Abstract
Lyme disease is often identified by the hallmark erythema migrans rash, but not all early cases present with a rash. In other cases the rash may be unseen or unrecognized by a physician. In these situations, Lyme disease is difficult to diagnose because it masquerades as a non-specific viral-like illness. The seasonal peak of Lyme disease ranging from May through September overlaps with that of viral illnesses such as enteroviral infections, West Nile virus, and in rare years such as 2009, early influenza season. We present a case of a patient with Lyme disease who was initially misdiagnosed with influenza A during the summer of 2009. Because of the diagnostic importance of recognizing the erythema migrans rash, physicians in endemic regions should always ask about new rashes or skin lesions and perform a thorough physical examination when patients present over the summer with viral-like symptoms. Even when no rash is evident, Lyme disease should be considered if these symptoms persist or worsen without a specific diagnosis.
Key words: Lyme disease, summer flu, viral-like illness, diagnosis
Introduction
Lyme disease is the most common vector-borne illness in North America with over 35,000 new cases reported annually to the Center for Disease Control (CDC), a nearly 300% increase since reporting began in 1992.1 Even so, Lyme disease is substantially underreported to the CDC by as much as 6 to 12 fold.2,3 Acute cases predominantly occur between the months of May and September. The seasonal variation is a function of the tick vector and its life cycle, as well as the outdoor activities of humans that determine exposure to the environments containing ticks.4 Acute cases of Lyme disease are uncommon in the winter months but do rarely occur.1 Untreated Lyme disease may progress to later stages of Lyme disease involving the joints or neurologic system that may manifest at any time of year.
The diagnosis of acute Lyme disease is primarily based on the identification of the hallmark erythema migrans rash, which is usually greater than 5 cm in diameter. Multiple lesions remote from the site of initial cutaneous infection may be present in early disseminated cutaneous disease. The classic bulls-eye rash with central clearing and peripheral erythema actually appears in only a minority of cases; most erythema migrans rashes are round, uniformly red or redish-purple lesions, although 1–2% manifest vesiculopustular lesions.5,6 In up to 16% of cases, the patient does not have a rash.7 In these cases, the primary manifestations of acute illness include flu-like or viral-like systemic patient-reported symptoms such as fever, chills, malaise, fatigue, generalized achiness, and head and neck pain. Early Lyme disease is not typically associated with respiratory symptoms such as rhinitis or cough. In the absence of respiratory symptoms, viral-like presentations of Lyme disease have symptoms virtually identical to those of actual viral infection such as influenza and other common community-acquired viral diseases, which may increase the risk of initial misdiagnosis.8 Furthermore, serologic testing for B. Burgdorferi during acute illness onset may be unhelpful or even misleading, as false-negative rates are as high as 60% in the first 2 to 4 weeks of infection.9
We present a patient with early Lyme disease who was initially misdiagnosed with influenza. These cases illustrate the overlap in the symptoms of early Lyme disease with viral infections such as parvovirus, enterovirus, West Nile virus, and influenza, especially when the characteristic rash is undetected, unrecognized, or entirely absent. This is further compounded in the setting of widespread community outbreaks of viral infection such as seen in the summer and fall 2009 influenza season, during which patients with suspected early influenza are commonly managed over the phone without direct physician contact and opportunity for observation and examination. An informal literature review found no previously reported cases of Lyme disease initially misdiagnosed as influenza.
Case Report
A 58-year-old woman in previously excellent health called her physician on October 8, 2009, with complaints of flu-like symptoms including generalized achiness, temperature of 101.2, and headaches. She did not report a cough. Tamiflu 75 mg twice a day was prescribed over the phone for presumed acute influenza. Two days later, the patient called with temperatures up to 101.2 each night. She continued to have general achiness and a slight sore throat but otherwise had no new or localizing symptoms and she was continued on tamiflu. The patient called two days later and reported a new welt on her stomach and new generalized joint pain. On further questioning over the phone, she recalled 3 weeks previously some sort of irritation or bite behind her knee that she could not see.
The patient was seen with continued systemic symptoms, and the exam revealed a primary erythema migrans lesion behind her knee, as well as two disseminated lesions (Figure 1). The remainder of her exam was unremarkable with no evidence for meningismus, normal pharyngeal exam without exudates, and no lymphadenopathy, splenomegaly, or hepatomegaly. The WBC was 5.7 with a normal differential and the platelet count was 245,000. Metabolic panel showed an AST of 413, ALT of 704, alkaline phosphatase of 282, and a total bilirubin of 0.7. Lyme serology was positive with ELISA of 2.86 and two of three bands positive on the IgM Western blot and 2 bands on the IgG Western blot.
Figure 1.
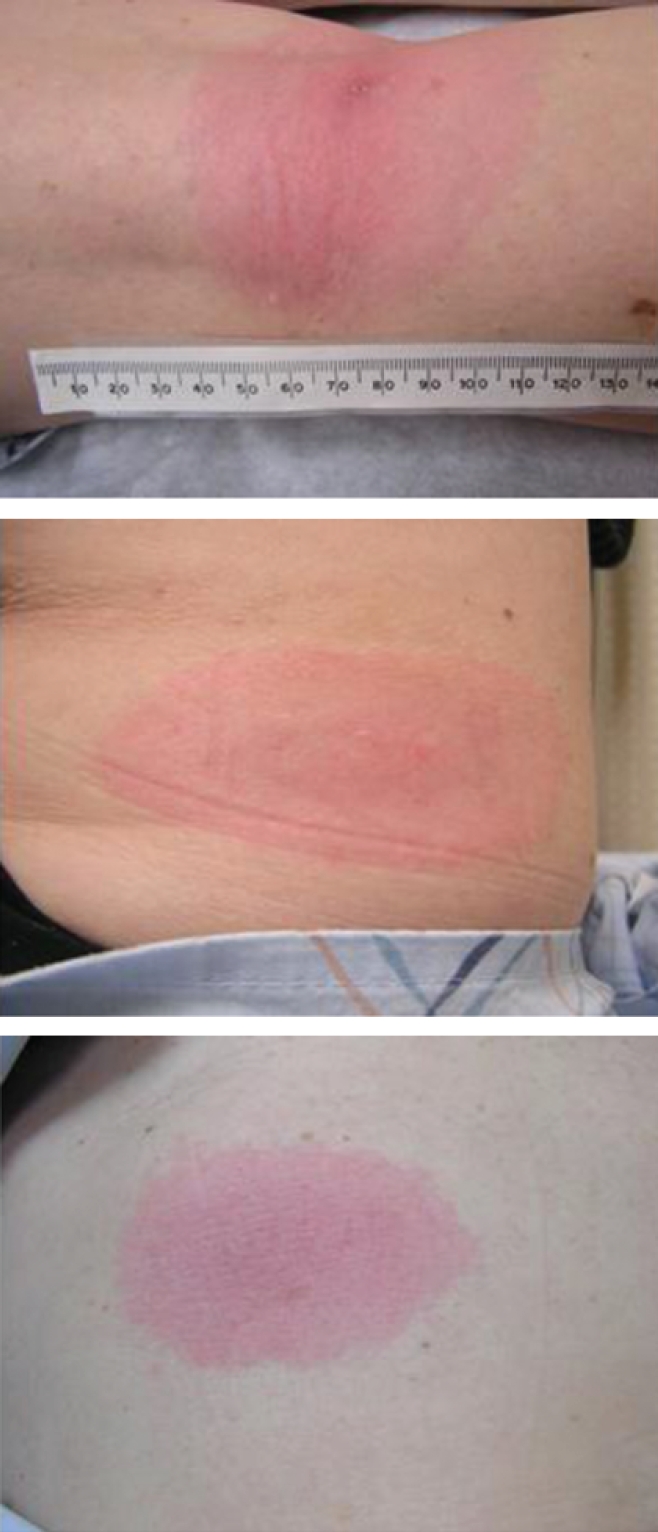
Erythema migrans lesions observed upon physical examination of patient. Primary lesion behind knee (left), along with two disseminated lesions (middle and right).
The patient's rash responded quickly to doxycycline and resolved over the following week. Her systemic symptoms of fever and achiness resolved as well. Repeat liver function testing showed resolution of her AST and ALT over the following two weeks.
Discussion
This case report illustrates the difficulty in distinguishing patients with early Lyme disease from those with an undifferentiated viral illness. The differential diagnosis and management of the summer viral-like illness remains a challenge. The viral-like symptoms of fever, headache, stiff neck, body aches, and fatigue are the common presenting features of many infections, both viral and non-viral. These illnesses often lack localizing symptoms such as cough, diarrhea, or urinary symptoms that point to more specific diagnosis such as pneumonia, gastroenteritis or genitorurinary infection. Undifferentiated viral illnesses also lack prominent lymphadenopathy that is characteristic of mono-like illnesses due to EBV, acute HIV, and HH6. Geographic considerations are very important in identifying patients at risk for tick-borne infections with over 95% of Lyme disease cases occurring in three distinct regions of the United States with sporadic cases in the remainder of the other states. Travel history is important as Lyme disease can be acquired in high risk regions of the United States and Europe and the patient may present with symptoms in a different region where physicians are unfamiliar with the disease and the diagnostic erythema migrans rash. Finally, seasonal considerations may point toward the more likely infections present at a particular time of the year.
In certain regions of the United States, such as the upper mid-west and northeastern and mid-Atlantic states, tick-borne infections account for a significant percentage of summer viral-like illnesses. One study examining the distribution of etiologies in community-acquired febrile illnesses in a Lyme endemic area found that 27% of patients with summer flu had laboratory evidence of probable tick-borne infection, including 11% with Lyme disease alone, 13% with anaplasmosis alone, and 3% with evidence of coinfection.8 Tick-borne infections include bacterial diseases such as Lyme disease, anaplasmosis, and ehrlichiosis, a protozoal disease called babesiosis, and the viral infections Powassan virus in North America and Tick-borne encephalitis in Europe. Tick-borne infections often present with indistinguishable viral–like symptoms, without respiratory symptoms such as rhinitis and cough that point toward a diagnosis of a viral respiratory infection. Other respiratory symptoms such as sore throat may occur in non-viral summer infections such as Lyme disease and babesiosis.10
The categories of summer viral-like infections include parvovirus, enterovirus, and West Nile virus as well as summer presentations of typical winter respiratory viruses like influenza A. Lyme disease shares its characteristic rheumatic and neurologic symptoms with several common viral illnesses. Joint pain is an important symptom of Lyme disease that is potentially confused with symptoms of viral infection, the best example of which is parvoviral infection. Parvovirus B19 season peaks in late spring and early summer, overlapping with the beginning of Lyme disease season.11 The clinical manifestations of parvovirus infection vary greatly depending on the host. In addition to general viral-like symptoms such as fever, myalgia, and malaise, both parvovirus and Lyme disease may have prominent, persistent arthralgias as part of their early presentations.12,13 The second half of Lyme disease season in late summer and early fall coincides with the peak seasons for enterovirus and West Nile virus, which both cause viral-like symptoms that may be accompanied by asceptic meningitis.14,15 Many enterovirus and West Nile virus infections are either asymptomatic or result in an undifferentiated febrile illness.16,17 Both diseases also commonly manifest non-diagnostic maculopapular eruptions.12,18 A minority of enterovirus and West Nile virus cases infect the central nervous system and may be accompanied by neuroinvasive symptoms such as aseptic meningitis, encephalitis, and paralysis.17,19–21 Since neuroborreliosis may also cause neurologic symptoms, Lyme disease is an important cause of treatable bacterial meningitis that must be considered.
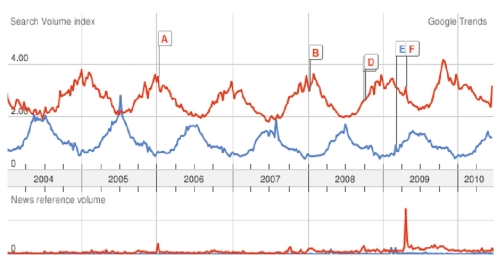
This case report also demonstrates the confounding effect of an abnormally early flu season on diagnosis of Lyme disease. The seasonality of influenza typically centers in the winter months, but cases may occur in the fall and spring. In some seasons such as in 2009, the peak of influenza cases occurs much earlier.22 Trend data from internet searches may be analyzed over time to identify disease outbreaks and supplement traditional surveillance methods.23 A Google Trends comparison suggests that Lyme disease and flu seasons did in fact have significant overlap in early fall 2009 (Figure 2). The 2009 influenza season afforded the opportunity to examine the rare instance in which influenza season had a significant overlap with Lyme disease season.
Figure 2.
Google Trends graph depicting tendency over time to search for Lyme disease and cough. Searches for Lyme disease (blue) and cough (red). Cough used as a search term to capture respiratory viral infections more likely to manifest during the winter months. In 2009, searches for cough increased earlier than usual, corresponding to an early flu season and thus overlapping with Lyme disease season. Search volume index refers to the number of searches for these terms, relative to the total number of searches done on Google over time. News reference volume refers to the number of times these terms appeared in a Google News story.
Because not all cases of influenza have distinguishing symptoms, there is a distinct possibility that some cases of early Lyme disease could have been mistaken for influenza. The likelihood of misdiagnosis was likely exacerbated by the heightened awareness and general media awareness afforded to influenza in 2009. Symptoms of influenza, including fever, myalgias, and fatigue were widely advertised, which placed the diagnosis of influenza first and foremost in the minds of both patients and physicians. In addition, physicians were instructed to attempt phone triage of patients with influenza in order to conserve resources during the potential epidemic outbreak. This discouraged physicians from bringing patients into the office for a full examination, which may be the only opportunity to observe the characteristic Lyme disease rash. This case emphasizes the importance of recognizing the rash to help distinguish Lyme disease from influenza or other flu-like illnesses.
Lyme disease typically does not manifest the leukocytosis often seen in typical bacterial infections, whereas lymphopenia has been reported in Lyme disease, mimicking a viral infection.24 The presence of anemia or signs of hemolysis should raise the possibility of babesiosis and prompt examination of the blood smear for parasites and PCR testing. A significant minority of patients with early Lyme disease may have elevated AST and ALT labs consistent with mild hepatocellular inflammation.25 Transaminase elevations are also seen in another tick-borne infection, anaplasmosis, as well as with many viral infections, including cytomegalovirus, Epstein-Barr virus, enterovirus, and even influenza.
Unfortunately, false negative Lyme serologies often occur early in the course of Lyme disease, confounding an early diagnosis. False negative rates as high as 60% are commonly seen in the first two to four weeks of infection.9 Because of the insensitivity of the serology, the diagnosis of early Lyme disease still remains highly dependent on observation and recognition of the erythema migrans rash. The ability of patient and physicians to recognize the diagnostic erythema migrans rash of early Lyme disease has never been studied. It is known that misdiagnosis does occur, especially when the lesions lack the stereotypical target or bull’s eye appearance with central clearing and peripheral erythema.
The CDC surveillance criteria for Lyme disease were recently modified in 2008 to include patients with viral-like presentations confirmed by a positive serology. Despite the official recognition of this presentation of early Lyme disease, it remains unclear how to manage patients with undifferentiated viral-like symptoms who may have early Lyme disease. The current Infectious Disease Society of America treatment guidelines for early Lyme disease do not offer specific recommendations on how to approach treatment decisions in the viral-like presentation of early Lyme disease.26
One approach on management is to exclude patients with viral-like symptoms with respiratory features such as rhinitis and severe cough suggestive of viral causes. In patients with truly undifferentiated viral-like symptoms, seasonal and geographic risk factors should be taken into consideration. When tick-borne disease is a significant risk, the physician should discuss the pros and cons of watchful waiting with acute/convalescent serology vs. empiric therapy with doxycycline. The patient may be involved in the decision making after explaining the risks and benefits of both approaches. Patients who appear ill with any signs of internal organ illness should be treated with doxycycline, which also treats for the possibility of anaplasma or Rocky Mountain Spotted Fever. Patients who have travelled to New England or who have a history of splenectomy or hematologic malignancy should be examined for babesia and treated if this is discovered.
The reported cases illustrate the ongoing importance of careful physician history and in-person physical examination in the diagnosis of early Lyme disease. Physicians should be aware of the potential for confusion between early Lyme disease and viral-like illnesses during late spring and summer months. There may also be occasions, such as in 2009, in which there is potential for misdiagnosis of Lyme disease during influenza outbreaks. Considering the importance of documenting early erythema migrans as a primary criterion for early diagnosis of Lyme disease, physicians are reminded of the importance of complete skin examination and evaluation in patients with febrile viral-like illnesses. Because of the limitations of laboratory evaluation, careful physician history and physical examination remain cornerstones of accurate diagnosis of early Lyme disease.
References
- 1.Bacon RM, Kugeler KJ, Mead PS. Surveillance for Lyme disease--United States, 1992–2006. MMWR Surveill Summ. 2008;57:1–9. [PubMed] [Google Scholar]
- 2.Meek JI, Roberts CL, Smith EV, Jr, Cartter ML. Underreporting of Lyme disease by Connecticut physicians, 1992. J Public Health Manag Pract. 1996;2:61–5. doi: 10.1097/00124784-199623000-00017. [DOI] [PubMed] [Google Scholar]
- 3.Coyle BS, Strickland GT, Liang YY, et al. The public health impact of Lyme disease in Maryland. J Infect Dis. 1996;173:1260–2. doi: 10.1093/infdis/173.5.1260. [DOI] [PubMed] [Google Scholar]
- 4.Piesman J, Mather TN, Dammin GJ, et al. Seasonal variation of transmission risk of Lyme disease and human babesiosis. Am J Epidemiol. 1987;126:1187–9. doi: 10.1093/oxfordjournals.aje.a114757. [DOI] [PubMed] [Google Scholar]
- 5.Tibbles CD, Edlow JA. Does this patient have erythema migrans? JAMA. 2007;297:2617–27. doi: 10.1001/jama.297.23.2617. [DOI] [PubMed] [Google Scholar]
- 6.Goldberg NS, Forseter G, Nadelman RB, et al. Vesicular erythema migrans. Arch Dermatol. 1992;128:1495–8. [PubMed] [Google Scholar]
- 7.Steere AC, Dhar A, Hernandez J, et al. Systemic symptoms without erythema migrans as the presenting picture of early Lyme disease. Am J Med. 2003;114:58–62. doi: 10.1016/s0002-9343(02)01440-7. [DOI] [PubMed] [Google Scholar]
- 8.Belongia EA, Reed KD, Mitchell PD, et al. Tickborne infections as a cause of nonspecific febrile illness in Wisconsin. Clin Infect Dis. 2001;32:1434–9. doi: 10.1086/320160. [DOI] [PubMed] [Google Scholar]
- 9.Nowakowski J, Schwartz I, Liveris D, et al. Laboratory diagnostic techniques for patients with early Lyme disease associated with erythema migrans: a comparison of different techniques. Clin Infect Dis. 2001;33:2023–7. doi: 10.1086/324490. [DOI] [PubMed] [Google Scholar]
- 10.Krause PJ, McKay K, Thompson CA, et al. Disease-specific diagnosis of coinfecting tickborne zoonoses: babesiosis, human granulocytic ehrlichiosis, and Lyme disease. Clin Infect Dis. 2002;34:1184–91. doi: 10.1086/339813. [DOI] [PubMed] [Google Scholar]
- 11.Nicolay N, Cotter S. Clinical and epidemiological aspects of parvovirus B19 infections in Ireland, January 1996–June 2008. Euro Surveill. 2009;14(25) pii:19249. [PubMed] [Google Scholar]
- 12.Woolf AD, Campion GV, Chishick A, et al. Clinical manifestations of human parvovirus B19 in adults. Arch Intern Med. 1989;149:1153–6. [PubMed] [Google Scholar]
- 13.Nikkari S, Roivainen A, Hannonen P, et al. Persistence of parvovirus B19 in synovial fluid and bone marrow. Ann Rheum Dis. 1995;54:597–600. doi: 10.1136/ard.54.7.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Khetsuriani N, Lamonte-Fowlkes A, Oberst S, Pallansch MA. Enterovirus surveillance--United States, 1970–2005. MMWR Surveill Summ. 2006;55:1–20. [PubMed] [Google Scholar]
- 15.Lindsey NP, Staples JE, Lehman JA, Fischer M. Surveillance for human West Nile virus disease - United States, 1999–2008. MMWR Surveill Summ. 2010;59:1–17. [PubMed] [Google Scholar]
- 16.Brown KE. Variants of B19. Dev Biol (Basel) 2004;118:71–7. [PubMed] [Google Scholar]
- 17.Sejvar JJ, Haddad MB, Tierney BC, et al. Neurologic manifestations and outcome of West Nile virus infection. JAMA. 2003;290:511–5. doi: 10.1001/jama.290.4.511. [DOI] [PubMed] [Google Scholar]
- 18.Petersen LR, Marfin AA. West Nile virus: a primer for the clinician. Ann Intern Med. 2002;137:173–9. doi: 10.7326/0003-4819-137-3-200208060-00009. [DOI] [PubMed] [Google Scholar]
- 19.Huang C, Morse D, Slater B, et al. Multiple-year experience in the diagnosis of viral central nervous system infections with a panel of polymerase chain reaction assays for detection of 11 viruses. Clin Infect Dis. 2004;39:630–5. doi: 10.1086/422650. [DOI] [PubMed] [Google Scholar]
- 20.Fowlkes AL, Honarmand S, Glaser C, et al. Enterovirus-associated encephalitis in the California encephalitis project, 1998–2005. J Infect Dis. 2008;198:1685–91. doi: 10.1086/592988. [DOI] [PubMed] [Google Scholar]
- 21.Alexander JP, Jr, Baden L, Pallansch MA, Anderson LJ. Enterovirus 71 infections and neurologic disease--United States, 1977–1991. J Infect Dis. 1994;169:905–8. doi: 10.1093/infdis/169.4.905. [DOI] [PubMed] [Google Scholar]
- 22.CDC. Update: influenza activity--United States, August 30, 2009–January 9, 2010. MMWR Morb Mortal Wkly Rep. 2010;59:38–43. [PubMed] [Google Scholar]
- 23.Seifter A, Schwarzwalder A, Geis K, Aucott J. The utility of “Google Trends” for epidemiological research: Lyme disease as an example. Geospat Health. 2010;4:135–7. doi: 10.4081/gh.2010.195. [DOI] [PubMed] [Google Scholar]
- 24.Steere AC, Malawista SE, Hardin JA, et al. Erythema chronicum migrans and Lyme arthritis. The enlarging clinical spectrum. Ann Intern Med. 1977;86:685–98. doi: 10.7326/0003-4819-86-6-685. [DOI] [PubMed] [Google Scholar]
- 25.Horowitz HW, Dworkin B, Forseter G, et al. Liver function in early Lyme disease. Hepatology. 1996;23:1412–7. doi: 10.1002/hep.510230617. [DOI] [PubMed] [Google Scholar]
- 26.Wormser GP, Dattwyler RJ, Shapiro ED, et al. The clinical assessment, treatment, and prevention of lyme disease, human granulocytic anaplasmosis, and babesiosis: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis. 2006;43:1089–134. doi: 10.1086/508667. [DOI] [PubMed] [Google Scholar]