Abstract
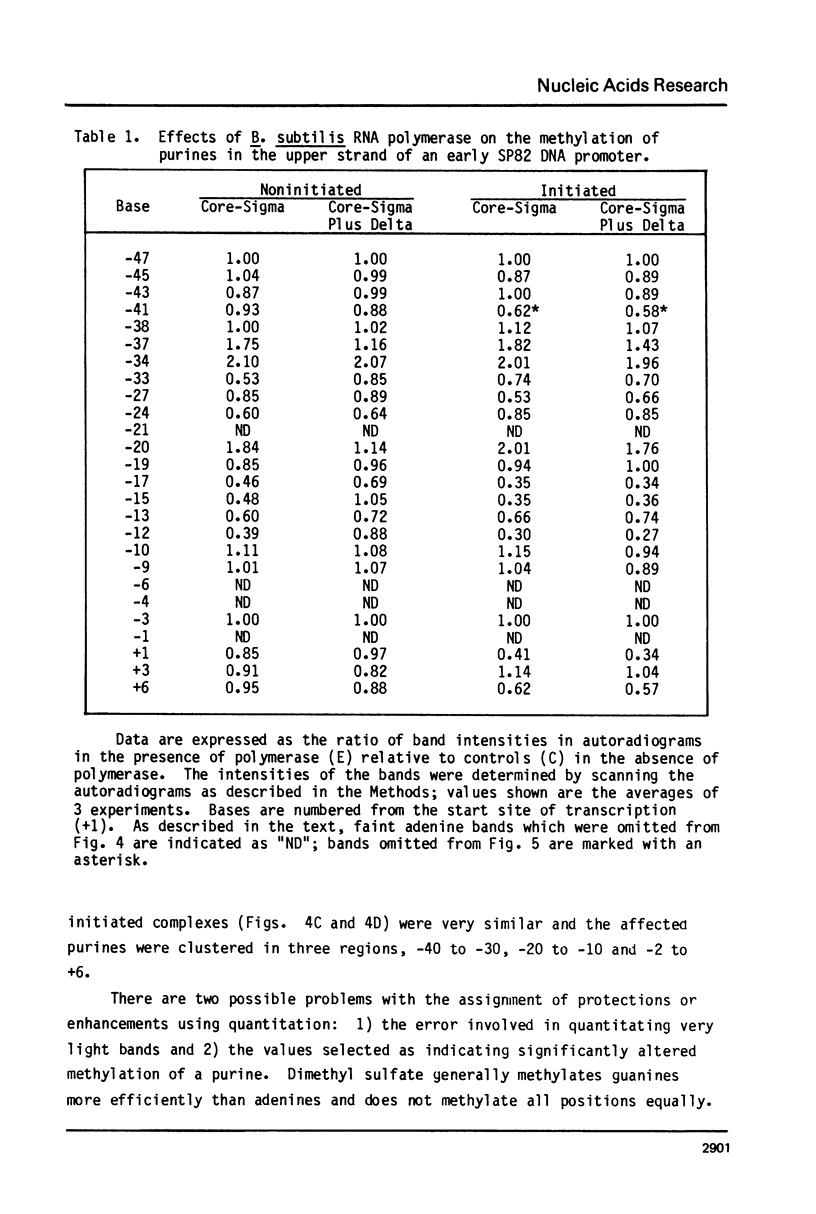
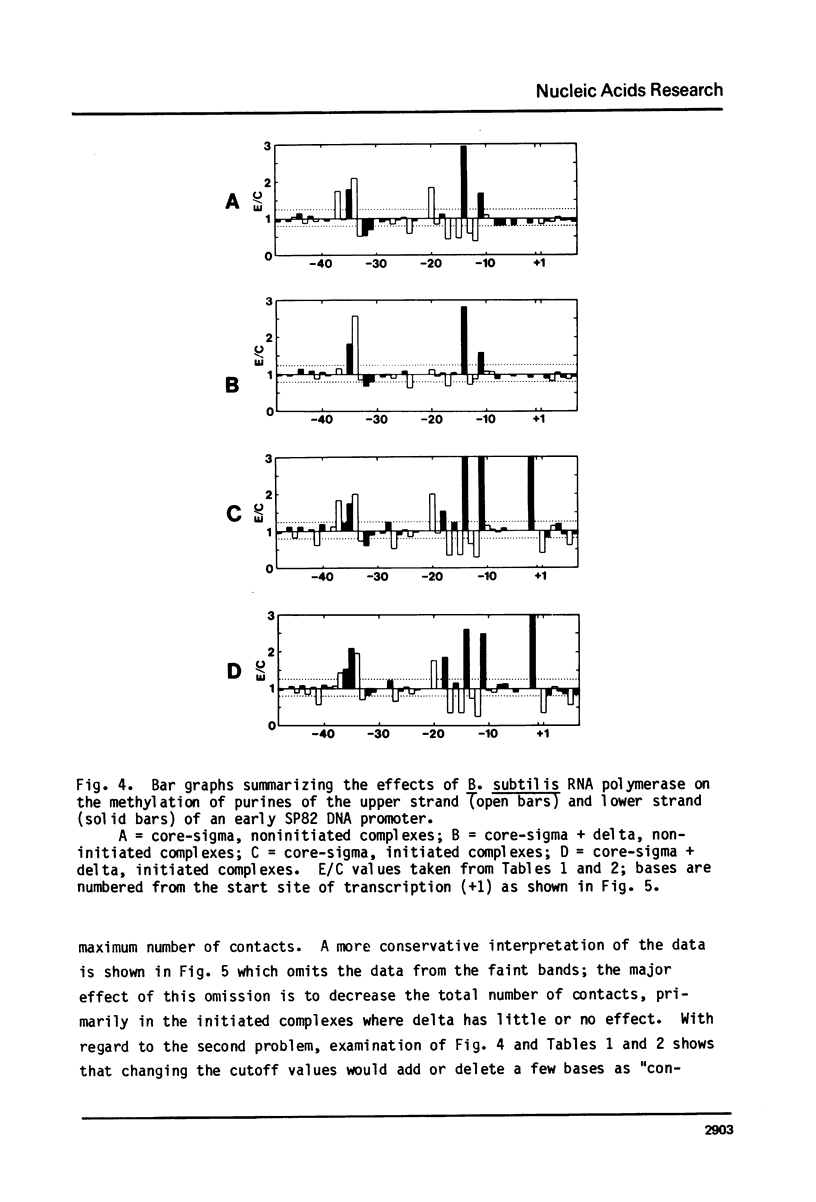
We have examined the effect of the delta subunit on the interaction of the Bacillus subtilis RNA polymerase with an early gene promotor of phase SP82. Methylation by dimethyl sulfate, used to probe close approaches of polymerase to purines, revealed that noninitiated complexes formed by holo-enzyme (core-sigma-delta) had significantly fewer contacts than complexes formed by core-sigma. The presence or absence of delta had little or no effect on close approaches to purines in initiated complexes. DNAase I footprinting indicated that core-sigma was bound to the same region regardless of whether delta, initiating nucleotides, or both, were present. These data support the conclusion that delta acts prior to initiation to enhance promoter selectivity by limiting the number of possible interactions that the polymerase can make with DNA.
Full text
PDF





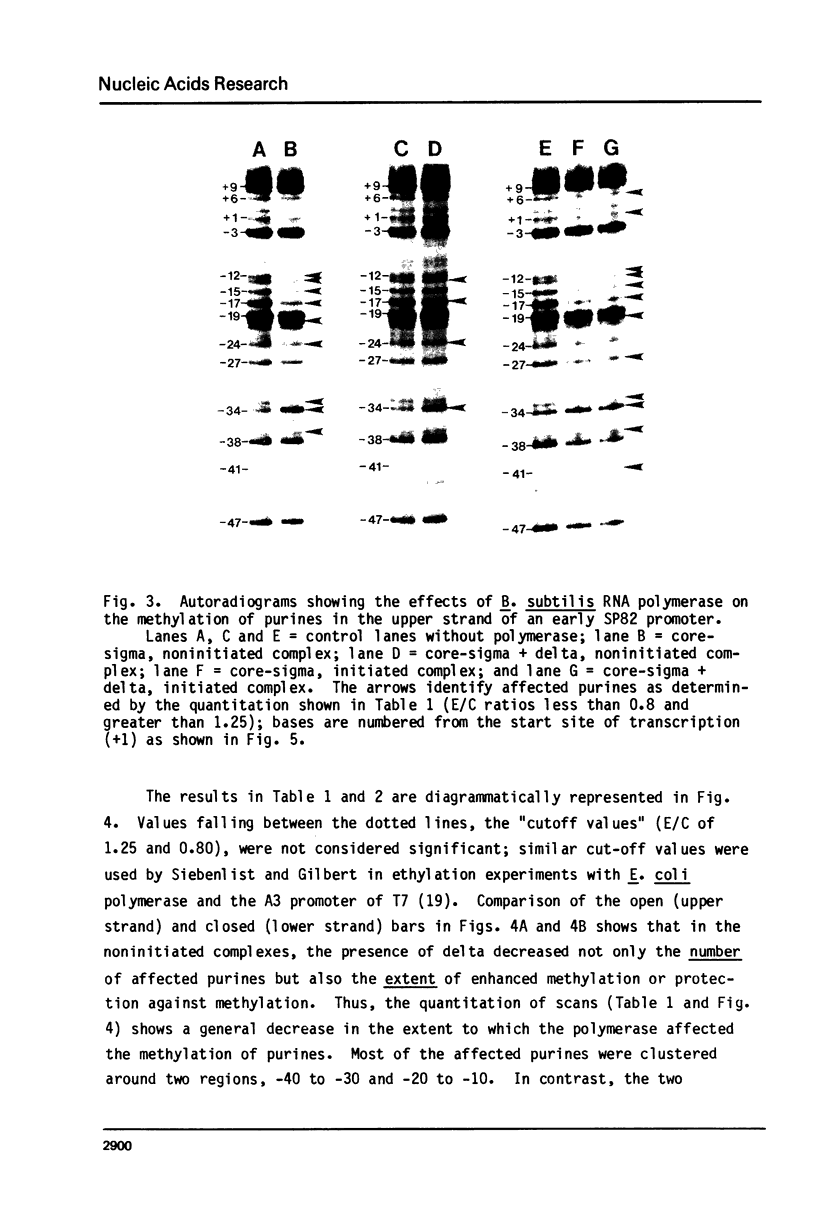








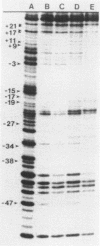
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Achberger E. C., Whiteley H. R. The interaction of Escherichia coli core RNA polymerase with specificity-determining subunits derived from unmodified and SP82-modified Bacillus subtilis RNA polymerase. J Biol Chem. 1980 Dec 25;255(24):11957–11964. [PubMed] [Google Scholar]
- Achberger E. C., Whiteley H. R. The role of the delta peptide of the Bacillus subtilis RNA polymerase in promoter selection. J Biol Chem. 1981 Jul 25;256(14):7424–7432. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Donis-Keller H., Maxam A. M., Gilbert W. Mapping adenines, guanines, and pyrimidines in RNA. Nucleic Acids Res. 1977 Aug;4(8):2527–2538. doi: 10.1093/nar/4.8.2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galas D. J., Schmitz A. DNAse footprinting: a simple method for the detection of protein-DNA binding specificity. Nucleic Acids Res. 1978 Sep;5(9):3157–3170. doi: 10.1093/nar/5.9.3157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen U. M., McClure W. R. A noncycling activity assay for the omega subunit of Escherichia coli RNA polymerase. J Biol Chem. 1979 Jul 10;254(13):5713–5717. [PubMed] [Google Scholar]
- Johnsrud L. Contacts between Escherichia coli RNA polymerase and a lac operon promoter. Proc Natl Acad Sci U S A. 1978 Nov;75(11):5314–5318. doi: 10.1073/pnas.75.11.5314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones B. B., Chan H., Rothstein S., Wells R. D., Reznikoff W. S. RNA polymerase binding sites in lambdaplac5 DNA. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4914–4918. doi: 10.1073/pnas.74.11.4914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAWLEY P. D., BROOKES P. FURTHER STUDIES ON THE ALKYLATION OF NUCLEIC ACIDS AND THEIR CONSTITUENT NUCLEOTIDES. Biochem J. 1963 Oct;89:127–138. doi: 10.1042/bj0890127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrie J. M., Spiegelman G. B., Whiteley H. R. DNA strand specificity of temporal RNA classes produced during infection of Bacillus subtilis by SP82. J Virol. 1976 Aug;19(2):359–373. doi: 10.1128/jvi.19.2.359-373.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee G., Talkington C., Pero J. Nucleotide sequence of a promoter recognized by Bacillus subtilis RNA polymerase. Mol Gen Genet. 1980;180(1):57–65. doi: 10.1007/BF00267352. [DOI] [PubMed] [Google Scholar]
- Mangel W. F., Chamberlin M. J. Studies of ribonucleic acid chain initiation by Escherichia coli ribonucleic acid polymerase bound to T7 deoxyribonucleic acid. I. An assay for the rate and extent of ribonucleic acid chain initiation. J Biol Chem. 1974 May 25;249(10):2995–3001. [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Panganiban A. T., Whiteley H. R. Analysis of bacteriophage SP82 major "early" in vitro transcripts. J Virol. 1981 Jan;37(1):372–382. doi: 10.1128/jvi.37.1.372-382.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pero J., Nelson J., Fox T. D. Highly asymmetric transcription by RNA polymerase containing phage-SP01-induced polypeptides and a new host protein. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1589–1593. doi: 10.1073/pnas.72.4.1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz A., Galas D. J. The interaction of RNA polymerase and lac repressor with the lac control region. Nucleic Acids Res. 1979 Jan;6(1):111–137. doi: 10.1093/nar/6.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siebenlist U., Gilbert W. Contacts between Escherichia coli RNA polymerase and an early promoter of phage T7. Proc Natl Acad Sci U S A. 1980 Jan;77(1):122–126. doi: 10.1073/pnas.77.1.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siebenlist U., Simpson R. B., Gilbert W. E. coli RNA polymerase interacts homologously with two different promoters. Cell. 1980 Jun;20(2):269–281. doi: 10.1016/0092-8674(80)90613-3. [DOI] [PubMed] [Google Scholar]
- Spiegelman G. B., Hiatt W. R., Whiteley H. R. Role of the 21,000 molecular weight polypeptide of Bacillus subtilis RNA polymerase in RNA synthesis. J Biol Chem. 1978 Mar 25;253(6):1756–1765. [PubMed] [Google Scholar]