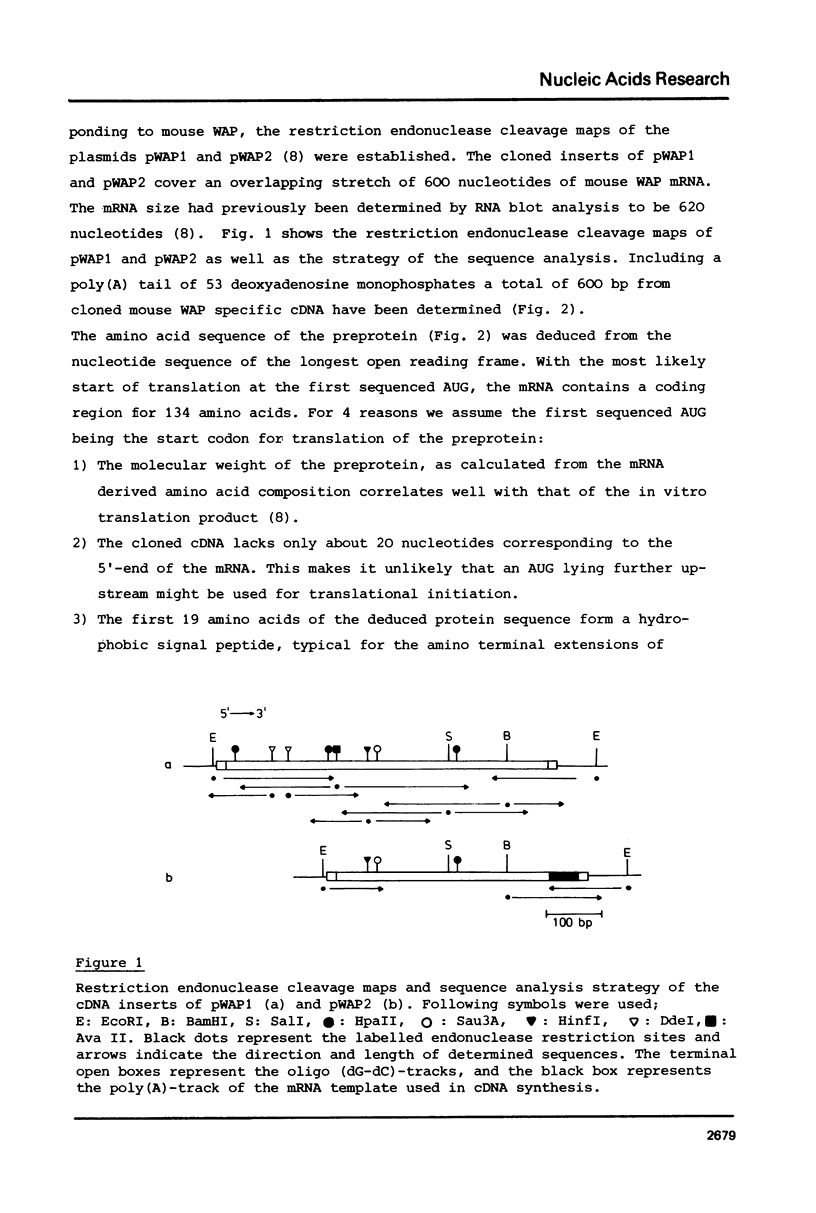
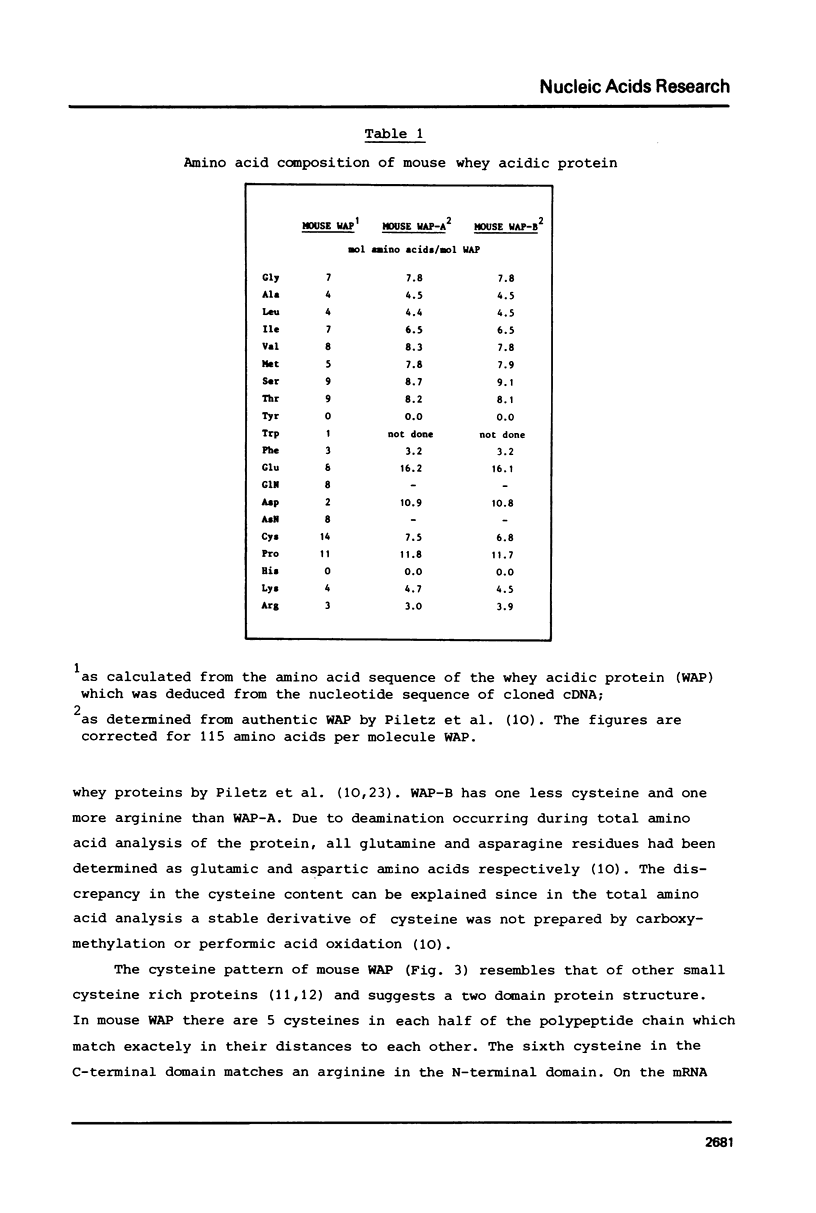
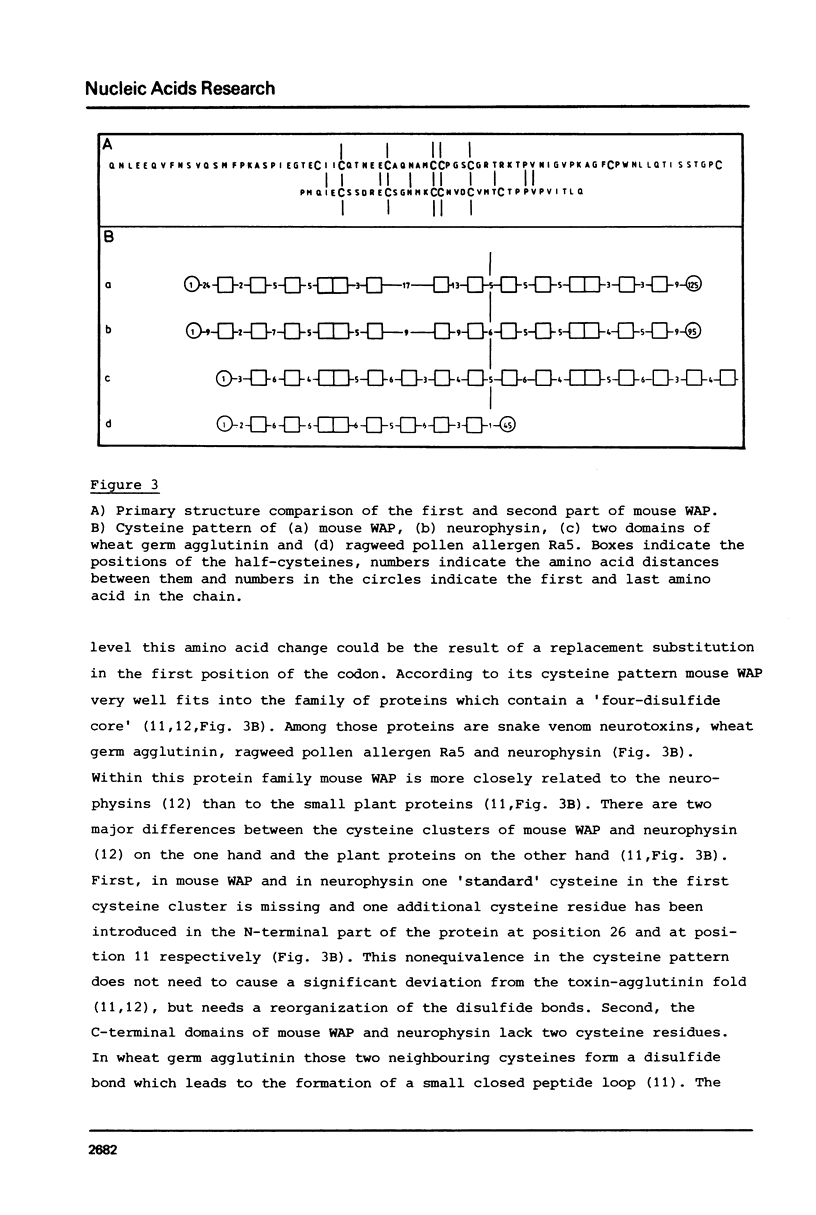
Abstract
Unlike in other mammalian species, the major whey protein in mouse is not alpha-lactalbumin, but a cysteine rich, acidic protein with a molecular weight of 14.0 kDa. We have deduced the amino acid sequence of this mouse acidic of whey protein from the nucleotide sequence of cloned cDNA. The positions of the half cysteines suggest that mouse whey acidic protein (WAP) is a two domain protein, very similar in structure to the plant lectin wheat germ agglutinin and the hypothalamic carrier protein neurophysin.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blobel G., Dobberstein B. Transfer of proteins across membranes. I. Presence of proteolytically processed and unprocessed nascent immunoglobulin light chains on membrane-bound ribosomes of murine myeloma. J Cell Biol. 1975 Dec;67(3):835–851. doi: 10.1083/jcb.67.3.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breslow E. Chemistry and biology of the neurophysins. Annu Rev Biochem. 1979;48:251–274. doi: 10.1146/annurev.bi.48.070179.001343. [DOI] [PubMed] [Google Scholar]
- Brignon G., Ribadeau Dumas B., Mercier J. C., Pelissier J. P., Das B. C. Complete amino acid sequence of bovine alphaS2-casein. FEBS Lett. 1977 Apr 15;76(2):274–279. doi: 10.1016/0014-5793(77)80167-1. [DOI] [PubMed] [Google Scholar]
- Craig R. K., Brown P. A., Harrison O. S., McIlreavy D., Campbell P. N. Guinea-pig milk-protein synthesis. Isolation and characterization of messenger ribonucleic acids from lactating mammary gland and identification of caseins and pre-alpha-lactalbumin as translation products in heterologous cell-free systems. Biochem J. 1976 Oct 15;160(1):57–74. doi: 10.1042/bj1600057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig R. K., McIlreavy D., Hall R. L. Separation and partial characterization of guinea-pig caseins. Biochem J. 1978 Aug 1;173(2):633–641. doi: 10.1042/bj1730633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis B. D., Tai P. C. The mechanism of protein secretion across membranes. Nature. 1980 Jan 31;283(5746):433–438. doi: 10.1038/283433a0. [DOI] [PubMed] [Google Scholar]
- Drenth J., Low B. W., Richardson J. S., Wright C. S. The toxin-agglutinin fold. A new group of small protein structures organized around a four-disulfide core. J Biol Chem. 1980 Apr 10;255(7):2652–2655. [PubMed] [Google Scholar]
- Drenth J. The structure of neurophysin. J Biol Chem. 1981 Mar 25;256(6):2601–2602. [PubMed] [Google Scholar]
- Gaye P., Gautron J. P., Mercier J. C., Hazé G. Amino terminal sequences of the precursors of ovine caseins. Biochem Biophys Res Commun. 1977 Dec 7;79(3):903–911. doi: 10.1016/0006-291x(77)91196-2. [DOI] [PubMed] [Google Scholar]
- Ginsburg M., Jayasena K. The distribution of proteins that bind neurohypophysial hormones. J Physiol. 1968 Jul;197(1):65–76. doi: 10.1113/jphysiol.1968.sp008546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legros J. J., Louis F., Grötschel-Stewart U., Franchimont P. Presence of immunoreactive neurophysin-like material in human target organs and pineal gland: physiological meaning. Ann N Y Acad Sci. 1975 Feb 21;248:157–171. doi: 10.1111/j.1749-6632.1975.tb34183.x. [DOI] [PubMed] [Google Scholar]
- Low B. W., Preston H. S., Sato A., Rosen L. S., Searl J. E., Rudko A. D., Richardson J. S. Three dimensional structure of erabutoxin b neurotoxic protein: inhibitor of acetylcholine receptor. Proc Natl Acad Sci U S A. 1976 Sep;73(9):2991–2994. doi: 10.1073/pnas.73.9.2991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- McKenzie R. M., Larson B. L. Purification and partial characterization of a unique group of phosphoproteins from rat milk whey. J Dairy Sci. 1978 Jun;61(6):723–728. doi: 10.3168/jds.S0022-0302(78)83639-X. [DOI] [PubMed] [Google Scholar]
- Piletz J. E., Ganschow R. E. Genetic variation of milk proteins in mice. Biochem Genet. 1981 Oct;19(9-10):1023–1030. doi: 10.1007/BF00504265. [DOI] [PubMed] [Google Scholar]
- Piletz J. E., Heinlen M., Ganschow R. E. Biochemical characterization of a novel whey protein from murine milk. J Biol Chem. 1981 Nov 25;256(22):11509–11516. [PubMed] [Google Scholar]
- Rosen J. M., Woo S. L., Comstock J. P. Regulation of casein messenger RNA during the development of the rat mammary gland. Biochemistry. 1975 Jul;14(13):2895–2903. doi: 10.1021/bi00684a016. [DOI] [PubMed] [Google Scholar]
- Rüther U. Construction and properties of a new cloning vehicle, allowing direct screening for recombinant plasmids. Mol Gen Genet. 1980;178(2):475–477. doi: 10.1007/BF00270503. [DOI] [PubMed] [Google Scholar]
- Sinding C., Robinson A. G. A review of neurophysins. Metabolism. 1977 Dec;26(12):1355–1370. doi: 10.1016/0026-0495(77)90031-2. [DOI] [PubMed] [Google Scholar]
- Wright C. S. The crystal structure of wheat germ agglutinin at 2-2 A resolution. J Mol Biol. 1977 Apr 25;111(4):439–457. doi: 10.1016/s0022-2836(77)80063-6. [DOI] [PubMed] [Google Scholar]
- Zamierowski M. M., Ebner K. E. A radioimmunoassay for mouse alpha-lactalbumin. J Immunol Methods. 1980;36(3-4):211–220. doi: 10.1016/0022-1759(80)90126-x. [DOI] [PubMed] [Google Scholar]


