Abstract
ABCA4 is a photoreceptor-specific ATP-binding cassette transporter implicated in the clearance of all-trans-retinal produced in the retina during light perception. Multiple mutations in this protein have been linked to Stargardt disease and other visual disorders. Here we report the first systematic study of posttranslational modifications in native ABCA4 purified from bovine rod outer segments. Seven N-glycosylation sites were detected in exocytoplasmic domains 1 and 2 by mass spectrometry, confirming the topological model of ABCA4 proposed previously. The modifying oligosaccharides were relatively short and homogeneous, predominantly representing a high-mannose type of N-glycosylation. Five phosphorylation sites were detected in cytoplasmic domain 1, with four of them located in the linker “regulatory-like” region conserved among ABCA subfamily members. Contrary to published results, phosphorylation of ABCA4 was found to be independent of light. Using human ABCA4 mutants heterologously expressed in mammalian cells, we showed that the Stargardt disease-associated alanine mutation in the phosphorylation site at position 901 led to protein misfolding and degradation. Furthermore, replacing the S1317 phosphorylation site reduced the basal ATPase activity of ABCA4, whereas an alanine mutation in either the S1185 or T1313 phosphorylation site resulted in a significant decrease in the all-trans-retinal-stimulated ATPase activity without affecting the basal activity, protein expression, or localization. In agreement with this observation, partial dephosphorylation of native bovine ABCA4 led to reduction of both basal and stimulated ATPase activity. Thus, we present the first evidence that phosphorylation of ABCA4 can regulate its function.
The purpose of a complex set of enzymatic reactions constituting the retinoid cycle in vertebrates is continuous regeneration of the visual chromophore, 11-cis-retinal, after it is transformed to its all-trans isomer by the absorption of light.1 Rapid clearance of all-trans-retinal is critical for maintaining healthy vision because accumulation of this chemically reactive compound mediates retinal degeneration either directly2–5 or through formation of a number of toxic byproducts.6–9 Understanding the fundamental biochemical processes involved in all-trans-retinal detoxification is essential for the development of effective therapeutics for various blinding diseases.
A key protein involved in all-trans-retinal clearance is ABCA4, an ABC transporter expressed predominantly in vertebrate photo-receptors. ABCA4 is localized to rod and cone photoreceptor outer segments where this membrane protein resides in rims and incisures of the disks (refs 10–12 and reviewed in ref 13). The primary structure of ABCA4 consists of ~2300 amino acids in a single polypeptide chain organized in two symmetrical nonidentical parts.14 The most plausible topological model of ABCA4, based on its glycosylation pattern, sequence alignments, and computational prediction of its transmembrane helices, suggests that each of these parts consists of six membrane-spanning helices, an exocytoplasmic domain situated in the disk lumen, and a cytoplasmic domain containing an ATP-binding cassette.15 A combination of animal model, biochemical, and human genetic studies indicates that the transported substrates of ABCA4 are likely to be all-trans-retinal and retinylidene-phosphatidylethanolamine (N-retinylidene-PE), a product of reversible covalent binding of all-trans-retinal to phosphatidylethanolamine and a precursor of the potentially toxic retinoidpyridinium-ethanolamine (A2E).4,8,11,16–21 ABCA4 is thought to reduce the concentration of all-trans-retinal and N-retinylidene-PE inside ROS disks by flipping these substances to the outer surface of the membrane, thereby preventing their accumulation (reviewed in refs 13 and 22–24). Once transported to the outer surface of the disk, liberated all-trans-retinal can re-enter the visual cycle with the help of several enzymes from the retinol dehydrogenase family.25,26
More than 400 mutations in the Abca4 gene have been linked to Stargardt disease, an autosomal recessive form of juvenile macular degeneration with a prevalence of ~1 in 10000 individuals.27–30 Stargardt disease is characterized by rapid irreversible loss of central vision with bilateral atrophy of photo-receptors and retinal pigment epithelial cells of the central retina. Degeneration of photoreceptors is thought to be associated with lost function of the mutant ABCA4 protein as well as cellular stress resulting from its misfolding and mislocalization.31,32 Some studies also link defects in Abca4 with cone–rod dystrophy,33 retinitis pigmentosa,34 and increased susceptibility to the development of age-related macular degeneration (AMD), the leading cause of vision loss in elderly individuals.8,9,35 Notably, the dry form of AMD and Stargardt disease display significant phenotypic similarities.36 Unfortunately, understanding and developing therapies for AMD are impeded by the multifactorial nature of this disease that involves both complex genetic and environmental components.37 In contrast, most cases of Stargardt disease are associated with mutations in the single Abca4 gene,30,38 which makes Stargardt disease a simplified model of AMD in human patients. In this regard, an AMD phenotype has been reproduced in mice by knocking out only two genes, Abca4 and Rdh8.39
Despite the biological role of ABCA4 and its relevance to serious visual disorders, knowledge of the biochemical, structural, and functional properties of this membrane protein remains very limited (reviewed in ref 13). Reasons for this include its large size, low abundance, and rapid loss of its stability and activity after removal from native ROS disk membranes. The hydrophobicity of all-trans-retinal and the instability of N-retinylidene-PE prevent direct measurements of their transport, leaving stimulation of ABC cassette ATPase activity in the presence of these potential substrates as the only available functional assay.16,17,19 Furthermore, a multitude of posttranslational modifications of ABCA4 remains to be explored. In particular, eight glycosylation sites in human ABCA4 demonstrated by mutagenesis studies15 have not been confirmed in the native protein. Moreover, light-dependent phosphorylation has been suggested for frog ABCA4,40 but its role as well as the number and positions of phosphorylation sites remains unknown. Finally, possible mechanisms of regulating ABCA4 transport activity still remain to be investigated.
Here we present a systematic study of posttranslational modifications of ABCA4 purified from a native source. Using mass spectrometry, we detected seven glycosylation sites in bovine ABCA4 and determined their oligosaccharide compositions. Localization of all glycosylation sites in exocytoplasmic domains 1 and 2 confirmed the current topological model of ABCA4. Furthermore, we detected five phosphorylation sites in cytoplasmic domain 1 and investigated their properties using heterologously expressed ABCA4 mutants as well as dephosphorylation of the native protein. Contrary to previously published results,40 we did not observe the light dependence of ABCA4 phosphorylation in either animal studies or isolated bovine retina. Our results suggest that phosphorylation significantly increases the basal and all-trans-retinal-stimulated ATPase activity of ABCA4. Thus, although phosphorylation is not an essential requirement for the biological activity of ABCA4, it likely represents a mechanism that modulates the function of this complex protein.
EXPERIMENTAL PROCEDURES
Materials
Frozen bovine retinas were purchased from W. L. Lawson Co. (Lincoln, NE), and fresh bovine eyes were obtained from a local slaughterhouse. Cyanogen bromide-activated agarose was provided by Santa Cruz Biotechnology (Santa Cruz, CA). DE52 resin was from GE Healthcare. Porcine brain polar lipids were purchased from Avanti Polar Lipids (Alabaster, AL). PNGase F and CHAPS were from Sigma-Aldrich. Human recombinant phosphatase 2A subunit C (L309 deletion) was from Cayman Chemical (Ann Arbor, MI). The DIG glycan differentiation kit and Complete Protease Inhibitor cocktail were from Roche Applied Science. Fluoresceine lectin kits I and II were purchased from Vector Laboratories (Burlingame, CA). Okadaic acid was obtained from Tocris Bioscience (Ellisville, MO). Protein G agarose was from Pierce. Pfu DNA polymerase was purchased from Fermentas (Burlington, ON). DDM detergent was provided by Affymetrix (Santa Clara, CA).
Animals
C57BL/6 mice were purchased from The Jackson Laboratory. All mice were maintained on a normal chow diet in a 12 h light–12 h dark cyclic environment. All experimental procedures involving animals were approved by the Case Western Reserve University Animal Care Committee and conformed to the recommendations of the American Veterinary Medical Association Panel on Euthanasia and the Association for Research in Vision and Ophthalmology (ARVO) Statement for the Use of Animals in Ophthalmic and Vision Research.
Purification and Reconstitution of ABCA4 from Bovine ROS
Bovine ROS were isolated from frozen retinas under dim red light transmitted through a Kodak No. 1 safelight filter (transmittance of >560 nm) using the procedure of Paper-master.41 ROS membranes were prepared by homogenization of ROS in hypotonic buffer [5 mM Bis-tris propane (BTP) (pH 7.5) and 1 mM DTT] followed by centrifugation at 20000g for 30 min. The pellet was solubilized with buffer containing 20 mM BTP (pH 7.5), 10% glycerol, 30 mM NaCl, 1 mM DTT, 20 mM DDM, and 0.2 mg/mL porcine brain polar lipids. Solubilized membranes were centrifuged at 40000g for 30 min to remove insoluble material. ABCA4 was purified at 4 °C under dim red light in two steps. For experiments involving measurements of ATPase activity, all buffers included 0.2 mg/mL porcine brain lipids. In the first step, solubilized membranes were applied to a DE52 anion exchange column equilibrated with 20 mM BTP (pH 7.5), 10% glycerol, 30 mM NaCl, 1 mM DTT, and 1 mM DDM (buffer A). The column was washed with the same buffer, and bound proteins were eluted with 20 mM BTP (pH 7.5), 10% glycerol, 150 mM NaCl, 1 mM DTT, and 1 mM DDM (buffer B). This partially purified ABCA4 preparation was used for in-gel digests followed by mass spectrometry experiments. In the second step, eluted proteins were incubated with gentle rocking for 2 h with the Rim3F4 monoclonal antibody coupled to cyanogen bromide-activated agarose. The resin was then packed into a column and washed with buffer B. To elute ABCA4, the resin was incubated in the same manner with buffer B containing 1 mg/mL YDLPLHPRT peptide.
To reconstitute ABCA4 into lipid vesicles, detergent was exchanged with 10 mM CHAPS during the immunoaffinity chromatography wash and elution steps. Reconstitution in porcine brain polar lipids was performed immediately after purification as described previously.16 Detergent was removed by overnight dialysis at 4 °C against buffer containing 20 mM BTP (pH 7.5), 10% glycerol, 150 mM NaCl, and 1 mM DTT.
Purification of ABCA4 from Mouse ROS
Mouse ROS and ROS membranes were isolated as previously described.42 Purification of ABCA4 was performed by immunoprecipitation on ice under dim red light. ROS from 10 mouse retinas were solubilized with 1 mL of buffer containing 10 mM sodium phosphate (pH 7.6), 50 mM NaCl, 50 mM NaF, 10 mM EDTA, 30 mM DDM, 10% glycerol, 0.1 mM DTT, and 10 nM okadaic acid. Insoluble material was removed by centrifugation at 16000g for 5 min. Then 50 μg of the Rim3F4 antibody was added followed by incubation for 1 h with gentle rocking. Fifty microliters of settled protein G agarose was added, and the sample was incubated for 1 h at room temperature. The resin was washed with buffer containing 10 mM sodium phosphate (pH 7.6), 50 mM NaCl, 50 mM NaF, 10 mM EDTA, 1 mM DDM, 10% glycerol, and 0.1 mM DTT. ABCA4 was eluted by incubation for 30 min at room temperature in the same buffer supplemented with 5 mg/mL YDLPLHPRT peptide.
Deglycosylation and Dephosphorylation of ABCA4
For mass spectrometry studies, bovine ABCA4 was deglycosylated with commercially obtained PNGase F from Elizabethkingia meningoseptica under denaturing conditions according to the manufacturer’s protocol. To deglycosylate ABCA4 under nondenaturing conditions, we incubated protein samples for 2 h at 4 °C with a 10-fold molar excess of PNGase F heterologously expressed in Escherichia coli from our laboratory. Cleavage of oligosaccharides was confirmed by a slight increase in ABCA4 mobility in sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS–PAGE) gels29 (data not shown).
To dephosphorylate ABCA4, 120 μL of the purified protein was incubated with 4 units of commercially available phosphatase 2A subunit C (PP2A) in the presence of 34 mM MgCl2, 2 mM DTT, and 2 mM EDTA for 1 h at room temperature or overnight at 4 °C.
Phosphoprotein Staining and Quantification
Phosphoproteins separated by SDS–PAGE were stained with Pro-Q Diamond gel stain according to the manufacturer’s protocol. Gels were imaged with a Typhoon 9410 scanner (GE Healthcare) with excitation at 532 nm and a 580 ± 30 nm emission filter. For total protein visualization, the same gels were then stained with SYPRO Ruby protein gel stain according to manufacturer’s instructions and imaged by the same instrument with excitation at 488 nm and a 580 ± 30 nm emission filter. Protein quantification was performed with ImageQuant TL (GE Healthcare).
Generation of ABCA4 Mutant Constructs
Human ABCA4 with a C-terminal 1D4 tag subcloned into a pCEP4 vector (Invitrogen) at its NotI and AsiSI sites was used as a template for site-directed mutagenesis. T901A, S1185A, T1313A, and S1317A mutations were introduced by overlap extension polymerase chain reaction using Pfu DNA polymerase and the following mutagenic primers (with introduced mutations shown in bold): T901Af, gagcccctagccgaggaaacg; T901Ar, cgtttcctcggctaggggctc; S1185Af, ctaagggtttcgccaccacgtgt; S1185Ar, acacgtggtggcgaaacccttag; T1313Af, gctggacaggccccccaggac; T1313Ar, gtcctggggggcctgtccagc; S1317Af, gacaccccaggacgccaatgtctgc; S1317Ar, gcagacattggcgtcctggggtgtc. T901A was constructed with ABCA4-fwd (aatattgcggccgccaccatgggcttcgtgagac) and ABCA4-FseI-rev (gccacagggctcaaaaatct) primers and subcloned into the NotI and FseI sites of the ABCA4 construct. S1185A, T1313A, and S1317A were constructed with ABCA4 FseI-Fwd (agatttttgagccctgtggc) and ABCA4-Sbf I-rev (ccctggtgctgcacctgc) primers and subcloned into the FseI and Sbf I sites. The presence of these mutations was confirmed by DNA sequencing.
Transfection of HEK-293T and COS-7 Cells
HEK-293T and COS-7 cells were grown in Dulbecco’s modified Eagle’s medium supplemented with 10% bovine growth serum, 100 units/mL penicillin, 100 μg/mL streptomycin, 2 mM L-glutamine, and 1.25 μg/mL fungizone. Typically, each 10 cm dish of cells at 30% confluency was transfected with 20 μg of plasmid DNA by using the calcium phosphate method.
Purification and Reconstitution of Heterologously Expressed ABCA4
ABCA4 was purified from HEK-293T or COS-7 cells and reconstituted in lipid vesicles following previously published procedures16 with certain modifications. The cell suspension (from two 10 cm dishes) was added slowly to 1.0 mL of buffer A [1 mg/mL porcine brain polar lipids, 10% glycerol, 1 mM DTT, 100 mM NaCl, 3 mM MgCl2, and 50 mM HEPES (pH 7.4)] containing 18 mM CHAPS and protease inhibitor cocktail and stirred for 1 h at 4 °C. After a 10 min centrifugation at 100000g, the supernatant was mixed with 70 μL of Rim1D4-Sepharose 2B beads for 1 h at 4 °C. The beads were washed six times in buffer B (10 mM HEPES, 100 mM NaCl, 5 mM MgCl2, and 1 mM DTT) containing 10 mM CHAPS and eluted with 0.2 mg/mL Rim1D4 peptide in the same buffer. Purified protein (160 μL) was mixed with an equal volume of 5 mg/mL porcine brain polar lipids (dissolved in 10 mM CHAPS), incubated on ice for 1 h, and dialyzed against three changes of buffer B overnight.
Immunofluorescent Labeling of Cells
COS-7 cells were grown on glass coverslips and transfected as described above. After 24 h, cells were washed in 0.1 M phosphate buffer (PB, pH 7.4) and fixed in 4% paraformaldehyde. Cells were then blocked and permeabilized for 15 min in PB containing 10% (v/v) normal goat serum and 0.2% (v/v) Triton X-100. Sections were stained with DAPI nuclear stain (Molecular Probes). Double labeling experiments were conducted on cells treated with Rho 1D4 antibody (UILO) followed by goat anti-mouse Ig conjugated to Alexa 596 (Molecular Probes) and calnexin labeled with a rabbit anti-calnexin polyclonal antibody (Abcam, Cambridge, MA) followed by goat anti-rabbit Ig conjugated to Alexa 488 (Molecular Probes). Stained sections were washed three times for 30 min each in PB and visualized with a Zeiss LSM700 confocal microscope (Carl Zeiss AG, Jena, Germany) and processed with Zeiss Zen Image Browser.
Autophosphorylation Assay
Purified bovine ABCA4 was dephosphorylated as described above. His-tagged PP2A was removed by passing the sample through a spin column containing NiNTA resin. The unbound fraction was supplemented with 1 mM ATP and incubated for 1 h at room temperature. Phosphoprotein staining was then performed as described above.
Lectin Binding Assays
Purified bovine ABCA4 was subjected to SDS–PAGE and transferred to a PVDF membrane. The membrane was incubated with blocking solution from a DIG glycan differentiation kit according to the manufacturer’s instructions followed by incubation at room temperature for 1.5 h in the dark with various fluorescein-labeled lectins (1:100 dilution) in Tris-buffered saline with 1 mM MgCl2, 1 mM MnCl2, and 1 mM CaCl2. After three 10 min washes with the same buffer, the membrane was dried and fluorescein fluorescence was measured with a Typhoon 9410 scanner (GE Healthcare) with excitation at 488 nm and a 526 nm emission filter. Alternatively, the membrane was stained by using the DIG glycan differentiation kit according to the manufacturer’s instructions.
ATPase Activity Assays
ABCA4 purified from bovine retina and reconstituted in lipid vesicles was supplemented with MgCl2 to a final concentration of 1 mM. To measure all-trans-retinal-stimulated activity, samples were preincubated with 100 μM all-trans-retinal for 5 min. The ATPase reaction was initiated via addition of 10 μL of a 10× ATP stock (1 μCi of [33P]ATP) to 90 μL of protein sample to reach a final ATP concentration of 50 μM. Samples were incubated in the dark at 37 °C. After 2 h, samples were added to 1 mL of 10% activated charcoal in 10 mM HCl, vortexed, and centrifuged. Radioactivity in the resulting supernatant was counted in an LS 6500 multipurpose scintillation counter (Beckman Coulter).
To determine the ATPase activity of heterologously expressed ABCA4 mutants, hydrolysis of [α-32P]ATP (Perkin-Elmer) in a 10 μL reaction volume was detected by thin layer chromatography as previously described.16 Final concentrations of both ATP and all-trans-retinal were 50 μM. Briefly, the reaction was initiated via addition of 1 μL of a 10× ATP solution (0.2 μCi) to 8 μL (20–40 ng) of the reconstituted sample. After 30 min at 37 °C, 4 μL of 10% SDS was added and the tube was briefly centrifuged. One microliter of the reaction mixture was spotted onto a polyethyleneimine cellulose plate (Sigma) and chromatographed in a 0.5 M LiCl/1 M formic acid mixture. The plate was exposed to a storage phosphor screen overnight and then scanned with a Phosphor Imager SI (Molecular Dynamics).
Light Dependence of ABCA4 Phosphorylation
Twenty female 6-week-old mice were dark-adapted for 16 h and divided into two equal groups. The first group was left in the dark, whereas the second group was anesthetized by intra-peritoneal injection of 20 μL/g of body weight of 6 mg/mL ketamine and 0.44 mg/mL xylazine diluted with 10 mM sodium phosphate (pH 7.2) and 100 mM NaCl. Mice were exposed to 100 W bulb light for 10 min and then sacrificed. Retinas were immediately removed from the eyeballs through an incision in the cornea and immersed in buffer containing 10 mM sodium phosphate (pH 7.6), 50 mM NaCl, 50 mM NaF, 10 mM EDTA, 0.1 mM DTT, and 10 nM okadaic acid. ABCA4 was then purified by immunoprecipitation as described above.
To test the light dependence of ABCA4 phosphorylation in bovine photoreceptors, isolated ROS were brought to room temperature and supplemented with 1 mM ATP and 1 mM MgCl2. ROS were then kept in the dark or illuminated with the fiber light (Fiber-Light 180 Illuminator, 150 W, Dolan Jenner Industries Inc.) through a 480–520 nm band-pass filter at a distance of 10 cm for 1 min. Samples were kept in the dark at room temperature for 30 min followed by ROS membrane isolation as described above. ABCA4 was partially purified by ion exchange chromatography (see above) and subjected to phosphoprotein staining.
Mass Spectrometry
Coomassie-stained SDS–PAGE gel pieces containing intact or PNGase F-treated bovine ABCA4 were destained with 50% acetonitrile in 100 mM ammonium bicarbonate followed by 100% acetonitrile. Next, Cys residues were reduced by incubating the sample with 20 mM DTT at room temperature for 60 min followed by alkylation with 50 mM iodoacetamide for 30 min in the dark. Reaction reagents were removed, and the gel pieces were washed with 100 mM ammonium bicarbonate and dehydrated in acetonitrile. Gel pieces were dried in a SpeedVac centrifuge, rehydrated in 50 mM ammonium bicarbonate containing sequencing grade modified trypsin or chymotrypsin, and left for overnight digestion. Proteolytic peptides were extracted from gels with 50% acetonitrile in 5% formic acid. Identification of ABCA4 phosphorylation was facilitated by removal of sugar chains from the protein with PNGase F (see above), as well as by phosphopeptide enrichment with a TiO2 MonoTip column (GL Science Inc.) used according to the manufacturer’s protocol. Liquid chromatography–tandem mass spectrometry (LC–MS/MS) analysis of the resulting peptides was performed with a LTQ Orbitrap XL linear ion trap mass spectrometer (Thermo Fisher Scientific, Waltham, MA) coupled to an Ultimate 3000 HPLC system (Dionex, Sunnyvale, CA). Spectra were recorded by data dependent methods consisting of a full scan and MS/MS of the five most abundant precursor ions at the normalized collision energy of 30%. Obtained data were submitted to a database search with Mascot Daemon (Matrix Science, Boston, MA). The search was performed by setting the variable modification to phosphorylation on Ser, Thr, and Tyr residues. Phosphorylation sites were then verified by manual examination of each tandem mass spectrum of phosphopeptides. Candidate N-glycosylation sites were initially identified with sugar-free peptides based on conversion of Asn residues to Asp by PNGase F. Corresponding glycosylated peptides were then identified in the spectra of ABCA4 not treated with PNGase F. The sugar composition of glycopeptides was determined by manual interpretation of their MS/MS spectra.
RESULTS
Purification of ABCA4 from Bovine ROS Membranes
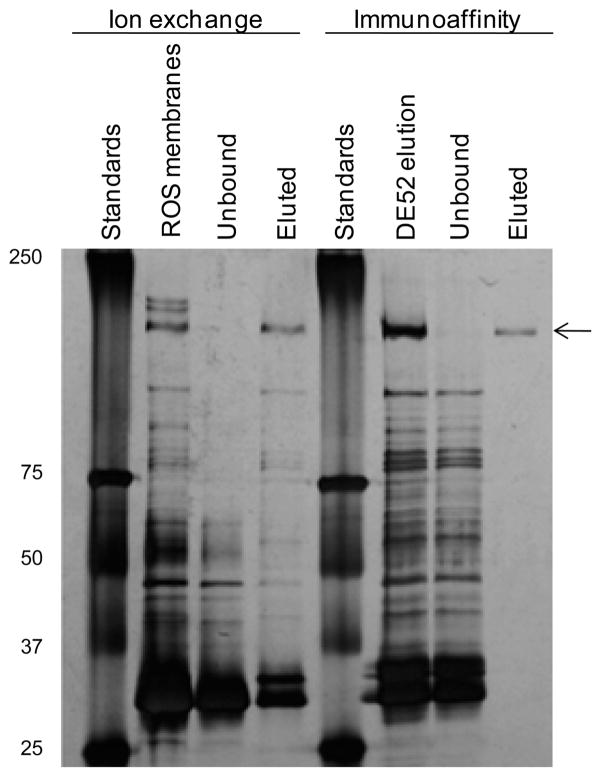
ABCA4 is commonly purified in one step by immunoaffinity chromatography with the immobilized Rim3F4 monoclonal antibody.29 This method, however, requires a relatively high antibody:ABCA4 ratio, making it more suitable for obtaining analytical quantities of ABCA4. We modified this procedure to obtain larger yields of native bovine protein required to determine the glycosylation and phosphorylation sites and glycan composition by mass spectrometry. The new ion exchange chromatography step enriched ABCA4 ~10-fold (from ~1 to ~10%) starting from solubilized ROS membranes (Figure 1). Furthermore, it efficiently separated high-molecular weight contaminating proteins, leading to high peptide sequence coverage after ABCA4 band excision and in-gel digestion with trypsin or chymotrypsin. For applications demanding a higher purity, the protein was further purified by immunoaffinity chromatography (Figure 1).
Figure 1.
Purification of ABCA4 from membranes of bovine rod outer segments. The silver-stained SDS–PAGE gel illustrates the two purification steps applied sequentially: ion exchange chromatography on DE52 resin (left) followed by immunoaffinity chromatography on agarose-coupled Rim3F4 monoclonal anti-ABCA4 antibody (right). The position of ABCA4 is marked with an arrow. See Experimental Procedures for details.
Glycosylation Sites of Native Bovine ABCA4
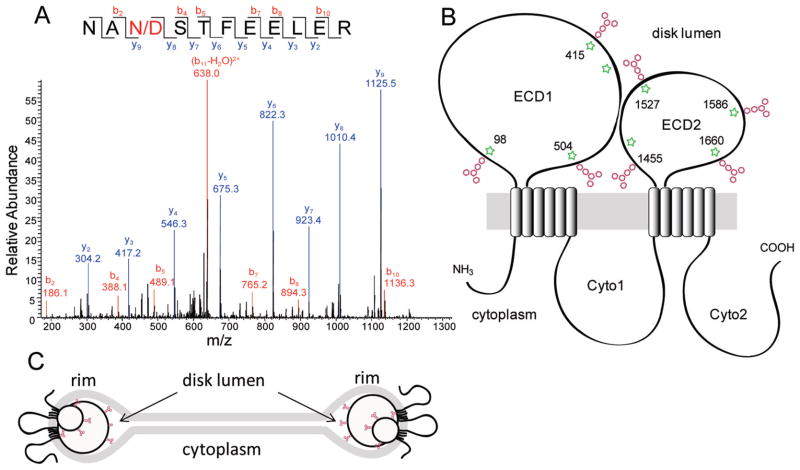
The overall peptide sequence coverage for ABCA4 purified from bovine retina in this study was 84%, with unidentified regions located mostly in transmembrane domains (Table S1 and Figures S1 and S2 of the Supporting Information). We identified seven N-linked glycosylation sites on the basis of characteristic 1 Da mass increases resulting from transformation of asparagine residues to aspartates by PNGase F during cleavage of β-aspartylglycosylamine linkages (Figure 2A,B). Consistent with the current topological model of ABCA4,15 all these sites were located in exocytoplasmic domains 1 (three sites) and 2 (four sites) (Figure 2B). These experimentally determined sites coincide with seven sites predicted in these domains on the basis of the Asn-X-Ser/Thr sequence motif of N-linked glycosylation. Notably, peptides carrying glycosylation sites at positions 98, 415, 504, 1527, 1586, and 1660 were not detected in a free form in glycosylated ABCA4 digests, which indicates nearly 100% glycosylation. The glycosylated fraction for the site at position 1455 was estimated to be 98% on the basis of comparison of areas under ion signals corresponding to the glycosylated and unglycosylated forms (data not shown). As expected, N-linked glycosylation sites were not detected in the cytoplasmic regions of ABCA4.
Figure 2.
Identification of N-glycosylation sites in native bovine ABCA4 by mass spectrometry. (A) Representative MS/MS spectrum of a peptide (NANSTFEELER, residues 413–423) obtained after trypsin digestion of ABCA4 deglycosylated with PNGase F. Treatment with PNGase F converts glycosylated asparagines to aspartates as a result of the glycoaminidase activity of this enzyme. (B) Topological model of ABCA4 illustrating N-glycosylation sites detected in this study. ECD1 and ECD2 are exocytoplasmic domains 1 and 2, respectively. Cyto1 and Cyto2 are cytoplasmic domains 1 and 2, respectively. Positions of glycosylation sites of human ABCA415 are denoted with green stars. (C) Glycosylation of the exocytoplasmic domains can contribute to localization of ABCA4 restricted to the rims of ROS disks.
Glycosylation Types and Glycan Composition
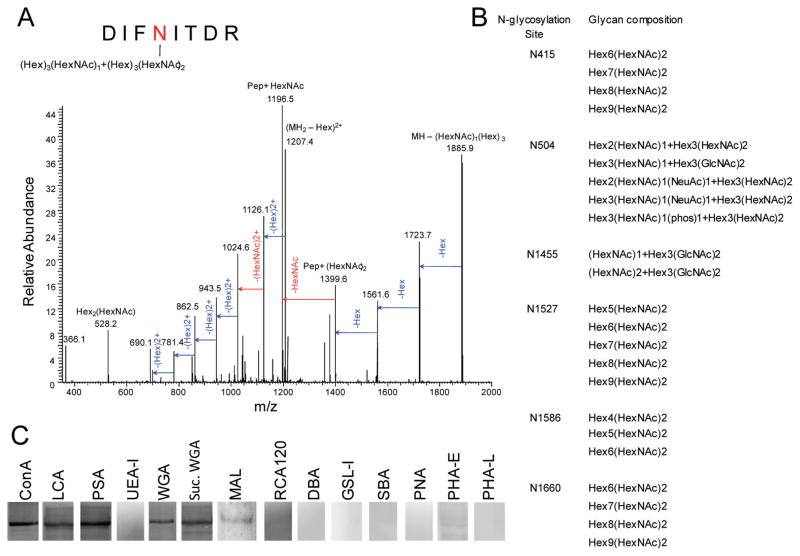
We determined the composition of six of seven ABCA4 oligosaccharides based on patterns of their collision-induced fragmentation observed in tandem mass (MS/MS) spectra of the corresponding glycopeptides (Figure 3A and Figure S3 and Table S2 of the Supporting Information). The sugar chains attached to the protein were relatively short and homogeneous in composition (Figure 3B). This agrees with the single sharp ABCA4 band observed in SDS–PAGE gels (Figure 1) with only a slight shift in mobility detected after protein deglycosylation.15,29 The mild heterogeneity observed in MS experiments could be partially induced by in-source glycan fragmentation.43 Deglycosylation of ABCA4 with PNGase F did not affect the basal and all-trans-retinal-stimulated ATPase activities of the protein (data not shown).
Figure 3.
Determination of glycan compositions and N-glycosylation types of native bovine ABCA4. (A) An MS/MS spectrum of glycosylated peptide DIFNITDR (residues 501–508) reflects collision-induced dissociation of the attached polysaccharide. (B) List of glycans detected by mass spectrometry for each N-glycosylation site except N98. Abbreviations: Hex, hexose; HexNAc, N-acetylhexosamine; NeuAc, N-acetylneuraminic acid. (C) Staining of a PVDF membrane containing ABCA4 with lectins of various specificities. Abbreviations: ConA, concanavalin A; LCA, Lens culinaris agglutinin; PSA, Pisum sativum agglutinin; UEA-I, Ulex europaeus agglutinin I; WGA, wheat germ agglutinin; Suc. WGA, succinylated WGA; MAL, Maackia amurensis lectin II; RCA120, Ricinus communis agglutinin I; DBA, Dolichos biflorus agglutinin; GSL-I, Griffonia (Bandeiraea) simplicifolia lectin I; SBA, soybean agglutinin; PNA, peanut agglutinin; PHA-E, Phaseolus vulgaris agglutinin E; PHA-L, P. vulgaris agglutinin L. See the Supporting Information for lectin specificities.
The types of N-linked glycosylation were determined by testing the binding of a diverse panel of lectins to native bovine ABCA4 (Figure 3C and Table S3 of the Supporting Information). Binding was detected for several lectins specific to α-linked mannose (ConA, LCA, and PSA), N-acetylglucos-amine (succinylated WGA and WGA) and sialic (N-acetylneuraminic) acid (MAL and WGA). In contrast, lectins specific to α-linked fucose, galactose, and N-acetylgalactos-amine did not bind ABCA4. In conjunction with the mass spectrometry data, this suggests that oligosaccharides of native bovine ABCA4 represent high-mannose (sites at positions 415, 1527, 1586, and 1660) and hybrid/complex (sites at positions 504 and 1455) types of N-glycosylation.
Identification of Phosphorylation Sites in Native Bovine ABCA4
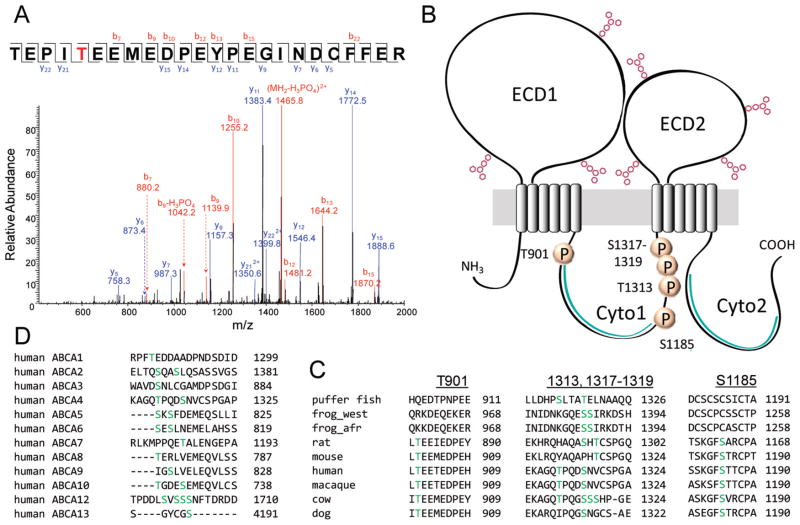
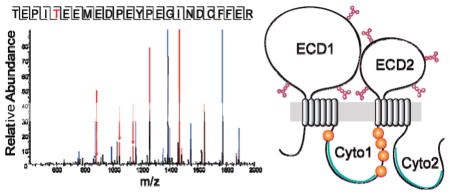
Five phosphorylated residues were detected in bovine ABCA4 by mass spectrometry after phosphopeptide enrichment with TiO2 chromatography (Figure 4A and Figures S4–S8 of the Supporting Information). All were found in cytoplasmic domain 1 (Cyto1), with one site (T901) located in the N-terminal part before the nucleotide binding cassette and the four remaining sites (S1185, T1313, and two of three Ser residues at positions 1317–1319) situated in a large region of the C-terminal part with an unknown biological function (Figure 4B). Sequence alignment showed conservation of the phosphorylation sites at positions 901 and 1185 among mammalian but not frog and fish ABCA4 transporters, whereas the sites at positions 1313 and 1317–1319 were partially conserved among all ABCA4 orthologs with known primary structures (Figure 4C). The position 1313 site is also largely conserved among human transporters belonging to the ABCA subfamily (Figure 4D).
Figure 4.
Phosphorylation sites of native bovine ABCA4. (A) MS/MS spectrum of a phosphorylated peptide (TEPITEEMEDPEYPEGINDCFFER, residues 897–920). T901 (red) is phosphorylated. (B) Topological model of ABCA4 illustrating detected phosphorylation sites (marked with P). Positions of ATP-binding cassettes within the cytoplasmic domains are colored green. (C) Sequence alignments showing conservation of detected phosphorylation sites among ABCA4 transporters from different species. Definitions: puffer fish, Takifugu rubripes; frog_west, western clawed frog [Xenopus (Silurana) tropicalis]; frog_afr, African clawed frog (Xenopus laevis). (D) Sequence alignment showing conservation of the S1185 phosphorylation site among human ABC transporters of the ABCA subfamily.
Employment of the TiO2 enrichment procedure to facilitate detection of phosphorylated peptides (see Experimental Procedures) precludes estimation of the extent of phosphorylation. However, we did make such an estimate for the AGQTPQGSSSHPGEPAAHPEGQPPPER peptide (residues 1310–1336) that can be reliably detected without enrichment. This peptide carries three phosphorylation sites, and the ratios of the unmodified peptide to peptides carrying one, two, and three phosphate groups were found to be 1:0.29, 1:0.11, and 1:0.03, respectively (Figure S9 of the Supporting Information). These values represent only rough estimates because of known differences in the ionization efficiency of phosphorylated and unphosphorylated peptides.44 Nevertheless, it is reasonable to conclude that the phosphorylation sites of native ABCA4 are only partially populated.
Mutations in Phosphorylation Sites Alter the Activity and Stability of Heterologously Expressed Human ABCA4
To reveal possible biological roles of ABCA4 phosphorylation, we created ABCA4 constructs with alanine mutations in the most conserved phosphorylation sites, namely, T901A, S1185A, T1313A, and S1317A. These proteins were heterologously expressed in mammalian cells, and their expression levels, cellular localizations, and basal and all-trans-retinal-stimulated ATPase activities were determined. The S1185A, T1313A, and, to a lesser extent, S1317A mutants localized to intracellular vesicles like wild-type ABCA445 and demonstrated comparable expression levels (Figure 5A,B). In contrast, replacement of Thr901 with an alanine resulted in a poor expression level along with retention of the protein in the endoplasmic reticulum, indicative of misfolding (Figure 5A,B).
Figure 5.
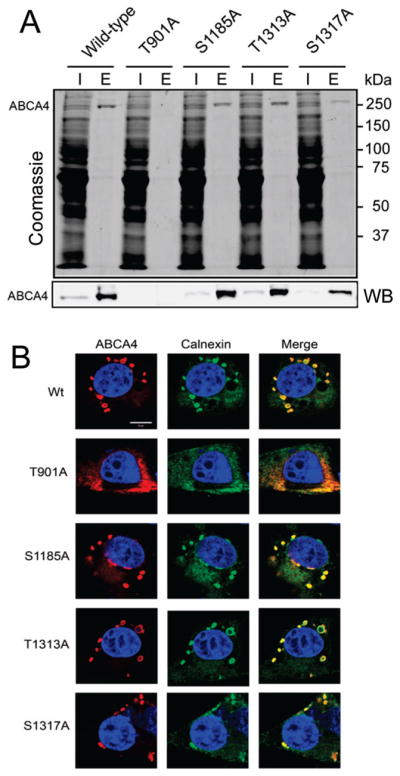
Effect of mutations in phosphorylation sites of human ABCA4 transiently expressed in COS-7 cells on expression levels and cellular localization of this protein. (A) Purification of 1D4-tagged wild-type and mutant ABCA4 from cell membranes illustrated by Coomassie-stained SDS–PAGE (top) and Western blotting (bottom). Abbreviations: I, input; E, elution from the 1D4 immunoaffinity column. The T901A mutant is poorly expressed, and the level of expression of the S1317A mutant is reduced compared to those of wild-type ABCA4 and the other ABCA4 mutants. (B) Cellular localization of wild-type and mutated ABCA4 assessed by confocal microscopy. WT and mutants S1185A, T1313A, and, to a lesser extent, S1317A localize to intracellular vesicles. Mutant T901A is retained in the ER. Calnexin was used as an ER marker.
Basal ATPase activities of the S1185A and T1313A mutants were identical to that of wild-type ABCA4 (Table 1). However, elimination of phosphorylation sites at these positions reduced the known stimulating effect of all-trans-retinal on ATP hydrolysis. In the presence of 50 μM all-trans-retinal, the ATPase activity of S1185A and T1313A mutants was increased by only 60 and 100%, respectively, relative to the roughly 150% stimulation observed for the wild-type protein. Furthermore, introducing an alanine residue at position 1317 affected both the basal and stimulated ATPase activity. As a result of this mutation, the basal activity was reduced approximately 5-fold. The ATPase activity of this mutant was stimulated ~60% by addition of all-trans-retinal but still was almost 7 times lower than wild-type ABCA4 activity.
Table 1.
Basal and All-trans-Retinal-Stimulated Activity of Human ABCA4 Variants with Mutated Phosphorylation Sites
| sample | basal activity (nmol mg−1 min−1) | retinal-stimulated activity (nmol mg−1 min−1) |
|---|---|---|
| wild-type | 37.5 ± 1.1 | 91.7 ± 1.5 |
| S1185A | 39.0 ± 1.4 | 61.6 ± 2.2 |
| T1313A | 37.5 ± 1.6 | 74.5 ± 2.4 |
| S1317A | 8.5 ± 0.9 | 13.5 ± 1.1 |
ABCA4 Does Not Possess Autophosphorylation Activity
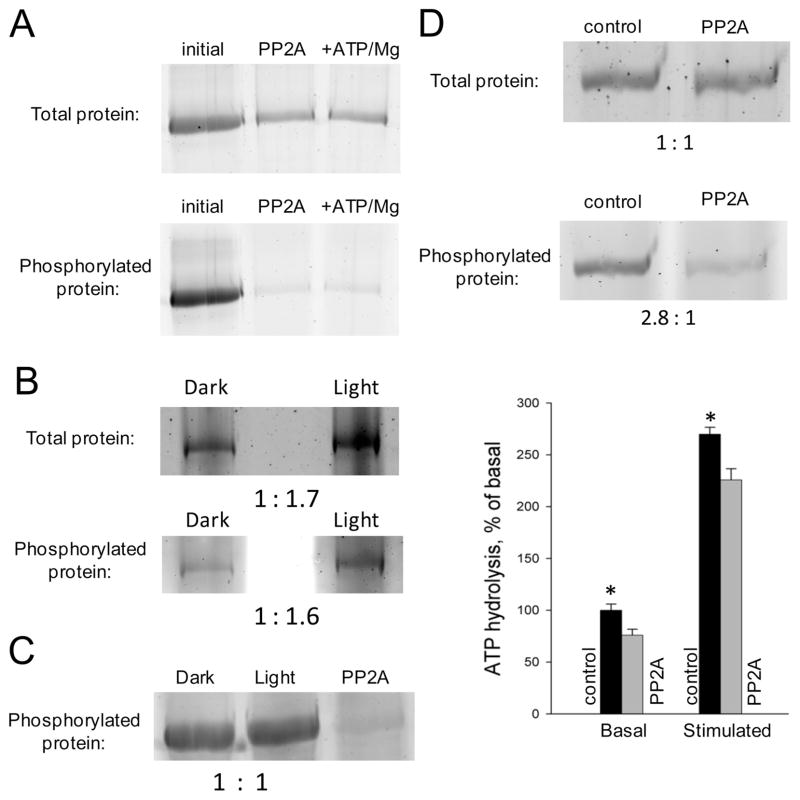
We investigated whether ABCA4 undergoes auto-phosphorylation. To do this, we first established that ABCA4 is a good substrate for the catalytic subunit of protein phosphatase 2 (PP2A). Treatment with PP2A indeed reduced the phosphorylation level of ABCA4 in a time-dependent manner (Figure S10 of the Supporting Information). To study autophosphorylation, we incubated purified ABCA4 with PP2A in the presence of detergent. The His-tagged phosphatase then was removed by passage through a Ni-NTA spin column. A subsequent incubation with ATP and magnesium did not alter the phosphorylation level of ABCA4 (Figure 6A). We conclude that ABCA4 does not have autophosphorylation activity under the described experimental conditions.
Figure 6.
Properties of ABCA4 phosphorylation. (A) Autophosphorylation of ABCA4 from bovine retina. Conditions: initial, ABCA4 before treatment with protein phosphatase 2 (PP2A); PP2A, ABCA4 after dephosphorylation with PP2A; +ATP/Mg, dephosphorylated protein incubated in the presence of ATP and magnesium. (B) Phosphorylation levels of ABCA4 purified from retina of dark- and light-adapted mice. (C) Phosphorylation levels of ABCA4 purified from bovine retina before and after photobleaching. (D) Influence of phosphorylation level on basal and all-trans-retinal-stimulated ATPase activity of native bovine ABCA4. Conditions: control, ABCA4 not treated with PP2A; PP2A, dephosphorylated ABCA4. Band intensity ratios shown below each gel were obtained by densitometry. Bars reflect relative levels of ATPase activity. Data shown represent means ± the standard deviation of six measurements. Statistically significant differences (p < 0.05) are denoted with asterisks.
ABCA4 Phosphorylation Is Independent of Light Exposure
An increase in the phosphorylation level of ABCA4 upon light exposure was reported earlier in freshly isolated bullfrog retinas.40 Therefore, we hypothesized that phosphorylation of ABCA4 in other species could also be light-dependent. To test this hypothesis, we first compared phosphorylation levels of ABCA4 from dark-adapted and light-exposed mice (see Experimental Procedures). ABCA4 from retinas of both dark-adapted mice in a darkroom and light-exposed mice under ambient light was purified by immunoprecipitation, and SDS-PAGE gels containing these proteins were exposed to a quantitative phosphoprotein-specific stain (Pro-Q Diamond). The total amount of protein in the bands was then quantified by staining with SYPRO Ruby (Figure 6B). This analysis did not reveal any effect of light on ABCA4 phosphorylation. In another approach, we prepared ROS from dark-adapted bovine retinas and exposed them to light in the presence of ATP following the general procedure of Szuts.40 ABCA4 then was purified from ROS membranes, and its level of phosphorylation was compared to that of the unilluminated protein isolated from ROS (Figure 6C). In agreement with results obtained in mice, we did not observe light-dependent changes in ABCA4 phosphorylation. Both ABCA4 samples demonstrated significant phosphorylation levels relative to that of the same protein treated with PP2A (Figure 6C).
ATPase Activity of Native ABCA4 Is Modulated by Phosphorylation
To determine whether the ATPase activity of native bovine ABCA4 depends on phosphorylation, we incubated purified ABCA4 with PP2A for 1 h at room temperature prior to lipid reconstitution and activity measurements. A control sample lacking PP2A was handled in the same way. We chose to limit the dephosphorylation step to 1 h because of previous evidence that ABCA4 quickly loses ATPase activity if not reconstituted in lipid vesicles immediately after purification.16 Treatment with PP2A reduced the phosphorylation level to ~35% of the initial value (Figure 6D). This partial dephosphorylation resulted in statistically significant reductions in the basal as well as all-trans-retinal-stimulated activity of the treated sample as compared to the control (Figure 6D). These results agree well with the reduced levels of basal or all-trans-retinal-stimulated activity shown for S1317A or S1185A and T1313A mutants, respectively, of ABCA4 expressed in mammalian cells (Table 1).
DISCUSSION
In this study, we characterize posttranslational modifications of the photoreceptor-specific ABC transporter, ABCA4. We detected seven N-linked glycosylation sites in the exocytoplasmic domains of native bovine ABCA4 and determined the composition of the modifying sugars. In addition, five phosphorylation sites were found on the first of two cytoplasmic domains. Our results also suggest that ABCA4 phosphorylation is light-independent and could regulate the transport activity of this protein.
N-Linked Glycosylation Sites and ABCA4 Membrane Topology
In the absence of direct structural information, several conflicting topological models of ABCA4 were initially proposed on the basis of indirect evidence.21,28,29 In a subsequent study, Bungert et al. demonstrated by systematically mutating predicted N-linked glycosylation sites in heterologously expressed human ABCA4 that glycosylation occurs in two large regions flanked by transmembrane α-helices.15 This resulted in the currently accepted topological model of ABCA4 with the two glycosylated domains (ECD-1 and ECD-2) situated outside of the cytoplasm in the ROS disk lumen (rods) or extracellular space (cones) (Figure 2B). In this study, we determined the N-glycosylation sites of native ABCA4 purified from bovine retina. There are 16 consensus NX(S/T) N-glycosylation sequences, where X is any amino acid except proline, in the primary structure, of which only seven undergo glycosylation. Six of these sites coincide with those discovered in human ABCA4; in the bovine protein, one site (S1455) is shifted and one site is missing (Figure 2B). Importantly, all modified residues are located in the exocytoplasmic domains. Thus, our results with native ABCA4 confirm the topological model of Bungert and colleagues.15
Composition of Oligosaccharides
Previously, it was shown that ABCA4 can be labeled with concanavalin A, a lectin specific to α-linked mannose, and that deglycosylation of the protein induced only a small mobility shift on SDS–PAGE.29 However, the types and composition of modifying oligosaccharides were unknown. This study directly demonstrates that the sugar groups attached to native bovine ABCA4 are small in size and have a homogeneous composition. Four of six oligosaccharides with determined compositions represent the high-mannose type of N-glycosylation, whereas the remaining two also contain N-acetylglucosamine and sialic acid groups (Figure 3A–C). This detailed characterization will be important for further structural studies of ABCA4.
Possible Roles of Glycosylation
Glycosylation is commonly observed in eukaryotic ABC transporters.46–48 It has been proposed that glycosylation can be needed for proper folding, stability, trafficking, and correct insertion of these proteins in the membrane,46,47 but in studies of P-glycoprotein, inhibition of N-glycosylation or removal of N-glycosylation sites by mutagenesis did not affect the level of drug resistance in transfected cells, suggesting that N-glycosylation is not important for substrate transport.46,49 In agreement with the latter observation, we did not find differences in either basal or all-trans-retinal-stimulated ATPase activity between intact and deglycosylated native ABCA4. Interestingly, a Stargardt disease-associated missense mutation in ABCA4, S100P,31,50 removes a glycosylation site in the ECD-1 domain (Figure 2B). In this case, mislocalization or incorrect insertion of ABCA4 into the membrane can relate to the observed photoreceptor degeneration.
A unique feature of ABCA4 among other ABC transporters is that this protein in rod cells resides almost exclusively in rims and incisures of the outer segment disks29,51 (Figure 2C). It has been suggested that such a confined localization can be dictated by the large size of exocytoplasmic domains of ABCA4 relative to known values of the distance between the membranes of a ROS disk in different species.13,52 In particular, the diameter of the larger ECD-1 was roughly estimated to be 5–6 nm,13 which is comparable to or greater than the measured values of the distance between membranes of the same disk.52 Several sugar groups decorating the exocytoplasmic domains of bovine ABCA4 contain more than 10 monosaccharides and would make this domain even larger (Figure 3B). Although the exact structures of these sugar groups are not yet known, we speculate that their size and hydrophilic character can contribute to the restricted localization of ABCA4.
ABCA4 Phosphorylation Sites
All five phosphorylation sites detected in this study are located in cytoplasmic domain 1 of bovine ABCA4 (Figure 4B). The T901 site precedes the ABC cassette, whereas the four other sites are segregated in the so-called linker region, a stretch of amino acids separating the two symmetrical parts of this transporter. Interestingly, phosphorylation in this region was found in many other mammalian ABC transporters, including ABCA1,53,54 P-glycoprotein,55–57 and cystic fibrosis transmembrane conductance regulator (ABCC7).56 In the most closely related ABCA1 with a sequence that is ~50% identical, phosphorylation of a PEST sequence58 located in this linker region initiates protein degradation by calpain protease,54 which explains the rapid turnover of ABCA1. Using the PESTfind server,59 we found that this PEST sequence is absent in ABCA4.
Reportedly, Ala and Arg mutations in the T901 phosphorylation site are associated with Stargardt disease,50,60 cone–rod dystrophy,61,62 and AMD.63 Retention of the T901A mutant in the endoplasmic reticulum (Figure 5B) as well as its drastically reduced level of expression (Figure 5A) demonstrated in this study suggest that replacement of the threonine at this position leads to misfolding and protein degradation, but it is not yet clear if this is caused by a lack of phosphorylation or replacement of the Thr side chain.
ABCA4 Phosphorylation Is Independent of Light
Because ABCA4 is involved in recycling of the byproduct of the visual cycle, we wanted to know whether its phosphorylation is regulated by light. In this regard, an early study showed that light exposure of bullfrog retinas as well as isolated fragmented rods significantly increased the total phosphorylation level of ABCA4, then known as the 220 kDa rim protein.40 To verify this finding, we compared total phosphorylation levels of ABCA4 purified by immunoprecipitation from retinas of dark-adapted and light-exposed mice. Although we cannot exclude the possibility of small changes in phosphorylation levels of individual sites in response to light exposure, the overall levels were found to be virtually identical (Figure 6B), an observation further confirmed by using ABCA4 purified from rods of bovine retinas that were either kept in the dark or exposed to light (Figure 6C). This contradiction with the previously published results could originate from differences in experimental techniques employed to detect phosphorylation or from the fact that the study of Szuts identified ABCA4 solely on the basis of the position of the band in an SDS–PAGE lane containing total ROS membrane proteins.40 Alternatively, regulation of ABCA4 phosphorylation by light can be restricted to selected species. In this regard, sequence alignments demonstrated that phosphorylation sites at positions 901 and 1185 are invariant in mammals but absent in puffer fish and two species of frogs (Figure 4C). Thus, we expect that the phosphorylation profile of ABCA4 differs in amphibia.
Phosphorylation and ATPase Activity of ABCA4
A large number of studies have implicated phosphorylation of human ABC transporters in the regulation of their activity (reviewed in ref 64). However, the only evidence available for the ABCA subfamily members is the regulation of ABCA1 activity by protein kinases A and CK2.65,66 We investigated the effect of alanine substitutions in phosphorylation sites of human ABCA4 expressed in COS-7 cells. The S1185A, T1313A, and S1317A mutants localized to intracellular vesicles (Figure 5B), suggestive of nativelike folding, although the lower expression level of T1317A could indicate possible destabilization. The markedly lower basal ATPase activity of this mutant (Table 1) can also suggest that phosphorylation at this position is involved in regulation of futile cycles of ATP hydrolysis in the absence of bound transported substrate. In contrast, the S1185A and T1313A mutants demonstrated native levels of basal ATPase activity, but their all-trans-retinal-stimulated ATPase activities were reduced 1.5- and 1.2-fold, respectively (Table 1). This implies that phosphorylation at these positions could regulate the transport activity of ABCA4. We cannot exclude the possibility that the reduced activity in response to the mutations in the linker region could result from structural alterations. Thus, it is not yet clear if the observed effects reflect a regulatory role of phosphorylation or a general sensitivity of this region to amino acid substitutions, although reduction of the basal and all-trans-retinal-stimulated activity of partially dephosphorylated native bovine ABCA4 (Figure 6D) favors a regulatory role. On the basis of relatively mild effects of mutations and dephosphorylation on the rates of ATP hydrolysis, we suggest that phosphorylation could have a regulatory function, but it is unlikely that this is critical for the biological activity of ABCA4.
To summarize, we conducted a comprehensive study of posttranslational modifications of the photoreceptor-specific ABC transporter ABCA4 from bovine retina. We confirmed the suggested topological model of ABCA4 by identifying seven N-glycosylation sites in exocytoplasmic domains 1 and 2. Furthermore, this study provides the first evidence that the transport activity of this complex protein can be regulated by phosphorylation of several residues located in cytoplasmic domain 1. Together with the results obtained for ABCA1,65,66 this indicates that phosphorylation can be universally involved in modulating the activity of ABCA subfamily members. Contrary to previously published results,40 we suggest that ABCA4 phosphorylation is light-independent. These findings contribute to our understanding of the structure and function of ABCA4, providing valuable information for future studies.
Supplementary Material
Acknowledgments
Funding
This research was supported, in whole or in part, by National Institutes of Health Grants EY009339, R24 EY021126, EY013203, EY02422, and P30 EY11373 and by the Foundation Fighting Blindness. K.P. is John H. Hord Professor of Pharmacology, and R.S.M. is a Canada Research Chair in Vision and Macular Degeneration.
We are grateful to Dr. Wendy Sun (Polgenix Inc.) for providing recombinant PNGase F and to Dr. Marcin Golczak and Dr. Laurie Molday for technical assistance and fruitful discussions. We thank Dr. Leslie T. Webster, Jr., and Dr. Michael Maguire for critical comments.
ABBREVIATIONS
- A2E
retinoid-pyridinium-ethanolamine
- ABC transporter
ATP-binding cassette transporter
- AMD
age-related macular degeneration
- CHAPS
3-[(3-holamidopropyl)dimethy-lam-monio]-1-propanesulfonate
- DDM
n-dodecyl β-D-maltopyranoside
- ECD-1 and ECD-2
exocytoplasmic domains 1 and 2, respectively
- PP2A
human recombinant phosphatase 2A subunit C
- ROS
rod outer segment(s)
Footnotes
(i) ABCA4 sequence coverage obtained by mass spectrometry, (ii) determination of glycan composition and phosphorylation sites, (iii) specificities of lectins used in this study, and (iv) dephosphorylation of ABCA4 with PP2A. This material is available free of charge via the Internet at http://pubs.acs.org.
Author Contributions
Y.T. and B.W. contributed equally to this work.
References
- 1.Kiser PD, Golczak M, Maeda A, Palczewski K. Key enzymes of the retinoid (visual) cycle in vertebrate retina. Biochim Biophys Acta. 2011 doi: 10.1016/j.bbalip.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sun H, Nathans J. ABCR, the ATP-binding cassette transporter responsible for Stargardt macular dystrophy, is an efficient target of all-trans-retinal-mediated photooxidative damage in vitro. Implications for retinal disease. J Biol Chem. 2001;276:11766–11774. doi: 10.1074/jbc.M010152200. [DOI] [PubMed] [Google Scholar]
- 3.Maeda A, Maeda T, Golczak M, Chou S, Desai A, Hoppel CL, Matsuyama S, Palczewski K. Involvement of all-trans-retinal in acute light-induced retinopathy of mice. J Biol Chem. 2009;284:15173–15183. doi: 10.1074/jbc.M900322200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maeda A, Golczak M, Maeda T, Palczewski K. Limited Roles of Rdh8, Rdh12 and Abca4 on All-Trans-Retinal Clearance in Mouse Retina. Invest Ophthalmol Visual Sci. 2009;50:5435–5443. doi: 10.1167/iovs.09-3944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shiose S, Chen Y, Okano K, Roy S, Kohno H, Tang J, Pearlman E, Maeda T, Palczewski K, Maeda A. Toll-like receptor 3 is required for development of retinopathy caused by impaired all-trans-retinal clearance in mice. J Biol Chem. 2011;286:15543–15555. doi: 10.1074/jbc.M111.228551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sparrow JR, Wu Y, Kim CY, Zhou J. Phospholipid meets all-trans-retinal: The making of RPE bisretinoids. J Lipid Res. 2010;51:247–261. doi: 10.1194/jlr.R000687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mata NL, Weng J, Travis GH. Biosynthesis of a major lipofuscin fluorophore in mice and humans with ABCR-mediated retinal and macular degeneration. Proc Natl Acad Sci USA. 2000;97:7154–7159. doi: 10.1073/pnas.130110497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mata NL, Tzekov RT, Liu X, Weng J, Birch DG, Travis GH. Delayed dark-adaptation and lipofuscin accumulation in abcr± mice: Implications for involvement of ABCR in age-related macular degeneration. Invest Ophthalmol Visual Sci. 2001;42:1685–1690. [PubMed] [Google Scholar]
- 9.Radu RA, Mata NL, Bagla A, Travis GH. Light exposure stimulates formation of A2E oxiranes in a mouse model of Stargardt’s macular degeneration. Proc Natl Acad Sci USA. 2004;101:5928–5933. doi: 10.1073/pnas.0308302101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Molday LL, Rabin AR, Molday RS. ABCR expression in foveal cone photoreceptors and its role in Stargardt macular dystrophy. Nat Genet. 2000;25:257–258. doi: 10.1038/77004. [DOI] [PubMed] [Google Scholar]
- 11.Sun H, Nathans J. Stargardt’s ABCR is localized to the disc membrane of retinal rod outer segments. Nat Genet. 1997;17:15–16. doi: 10.1038/ng0997-15. [DOI] [PubMed] [Google Scholar]
- 12.Papermaster DS, Reilly P, Schneider BG. Cone lamellae and red and green rod outer segment disks contain a large intrinsic membrane protein on their margins: An ultrastructural immunocytochemical study of frog retinas. Vision Res. 1982;22:1417–1428. doi: 10.1016/0042-6989(82)90204-8. [DOI] [PubMed] [Google Scholar]
- 13.Tsybovsky Y, Molday RS, Palczewski K. The ATP-binding cassette transporter ABCA4: Structural and functional properties and role in retinal disease. Adv Exp Med Biol. 2010;703:105–125. doi: 10.1007/978-1-4419-5635-4_8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peelman F, Labeur C, Vanloo B, Roosbeek S, Devaud C, Duverger N, Denefle P, Rosier M, Vandekerckhove J, Rosseneu M. Characterization of the ABCA transporter subfamily: Identification of prokaryotic and eukaryotic members, phylogeny and topology. J Mol Biol. 2003;325:259–274. doi: 10.1016/s0022-2836(02)01105-1. [DOI] [PubMed] [Google Scholar]
- 15.Bungert S, Molday LL, Molday RS. Membrane topology of the ATP binding cassette transporter ABCR and its relationship to ABC1 and related ABCA transporters: Identification of N-linked glycosylation sites. J Biol Chem. 2001;276:23539–23546. doi: 10.1074/jbc.M101902200. [DOI] [PubMed] [Google Scholar]
- 16.Ahn J, Wong JT, Molday RS. The effect of lipid environment and retinoids on the ATPase activity of ABCR, the photoreceptor ABC transporter responsible for Stargardt macular dystrophy. J Biol Chem. 2000;275:20399–20405. doi: 10.1074/jbc.M000555200. [DOI] [PubMed] [Google Scholar]
- 17.Beharry S, Zhong M, Molday RS. N-Retinylidene-phosphatidylethanolamine is the preferred retinoid substrate for the photoreceptor-specific ABC transporter ABCA4 (ABCR) J Biol Chem. 2004;279:53972–53979. doi: 10.1074/jbc.M405216200. [DOI] [PubMed] [Google Scholar]
- 18.Molday LL, Rabin AR, Molday RS. ABCR expression in foveal cone photoreceptors and its role in stargardt macular dystrophy. Am J Ophthalmol. 2000;130:689. doi: 10.1016/s0002-9394(00)00756-x. [DOI] [PubMed] [Google Scholar]
- 19.Sun H, Molday RS, Nathans J. Retinal stimulates ATP hydrolysis by purified and reconstituted ABCR, the photoreceptor-specific ATP-binding cassette transporter responsible for Stargardt disease. J Biol Chem. 1999;274:8269–8281. doi: 10.1074/jbc.274.12.8269. [DOI] [PubMed] [Google Scholar]
- 20.Weng J, Mata NL, Azarian SM, Tzekov RT, Birch DG, Travis GH. Insights into the function of Rim protein in photoreceptors and etiology of Stargardt’s disease from the phenotype in abcr knockout mice. Cell. 1999;98:13–23. doi: 10.1016/S0092-8674(00)80602-9. [DOI] [PubMed] [Google Scholar]
- 21.Sun H, Smallwood PM, Nathans J. Biochemical defects in ABCR protein variants associated with human retinopathies. Nat Genet. 2000;26:242–246. doi: 10.1038/79994. [DOI] [PubMed] [Google Scholar]
- 22.Molday RS. ATP-binding cassette transporter ABCA4: Molecular properties and role in vision and macular degeneration. J Bioenerg Biomembr. 2007;39:507–517. doi: 10.1007/s10863-007-9118-6. [DOI] [PubMed] [Google Scholar]
- 23.Molday RS, Zhong M, Quazi F. The role of the photoreceptor ABC transporter ABCA4 in lipid transport and Stargardt macular degeneration. Biochim Biophys Acta. 2009;1791:573–583. doi: 10.1016/j.bbalip.2009.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sullivan JM. Focus on molecules: ABCA4 (ABCR)—an import-directed photoreceptor retinoid flipase. Exp Eye Res. 2009;89:602–603. doi: 10.1016/j.exer.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Parker RO, Crouch RK. Retinol dehydrogenases (RDHs) in the visual cycle. Exp Eye Res. 2010;91:788–792. doi: 10.1016/j.exer.2010.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maeda A, Maeda T, Sun W, Zhang H, Baehr W, Palczewski K. Redundant and unique roles of retinol dehydrogenases in the mouse retina. Proc Natl Acad Sci USA. 2007;104:19565–19570. doi: 10.1073/pnas.0707477104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Allikmets R, Singh N, Sun H, Shroyer NF, Hutchinson A, Chidambaram A, Gerrard B, Baird L, Stauffer D, Peiffer A, Rattner A, Smallwood P, Li Y, Anderson KL, Lewis RA, Nathans J, Leppert M, Dean M, Lupski JR. A photoreceptor cell-specific ATP-binding transporter gene (ABCR) is mutated in recessive Stargardt macular dystrophy. Nat Genet. 1997;15:236–246. doi: 10.1038/ng0397-236. [DOI] [PubMed] [Google Scholar]
- 28.Azarian SM, Travis GH. The photoreceptor rim protein is an ABC transporter encoded by the gene for recessive Stargardt’s disease (ABCR) FEBS Lett. 1997;409:247–252. doi: 10.1016/s0014-5793(97)00517-6. [DOI] [PubMed] [Google Scholar]
- 29.Illing M, Molday LL, Molday RS. The 220-kDa rim protein of retinal rod outer segments is a member of the ABC transporter superfamily. J Biol Chem. 1997;272:10303–10310. doi: 10.1074/jbc.272.15.10303. [DOI] [PubMed] [Google Scholar]
- 30.Westerfeld C, Mukai S. Stargardt’s disease and the ABCR gene. Semin Ophthalmol. 2008;23:59–65. doi: 10.1080/08820530701745249. [DOI] [PubMed] [Google Scholar]
- 31.Cideciyan AV, Swider M, Aleman TS, Tsybovsky Y, Schwartz SB, Windsor EA, Roman AJ, Sumaroka A, Steinberg JD, Jacobson SG, Stone EM, Palczewski K. ABCA4 disease progression and a proposed strategy for gene therapy. Hum Mol Genet. 2009;18:931–941. doi: 10.1093/hmg/ddn421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wiszniewski W, Zaremba CM, Yatsenko AN, Jamrich M, Wensel TG, Lewis RA, Lupski JR. ABCA4 mutations causing mislocalization are found frequently in patients with severe retinal dystrophies. Hum Mol Genet. 2005;14:2769–2778. doi: 10.1093/hmg/ddi310. [DOI] [PubMed] [Google Scholar]
- 33.Hamel CP. Cone rod dystrophies. Orphanet J Rare Dis. 2007;2:7. doi: 10.1186/1750-1172-2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Martinez-Mir A, Paloma E, Allikmets R, Ayuso C, del Rio T, Dean M, Vilageliu L, Gonzalez-Duarte R, Balcells S. Retinitis pigmentosa caused by a homozygous mutation in the Stargardt disease gene ABCR. Nat Genet. 1998;18:11–12. doi: 10.1038/ng0198-11. [DOI] [PubMed] [Google Scholar]
- 35.Allikmets R, Shroyer NF, Singh N, Seddon JM, Lewis RA, Bernstein PS, Peiffer A, Zabriskie NA, Li Y, Hutchinson A, Dean M, Lupski JR, Leppert M. Mutation of the Stargardt disease gene (ABCR) in age-related macular degeneration. Science. 1997;277:1805–1807. doi: 10.1126/science.277.5333.1805. [DOI] [PubMed] [Google Scholar]
- 36.Swaroop A, Branham KE, Chen W, Abecasis G. Genetic susceptibility to age-related macular degeneration: A paradigm for dissecting complex disease traits. Hum Mol Genet. 2007;16(2):R174–R182. doi: 10.1093/hmg/ddm212. [DOI] [PubMed] [Google Scholar]
- 37.Swaroop A, Chew EY, Rickman CB, Abecasis GR. Unraveling a multifactorial late-onset disease: From genetic susceptibility to disease mechanisms for age-related macular degeneration. Annu Rev Genomics Hum Genet. 2009;10:19–43. doi: 10.1146/annurev.genom.9.081307.164350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Koenekoop RK. The gene for Stargardt disease, ABCA4, is a major retinal gene: A mini-review. Ophthalmic Genet. 2003;24:75–80. doi: 10.1076/opge.24.2.75.13996. [DOI] [PubMed] [Google Scholar]
- 39.Maeda A, Maeda T, Golczak M, Palczewski K. Retinopathy in mice induced by disrupted all-trans-retinal clearance. J Biol Chem. 2008;283:26684–26693. doi: 10.1074/jbc.M804505200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Szuts EZ. Light stimulates phosphorylation of two large membrane proteins in frog photoreceptors. Biochemistry. 1985;24:4176–4184. doi: 10.1021/bi00336a054. [DOI] [PubMed] [Google Scholar]
- 41.Papermaster DS. Preparation of retinal rod outer segments. Methods Enzymol. 1982;81:48–52. doi: 10.1016/s0076-6879(82)81010-0. [DOI] [PubMed] [Google Scholar]
- 42.Bereta G, Wang B, Kiser PD, Baehr W, Jang GF, Palczewski K. A functional kinase homology domain is essential for the activity of photoreceptor guanylate cyclase 1. J Biol Chem. 2010;285:1899–1908. doi: 10.1074/jbc.M109.061713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Peterman SM, Mulholland JJ. A novel approach for identification and characterization of glycoproteins using a hybrid linear ion trap/FT-ICR mass spectrometer. J Am Soc Mass Spectrom. 2006;17:168–179. doi: 10.1016/j.jasms.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 44.Gao Y, Wang Y. A method to determine the ionization efficiency change of peptides caused by phosphorylation. J Am Soc Mass Spectrom. 2007;18:1973–1976. doi: 10.1016/j.jasms.2007.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhong M, Molday LL, Molday RS. Role of the C terminus of the photoreceptor ABCA4 transporter in protein folding, function, and retinal degenerative diseases. J Biol Chem. 2009;284:3640–3649. doi: 10.1074/jbc.M806580200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schinkel AH, Kemp S, Dolle M, Rudenko G, Wagenaar E. N-Glycosylation and deletion mutants of the human MDR1 P-glycoprotein. J Biol Chem. 1993;268:7474–7481. [PubMed] [Google Scholar]
- 47.Fitzgerald ML, Mendez AJ, Moore KJ, Andersson LP, Panjeton HA, Freeman MW. ATP-binding cassette transporter A1 contains an NH2-terminal signal anchor sequence that translocates the protein’s first hydrophilic domain to the exoplasmic space. J Biol Chem. 2001;276:15137–15145. doi: 10.1074/jbc.m100474200. [DOI] [PubMed] [Google Scholar]
- 48.Mengerink KJ, Vacquier VD. An ATP-binding cassette transporter is a major glycoprotein of sea urchin sperm membranes. J Biol Chem. 2002;277:40729–40734. doi: 10.1074/jbc.M207184200. [DOI] [PubMed] [Google Scholar]
- 49.Bakos E, Hegedus T, Hollo Z, Welker E, Tusnady GE, Zaman GJ, Flens MJ, Varadi A, Sarkadi B. Membrane topology and glycosylation of the human multidrug resistance-associated protein. J Biol Chem. 1996;271:12322–12326. doi: 10.1074/jbc.271.21.12322. [DOI] [PubMed] [Google Scholar]
- 50.Webster AR, Heon E, Lotery AJ, Vandenburgh K, Casavant TL, Oh KT, Beck G, Fishman GA, Lam BL, Levin A, Heckenlively JR, Jacobson SG, Weleber RG, Sheffield VC, Stone EM. An analysis of allelic variation in the ABCA4 gene. Invest Ophthalmol Visual Sci. 2001;42:1179–1189. [PubMed] [Google Scholar]
- 51.Papermaster DS, Schneider BG, Zorn MA, Kraehenbuhl JP. Immunocytochemical localization of a large intrinsic membrane protein to the incisures and margins of frog rod outer segment disks. J Cell Biol. 1978;78:415–425. doi: 10.1083/jcb.78.2.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nickell S, Park PS, Baumeister W, Palczewski K. Three-dimensional architecture of murine rod outer segments determined by cryoelectron tomography. J Cell Biol. 2007;177:917–925. doi: 10.1083/jcb.200612010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang N, Chen W, Linsel-Nitschke P, Martinez LO, Agerholm-Larsen B, Silver DL, Tall AR. A PEST sequence in ABCA1 regulates degradation by calpain protease and stabilization of ABCA1 by apoA-I. J Clin Invest. 2003;111:99–107. doi: 10.1172/JCI16808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Martinez LO, Agerholm-Larsen B, Wang N, Chen W, Tall AR. Phosphorylation of a pest sequence in ABCA1 promotes calpain degradation and is reversed by ApoA-I. J Biol Chem. 2003;278:37368–37374. doi: 10.1074/jbc.M307161200. [DOI] [PubMed] [Google Scholar]
- 55.Germann UA, Chambers TC, Ambudkar SV, Pastan I, Gottesman MM. Effects of phosphorylation of P-glycoprotein on multidrug resistance. J Bioenerg Biomembr. 1995;27:53–61. doi: 10.1007/BF02110331. [DOI] [PubMed] [Google Scholar]
- 56.Rich DP, Berger HA, Cheng SH, Travis SM, Saxena M, Smith AE, Welsh MJ. Regulation of the cystic fibrosis transmembrane conductance regulator Cl− channel by negative charge in the R domain. J Biol Chem. 1993;268:20259–20267. [PubMed] [Google Scholar]
- 57.Chambers TC, Germann UA, Gottesman MM, Pastan I, Kuo JF, Ambudkar SV. Bacterial expression of the linker region of human MDR1 P-glycoprotein and mutational analysis of phosphorylation sites. Biochemistry. 1995;34:14156–14162. doi: 10.1021/bi00043a021. [DOI] [PubMed] [Google Scholar]
- 58.Rechsteiner M, Rogers SW. PEST sequences and regulation by proteolysis. Trends Biochem Sci. 1996;21:267–271. [PubMed] [Google Scholar]
- 59.Rice P, Longden I, Bleasby A. EMBOSS: The European Molecular Biology Open Software Suite. Trends Genet. 2000;16:276–277. doi: 10.1016/s0168-9525(00)02024-2. [DOI] [PubMed] [Google Scholar]
- 60.Valverde D, Riveiro-Alvarez R, Bernal S, Jaakson K, Baiget M, Navarro R, Ayuso C. Microarray-based mutation analysis of the ABCA4 gene in Spanish patients with Stargardt disease: Evidence of a prevalent mutated allele. Mol Vision. 2006;12:902–908. [PubMed] [Google Scholar]
- 61.Aguirre-Lamban J, Riveiro-Alvarez R, Maia-Lopes S, Cantalapiedra D, Vallespin E, Avila-Fernandez A, Villaverde-Montero C, Trujillo-Tiebas MJ, Ramos C, Ayuso C. Molecular analysis of the ABCA4 gene for reliable detection of allelic variations in Spanish patients: Identification of 21 novel variants. Br J Ophthalmol. 2009;93:614–621. doi: 10.1136/bjo.2008.145193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Valverde D, Riveiro-Alvarez R, Aguirre-Lamban J, Baiget M, Carballo M, Antinolo G, Millan JM, Garcia Sandoval B, Ayuso C. Spectrum of the ABCA4 gene mutations implicated in severe retinopathies in Spanish patients. Invest Ophthalmol Visual Sci. 2007;48:985–990. doi: 10.1167/iovs.06-0307. [DOI] [PubMed] [Google Scholar]
- 63.Rivera A, White K, Stohr H, Steiner K, Hemmrich N, Grimm T, Jurklies B, Lorenz B, Scholl HP, Apfelstedt-Sylla E, Weber BH. A comprehensive survey of sequence variation in the ABCA4 (ABCR) gene in Stargardt disease and age-related macular degeneration. Am J Hum Genet. 2000;67:800–813. doi: 10.1086/303090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Stolarczyk EI, Reiling CJ, Paumi CM. Regulation of ABC Transporter Function via Phosphorylation by Protein Kinases. Curr Pharm Biotechnol. 2011;12:621–635. doi: 10.2174/138920111795164075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Roosbeek S, Peelman F, Verhee A, Labeur C, Caster H, Lensink MF, Cirulli C, Grooten J, Cochet C, Vandekerckhove J, Amoresano A, Chimini G, Tavernier J, Rosseneu M. Phosphorylation by protein kinase CK2 modulates the activity of the ATP binding cassette A1 transporter. J Biol Chem. 2004;279:37779–37788. doi: 10.1074/jbc.M401821200. [DOI] [PubMed] [Google Scholar]
- 66.See RH, Caday-Malcolm RA, Singaraja RR, Zhou S, Silverston A, Huber MT, Moran J, James ER, Janoo R, Savill JM, Rigot V, Zhang LH, Wang M, Chimini G, Wellington CL, Tafuri SR, Hayden MR. Protein kinase A site-specific phosphorylation regulates ATP-binding cassette A1 (ABCA1)-mediated phospholipid efflux. J Biol Chem. 2002;277:41835–41842. doi: 10.1074/jbc.M204923200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.