Abstract
Aims
The oxidized low-density lipoprotein receptor LOX-1 is up-regulated on activated endothelial cells, for example, the endothelium of atherosclerosis-prone sites, in both human and animal models. We examined whether endothelial LOX-1 overexpression may contribute to atherogenesis.
Methods
Adenoviral vectors expressing LOX-1 or LOXIN (a splice variant of LOX-1 with inhibitory function) were created and used to transduce the normally lesion-free common carotid artery, in high fat-fed female ApoE−/− mice. Mice were placed on high-fat diet for 4 weeks prior to gene transfer with either LOX-1 or a combination of LOX-1 and LOXIN, and assessment of plaque development analyzed 6 weeks following gene transfer.
Results
Compared to controls, LOX-1 transduction induced a significant increase in plaque coverage within the common carotid artery to 91% compared to 50% after RAd66 control virus infection (P≤.05). This was inhibited by co-expression of LOXIN (62%).
Conclusions
These results demonstrate that up-regulation of LOX-1 promotes atherogenesis, highlighting LOX-1 function as a target for intervention. In addition, this study further demonstrated the inhibitory function of LOXIN.
Abbreviation: ROS, reactive oxygen species; LOX-1, lectin-like oxidized LDL receptor
Keywords: LOX-1, OLR1, Atherogenesis, LOXIN, Splice variant
1. Introduction
LOX-1 is a lectin-like oxidized LDL receptor (also known as oxidized LDL receptor 1—OLR1), which was initially described in endothelial cells by Sawamura et al. [1]. LOX-1 expression has subsequently been described in both smooth muscle cells and macrophage in atherosclerotic plaques [2] as well as in other cell types including adipocytes [3], platelets [4], and chondrocytes [5]. LOX-1 expression can be induced or up-regulated by a number of processes many of which are involved in the atherosclerotic process, including hypertension, sepsis, inflammatory mediators, dyslipidemia, advanced glycation end products, and fluid shear stress (reviewed in Ref. [6]). LOX-1 performs a number of functions in addition to oxidized LDL (oxLDL) binding, such as binding of apoptotic cell bodies and aged red blood cells [7] and acting as a leukocyte adhesion molecule [8]. Binding of oxLDL to LOX-1 induces endothelial dysfunction and apoptosis, stimulating reactive oxygen species (ROS) production and NFκB activation [9], strongly linking LOX-1 with the process of atherosclerosis [6,10].
Several studies in hyperlipidemic mice have demonstrated a link between LOX-1 and atherosclerosis. Mehta et al. [11] created a LOX-1−/−/LDLR−/− mouse, which on high-fat diet exhibited reduced plaque development in the aorta compared to controls. In addition, the LOX-1−/−/LDLR−/− mice also demonstrated a number of anti-atherosclerotic features, e.g., increased IL-10 levels and eNOS activity, with a concomitant reduction in MAPK p38 and NFκB activation. Inoue et al. [12] created a bovine LOX-1 transgenic mouse, where LOX-1 was overexpressed in multiple cell types including vascular and cardiac tissue. Among the pathologies displayed in this transgenic mouse was an increase in ox-LDL uptake and atheroma-like lesions in coronary arteries. In addition, Ishigaki et al. [13] used an adenoviral vector to overexpress LOX-1 in the liver, enhancing hepatic uptake of ox-LDL and reducing atheroma in the aorta. Taken together, these experiments clearly demonstrated a role for LOX-1 in atherosclerosis, although the contribution of endothelial vs. smooth muscle cell or macrophage expression has yet to be determined.
LOXIN is a splice variant of LOX-1, the expression of which is increased by single nucleotide polymorphisms (SNPs) which have been associated with a lower incidence of myocardial infarction [14]. LOXIN forms heterodimers with LOX-1, preventing cell surface localization and function [14,15].
To examine the consequence of selective endothelial expression of LOX-1 in atherosclerosis, we used adenoviral gene transfer of LOX-1 in the common carotid artery. We found that overexpression of LOX-1 enhances atherogenesis and that LOXIN inhibits the development of plaque induced by LOX-1 overexpression.
2. Materials and methods
2.1. Construction of viruses
Plasmids containing the cDNA for both LOX-1 and LOXIN were a generous gift from Prof. Giuseppe Novelli. The expression cassette from pCpG-mcs (InvivoGen, San Diego, CA, USA) containing the mCMV enhancer, EF1α promoter, small synthetic intron, and polyA signal was removed by EcoRI digest and cloned into pDC511 (Microbix Biosystems, Canada). The cDNAs for LOX-1 and LOXIN were amplified by PCR using KOD proofreading polymerase with primers SW187F 5′ GCGCAGGCCTCCCGCCATGACTTTTGATGACC, which created a StuI restriction site and optimized the KOZAK sequence, and SW188R 5′ CGGCGCTAGCTAAAATGCAGTTTTC, which created a NheI restriction site. The NcoI site within the multiple cloning site of the expression cassette was removed by digestion, blunting, and relegation, and the amplified cDNAs for LOX-1 and LOXIN cloned in StuI/NheI. Adenoviral vectors were produced using the Microbix Biosystems kit according to their protocols. RAd66 [16], an Ad-null empty virus, was used to control for virus-induced inflammation.
2.2. In vivo delivery of adenoviral vectors
All experiments were performed according to home office guidelines and approved by the local ethics committee for animal experimentation. Eight-week-old female ApoE−/− mice were placed on high-fat diet (containing 21% lard and 0.15% cholesterol) 4 weeks prior to gene delivery, to induce hypercholesterolemia and then maintained on high-fat diet for the remainder of the experiment (n=6 per group). Adenoviral transduction of carotid arteries was performed by luminal incubation of each vector for 10 min without silastic collar placement as described [17] (see Supplementary Information). Viruses were diluted to 1×1010 pfu/ml using the dialysis buffer used to prepare the adenoviral vector stocks [10 mM Tris (pH 7.5), 135 mM NaCl, 1 mM MgCl2, 10% v/v glycerol], to ensure that all transductions were performed under the same conditions, the vehicle control just contained dialysis buffer. For investigating the effects of LOXIN on LOX-1-induced atherogenesis, 1×1010 pfu/ml of each vector was used (total 2×1010 pfu/ml); hence 2×1010 pfu/ml of the control virus RAd66 was used as a control for this group (labelled RAd66 high). Six weeks following transduction, mice were sacrificed and perfusion fixed with 4% formaldehyde for 5 min. The carotids were exposed, cut longitudinally, and excised before being pinned out flat and fixed for a further 24 h. The fixed arteries were then immobilized in agar, processed, and paraffin embedded so that longitudinal sections of the carotids could be cut.
2.3. Measurement of plaque area
Longitudinal sections (3 μm) were cut through the whole width of the carotid artery. Measurement of the percentage of section covered by plaque was performed every 25 sections (75 μm) through the width of the artery. An average of 6.75 measurements was made per carotid. To standardize the analysis, measurement of plaque coverage was performed on the field of view 500 μm below the carotid bifurcation. This avoids the potential for plaque initiation due to either the turbulent shear stress experienced around the bifurcation or the mechanical damage to the endothelium during gene transfer. The average length analyzed for plaque coverage was ∼1400 μm the length of internal elastic lamina. The data was normally distributed within each group, and differences between groups were analyzed using one-way analysis of variance (ANOVA), using Tukey–Kramer multiple comparisons post hoc test.
2.4. Immunohistochemical analysis of LOX-1 expression
In a separate cohort of mice, gene transfer of either LOX-1 or RAd66 was performed and the mice sacrificed after 7 days. Both the transduced and nontransduced arteries were taken and snap frozen in OCT compound (BDH), orientated to allow transverse sections to be cut. Seven-micrometer-thick frozen sections were cut, air dried, and fixed in methanol with 0.3% H2O2 for 10 min. Human LOX-1 expression was visualized using goat anti-human LOX-1 antibody (5 μg/ml, AF1798, R&D Systems, Abingdon, UK) or matched nonimmune goat control, with 1/400 biotinylated rabbit anti-goat secondary antibody (DAKO, Ely, UK) and 1/200 extravidin HRP conjugate (Sigma, Poole, UK) with SIGMA FAST diaminobenzidine staining tablets (Sigma). Sections were counterstained with hematoxylin for 30 s.
3. Results
3.1. LOX-1 overexpression contributes to atherogenesis
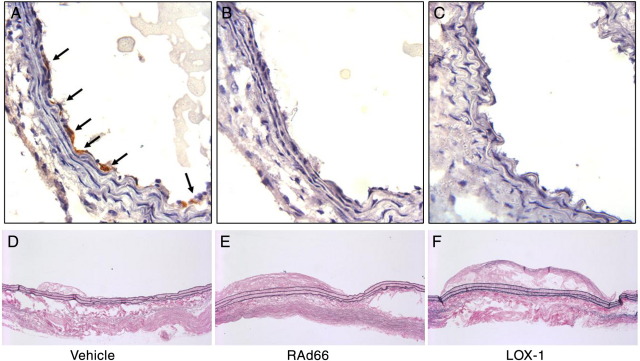
In order to test the potential of endothelial LOX-1 overexpression to contribute to atherogenesis, we performed luminal gene transfer using an adenoviral vector. Ten-minute luminal incubation of the vector, or an empty virus control (RAd66), was sufficient to achieve gene transfer, detected by immunohistochemistry on transduced vessels (Fig. 1A–C). Only cells on the surface of the lumen stained for human LOX-1, showing that the technique selectively transduces endothelial cells, in agreement with previous reports [18].
Fig. 1.
(A–C) Immunohistochemical analysis of LOX-1 expression, 1 week following gene transfer. (A) Anti-LOX-1 antibody; (B) nonimmune goat control; (C) anti-LOX-1 antibody on RAd66 control transduced artery. Intense staining of luminal cells was observed, which by position and shape were predominantly identified as endothelial cells. (D–F) Representative longitudinal sections of transduced arteries 6 weeks post gene transfer. (D) Vehicle control treated; (E) RAd66 control virus transduced; (F) AdLOX-1-transduced artery.
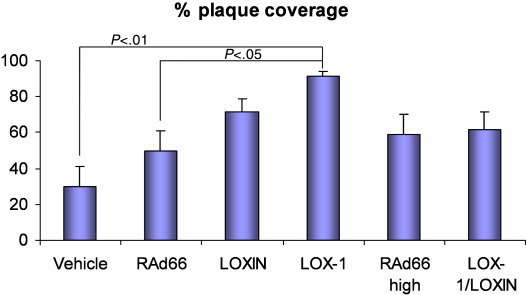
To assess the impact of endothelial LOX-1 overexpression on the development of atherosclerosis, carotid arteries were examined 6 weeks following gene transfer, in hyperlipidemic ApoE−/− mice, without the placement of any flow-modifying cuffs or collars. Transduced arteries were removed, opened up, and sectioned longitudinally to allow the area of the vessel surface covered by plaque to be assessed along the vessel (Fig. 1D–F). There was significantly more plaque coverage in arteries transduced by LOX-1 compared to controls, with an average of 91% coverage vs. 50% RAd66 control virus (Fig. 2, P≤.05). Infection with RAd66 alone increased plaque coverage (50% compared to 30%) compared to vehicle, although this failed to reach significance.
Fig. 2.
Measurement of plaque coverage in vehicle, RAd66 control (1×1010 pfu/ml), LOX-1 (1×1010 pfu/ml), LOXIN LOX-1 (1×1010 pfu/ml), RAd66 high control (2×1010 pfu/ml), and LOX-1/LOXIN (1×1010 pfu/ml of each vector) transduced arteries. Analysis of plaque coverage in the test region identified a significant increase in plaque coverage in the AdLOX-1-transduced carotid artery compared to RAd66 control and vehicle-transduced artery using one-way ANOVA (P>.05 and >.001, respectively).
3.2. LOXIN ameliorates LOX-1 contribution to atherogenesis
In order to assess the effect of co-expressing the ‘dominant-negative’ splice variant LOXIN on the LOX-1 enhancement of atherogenesis, we transduced arteries with both vectors as part of the same experiment. We chose to keep the concentration of LOX-1 vector the same (1×1010 pfu/ml) and supplement it with an equal concentration of LOXIN vector. As the total concentration of virus was double, a separate control group was used with 2×1010 pfu/ml RAd66 (Fig. 2). Carotid arteries transduced by LOX-1 and LOXIN together show no difference in plaque coverage compared to the high-dose RAd66 control (62% vs. 60%). Hence co-expression of LOXIN with LOX-1 abolishes its atherogenic effect. Again, a trend towards greater plaque coverage was observed in the high-dose RAd66 group compared to vehicle alone (30% vs. 60%; P=.09), presumably due to adenovirus-induced inflammation of the vessel wall. The higher dose of RAd66 produced a small nonsignificant increase in atherogenic effect compared to the lower dose (60% vs. 50%).
4. Discussion
We demonstrated here for the first time the ability of endothelial LOX-1 overexpression to promote atherogenesis in the common carotid artery of hyperlipidemic ApoE−/− mice. This amplifies the conclusions from LOX-1-null mice where the function of LOX-1 is deleted in other cell types, including macrophage and smooth muscle cells. LOX-1 is up-regulated in nondiseased but atheroprone arterial sites in hyperlipidemic rabbits, in addition to early atherosclerotic lesions in rabbits and humans [2,19]. The experiments performed here suggest that endothelial LOX-1 expression may have pathological consequences and is not simply a passive marker of disturbed flow in atheroprone vascular sites. We have also demonstrated experimentally for the first time in an in vivo model that LOXIN is capable of inhibiting the development of atherosclerosis that is induced by LOX-1 overexpression. This is in keeping with the human data, which shows that SNPs that increase LOXIN expression are linked to a lower event rate of acute coronary syndromes [14]. The interpretation of the LOXIN-alone group is difficult, as the overexpression of LOXIN in the absence of LOX-1 is an unphysiological situation. LOXIN naturally occurs at a roughly equivalent level compared to LOX-1 in humans [14] and is able to inhibit LOX-1 cell surface expression [14,15]; however, the effect of overexpressing LOXIN in the absence of LOX-1 overexpression is unknown and unphysiological. Mouse LOX-1 contains an exon not present in humans; thus it is unclear whether human LOXIN is able to interact with murine LOX-1. The presence of an equivalent murine LOXIN splice variant in the mouse has not been described.
The expression and action of LOX-1 have been widely investigated and are the subject of many publications (reviewed in Refs. [6,10]). One of the key mediators of LOX-1 signalling is the activation and nuclear localization of the transcription factor NFκB [9]. To assess NFκB activation in LOX-1-transduced arteries, we performed immunohistochemistry for VCAM-1 and LOX-1 in sequential sections, because VCAM-1 expression is NFκB dependent [20] and has a role in the development of atherosclerosis [21]. However, we were unable to demonstrate a specific differential up-regulation of VCAM-1 in LOX-1-transduced cells because VCAM-1 expression was detected in all endothelial cells, suggesting NFκB activation was ubiquitous in this model (this may also be due to the semiquantitative nature of immunohistochemistry limiting a difference in expression from being observed—data not shown). The precise mechanism(s) by which endothelial overexpression of LOX-1 enhances atherosclerosis in this model is undefined and is likely to be a combination of increased production of ROS, NFκB activation, adhesion molecule expression, and leukocyte binding and extravasation [6,10]. Thus a detailed study of the pro-atherogenic mechanisms of LOX-1 in endothelial cells in vivo is warranted.
We chose to perform these experiments in the common carotid artery of hyperlipidemic mice because this site normally remains free of atherosclerotic plaques even after months of high-fat feeding, due to its lack of curvature and side branches. Thus it is a good test site for the analysis of genes which may have pro-atherogenic function. Adenoviral vectors provide an efficient means of ectopically inducing gene expression in the carotid artery; however, strong expression from these vectors is not expected to last for more than 2–3 weeks. This makes them useful for studies looking at atherogenic gene function in the mouse hyperlipidemic model, where atherosclerosis develops rapidly, enabling even short-term transgene expression of proatherogenic genes to initiate a lesion. Fibrotic deposition around transduced arteries is observed in this model, as a response to surgically induced injury. En face oil red O staining was used to visualize lipid deposition in transduced and control arteries (see Supplementary Information); however, there was variable staining of the fibrotic tissue surrounding the artery, with some arteries exhibiting significant perivascular staining, presumably because of foam cell accumulation in the surrounding tissue. Because it was not possible to accurately discriminate between luminal and adventitial oil red O staining in all the transduced arteries, measurement of plaque area on longitudinal sections was used.
The approach used here worked well to examine the proatherogenic effect of a cell-surface molecule, without the need for creating a transgenic animal, allowing rapid analysis of gene function. The experimental design should also work for anti-atherogenic molecules, as the combination of surgery and control virus induced significant initiation of plaque coverage (no plaque is observed in unoperated vessels—S. White, unpublished data). This gives the possibility of a simple single procedure for observing either pro- or anti-atherogenic effects of gene overexpression, in the ApoE−/− mouse.
In conclusion, we have demonstrated that overexpression of LOX-1 in the hyperlipidemic mouse was capable of initiating atherosclerotic plaques in transduced arteries and that LOXIN was able to abrogate plaque induction.
Acknowledgments
The authors wish to thank Prof. Giuseppe Novelli for the provision of plasmids containing the cDNA of LOX-1 and LOXIN. The authors would also like to thank Dr. Chris Rogers for statistical analysis and Dr. Ray Bush, Paul Savage, and Yvonne Johnson for technical assistance.
Footnotes
Work was performed at the Bristol Heart Institute, University of Bristol, UK.
This study was funded by the British Heart Foundation (grant numbers FS/03/096/16318 and RG/04/009).
The authors have no conflict of interests.
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.carpatho.2010.08.007.
Appendix A.
Supplementary Information
References
- 1.Sawamura T, Kume N, Aoyama T, Moriwaki H, Hoshikawa H, Aiba Y. An endothelial receptor for oxidised low-density lipoprotein. Nature. 1997;386:73–77. doi: 10.1038/386073a0. [DOI] [PubMed] [Google Scholar]
- 2.Kataoka H, Kume N, Miyamoto S, Minami M, Moriwaki H, Murase T. Expression of lectinlike oxidized low-density lipoprotein receptor-1 in human atherosclerotic lesions. Circulation. 1999;99(24):3110–3117. doi: 10.1161/01.cir.99.24.3110. [DOI] [PubMed] [Google Scholar]
- 3.Chui PC, Guan HP, Lehrke M, Lazar MA. PPARgamma regulates adipocyte cholesterol metabolism via oxidized LDL receptor 1. J Clin Invest. 2005;115(8):2244–2256. doi: 10.1172/JCI24130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kakutani M, Masaki T, Sawamura T. A platelet–endothelium interaction mediated by lectin-like oxidized low-density lipoprotein receptor-1. Proc Natl Acad Sci U S A. 2000;97(1):360–364. doi: 10.1073/pnas.97.1.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Akagi M, Nishimura S, Yoshida K, Kakinuma T, Sawamura T, Munakata H. Cyclic tensile stretch load and oxidized low density lipoprotein synergistically induce lectin-like oxidized LDL receptor-1 in cultured bovine chondrocytes, resulting in decreased cell viability and proteoglycan synthesis. J Orthop Res. 2006;24(8):1782–1790. doi: 10.1002/jor.20211. [DOI] [PubMed] [Google Scholar]
- 6.Mehta JL, Chen J, Hermonat PL, Romeo F, Novelli G. Lectin-like, oxidized low-density lipoprotein receptor-1 (LOX-1): a critical player in the development of atherosclerosis and related disorders. Cardiovasc Res. 2006;69(1):36–45. doi: 10.1016/j.cardiores.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 7.Oka K, Sawamura T, Kikuta K-I, Itokawa S, Kume N, Kita T. Lectin-like oxidised low-density lipoprotein receptor 1 mediates phagocytosis of aged/apoptotic cells in endothelial cells. Proc Natl Acad Sci U S A. 1998;95:9535–9540. doi: 10.1073/pnas.95.16.9535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hayashida K, Kume N, Minami M, Kita T. Lectin-like oxidized low-density lipoprotein receptor-1 (LOX-1) supports adhesion of leukocytes under both static and flow conditions. Circulation. 2001;104(17):443. [Google Scholar]
- 9.Cominacini L, Pasini AF, Garbin U, Davoli A, Tosetti ML, Campagnola M. Oxidized low density lipoprotein (ox-LDL) binding to ox-LDL receptor-1 in endothelial cells induces the activation of NF-kappaB through an increased production of intracellular reactive oxygen species. J Biol Chem. 2000;275(17):12633–12638. doi: 10.1074/jbc.275.17.12633. [DOI] [PubMed] [Google Scholar]
- 10.Morawietz H. LOX-1 and atherosclerosis: proof of concept in LOX-1-knockout mice. Circ Res. 2007;100(11):1534–1536. doi: 10.1161/CIRCRESAHA.107.101105. [DOI] [PubMed] [Google Scholar]
- 11.Mehta JL, Sanada N, Hu CP, Chen J, Dandapat A, Sugawara F. Deletion of LOX-1 reduces atherogenesis in LDLR knockout mice fed high cholesterol diet. Circ Res. 2007;100(11):1634–1642. doi: 10.1161/CIRCRESAHA.107.149724. [DOI] [PubMed] [Google Scholar]
- 12.Inoue K, Arai Y, Kurihara H, Kita T, Sawamura T. Overexpression of lectin-like oxidized low-density lipoprotein receptor-1 induces intramyocardial vasculopathy in apolipoprotein E-null mice. Circ Res. 2005;97(2):176–184. doi: 10.1161/01.RES.0000174286.73200.d4. [DOI] [PubMed] [Google Scholar]
- 13.Ishigaki Y, Katagiri H, Gao J, Yamada T, Imai J, Uno K. Impact of plasma oxidized low-density lipoprotein removal on atherosclerosis. Circulation. 2008;118(1):75–83. doi: 10.1161/CIRCULATIONAHA.107.745174. [DOI] [PubMed] [Google Scholar]
- 14.Mango R, Biocca S, del Vecchio F, Clementi F, Sangiuolo F, Amati F. In vivo and in vitro studies support that a new splicing isoform of OLR1 gene is protective against acute myocardial infarction. Circ Res. 2005;97(2):152–158. doi: 10.1161/01.RES.0000174563.62625.8e. [DOI] [PubMed] [Google Scholar]
- 15.Biocca S, Filesi I, Mango R, Maggiore L, Baldini F, Vecchione L. The splice variant LOXIN inhibits LOX-1 receptor function through hetero-oligomerization. J Mol Cell Cardiol. 2008;44(3):561–570. doi: 10.1016/j.yjmcc.2007.11.017. [DOI] [PubMed] [Google Scholar]
- 16.Wilkinson GWG, Akrigg A. Constitutive and enhanced expression from the CMV major IE promoter in a defective adenovirus vector. Nucleic Acids Res. 1992;20:2233–2239. doi: 10.1093/nar/20.9.2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.von der Thusen JH, Kuiper J, Fekkes ML, de Vos P, van Berkel TJC, Biessen EAL. Attenuation of atherogenesis by systemic and local adenovirus-mediated gene transfer of interleukin-10 in LDLr−/− mice. Faseb Journal. 2001;15(12):U19–U37. doi: 10.1096/fj.01-0483fje. [DOI] [PubMed] [Google Scholar]
- 18.Pajusola K, Gruchala M, Joch H, Luscher TF, Yla-Herttuala S, Bueler H. Cell-type-specific characteristics modulate the transduction efficiency of adeno-associated virus type 2 and restrain infection of endothelial cells. J Virol. 2002;76(22):11530–11540. doi: 10.1128/JVI.76.22.11530-11540.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen M, Kakutani M, Minami M, Kataoka H, Kume N, Narumiya S. Increased expression of lectinlike oxidised low density lipoprotein receptor-1 in initial atherosclerotic lesions of Watanabe heritable hyperlipidemic rabbits. Arterioscler Thromb Vasc Biol. 2000;20:1107–1115. doi: 10.1161/01.atv.20.4.1107. [DOI] [PubMed] [Google Scholar]
- 20.Neish AS, Williams AJ, Palmer HJ, Whitley MZ, Collins T. Functional-analysis of the human vascular cell-adhesion molecule-1 promoter. J Exp Med. 1992;176(6):1583–1593. doi: 10.1084/jem.176.6.1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cybulsky MI, Iiyama K, Li H, Zhu S, Chen M, Iiyama M. A major role for VCAM-1, but not ICAM-1, in early atherosclerosis. J Clin Invest. 2001;107(10):1255–1262. doi: 10.1172/JCI11871. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information