrich) to ensure equal loading between wells. Experiments were repeated once to confirm results.
Immunoblot Analysis of Phosphatidylinositol-3-Kinase (PI3K), Akt, and Mammalian Target of Rapamycin (mTOR) Phosphorylation in Ovarian Cancer Cells
SKOV3ip1 and SKOV3TRip2 cells were plated at concentrations of 5 × 105 cells per 10-cm plate. Cells were treated with control or p130cas siRNA as described above and incubated for 72 hours at 37°C. Cell lysates were prepared for cells treated with control or p130cas siRNA as described above. Again, 50 μg lysate per lane was resolved by SDS-PAGE and transferred to nitrocellulose membranes. Membranes were incubated in 5% milk for 1 hour at room temperature to block nonspecific binding and then with the respective phosphoprotein antibody (1:1000 dilution; Cell Signaling) overnight at 4°C followed by an HRP-conjugated sheep anti-rabbit IgG antibody (1:2000 dilution; GE Healthcare) for 2 hours at room temperature. The membranes were washed, and immunoreactive proteins were detected with the use of an enhanced chemiluminescence Western Blotting Detection Kit (GE Healthcare). As a reference, blots were stripped and probed for the respective total protein (1:1000 dilution; Cell Signaling), or total actin (1:2000 dilution; Sigma-Aldrich) to ensure equal loading between wells. Experiments were repeated once to confirm results.
Immunoblot Analysis of light chain 3 (LC3) and Beclin-1 in Ovarian Cancer Cells
SKOV3ip1 cells were plated at concentrations of 5 × 105 cells per 10-cm plate. After cells were attached, cells were treated with control or p130cas siRNA as described above and incubated for 72 hours at 37°C. Cells were then incubated in trypsin–EDTA and collected by centrifugation, and whole-cell lysates were obtained using a lysis buffer as previously described (50). Aliquots containing 30 μg of total protein from each sample were subjected to SDS-PAGE with a 4%–20% gradient and electrotransferred to nitrocellulose membranes. The membranes were blocked with 5% dry milk in Tris-buffered saline Tween 20 (TBST), probed with primary antibodies anti-LC3 (Axxora, San Diego, CA) and Beclin-1 (Santa Cruz, Biotechnology, Inc., Santa Cruz, CA). The antibodies were diluted in TBST containing 2.5% dry milk and incubated with HRP-conjugated anti-rabbit or anti-mouse secondary antibody (Amersham Life Science, Cleveland, OH). Mouse anti-β-actin and donkey anti-mouse secondary antibodies (Sigma Chemical, St Louis, MO) were used to monitor β-actin expression and to ensure equal loading of proteins. Chemiluminescent detection was performed with Chemi-glow detection reagents (Alpha Innotech, San Leandro, CA). All blots were visualized with a FluorChem 8900 imager and quantified by a densitometer using the Alpha Imager application program (Alpha Innotech).
Transfections With green fluorescent protein (GFP)–LC3 Plasmid and Detection of Autophagosome Formation
Exponentially growing untreated SKOV3ip1 cells were plated 24 hours before transfection. Plated cells were transfected with double-stranded siRNA targeting p130cas mRNA or control siRNA and/or plasmid expressing the GFP and microtubule-associated protein 1 LC3 fusion vector (GFP-LC3) using the transfection reagent according to the manufacturer’s protocol. Untransfected cells and non-silencing control siRNA–transfected cells were used as negative controls. After 72 hours of treatment, the cells were photographed by phase contrast microscopy or fluorescent microscopy to determine the presence of autophagosomes.
Transmission Electron Microscopy
SKOV3ip1 cells were grown on six-well plates, treated with p130cas or control siRNA or left untreated for 72 hours. Cells were fixed for 2 hours with 2.5% glutaraldehyde in 0.1 M cacodylate buffer (pH 7.4), fixed again in 1% OsO4 in the same buffer and then subjected to the electron microscopic analysis as described previously (51). Representative areas were chosen for ultrathin sectioning and viewed with a Hitachi 7600 electron microscope (Hitachi High Technologies America, Inc., Pleasanton, CA).
Statistical Analysis
Fisher exact test was used to examine associations between p130cas expression in human samples and clinical variables. Kaplan–Meier survival curves and log-rank tests were used to examine the association between tumor expression of p130cas and patient disease–specific survival. Multivariable analysis was performed using the Cox proportional hazards model. The data for this analysis were tested (plot of differences in log of cumulative hazard rates of tested variable against time) and found to conform to proportional hazards assumptions. For animal experiments, 10 mice were assigned per treatment group. This sample size gave 80% power to detect a 50% reduction in tumor weight with 95% confidence. Mouse and tumor weights and the number of tumor nodules for each group were compared using the Mann–Whitney rank sum test. A P value less than .05 was deemed statistically significant. All statistical tests were two-sided. All statistical analyses were performed using SPSS version 12 for Windows statistical software (SPSS, Inc., Chicago, IL).
Results
Characterization and Clinical Significance of p130Cas Expression in Human Epithelial Ovarian Carcinoma
We first characterized the degree of p130cas protein expression in tumors from a cohort of ovarian cancer patients (n = 91) and examined associations between p130cas expression and clinical and pathological variables. The median age at diagnosis of our patient cohort was 61 years (range = 36–89 years), and the median follow up was 2.32 years (range = 0.07–13.01 years). Of the 91 cancer specimens evaluated, 70 (76%) had high p130cas expression and 21 (24%) had low expression (Table 1, Figure 1, A). Eighty percent of the examined patients had tumors with serous histology and 87% had high-grade carcinomas. Eighty-three percent of patients had advanced-stage disease (III or IV) at initial diagnosis and 74% had associated ascites. p130Cas overexpression was statistically significantly associated with advanced-stage disease (P < .001) and suboptimal cytoreduction (P = .007). There was no association between the level of p130cas expression and histology (P = .19), presence of ascites (P = .10), or distant metastasis at the time of initial diagnosis (P = .23).
Table 1.
Association of clinicopathologic variables with p130cas staining*
| p130Cas expression |
||||
| Variable | Low | High | P† | |
| Histological Grade | Low | 2 | 2 | |
| High | 19 | 68 | .19 | |
| Stage‡ | I/II | 6 | 2 | |
| III/IV | 15 | 68 | <.001 | |
| Ascites§ | Present | 6 | 9 | |
| Absent | 15 | 59 | .1 | |
| Cytoreduction | Optimal | 18 | 37 | |
| Suboptimal | 3 | 33 | .007 | |
| Distant metastasis§ | Absent | 18 | 51 | |
| Present | 3 | 17 | .23 | |
p130Cas expression in human ovarian carcinoma by immunohistochemical staining for p130cas.
Fisher exact test (two-sided).
Stage [International Federation of Gynecology and Obstetrics staging system (52)].
Data missing from two patients (ascites and distant metastases).
Figure 1.
p130Cas expression in human epithelial ovarian carcinoma. A) Representative images of low vs high levels of immunohistochemical peroxidase staining for p130cas. Both are shown at ×200 magnification (scale bar = 50 μm). The negative control (×100) represents a sample of ovarian cancer tissue used in this study that was processed by immunohistochemistry with the secondary antibody alone. B) Kaplan–Meier curves of overall survival of patients whose ovarian tumors expressed high vs low levels of p130cas. Two-sided comparisons were made using the log-rank statistic. Survival probabilities and 95% confidence intervals at 2 and 4 years for low p130cas expression were 95% (95% CI = 91% to 99%) and 80% (95% CI = 65% to 95%), respectively, and for high p130cas expression were 53% (95% CI = 42% to 65%) and 15% (95% CI = 3% to 27%), respectively. C) Kaplan–Meier curves of progression-free survival for patients whose ovarian tumors expressed high vs low levels of p130cas. Two-sided comparisons were made using the log-rank statistic. Survival probabilities and 95% confidence intervals at 2 and 4 years for low p130cas expression were 47% (95% CI = 34% to 60%) and 7% (95% CI = 2% to 12%), respectively, and for high p130cas expression were 13% (95% CI = 7% to 18%) and 3% (95% CI = 1% to 5%), respectively.
We next evaluated whether tumor expression of the p130cas protein was associated with disease-specific survival and time to recurrence in patients. Kaplan–Meier curves for disease-specific survival revealed that p130cas overexpression was associated with increased ovarian cancer mortality (median survival for patients with high vs low p130cas expression: 2.14 vs 9.1 years, difference = 6.96 years, 95% confidence interval [CI] = 1.69 to 9.48 years, P < .001) (Figure 1, B). In addition, Kaplan–Meier curves for progression-free survival revealed that p130cas overexpression was associated with decreased relapse-free survival (median progression-free survival for patients with high vs low p130cas expression: 1.04 vs 2.13 years, difference = 1.09 years, 95% CI = 0.47 to 2.60 years, P = .01) (Figure 1, C). To determine whether p130cas overexpression was independently associated with patient survival, a multivariable analysis was performed that controlled for patient age, tumor stage, histology, and degree of cytoreduction (Table 2). Similar to the results above, p130cas expression remained statistically significantly associated with patient survival when the analysis was limited to patients with advanced-stage serous ovarian cancer (P < .001). As expected, based on prior literature, advanced-stage (III or IV) disease and suboptimal cytoreduction were each associated with poor survival (hazard ratio [HR] of death from ovarian cancer comparing advanced-stage [III or IV] disease with early-stage disease [I or II] = 1.87, 95% CI = 1.09 to 3.23, P = .02 and HR [comparing suboptimal cytoreduction with optimal cytoreduction] = 2.62, 95% CI = 1.23 to 6.86, P = .002, respectively) (53). p130Cas expression remained statistically significantly associated with poor disease-free survival (HR of death from ovarian cancer = 4.89, 95% CI = 1.87 to 12.83; P = .001).
Table 2.
Multivariable analysis of associations between clinicopathologic variables and disease-specific survival in ovarian cancer patients*
| Variable | HR (95% CI) | P† |
| Age, y | ||
| <60 | 1.00 (referent) | |
| >60 | 1.03 (1.01 to 1.55) | .02 |
| Grade | ||
| Low | 1.00 (referent) | |
| High | 1.42 (0.96 to 17.39) | .42 |
| Stage | ||
| I/II | 1.00 (referent) | |
| III/IV | 1.87 (1.09 to 3.23) | .02 |
| Cytoreduction | ||
| Suboptimal | 1.00 (referent) | |
| Optimal | 2.62 (1.23 to 6.86) | .002 |
| p130cas expression | ||
| Low | 1.00 (referent) | |
| High | 4.89 (1.87 to 12.83) | .001 |
CI = confidence interval; HR = hazard ratio of death from ovarian cancer.
Two-sided, from Cox proportional hazards model (verified to conform to proportional hazards assumptions).
p130cas siRNA-DOPC Therapy in an Orthotopic Model of Advanced Ovarian Cancer
Because we had demonstrated an association between p130cas overexpression and poor overall and relapse-free survival in ovarian cancer patients, we next sought to examine the therapeutic efficacy of silencing p130cas gene expression with siRNA in a murine orthotopic model of advanced ovarian cancer. Before initiating therapy experiments, we first determined levels of p130cas protein expression in a panel of ovarian cancer cell lines (Figure 2, A) and then confirmed that the selected p130cas-targeted siRNA effectively silenced p130cas expression in HeyA8 cells in vitro (Figure 2, B). We observed no difference in p130cas protein expression in cells that were transfected with control siRNA.
Figure 2.
Silencing of p130cas gene expression with small interfering RNA (siRNA) in vitro and in vivo. A) Immunoblot analysis of p130cas protein expression in a panel of ovarian cancer cell lines. Equal amounts of total protein were loaded in each lane; β-actin was used as a loading control. B) Silencing of p130cas expression using siRNA. HeyA8 ovarian cancer cells were transfected with control siRNA or p130cas siRNA in vitro and analyzed after 48–72 hours for p130cas protein expression. β-actin was used as a loading control. C) Decreased p130cas protein expression in HeyA8 orthotopic ovarian tumors collected at the completion of a therapy experiment with p130cas siRNA in neutral nanoliposomes (siRNA-DOPC). β-actin was used as a loading control. D–F) p130cas siRNA-based therapies in nude mice injected with HeyA8 (D), SKOV3ip1 (E), or taxane-resistant HeyA8-MDR ovarian cancer cells (F). In each experiment, 10 mice per group were randomly assigned to receive therapy with control siRNA-DOPC, control siRNA-DOPC plus docetaxel, p130cas siRNA-DOPC, or p130cas siRNA-DOPC plus docetaxel. Mice were killed by cervical dislocation when the mice in the control group became moribund (HeyA8: 28 days; SKOV3ip1: 38 days; HeyA8-MDR: 32 days), and tumor weight and disease location were recorded. Mean tumor weight with 95% confidence intervals (left panels) and corresponding tumor weight distributions for each mouse model (right panels) are shown. Statistical analysis for tumor weights was performed by two-sided Mann–Whitney rank sum tests. Asterisks represent a statistically significant difference from the control siRNA-DOPC treatment group. P values for each comparison are listed above the brackets shown (for HeyA8: p130cas siRNA, P = .04; control siRNA-DOPC + docetaxel, P < .001; p130cas siRNA-DOPC + docetaxel, P < .001; for SKOV3ip1: control siRNA-DOPC + docetaxel, P = .02; p130cas siRNA-DOPC + docetaxel, P < .001; for HeyA8-MDR: p130cas siRNA + docetaxel, P = .002). Double dagger designates a statistically significant difference in tumor weights for mice treated with p130cas siRNA-DOPC plus docetaxel compared with control siRNA-DOPC plus docetaxel (SKOV3ip1, P < .001).
We subsequently evaluated the antitumor effects of p130cas gene silencing with p130cas siRNA-DOPC in mice bearing orthotopic HeyA8 and SKOV3ip1 tumors (Figure 2, D and E, respectively). One week following tumor cell injection, mice were randomized into one of four treatment groups (n = 10 mice per group) and treated with intraperitoneal injections of the following agents: control siRNA-DOPC, p130cas siRNA-DOPC, control siRNA-DOPC plus docetaxel, or p130cas siRNA-DOPC plus docetaxel. In the HeyA8 tumor model, p130cas siRNA-DOPC reduced tumor weight at the time of necropsy compared with control siRNA-DOPC therapy alone (HeyA8: 57% reduction in tumor weight, P = .04) (Figure 2, D). Control siRNA-DOPC in combination with docetaxel chemotherapy reduced tumor growth compared with control siRNA-DOPC in both the HeyA8 and SKOV3ip1models (HeyA8: 84% reduction in tumor weight, P < .001; SKOV3ip1: 69% reduction in tumor weight, P = .02). In both tumor models, the greatest reduction in tumor growth was noted in the p130cas siRNA-DOPC plus docetaxel treatment arm compared with control siRNA-DOPC alone (HeyA8 tumor weight at 28 days: control siRNA vs p130cas siRNA-DOPC plus docetaxel, 1.62 vs 0.13 g, difference = 1.49 g, 95% CI = 0.60 to 2.37 g, meaning a 92% reduction in tumor weight, P < .001; at 38 days, SKOV3ip1 tumors with control siRNA = 1.07 g, SKOV3ip1 tumors with p130cas siRNA-DOPC plus docetaxel = 0.05 g, difference = 1.02 g, 95% CI = 0.45 to 1.58 g, meaning a 95% reduction in tumor weight, P < .001). p130Cas siRNA-DOPC plus docetaxel was statistically significantly more effective than control siRNA-DOPC plus docetaxel in reducing tumor growth in the SKOV3ip1 model (84% reduction in tumor weight; P < .001).
To confirm that p130cas gene expression was silenced during these therapy experiments, tumors collected from the p130cas treatment arm (HeyA8) were compared with tumors collected from mice treated with control siRNA-DOPC. All samples for target modulation were collected at the completion of the therapy experiment. Analysis of protein expression by SDS-PAGE revealed that orthotopic tumors from mice treated with p130cas siRNA-DOPC expressed 95% less p130cas (by densitometry) than mice treated with control siRNA-DOPC (Figure 2, C).
Because most tumors ultimately develop resistance to chemotherapy, we next examined the effect of siRNA-mediated p130cas gene silencing on the growth of orthotopic tumors derived from the taxane-resistant ovarian cancer HeyA8-MDR cell line (Figure 2, F). As expected, control siRNA-DOPC plus docetaxel did not have a statistically significant effect on tumor growth compared with control siRNA-DOPC therapy alone (20% reduction; P = .84). p130Cas siRNA-DOPC alone resulted in a 54% reduction in tumor growth (P = .07) compared with control siRNA-DOPC therapy. In this model, p130cas siRNA-DOPC plus docetaxel had the greatest efficacy on tumor growth inhibition compared with control siRNA-DOPC therapy alone (at 32 days, HeyA8-MDR tumors with control siRNA = 1.01 g, Hey8MDR tumors with p130cas siRNA-DOPC plus docetaxel = 0.21 g, difference = 0.8 g, 95% CI = 0.21 to 1.40 g, meaning a 80% reduction in tumor weight, P = .002). In addition, combination therapy with p130cas siRNA-DOPC plus docetaxel resulted in a 56% reduction in tumor growth compared with p130cas siRNA-DOPC therapy alone (P = .09).
Among the tumor models examined, there was no statistically significant difference in tumor incidence among treatment groups (Table 3). However, in all three tumor models examined, mice treated with p130cas siRNA-DOPC plus docetaxel had less tumor burden; they had fewer tumor nodules than mice treated with control siRNA-DOPC (there were 6.5 HeyA8 tumors per mouse with control siRNA vs 1.7 tumors per mouse with p130cas siRNA plus docetaxel, difference = 4.8 tumors per mouse, 95% CI = 2.79 to 6.81, meaning a 74% reduction, P < .001; there were 16.3 SKOV3ip1 tumors per mouse with control siRNA vs 2.13 tumors per mouse with p130cas siRNA plus docetaxel, difference = 14.2 tumors per mouse, 95% CI = 3.37 to 24.98, meaning an 87% reduction, P < .001; there were 6.5 HeyA8-MDR tumors per mouse with control siRNA vs 2.56 with p130cas siRNA plus docetaxel, difference = 3.94 tumors per mouse, 95% CI = −0.09 to 7.98, meaning a 61% reduction, P < .05).
Table 3.
Orthotopic tumor development in mice treated with control or p130cas siRNA with or without docetaxel*
| Cell line and treatment group | Tumor incidence† | Mean No. of tumor nodules (95% CI) | P‡ |
| HeyA8 | |||
| Control siRNA | 80 | 6.5 (4.15 to 8.86) | referent |
| p130Cas siRNA | 100 | 3.3 (1.30 to 5.30) | .27 |
| Control siRNA plus docetaxel | 100 | 2.0 (1.49 to 2.31) | <.001 |
| p130Cas siRNA plus docetaxel | 100 | 1.7 (1.02 to 2.38) | <.001 |
| SKOV3ip1 | |||
| Control siRNA | 100 | 11.5 (6.07 to 26.5) | referent |
| p130Cas siRNA | 100 | 7.0 (2.80 to 13.20) | .28 |
| Control siRNA plus docetaxel | 90 | 3.0 (2.24 to 5.54) | .01 |
| p130Cas siRNA plus docetaxel | 80 | 2.0 (1.30 to 2.95) | <.001 |
| HeyA8-MDR | |||
| Control siRNA | 100 | 5.5 (3.09 to 9.96) | referent |
| p130Cas siRNA | 80 | 5.0 (1.43 to 6.82) | .24 |
| Control siRNA plus docetaxel | 90 | 3.0 (2.95 to 8.17) | .72 |
| p130Cas siRNA plus docetaxel | 90 | 1.0 (0.06 to 5.05) | .03 |
CI = confidence interval; siRNA = small interfering RNA.
Number of mice that developed tumors divided by the number of mice treated.
Mann–Whitney rank sum test (all P values are two-sided).
During therapy experiments, mice were closely monitored for behavioral changes in feeding habits and mobility that might be a sign of toxicity, and no such indications were identified. In addition, mean body weight did not statistically significantly differ among the treatment groups (data not shown), further confirming that the feeding habits of the mice were not affected by the respective treatments.
Effect of p130Cas Silencing on Downstream Signaling
RNA microarrays were performed to determine the effects of silencing p130cas on downstream signaling pathways. There were 645 genes that were differentially expressed in control siRNA–treated samples vs p130cas siRNA–treated samples (Figure 3, A). A complete list of genes that are differentially expressed in the presence of p130cas silencing is available online (Supplementary Table 1, available online). A comprehensive network analysis identified cell death pathways as being among those most affected by p130cas silencing (Supplementary Figure 1, available online). p130Cas gene silencing after p130cas siRNA treatment resulted in a substantial decrease in expression of the gene for the PI3K catalytic subunit, PIK3CA (Figure 3, B).
Figure 3.
p130Cas signaling detected using RNA microarray. A) Statistically significant differences in gene expression in Hey8 cells treated with p130cas small interfering RNA (siRNA) vs control siRNA. HeyA8 treated with p130cas siRNA vs control siRNA in vitro were harvested and RNA was isolated. Genes were selected by univariate tests (two-sided two-sample t tests, P < .001). Six hundred forty-five genes were differentially expressed between control siRNA– and p130cas siRNA–treated cells. The data are presented in matrix format in which rows represent individual genes and columns represent each sample. Each cell in the matrix represents the expression level of a gene feature in an individual sample. The red and green colors reflect high and low expression levels, respectively, as indicated in the scale bar (log2 transformed scale). B) Summary of RNA microarray results. The data revealed an abrogation in cell death pathways, specifically, a statistically significant decrease in phosphatidylinositol-3 kinase catalytic subunit (PI3KCA) levels (two-sided two-sample t test, P = .046). SI = small interfering.
Effect of p130cas siRNA-DOPC on Tumor Cell Death
Because we found decreased expression of Bcl-2 and PI3K with the RNA microarrays, we next evaluated the effects of p130cas (BCAR1) gene silencing on tumor cell apoptosis as determined by TUNEL staining of tumors obtained from the therapy experiments described above (Figure 4, A). In the SKOV3ip1 model, tumors from mice treated with p130cas siRNA-DOPC had a statistically significantly more TUNEL-positive nuclei than tumors from mice treated with control siRNA-DOPC (a mean of 17.4 TUNEL-positive cells per field with p130cas siRNA vs 7.16 TUNEL-positive cells per field with control siRNA, difference = 10.24 cells per field, 95% CI = 5.98 to 14.50, 143% increase, P < .001). Control siRNA-DOPC plus docetaxel resulted in a 41% increase in tumor cell apoptosis (10.08 TUNEL-positive cells per field, difference vs control = 2.92, 95% CI = −0.29 to 6.13, P = .17). In addition, tumor cell apoptosis was statistically significantly increased with p130cas siRNA-DOPC plus docetaxel compared with control siRNA-DOPC in the SKOV3ip1 model (25 TUNEL-positive cells per field with p130cas siRNA plus docetaxel vs 7.16 TUNEL-positive cells per field with control siRNA, difference = 17.84, 95% CI = 11.79 to 23.89, 250% increase, P < .001).
Figure 4.
Effect of p130cas small interfering RNA in neutral nanoliposomes (siRNA-DOPC) therapy on tumor cell apoptosis. A) Tumor cell apoptosis was assessed by immunofluorescent staining for terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate nick-end labeling (TUNEL) in tumors obtained from the SKOV3ip1 therapy experiment. Photomicrographs (×200 magnification) represent TUNEL-positive tumor cells (green) and tumor cell nuclei (blue); scale bar = 50 μm. The bar graph (right panel) shows the mean number of TUNEL-positive cells for each treatment group. The mean number of TUNEL-positive cells was calculated by averaging the TUNEL-positive cells from five random fields per slide (each slide represented a tumor from one mouse); five slides per treatment group were examined. Error bars represent 95% confidence intervals. Comparison between groups was performed by two-sided Mann–Whitney rank sum tests. Asterisks represent statistically significant increases in tumor cell apoptosis compared with siRNA-DOPC controls (p130cas siRNA-DOPC: P < .001; p130cas siRNA-DOPC + docetaxel: P < .001). Cell viability was assessed by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assays at various time points after treatment with p130cas siRNA and/or docetaxel. Mean values for the measured optical density (OD) at 570 nm for 12 replicates are shown. Error bars represent 95% confidence intervals. B) SKOV3ip1 cell viability was statistically significantly decreased following p130cas siRNA (P < .001). C) SKOV3TRip2 cell viability was statistically significantly decreased following p130cas siRNA (P = .01, two-sided Mann–Whitney rank sum tests). Cell death was assessed with Annexin V-phycoerythrin (Annexin V-PE) and 7-aminoactinomycin D (7AAD) by fluorescence-activated cell sorting analysis at various time points after treatment with p130cas siRNA and/or docetaxel. Mean values for the three replicates with 95% confidence intervals are shown. Error bars represent standard error of the mean. D) Graphical representation of Annexin V-PE and 7AAD apoptosis detection assay. SKOV3ip1 ovarian cancer cells were either left untreated or treated with p130cas siRNA and/or docetaxel for 48, 72, or 96 hours, and the percentage of apoptotic cells was determined. Values are the means of three replicates and 95% confidence intervals. Focusing on the 72-hour time point, when p130cas expression is at its lowest, the brackets represent comparisons made by a two-sided Mann–Whitney rank sum test. Asterisks represent statistically significant increases in tumor cell apoptosis compared with the untreated group (among Annexin V-PE [+] samples, p130cas siRNA: P < .001; docetaxel: P < .001; p130cas siRNA + docetaxel: P < .001; among Annexin V-PE/7AAD [+] samples, p130cas siRNA: P < .001; docetaxel: P < .001; p130cas siRNA + docetaxel: P < .001) Double dagger represents statistically significantly increased tumor cell apoptosis (Annexin V-PE/7-AAD) with p130cas siRNA plus docetaxel compared with docetaxel therapy alone (P = .01; two-sided Mann–Whitney rank sum tests). E) Graphical representation of Annexin V-PE apoptosis detection assay with benzyloxycarbonyl-Val-Ala-Asp (OMe) fluoromethylketone (ZVAD-fmk) pretreatment. SKOV3ip1 cells were left untreated or subjected to p130cas siRNA and/or ZVAD-fmk pretreatment for 72 hours, and the percentage of apoptotic cells was then determined. The values shown are the means of three replicates and 95% confidence intervals. Comparisons between treatment groups were performed by Mann–Whitney rank sum test (two-sided). Asterisks represent statistically significant increases in tumor cell apoptosis compared with the untreated group (among Annexin V-PE [+] samples, p130cas siRNA: P = .002 two-sided Mann–Whitney rank sum tests). No difference in percent apoptosis was visualized with ZVAD-fmk pretreatment. F) Graphical representation of tumor cell migration. HeyA8 cells were either untreated or subjected to control or p130cas siRNA, and the percentage of migratory cells per high-powered field (HPF) was determined. There was statistically significantly decreased HeyA8 tumor cell migration with p130Cas siRNA. Comparisons between treatment groups were performed by two-sided Mann–Whitney rank sum tests. The asterisk represents a statistically significant decrease in tumor cell migration compared with the untreated group (p130cas siRNA: P < .001). G) Graphical representation of tumor cell invasion. HeyA8 cells were either left untreated or subjected to control siRNA or p130cas siRNA, and the percentage of invasive cells per HPF was determined. HeyA8 tumor cell invasion was statistically significantly decreased in the presence of p130Cas siRNA. Comparisons between treatment groups were performed by two-sided Mann–Whitney rank sum tests. The asterisk represents a statistically significant decrease in tumor cell invasion compared with the untreated group (p130cas siRNA: P < .001).
The finding that p130cas siRNA-mediated therapy-modulated tumor cell death led us to further examine the underlying mechanisms. We first examined the effects of p130cas gene silencing on tumor cell viability in vitro in combination with docetaxel chemotherapy using a tumor cell viability assay. p130Cas siRNA treatment led to a 30% reduction (P < .001) in the number of SKOV3ip1 cells compared with untreated cells at 120 hours (Figure 4, B). p130Cas siRNA in combination with docetaxel resulted in a threefold decrease in the calculated IC50 when compared with control siRNA treatments or untreated cells (p130cas siRNA with docetaxel IC50 = 1 nM vs cells and control siRNA with docetaxel IC50 = 3 nM). Given the finding of potential sensitization to taxane chemotherapy with p130cas siRNA treatment, we next looked at cell viability in the taxane-resistant SKOV3TRip2 cells. Again, p130cas siRNA treatment led to a 21% reduction (P = 0.01) in cell number compared with untreated cells at 120 hours (Figure 4, C). p130Cas siRNA in combination with docetaxel resulted in a 2.5-fold decrease in the calculated IC50 of SKOV3TRip2 cells when compared with control siRNA or untreated cells (p130cas siRNA with docetaxel IC50 = 20 nM vs cells and control siRNA with docetaxel IC50 = 50 nM). Because we noted a statistically significant reduction in tumor cell viability with p130cas gene silencing alone, we next set out to confirm the results of the in vivo TUNEL staining by in vitro Annexin V PE and 7AAD staining and subsequent FACS analysis in SKOV3ip1 cells (Figure 4, D). The most important findings were noted at the 72-hour time point, when one would expect maximal decreases in p130cas expression. p130Cas siRNA therapy alone resulted in a 6.4-fold increase in early tumor cell apoptosis (mean number of Annexin V PE-positive cells with p130cas siRNA = 11.5; mean number of Annexin V PE-positive untreated cells = 1.8, difference = 9.7 positive cells, 95% CI = 6.8 to 12.6, P < .001) and a 2.2-fold increase in tumor cell necrosis (mean number of Annexin V PE and 7AAD dual stained cells with p130cas siRNA = 7.3, mean number of Annexin V PE and 7AAD dual stained untreated cells = 3.3, difference = 4.0 stained cells, 95% CI = 2.5 to 5.5, P < .001) (Figure 4, D). Docetaxel therapy alone at IC50 concentrations (in SKOV3ip1, 1 nM) resulted in a 6.6-fold increase in early tumor cell apoptosis and a 4.6-fold increase in tumor cell necrosis compared with untreated cells (as a measure of apoptosis, mean number of Annexin V PE-positive cells with docetaxel = 11.8, mean number of Annexin V PE-positive untreated cells = 1.8, difference = 10.0 positive cells, 95% CI = 5.9 to 14.1, P < .001; as a measure of necrosis, mean number of Annexin V PE and 7AAD dual stained cells with docetaxel = 15.1, mean number of Annexin V PE and 7AAD dual stained untreated cells = 3.3, difference = 11 dual stained cells, 95% CI = 6.8 to 16.9, P < .001). Combination therapy with p130cas siRNA plus docetaxel therapy (1 nM) resulted in 9.5-fold increase in early tumor cell apoptosis compared with untreated cells (mean number of Annexin V PE-positive cells with p130cas siRNA and docetaxel = 17.1, mean number of Annexin V PE-positive untreated cells = 1.8, difference = 15.3 positive cells, 95% CI = 10.3 to 20.4, P < .001). Combination therapy resulted in an 8.1-fold increase tumor cell necrosis compared with untreated cells (mean number of Annexin V PE and 7AAD dual stained cells with p130cas siRNA and docetaxel = 26.3, mean number of Annexin V PE and 7AAD dual stained untreated cells = 3.3, difference = 23.0 dual stained cells, 95% CI = 17.4 to 28.7, P < .001). Compared with docetaxel monotherapy, combination therapy resulted in a 1.7-fold increase in tumor cell necrosis (mean number of Annexin V PE and 7AAD dual stained cells with p130cas siRNA and docetaxel = 26.3, mean number of Annexin V PE and 7AAD dual stained cells with docetaxel = 7.3, difference = 11.2 dual stained cells, 95% CI = 3.7 to 18.7, P = .01). Two-dimensional histograms of the FACS analysis are available online (Supplementary Figure 3, available online). Collectively, these results confirm the findings of our in vivo therapy experiments and suggest a direct effect of p130cas gene silencing on tumor cell death.
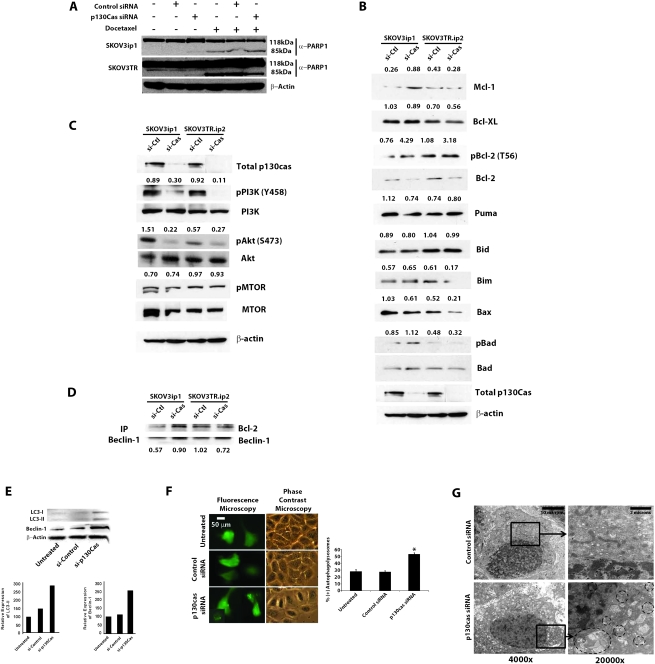
To investigate whether the observed apoptosis visualized with p130cas gene silencing was a result of caspase activation, a similar apoptosis assay experiment was performed after pretreatment of SKOV3ip1 cells with a pan-caspase inhibitor (ZVAD-fmk). Combination treatment with p130cas siRNA and ZVAD-fmk resulted in no statistically significant difference in the percentage of Annexin V-PE-positive cells as determined by FACS analysis compared with p130cas siRNA treatment alone (Figure 4, E). It is well known that in caspase-dependent apoptosis, PARP is cleaved into two fragments of 24 and 89 kDa (54). Thus, we next determined if p130cas gene silencing resulted in PARP cleavage. Immunoblot analysis revealed that PARP was not cleaved following p130cas siRNA treatment (Figure 5, A). By contrast, presence of the 89-kDa cleavage fragment of PARP was noted following docetaxel treatment alone and in combination with p130cas siRNA.
Figure 5.
Effect of p130cas small interfering RNA (siRNA) on tumor cell autophagy. A) Immunoblot analysis of cleaved poly-ADP ribose polymerase (PARP) at 72 hours following treatment of SKOV3ip1 and SKOV3TR cells with p130cas siRNA and/or docetaxel. Immunoblotting for total actin was performed to confirm equal loading. B) Immunoblot analysis of the Bcl-2 family members following treatment SKOV3ip1 and SKOV3TR cells with p130cas siRNA. Immunoblotting for total actin was performed to confirm equal loading of total protein. Densitometry was performed to objectively assess potential differences. When estimating relative amounts of phosphoproteins, equal loading was ensured by immunoblotting to determine amounts of the corresponding total proteins C) Immunoblot analysis of phosphorylated phosphatidylinositol-3-kinase (PI3K), total PI3K, phosphorylated Akt, total Akt, phosphorylated mammalian target of rapamycin (mTOR), and total mTOR following treatment with p130cas siRNA. When estimating relative amounts of phosphoproteins, equal loading of was ensured by immunoblotting to determine amounts of the corresponding total proteins. D) Co-immunoprecipitation and immunoblot analysis of Beclin-1 and Bcl-2, respectively, following p130cas silencing in SKOV3ip1 and SKOV3TR cells. Numbers below blots indicated densitometry for differences in Bcl-2 relative to Beclin-1. E) Immunoblot analysis for cleavage of LC3 and Beclin-1 following treatment with p130cas siRNA. Immunoblotting for total actin was performed to confirm equal loading. Densitometry results show relative expression of LC3-II and Beclin-1 each normalized to actin. F) Detection of autophagolysosomes by transfection with green fluorescent protein (GFP)–light chain 3 (LC3) following treatment with p130cas siRNA. SKOV3ip1 cells were transfected with GFP-LC3 plasmid and then treated with 1 μg of control siRNA or p130cas siRNA, Control siRNA–treated cells showed a diffuse expression pattern of GFP-LC3; however, autophagosomes formed in p130cas siRNA–treated cells. In fluorescence microscope images (×200 magnification), LC3 II had translocated and accumulated in the membranes of autophagosomes, signifying the induction of autophagy. About 200 cells were counted for each case and if more than five punctate pattern containing cells were present, the sample was considered to be positive for autophagy (scale bar = 50 μm). G) Detection of autophagolysosomes by transmission electron microscopy. SKOV3ip1 cells were grown on six-well plates, treated with p130cas siRNA or control siRNA or left untreated for 72 hours, fixed for 2 hours with 2.5% glutaraldehyde in 0.1 M cacodylate buffer (pH 7.4), fixed again in 1% OsO4 in the same buffer, and then subjected to the electron microscopic analysis (×4000 and ×20 000 magnification; scale bars = 10 μm [left panels] and 2 μm [right panels]). Representative areas were chosen for ultrathin sectioning.
Because our findings suggested the possibility of a caspase-independent process that resulted in cell death, we next evaluated cells treated with p130cas siRNA for mediators of cell survival and apoptosis. Both taxane-sensitive and taxane-resistant cell lines were treated with control or p130cas siRNA, and levels of activated PI3K, Akt, and mTOR were evaluated. p130Cas gene silencing reduced levels of activated PI3K (66% in SKOV3ip1 and 88% in SKOV3TRip2) and activated Akt (85% in SKOV3ip1; 53% in SKOV3TRip2) (Figure 5, C) with no impact on total PI3K protein expression. Furthermore, p130cas gene silencing resulted in increased levels of an inactive phosphorylated form of Bcl-2 (Figure 5, B). We next evaluated levels of total and activated mTOR in SKOV3ip1 and SKOV3TRip2 cell lines treated with control or p130cas siRNA and found no statistically significant difference in activated or total mTOR in the presence of p130cas siRNA (Figure 5, C). These findings suggested that the increased cell death seen with p130cas gene silencing may have been mediated by reduced Akt activation, the resultant withdrawal of cell survival signaling, and concomitant induction of cell death by autophagy. To explore these possibilities, we next treated SKOV3ip1 cells with control or p130cas siRNA and evaluated levels of Beclin-1 and LC3. Beclin-1, also known as autophagy-related gene 6 (ATG6), is required for initiation of the formation of autophagosomes (55) and during autophagy, the cytoplasmic form of LC3 (LC3 I) is processed and recruited to autophagosomes, where LC3 II is generated by site-specific proteolysis and lipidation near its C-terminus (56). p130Cas gene silencing resulted in increased expression of cleaved LC3 and Beclin-1 (Figure 5, E), further suggesting that the increased cell death seen with p130cas gene silencing may be attributable to induction of autophagy. Furthermore, co-immunoprecipitation experiments demonstrated decreased Bcl-2 and Beclin-1 interaction in SKOV3Trip2 cells treated with p130cas siRNA (Figure 5, D). These results were further confirmed by fluorescence microscopic analysis of GFP-labeled LC3 protein localization. After p130cas siRNA treatment of SKOV3ip1 cells, punctate GFP-LC3 fluorescent spots dramatically increased, whereas diffuse fluorescence of GFP-LC3 in the cytoplasm and nucleus disappeared (Figure 5, F), suggesting the formation of autophagolysosomes. p130Cas siRNA treatment resulted in 53% of SKOV3ip1 cells with punctate LC3, as opposed to only 28% in untreated or control siRNA–treated cells. Finally, the presence of p130cas siRNA-induced autophagy was confirmed by transmission electron microscopy. p130cas siRNA induced a dramatic increase in autophagosomes and autophagolysosomes in the cytoplasm of SKOV3ip1 cells. In contrast, control siRNA–treated cells exhibited only normal mitochondria and endoplasmic reticulum without autophagic vesicles (Figure 5, G).
Effect of p130Cas siRNA on Tumor Cell Proliferation, Migration, and Invasion
Finally, we examined the effects of p130cas gene silencing on tumor cell proliferation in vivo. Orthotopic tumors harvested at the completion of the SKOV3ip1 therapy experiment were stained with an antibody against PCNA (Supplementary Figure 2, available online). Compared with treatment with control siRNA-DOPC alone, treatment with p130cas siRNA-DOPC alone or with control siRNA-DOPC plus docetaxel resulted in a statistically significant decrease in tumor cell proliferation (at 37 days, cell number with control siRNA-DOPC treatment = 98.9, with p130Cas siRNA-DOPC treatment = 68.5, difference = 30.4, 95% CI = 14.13 to 46.59, 31% reduction, P < .001; cell number with control siRNA-DOPC plus docetaxel= 68.2, difference vs control siRNA-DOPC = 30.36, 95% CI = 15.70 to 45.66, 31% reduction, P < .001). The largest effect was observed in the p130cas siRNA-DOPC plus docetaxel combination therapy group (at 37 days, cell number = 56.1, difference vs control siRNA-DOPC = 42.8, 95% CI = 30.10 to 55.5, 43% reduction, P < .001).
In addition to tumor cell proliferation, acquisition of a motile phenotype is essential to cancer metastases and progression. p130Cas has been implicated in the process of cancer cell migration and invasion through its interaction with the focal adhesion complex (57). Consequently, the effects of p130cas silencing on in vitro measures of migration and invasion were examined. Compared with untreated cells and cells treated with control siRNA, p130cas siRNA resulted in statistically significant decreases in both tumor cell migration and invasion (migratory cells with p130cas siRNA = 682, with control siRNA = 925, difference = 243, 95% CI = 202 to 285, 26% reduction, P < .001; invasive cells with p130cas siRNA = 1169, with control siRNA = 4144, difference = 2975, 95% CI = 2867 to 3884, 72% reduction, P < .001) (Figure 4, F and G).
Discussion
Key findings from this study are that p130cas overexpression in ovarian tumors was associated with decreased patient overall and progression-free survival and was associated with poor clinical outcome in patients with epithelial ovarian cancer. In addition, p130cas gene silencing with p130cas siRNA-DOPC in combination with docetaxel chemotherapy reduced tumor growth in both chemotherapy-sensitive and chemotherapy-resistant orthotopic models of ovarian carcinoma. The observed antitumor effects were likely due to decreased tumor proliferation and increased tumor cell death following p130cas gene silencing. Our findings define p130cas as a viable and efficacious therapeutic target in ovarian carcinoma.
The results of this study are consistent with existing studies evaluating the degree of p130cas expression and clinical outcomes in patients with breast and prostate carcinomas. Van der Flier et al. (33) found that p130cas overexpression in tumors was an independent predictor of poor relapse-free and overall survival in 937 breast cancer patients. In addition, breast cancer patients with p130cas overexpression demonstrated a blunted response to tamoxifen independent of the usual predictors of response, such as age, menopausal status, and estrogen receptor status (33). These findings were further validated in a larger cohort of patients using an enzyme-linked immunosorbent assay (ELISA) specific for p130cas (30–32). Similarly, in prostate cancer, p130cas expression is associated with higher Gleason grade, higher preoperative PSA, hormone refractory cancer, and metastasis (29). To the best of our knowledge, this study is the first to link p130cas expression and increased ovarian cancer–specific mortality. Our findings, in conjunction with the previously known role of p130cas, provide strong rationale for therapeutic targeting of p130cas in ovarian carcinoma.
To date, no studies have reported successful therapeutic targeting of p130cas. Several potential reasons for this may exist. First, the complete three-dimensional structure of p130cas is not known (34,58). Second, p130cas is heavily tyrosine phosphorylated and the mechanistic significance of individual phosphorylation sites is not known (35). Despite several phosphorylation-dependent functions, p130cas has no intrinsic kinase activity preventing it from being readily targeted with small-molecule inhibitors (36). Last, given its large size, p130cas has an unstable conformational structure, making drugs dependent on the conformation of a given protein less desirable (37). When considering methods of targeting p130cas in vivo, siRNA therapy is able to overcome each of these limitations. More importantly, targeting the scaffolding function of p130cas with siRNA imparts the notable advantage of affecting multiple components of cancer progression and tumor growth with a single therapy. Consequently, we used systemic siRNA delivery, as previously described by our laboratory (39,40,59), to silence p130cas in an orthotopic murine model of ovarian carcinoma. In this study, p130cas siRNA-DOPC reduced tumor expression of p130cas. More importantly, we demonstrated that decreased p130cas expression, both alone and in combination with taxane-based chemotherapy, resulted in statistically significant reductions in orthotopic ovarian tumor growth. Early studies performed in rat fibroblasts have established a role for p130cas in apoptosis (60). p130Cas has 10 consensus sequences recognized as caspase-3 cleavage sites (DXXD). With apoptotic stimuli, such as etoposide, appearance of a 31-kDa fragment of p130cas can be detected by immunoblotting. The 31-kDa fragment of p130cas translocates to the nucleus and forms a heterodimer with E2A. In contrast to the typical binding partner of E2A, 31-kDa p130cas lacks a DNA binding domain, thereby inhibiting p21 transcription and potentiating apoptosis. In this study, our demonstration that treatment of mice with p130cas siRNA-DOPC alone and in combination with docetaxel increased the mean number of TUNEL-positive tumor cells suggests an alternate mechanism for the increased cell death in the presence of p130cas gene silencing. Transgenic mice with p130cas overexpression in the mammary fat pad demonstrate extensive epithelial hyperplasia during development and delayed involution, implicating p130cas in cell survival (25). Furthermore, Ta et al. (61) have suggested that p130cas overexpression indeed functions as a barrier to pro-apoptotic stimuli and may portend chemoresistance to cytotoxic agents. In this study, we demonstrate decreased tumor cell viability and increased tumor cell death with p130cas siRNA alone and in combination with docetaxel chemotherapy. When examining the mechanism of increased cell death, we noted an increase in several hallmarks of two forms of cell death, apoptosis and autophagy. A proportion of the increased cell death was independent of caspase activation and PARP cleavage. Both the abrogation of the PI3K-Akt pathway and an increase in Bcl-2 phosphorylation or inactivation led us to also evaluate whether induction of a toxic form of autophagy was responsible for the increased cell death that we observed with p130cas gene silencing. Autophagy is an evolutionarily conserved catabolic process in which cells lacking nutrients degrade their own components to sustain cell viability (62). There is increasing evidence that when autophagy is overstimulated, it can progress to autophagic cell death (63). Our data show increased expression of Beclin-1 and decreased Beclin-1 and Bcl-2 binding and autophagosome formation following p130cas gene silencing, thereby establishing a novel mechanism by which p130cas gene silencing increases tumor cell death. Together, these data suggest that p130cas exerts a direct effect on tumor cell survival, and p130cas gene silencing may play an important role in promoting tumor cell death in our preclinical murine model. What remains uncertain is the connection between the observed apoptotic and autophagic forms of cell death. Data from existing studies suggest that apoptosis and autophagy may be interconnected, but whether one mechanism of cell death is sufficient or mutually exclusive of the other is not known (64). Some researchers have demonstrated that execution of apoptosis is preceded by and dependent upon the occurrence of autophagy and as a result, inhibition of pan-caspase activation did not abrogate the proportion of cells undergoing autophagic cell death, and autophagy alone was not sufficient to cause maximal cell death (63). Alternatively, apoptosis and autophagy may occur in a mutually exclusive manner in which inhibition of autophagy to a given death signal may result in generation of apoptotic cell death and vice versa (65,66). In keeping with existing literature, our research suggests the possibility of concomitant apoptotic and autophagic cell death as a result of p130cas silencing because cell death was not abrogated with caspase inhibition.
This work is not without limitations, particularly those that relate to inherent weaknesses in use of cell lines and murine preclinical models of human malignancies. Cell lines, for example, do not represent the heterogeneity seen in human tumors. Tumors in mice may induce differing patterns of growth because of differences in the host immune response. Furthermore, the siRNA sequences used in this work were human specific and consequently bear no information on the potential toxicity of targeting p130cas in clinical models.
In summary, we have shown that p130cas overexpression was associated with poor outcome in a cohort of ovarian cancer patients and that targeted therapy with p130cas siRNA-DOPC was effective in reducing tumor growth in both chemotherapy-sensitive and chemotherapy-resistant models. Mechanisms for decreased in vivo ovarian cancer growth include decreased tumor cell proliferation and angiogenesis and increased tumor cell death via induction of apoptosis and a toxic form of autophagy, thereby identifying p130cas as an important therapeutic target in ovarian carcinoma.
Funding
Portions of this work were supported by the National Institutes of Health (CA 110793, 109298, P50 CA083639, P50 CA098258, CA128797, RC2GM092599, U54 CA151668), the Ovarian Cancer Research Fund, Inc (Program Project Development Grant), the Department of Defense (OC073399, W81XWH-10-1-0158, BC085265), the RGK Foundation, the Laura and John Arnold Foundation, the Zarrow Foundation, the Marcus Foundation, and the Betty Anne Asche Murray Distinguished Professorship. A.M.N., R.L.S., and W.S.G. are supported by National Cancer Institute, Department of Health & Human Services, National Institutes of Health T32 Training Grant (T32 CA101642).
Supplementary Material
Footnotes
The authors thank Donna Reynolds, Dr Robert Langley, and Fang Wang for their technical expertise and helpful discussion.
The study sponsors had no role in the design of the study; the collection, analysis, and interpretation of the data; the writing of the article; and the decision to submit the article for publication.
References
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58(2):71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Hogdall E. Cancer antigen 125 and prognosis. Curr Opin Obstet Gynecol. 2008;20(1):4–8. doi: 10.1097/GCO.0b013e3282f2b124. [DOI] [PubMed] [Google Scholar]
- 3.Bast RC, Jr, Badgwell D, Lu Z, et al. New tumor markers: CA125 and beyond. Int J Gynecol Cancer. 2005;15(suppl 3):274–281. doi: 10.1111/j.1525-1438.2005.00441.x. [DOI] [PubMed] [Google Scholar]
- 4.Jacobs I, Stablile I, Bridges J, et al. Multimodal approach to screening for ovarian cancer. Lancet. 1988;1(8580):268–271. doi: 10.1016/s0140-6736(88)90351-0. [DOI] [PubMed] [Google Scholar]
- 5.Moore RG, Brown AK, Miller MC, et al. The use of multiple novel tumor biomarkers for the detection of ovarian carcinoma in patients with a pelvic mass. Gynecol Oncol. 2008;108(2):402–408. doi: 10.1016/j.ygyno.2007.10.017. [DOI] [PubMed] [Google Scholar]
- 6.Bankhead CR, Kehoe ST, Austoker J. Symptoms associated with diagnosis of ovarian cancer: a systematic review. BJOG. 2005;112(7):857–865. doi: 10.1111/j.1471-0528.2005.00572.x. [DOI] [PubMed] [Google Scholar]
- 7.Ozols RF, Bundy BN, Greer BE, et al. Phase III trial of carboplatin and paclitaxel compared with cisplatin and paclitaxel in patients with optimally resected stage III ovarian cancer: a Gynecologic Oncology Group study. J Clin Oncol. 2003;21(17):3194–3200. doi: 10.1200/JCO.2003.02.153. [DOI] [PubMed] [Google Scholar]
- 8.McGuire WP, Hoskins WJ, Brady MF, et al. Cyclophosphamide and cisplatin compared with paclitaxel and cisplatin in patients with stage III and stage IV ovarian cancer. N Engl J Med. 1996;334(1):1–6. doi: 10.1056/NEJM199601043340101. [DOI] [PubMed] [Google Scholar]
- 9.Bookman MA. Developmental chemotherapy and management of recurrent ovarian cancer. J Clin Oncol. 2003;21(10 suppl):149s–167s. doi: 10.1200/jco.2003.02.553. [DOI] [PubMed] [Google Scholar]
- 10.Burger RA, Sill MW, Monk BJ, Greer BE, Soroski JI. Phase II trial of bevacizumab in persistent or recurrent epithelial ovarian cancer or primary peritoneal cancer: a Gynecologic Oncology Group Study. J Clin Oncol. 2007;25(33):5165–5171. doi: 10.1200/JCO.2007.11.5345. [DOI] [PubMed] [Google Scholar]
- 11.Cannistra SA, Matulonis UA, Penson RT, et al. Phase II study of bevacizumab in patients with platinum-resistant ovarian cancer or peritoneal serous cancer. J Clin Oncol. 2007;25(33):5180–5186. doi: 10.1200/JCO.2007.12.0782. [DOI] [PubMed] [Google Scholar]
- 12.Matulonis UA, Berlin S, Ivy P, et al. Cediranib, an oral inhibitor of vascular endothelial growth factor receptor kinases, is an active drug in recurrent epithelial ovarian, fallopian tube, and peritoneal cancer. J Clin Oncol. 2009;27(33):5601–5606. doi: 10.1200/JCO.2009.23.2777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fong PC, Yap TA, Boss DS, et al. Poly(ADP)-ribose polymerase inhibition: frequent durable responses in BRCA carrier ovarian cancer correlating with platinum-free interval. J Clin Oncol. 2010;28(15):2512–2519. doi: 10.1200/JCO.2009.26.9589. [DOI] [PubMed] [Google Scholar]
- 14.Sakai R, Iwamatsu A, Hirano N, et al. A novel signaling molecule, p130, forms stable complexes in vivo with v-Crk and v-Src in a tyrosine phosphorylation-dependent manner. EMBO J. 1994;13(16):3748–3756. doi: 10.1002/j.1460-2075.1994.tb06684.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Defilippi P, Di Stefano P, Cabodi S. p130Cas: a versatile scaffold in signaling networks. Trends Cell Biol. 2006;16(5):257–263. doi: 10.1016/j.tcb.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 16.Brabek J, Constancio SS, Siesser PF, Shin NY, Pozzi A, Hank SK. Crk-associated substrate tyrosine phosphorylation sites are critical for invasion and metastasis of SRC-transformed cells. Mol Cancer Res. 2005;3(6):307–315. doi: 10.1158/1541-7786.MCR-05-0015. [DOI] [PubMed] [Google Scholar]
- 17.Panetti TS. Tyrosine phosphorylation of paxillin, FAK, and p130CAS: effects on cell spreading and migration. Front Biosci. 2002;7:2143–2150. doi: 10.2741/A771. [DOI] [PubMed] [Google Scholar]
- 18.Sanders MA, Basson MD. p130cas but not paxillin is essential for Caco-2 intestinal epithelial cell spreading and migration on collagen IV. J Biol Chem. 2005;280(25):23516–23522. doi: 10.1074/jbc.M413165200. [DOI] [PubMed] [Google Scholar]
- 19.Chodniewicz D, Klemke RL. Regulation of integrin-mediated cellular responses through assembly of a CAS/Crk scaffold. Biochim Biophys Acta. 2004;1692(2–3):63–76. doi: 10.1016/j.bbamcr.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 20.O’Neill GM, Fashena SJ, Golemis EA. Integrin signalling: a new Cas(t) of characters enters the stage. Trends Cell Biol. 2000;10(3):111–119. doi: 10.1016/s0962-8924(99)01714-6. [DOI] [PubMed] [Google Scholar]
- 21.Klemke RL, Leng J, Molander R, Brooks PC, Vuori K, Cheresh DA. CAS/Crk coupling serves as a “molecular switch” for induction of cell migration. J Cell Biol. 1998;140(4):961–972. doi: 10.1083/jcb.140.4.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shim SR, Kook S, Kim JI, Song WK. Degradation of focal adhesion proteins paxillin and p130cas by caspases or calpains in apoptotic rat-1 and L929 cells. Biochem Biophys Res Commun. 2001;286(3):601–608. doi: 10.1006/bbrc.2001.5441. [DOI] [PubMed] [Google Scholar]
- 23.Kim W, Kook S, Kim DJ, Teodorof C, Song WK. The 31-kDa caspase-generated cleavage product of p130cas functions as a transcriptional repressor of E2A in apoptotic cells. J Biol Chem. 2004;279(9):8333–8342. doi: 10.1074/jbc.M312026200. [DOI] [PubMed] [Google Scholar]
- 24.Wei L, Yang Y, Zhang X, Yu Q. Cleavage of p130Cas in anoikis. J Cell Biochem. 2004;91(2):325–335. doi: 10.1002/jcb.10760. [DOI] [PubMed] [Google Scholar]
- 25.Cabodi S, Tinnirello A, Di Stefano P, et al. p130Cas as a new regulator of mammary epithelial cell proliferation, survival, and HER2-neu oncogene-dependent breast tumorigenesis. Cancer Res. 2006;66(9):4672–4680. doi: 10.1158/0008-5472.CAN-05-2909. [DOI] [PubMed] [Google Scholar]
- 26.Ambrogio C, Voena C, Manazza AD, et al. p130Cas mediates the transforming properties of the anaplastic lymphoma kinase. Blood. 2005;106(12):3907–3916. doi: 10.1182/blood-2005-03-1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Honda H, Oda H, Nakamoto T, et al. Cardiovascular anomaly, impaired actin bundling and resistance to Src-induced transformation in mice lacking p130Cas. Nat Genet. 1998;19(4):361–365. doi: 10.1038/1246. [DOI] [PubMed] [Google Scholar]
- 28.Tazaki T, Miyazaki K, Hiyama E, et al. Functional analysis of Src homology 3-encoding exon (exon 2) of p130Cas in primary fibroblasts derived from exon 2-specific knockout mice. Genes Cells. 2008;13(2):145–157. doi: 10.1111/j.1365-2443.2007.01156.x. [DOI] [PubMed] [Google Scholar]
- 29.Fromont G, Vallancien G, Validire P, Levillain P, Cussenot O. BCAR1 expression in prostate cancer: association with 16q23 LOH status, tumor progression and EGFR/KAI1 staining. Prostate. 2007;67(3):268–273. doi: 10.1002/pros.20516. [DOI] [PubMed] [Google Scholar]
- 30.Dorssers LC, Grebenchtchikov N, Brinkman A, et al. The prognostic value of BCAR1 in patients with primary breast cancer. Clin Cancer Res. 2004;10(18, pt 1):6194–6202. doi: 10.1158/1078-0432.CCR-04-0444. [DOI] [PubMed] [Google Scholar]
- 31.Grebenchtchikov N, Brinkman A, van Broekhoven SP, et al. Development of an ELISA for measurement of BCAR1 protein in human breast cancer tissue. Clin Chem. 2004;50(8):1356–1363. doi: 10.1373/clinchem.2003.029868. [DOI] [PubMed] [Google Scholar]
- 32.Dorssers LC, Grebenchtchikov N, Brinkman A, et al. Application of a newly developed ELISA for BCAR1 protein for prediction of clinical benefit of tamoxifen therapy in patients with advanced breast cancer. Clin Chem. 2004;50(8):1445–1447. doi: 10.1373/clinchem.2004.035493. [DOI] [PubMed] [Google Scholar]
- 33.van der Flier S, Brinkman A, Look MP, et al. Bcar1/p130Cas protein and primary breast cancer: prognosis and response to tamoxifen treatment. J Natl Cancer Inst. 2000;92(2):120–127. doi: 10.1093/jnci/92.2.120. [DOI] [PubMed] [Google Scholar]
- 34.Nasertorabi F, Garcia-Guzman M, Briknarova K, et al. Organization of functional domains in the docking protein p130Cas. Biochem Biophys Res Commun. 2004;324(3):993–998. doi: 10.1016/j.bbrc.2004.09.148. [DOI] [PubMed] [Google Scholar]
- 35.Patwardhan P, Shen Y, Goldberg GS, Miller WT. Individual Cas phosphorylation sites are dispensable for processive phosphorylation by Src and anchorage-independent cell growth. J Biol Chem. 2006;281(30):20689–20697. doi: 10.1074/jbc.M602311200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ohmori T, Yatomi Y, Inoue K, Satoh K, Ozaki Y. Tyrosine dephosphorylation, but not phosphorylation, of p130Cas is dependent on integrin alpha IIb beta 3-mediated aggregation in platelets: implication of p130Cas involvement in pathways unrelated to cytoskeletal reorganization. Biochemistry. 2000;39(19):5797–5807. doi: 10.1021/bi991849z. [DOI] [PubMed] [Google Scholar]
- 37.Wisniewska M, Bossenmaier B, Georges G, et al. The 1.1 A resolution crystal structure of the p130cas SH3 domain and ramifications for ligand selectivity. J Mol Biol. 2005;347(5):1005–1014. doi: 10.1016/j.jmb.2005.02.017. [DOI] [PubMed] [Google Scholar]
- 38.Thaker PH, Han LY, Kamat AA, et al. Chronic stress promotes tumor growth and angiogenesis in a mouse model of ovarian carcinoma. Nat Med. 2006;12(8):939–944. doi: 10.1038/nm1447. [DOI] [PubMed] [Google Scholar]
- 39.Landen CN, Jr, Chavez-Reyes A, Bucana C, et al. Therapeutic EphA2 gene targeting in vivo using neutral liposomal small interfering RNA delivery. Cancer Res. 2005;65(15):6910–6918. doi: 10.1158/0008-5472.CAN-05-0530. [DOI] [PubMed] [Google Scholar]
- 40.Halder J, Kamat AA, Landen CN, Jr, et al. Focal adhesion kinase targeting using in vivo short interfering RNA delivery in neutral liposomes for ovarian carcinoma therapy. Clin Cancer Res. 2006;12(16):4916–4924. doi: 10.1158/1078-0432.CCR-06-0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hwang JY, Mangala LS, Fok JY, et al. Clinical and biological significance of tissue transglutaminase in ovarian carcinoma. Cancer Res. 2008;68(14):5849–5858. doi: 10.1158/0008-5472.CAN-07-6130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thaker PH, Yazici S, Nilsson MB, et al. Antivascular therapy for orthotopic human ovarian carcinoma through blockade of the vascular endothelial growth factor and epidermal growth factor receptors. Clin Cancer Res. 2005;11(13):4923–4933. doi: 10.1158/1078-0432.CCR-04-2060. [DOI] [PubMed] [Google Scholar]
- 43.Hendrix MJ, Seftor EA, Seftor RE, Fidler IJ. A simple quantitative assay for studying the invasive potential of high and low human metastatic variants. Cancer Lett. 1987;38(1–2):137–147. doi: 10.1016/0304-3835(87)90209-6. [DOI] [PubMed] [Google Scholar]
- 44.Maglott D, Ostell J, Pruitt KD, Tatusova T. Entrez Gene: gene-centered information at NCBI (Translated from English) Nucleic Acids Res. 2007;35(Database issue):D26–D31. doi: 10.1093/nar/gkl993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bader G. BIND—The biomolecular interaction network database (Translated from English) Nucleic Acids Res. 2001;29(1):242–245. doi: 10.1093/nar/29.1.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kanehisa M, Goto S KEGG: kyoto encyclopedia of genes and genomes (Translated from English) Nucleic Acids Res. 2000;28(1):27–30. doi: 10.1093/nar/28.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Griffith O. ORegAnno: an open-access community-driven resource for regulator annontation (Translated from English) Nucleic Acids Res. 2008;36(D):107–113. doi: 10.1093/nar/gkm967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ashburner M. Gene ontology: tool for the unification of biology. The Gene Ontology Consortium. (Translated from English) Nat Genet. 2000;25(1):25–29. doi: 10.1038/75556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lovasz L. Random walks on graphs: a survey. Bolyayu Soc Math Stud. 1993;2(2):1–46. [Google Scholar]
- 50.Akar U, Chaves-Reyez A, Barria M, et al. Silencing of Bcl-2 expression by small interfering RNA induces autophagic cell death in MCF-7 breast cancer cells. Autophagy. 2008;4(5):669–679. doi: 10.4161/auto.6083. [DOI] [PubMed] [Google Scholar]
- 51.Klionsky DJ, Abellovich H, Agostinis P, et al. Guidelines for the use and interpretation of assays for monitoring autophagy in higher eukaryotes. Autophagy. 2008;4(2):151–175. doi: 10.4161/auto.5338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hoskins W, Perez C, Young R. Principles and Practice of Gynecologic Oncology. Riverwoods, IL: Lippincott Williams & Wilkins; 2005. [Google Scholar]
- 53.Van der Burg ME. Advanced ovarian cancer. Curr Treat Options Oncol. 2001;2(2):109–118. doi: 10.1007/s11864-001-0053-1. [DOI] [PubMed] [Google Scholar]
- 54.Bouchard VJ, Rouleau M, Poirier GG. PARP-1, a determinant of cell survival in response to DNA damage. Exp Hematol. 2003;31(6):446–454. doi: 10.1016/s0301-472x(03)00083-3. [DOI] [PubMed] [Google Scholar]
- 55.Levine B, Sinha S, Kroemer G. Bcl-2 family members: dual regulators of apoptosis and autophagy. Autophagy. 2008;4(5):600–606. doi: 10.4161/auto.6260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tan ML, Ooi JP, Ismail N, Moad AI, Muhammad TS. Programmed cell death pathways and current antitumor targets. Pharm Res. 2009;26(7):1547–1560. doi: 10.1007/s11095-009-9895-1. [DOI] [PubMed] [Google Scholar]
- 57.Zouq NK, Keeble JA, Lindsay J, et al. FAK engages multiple pathways to maintain survival of fibroblasts and epithelia: differential roles for paxillin and p130Cas. J Cell Sci. 2009;122(pt 3):357–367. doi: 10.1242/jcs.030478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nasertorabi F, Alonso A, Rogers SW, et al. Crystallization of the SH2-binding site of p130Cas in complex with Lck, a Src-family kinase. Acta Crystallogr Sect F Struct Biol Cryst Commun. 2005;61(pt 2):174–177. doi: 10.1107/S1744309104034177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Landen CN, Merritt WM, Mangala LS, et al. Intraperitoneal delivery of liposomal siRNA for therapy of advanced ovarian cancer. Cancer Biol Ther. 2006;5(12):1708–1713. doi: 10.4161/cbt.5.12.3468. [DOI] [PubMed] [Google Scholar]
- 60.Kook S, Shim SR, Choi SJ, et al. Caspase-mediated cleavage of p130cas in etoposide-induced apoptotic Rat-1 cells. Mol Biol Cell. 2000;11(3):929–939. doi: 10.1091/mbc.11.3.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ta HQ, Thomas KS, Schrecengost RS, Bouton AH. A novel association between p130Cas and resistance to the chemotherapeutic drug adriamycin in human breast cancer cells. Cancer Res. 2008;68(21):8796–8804. doi: 10.1158/0008-5472.CAN-08-2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Martin AP, Mitchell C, Rahmani M, Nephew KP, Grant S, Dent P. Inhibition of MCL-1 enhances lapatinib toxicity and overcomes lapatinib resistance via BAK-dependent autophagy. Cancer Biol Ther. 2009;8(21):2084–2096. doi: 10.4161/cbt.8.21.9895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Xue L, Fletcher GC, Tolkovsky AM. Autophagy is activated by apoptotic signalling in sympathetic neurons: an alternative mechanism of death execution. Mol Cell Neurosci. 1999;14(3):180–198. doi: 10.1006/mcne.1999.0780. [DOI] [PubMed] [Google Scholar]
- 64.Gozuacik D, Kimchi A. Autophagy as a cell death and tumor suppressor mechanism. Oncogene. 2004;23(16):2891–2906. doi: 10.1038/sj.onc.1207521. [DOI] [PubMed] [Google Scholar]
- 65.Kanzawa T, Kondo Y, Ito H, Kondo S, Germano I. Induction of autophagic cell death in malignant glioma cells by arsenic trioxide. Cancer Res. 2003;63(9):2103–2108. [PubMed] [Google Scholar]
- 66.Xue L, Fletcher GC, Tolkovsky AM. Mitochondria are selectively eliminated from eukaryotic cells after blockade of caspases during apoptosis. Curr Biol. 2001;11(5):361–365. doi: 10.1016/s0960-9822(01)00100-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.