Abstract
Neuronal activity leads to arteriole dilation and increased blood flow in retinal vessels. This response, termed functional hyperemia, is diminished in the retinas of diabetic patients, possibly contributing to the development of diabetic retinopathy. The mechanism responsible for this loss is unknown. Here we show that light-evoked arteriole dilation was reduced by 58% in a streptozotocin-induced rat model of type 1 diabetes. Functional hyperemia is believed to be mediated by glial cells and we found that glial-evoked vasodilation was reduced by 60% in diabetic animals. The diabetic retinas showed neither a decrease in the thickness of the retinal layers nor an increase in neuronal loss, although signs of early glial reactivity and an upregulation of inducible nitric oxide synthase (iNOS) were detected. Inhibition of iNOS restored both light- and glial-evoked dilations to control levels. These findings suggest that high NO levels resulting from iNOS upregulation alters glial control of vessel diameter and may underlie the loss of functional hyperemia observed in diabetic retinopathy. Restoring functional hyperemia by iNOS inhibition may limit the progression of retinopathy in diabetic patients.
Keywords: iNOS, glial cells, neurovascular coupling, diabetes, retina
INTRODUCTION
Diabetic retinopathy is a leading cause of blindness in the developed world. It has traditionally been considered a disease of the retinal vasculature. In its later stages, diabetic retinopathy is characterized by capillary occlusions, microaneurysms, edema, and neovascularization (Antonetti et al., 2006), leading to the loss of vision in many patients. The contribution of nonvascular cells to the development of diabetic retinopathy has only recently been explored. In early stages of the disease, neurons within the inner retina die (Barber et al., 1998), and astrocytes and Müller cells (the two macroglial cells of the retina) undergo significant pathological changes (Lieth et al., 1998; Mizutani et al., 1998). Increased apoptosis is also observed in pericytes and vascular endothelial cells (Mizutani et al., 1996). Although both vascular and nonvascular cells are affected in diabetic retinopathy, it is not clear whether the vascular pathology is a product or cause of the neuronal and glial dysfunction (Antonetti et al., 2006).
In the healthy retina, light-evoked neuronal activity leads to increased blood flow in retinal vessels. This response, termed functional hyperemia, fine tunes the retinal circulation, bringing needed oxygen and nutrients to active neurons (Riva et al., 2005). Recent studies demonstrate that functional hyperemia in both the retina and the brain is mediated, in large part, by glial cells (Metea and Newman, 2006; Mulligan and MacVicar, 2004; Takano et al., 2006; Zonta et al., 2003). The release of transmitters from neurons stimulates Ca2+ increases within glial cells (Porter and McCarthy, 1996), leading to the activation of phospholipase A2 and the production of arachidonic acid (AA) (Koehler et al., 2009). Arachidonic acid, in turn, is metabolized into a number of vasoactive compounds, including prostaglandin E2 (PGE2) and epoxyeicosatrienoic acids (EETs), which dilate vessels, and 20-hydroxyeicosatetraenoic acid (20-HETE), which constricts vessels (Amruthesh et al., 1992; Ellis et al., 1979; Harder et al., 1994).
Two recent studies reported a dramatic reduction in functional hyperemia in diabetic patients (Garhofer et al., 2004; Mandecka et al., 2007). The loss of this vascular response could starve the retina of needed oxygen and glucose, putting neurons at risk and contributing to retinal pathology. The cellular and molecular mechanisms responsible for the decrease in functional hyperemia in diabetic patients are not known. It is possible that the neuronal or glial dysfunctions observed in early stages of the disease are responsible for altered neurovascular signaling, leading to the loss of functional hyperemia. In this study, we investigate the mechanisms underlying this loss in an animal model of diabetic retinopathy and test a treatment for restoring the response. We find that altered glia-to-vessel signaling is responsible for the loss of functional hyperemia in the diabetic retina and that inhibiting inducible nitric oxide synthase (iNOS) restores the response.
MATERIALS AND METHODS
Animals
Male Long-Evans rats were obtained from Harlan (Indianapolis, IN) and treated in accordance with the guidelines of the Institutional Animal Care and Use Committee of the University of Minnesota.
Induction of Diabetic Retinopathy
The streptozotocin (STZ) model of type 1 diabetes (Rungger-Brandle and Dosso, 2003; Yu et al., 2001) was used. Two-month-old rats were anesthetized with isoflurane and injected IP with streptozotocin (70 mg/kg; freshly prepared in citrate buffer). Blood glucose levels were measured three days later to ensure successful induction of diabetes (glucose >250 mg/dL; OneTouch Ultra, Life-Scan). Animals were given a low level of supplemental insulin (1.5 U of Lantus insulin glargine subcutaneously, thrice a week (Du et al., 2002) to prevent excessive weight loss and a catabolic response while maintaining high glucose levels. Body weight and blood glucose were monitored biweekly. Blood glucose averaged 484 ± 9 mg/dL during the survival period and 562 ± 17 mg/dL at the time of sacrifice. Vehicle-injected, age-matched controls had blood glucose averaging 139 ± 2 mg/dL during the survival period, and 204 ± 8 mg/dL at sacrifice.
Isolated Retina Preparation
The isolated retina preparation has been described previously (Newman, 2001). Briefly, animals were killed by an overdose of isoflurane and bilateral pneumothorax, and eyes enucleated. Following removal of the vitreous humor, pieces of retinas were placed in a chamber and superfused at 2–3 mL/min with HEPES-buffered saline equilibrated with air. Arterioles were preconstricted with the thromboxane analog U-46619 (100 nM) for 10 min (Filosa et al., 2004) or until stable to achieve comparable tone in all arterioles studied. At this concentration, U-46619 constricted arterioles moderately, rendering them responsive to both vasodilating and vasoconstricting signals. For iNOS inhibition experiments, retinas were pre-incubated in iNOS blockers for a minimum of 90 min.
Retinal Imaging
Retinas were imaged with a 40X water immersion objective, infrared differential interference contrast (IR-DIC) optics, and a cooled CCD camera (CoolSnap ES; Roper Scientific, Duluth, GA). Images were captured and analyzed using MetaMorph image processing software (Molecular Devices, Downingtown, PA). Techniques used to identify retinal arterioles have been previously described (Metea and Newman, 2006). Arteriole responses were quantified as the percent difference between the largest change in vessel diameter measured during stimulation and the average prestimulus resting diameter. Diffuse flickering white light (250 ms flashes repeated twice a second) was used to stimulate the retina.
Calcium Imaging and Glial Stimulation
Following removal of the vitreous humor, retinal pieces were incubated in the Ca2+-indicator dye fluo-4 AM (37.5 µg/mL), the caged Ca2+ compound o-nitrophenyl EGTA AM (9.4 µg/mL) and pluronic F-127 (2.6 mg/mL) for 30 min at room temperature to selectively label retinal glia as described previously (Newman, 2001). Glial Ca2+ and vessel diameter were recorded concurrently by alternate epifluorescence and IR-DIC imaging. Photo-release of Ca2+ was achieved by focusing 4 ns, 5 µm diameter flashes of 337 nm UV light (VSL-337ND photolysis unit; Prairie Technologies, Middleton, WI) onto individual astrocytes or Müller cells. The UV light was pulsed 200 times at 330 Hz and repeated every second for 5–10 s until an intercellular Ca2+ wave was initiated.
Histology
Retinas were fixed in 4% paraformaldehyde in PBS for 1 h. Fixed retinas were shock-frozen in a 50%–50% mixture of OCT compound (Sakura Tissue-Tek) and Aquamount (Lerner Laboratories). Twelve-µm-thick cryostat sections were cut and mounted using Vectashield containing DAPI (Vector Laboratories). Sections were imaged using confocal microscopy.
Cell Death Detection
Apoptotic cells were detected by TUNEL labeling using the In Situ Cell Death Detection Kit, Fluorescein (Roche, Basel, Switzerland) according to the protocol provided by the supplier. Briefly, fixed sections were permeabilized in PBS containing Triton X-100 (Sigma) and then incubated in the reaction mixture for 60 min at 37°C. TUNEL positive cells were counted manually and analyzed as the number of positive cells per 10-µm segment of retinal section.
Immunolabeling
Retinal sections were blocked for 30 min with 10% normal goat serum (NGS), 0.5% Triton X-100, and 1% bovine serum albumin (BSA) in PBS, pH 7.0. All subsequent steps were conducted in PBS containing 1% NGS, 0.5% triton X, and 1% BSA. Sections were stained with mouse anti-GFAP (1:500) for 1 h at room temperature (RT) or with rabbit anti-iNOS (1:100), rabbit anti-eNOS (1:2,000) or rabbit anti-nNOS (1:2,000) overnight at 4°C. After washing, sections were incubated with the secondary for 1 h at RT. Alexa-Fluor® 594 conjugated goat anti-mouse (1:400) was used for GFAP and Alexa Fluor® 488 conjugated goat anti-rabbit (1:1,000) was used for all others.
Statistics
Statistical significance for vasomotor responses was determined by one-tailed Mann-Whitney-Wilcoxon rank sum test for non-normal distributions. Proportions test was used for binomial data (e.g., whether a dilation or constriction occurred). Homoscedastic two-tailed Student’s t-test was used for all other analyses. α = 0.05 for all analyses.
Solutions and Drugs
HEPES-buffered saline contained (in mM): 128 NaCl, 3.0 KCl, 2.0 CaCl2, 1.0 MgSO4, 0.5 NaH2PO4, 15.0 D-glucose, and 20 HEPES, pH 7.4, equilibrated with air. o-nitrophenyl EGTA AM, fluo-4 AM, and pluronic F-127 were from Invitrogen (San Diego, CA). 1400W (N-[[3-(aminomethyl)phenyl]methyl]-ethanim idamide dihydrochloride), U-46619 (9,11-dideoxy-9_, 11_methanoepoxyprosta5Z,13E-dien-1-oic acid), and PGE2 (9-oxo-11α,15S-dihydroxy-prosta-5Z,13E-dien-1-oic acid) were from Cayman Chemicals (Ann Arbor, MI). Aminoguanidine hydrochloride and rabbit anti-eNOS were from Sigma-Aldrich (St. Louis, MO). Rabbit anti-nNOS and mouse anti-GFAP were from Millipore (Bedford, MA) and rabbit anti-iNOS from Santa Cruz Biotechnology (Santa Cruz, CA).
RESULTS
Light-Evoked Vasodilations Are Reduced in the Diabetic Retina
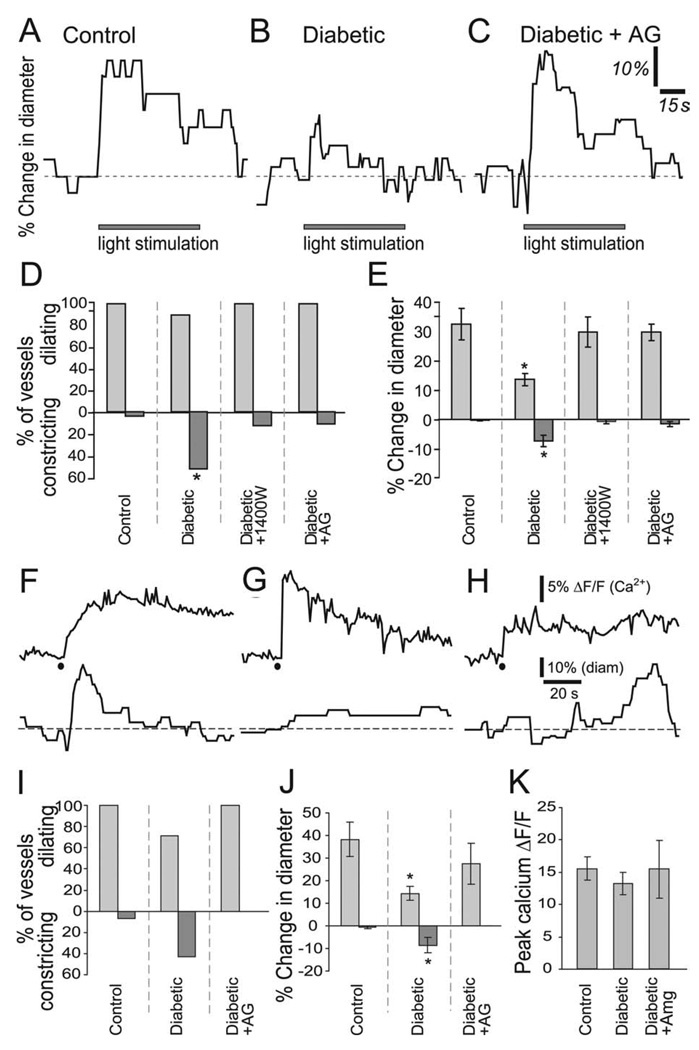
We first examined whether arteriole responses to light stimulation were altered in the streptozotocin (STZ) induced model of type 1 diabetes, which has been used extensively to study diabetic retinopathy (Rungger-Brandle and Dosso, 2003; Yu et al., 2001). Isolated rat retinas were stimulated with diffuse flickering white light while monitoring the diameter of arterioles on the vitreal surface of the retina. Four months after induction of diabetes, light-evoked arteriole dilation was similar in diabetic (25.8 ± 4.7%; n = 26 vessels) and control (22.5 ± 3.1%; n = 11) groups. However, significant changes in neurovascular coupling were observed at seven months. At this time, light stimulation of normal retinas resulted in dilations averaging 30.9 ± 5.2% (Fig. 1A,C; n = 23) while dilations were reduced to 12.9 ± 1.9% (Fig. 1B,D; n = 19, p < 0.001) in diabetic retinas. Although light-evoked vasoconstrictions were rarely observed in normal retinas (4% of vessels), they occurred frequently in diabetic retinas (53% of vessels, p < 0.005; results summarized in Fig. 5D,E). Vasoconstrictions typically followed transient vasodilations (Fig. 1D). All subsequent experiments were conducted on seven-month survival animals.
Fig. 1. Light-evoked vasodilation is reduced in diabetic retinas.
A,B: IR-DIC images of the vitreal surface of the retina, illustrating the light-evoked responses of small arterioles. In a control retina (A), light stimulation evokes a large vasodilation (at 17 and 45 s after onset of the light stimulus). In a diabetic retina (B), light evokes a smaller dilation (at 21 s), followed by a constriction (at 27 s). The diameter of both control and diabetic vessels recover to baseline after light stimulation ends. Solid white lines indicate baseline vessel diameter; dashed lines indicate changed diameter. Scale bar, 10 µm. C,D: Light-evoked arteriole dilation in a normal (C) and a diabetic (D) retina. Light stimulation evokes a smaller dilation, followed by a constriction, in the diabetic retina.
Fig. 5. Inhibition of iNOS activity restores light- and glial-evoked vasodilation in diabetic retinas.
A–C: Light-evoked vasomotor responses in a control retina (A), diabetic retina (B) and a diabetic retina treated with aminoguanidine (AG, 100 µM; C). D: The incidence of light-evoked arteriole dilations and constrictions. The incidence of vasoconstrictions is increased in diabetic retinas and is restored to control levels by 1400W (1 µM) and AG (100 µM), two inhibitors of iNOS. E: The magnitude of light-evoked arteriole dilations and constrictions. The magnitude of vasodilations is reduced while that of vasoconstrictions is increased in diabetic retinas. 1400W and AG restore these vasomotor responses to control levels. F–H: Photolysis-evoked glial Ca2+ increases (upper traces) and the resulting vascular responses (lower traces) in a control retina (F), diabetic retina (G) and a diabetic retina treated with AG (100 µM; H). Black dots indicate photolysis of caged Ca2+. I: The incidence of glial-evoked arteriole dilations and constrictions. J. The magnitude of glial-evoked vasodilations and constrictions. The magnitude of vasodilations is reduced and vasoconstrictions increased in diabetic retinas. Both are restored to control levels by AG. K: Photolysis-evoked peak glial Ca2+ responses (measured in processes surrounding vessels) are not different between control, diabetic and AG-treated diabetic retinas. *p < 0.02.
Response differences between control and diabetic retinas could be due to differences in the resting diameter of the vessels (Blanco et al., 2008). This was not the case, however. The mean resting diameter for all arterioles analyzed (after treatment with the thromboxane analog U-46619) was similar in control (25.3 ± 1.6 µm, n = 52) and diabetic (26.7 ± 1.1 µm, n = 85) retinas (p > 0.4).
We questioned whether the decrease in functional hyperemia in diabetic animals was due to decreased responsiveness of retinal vessels. We tested this by directly applying the vasodilating agent PGE2 to retinal arterioles that were responsive to light stimulation. We used a relatively high concentration of PGE2 (200 µM) in order to evoke rapid, short latency responses. Rapid superfusion of PGE2 produced similar dilations in control and diabetic arterioles (controls: 13 of 17 vessels dilated, 24.4 ± 19% dilation; diabetics: 10 of 11 vessels, 24.3 ± 14% dilation, p > 0.2; Supp. Info. Fig. 1), irrespective of whether they dilated or constricted to light. The results demonstrate that vascular responsiveness is not compromised in the diabetic retina.
Our observation that light stimulation evokes arteriole vasodilation but rarely evokes vasoconstriction in healthy retinas differs from our previous finding (Metea and Newman, 2006) that stimulation typically evoked a biphasic response consisting of a transient dilation followed by a constriction. The difference in vessel behavior in the two sets of experiments is due to the oxygen levels used. Earlier experiments were performed under hyperoxic conditions (95% oxygen) while the present experiments were conducted under conditions similar to those in vivo (21% oxygen). Recent studies have demonstrated that hyperoxia blocks PGE2-mediated vasodilation and enhances vasoconstriction mediated by 20-HETE (Gordon et al., 2008; Mishra et al., 2010). In the present experiments, conducted in 21% oxygen, vasodilations in healthy retinas were larger because they were mediated by both PGE2 and EETs, while vasoconstrictions were masked.
Few Overt Signs of Retinopathy Are Seen in the Diabetic Retina
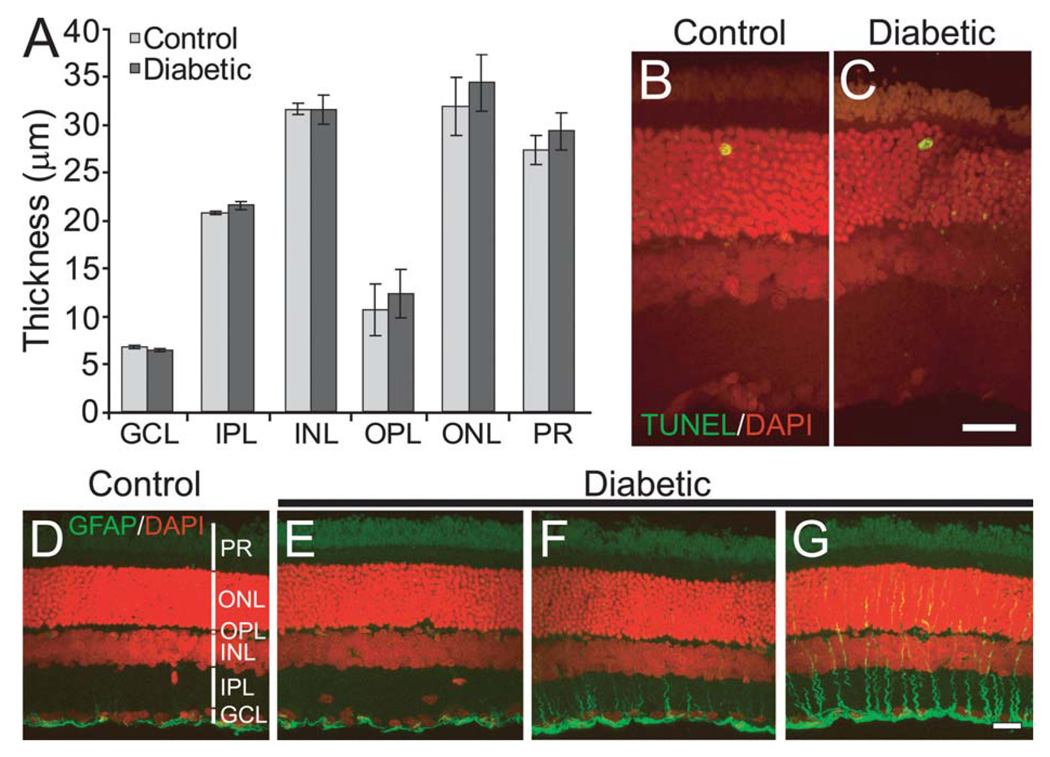
A loss of retinal neurons, demonstrated by a decrease in the thickness of retinal layers and by increased TUNEL staining, has been reported in both diabetic patients and in STZ-treated diabetic rats (Barber et al., 1998; van Dijk et al., 2009). This neuronal loss could, in theory, account for the reduction in functional hyperemia observed in our experiments. We examined whether there was a loss of neurons in our diabetic animals by measuring the thickness of retinal layers in DAPI-labeled sections (n = 3 animals for controls and 4 for diabetics). There was no reduction in thickness of any of the retinal layers (Fig. 2A). We also examined cell death directly by TUNEL staining (Fig. 2B,C). There was no difference in the number of TUNEL positive cells in the control (0.17 ± 0.11 cells/mm; two sections each from three animals) and diabetic (0.13 ± 0.13 cells/mm; p > 0.8; two sections each from four animals) retinas. We also examined TUNEL staining one week after STZ injection to test whether the induction of diabetes increases apoptosis early on, as observed previously (Feit-Leichman et al., 2005). We observed no difference in TUNEL staining between controls (0.10 ± 0.05 cells/mm) and diabetics (0.07 ± 0.06 cells/mm; data not shown). These results demonstrate that there was no significant loss of neurons in our diabetic animals.
Fig. 2. Few overt signs of retinopathy are seen in the diabetic retina.
A: Mean thickness of retinal layers is not reduced in diabetic animals. GCL, ganglion cell layer; IPL, inner plexiform layer; INL, inner nuclear layer; OPL, outer plexiform layer; ONL, outer nuclear layer; PR, photoreceptors. B,C: Cell death was not increased in diabetic retinas. Very few TUNEL-positive cells (green/yellow profiles) were observed in both control (B) and diabetic (C) retinas. DAPI-labeled cell nuclei are shown in red. D–G: Immunostaining shows the expression of GFAP (green) in control (D) and three diabetic (E–G) retinas. A range of GFAP expression was observed in the vertically oriented Müller cells in diabetic retinas. Retinas from some animals were similar to controls (E), some showed a minor increase (F) while some showed substantial upregulation (G). GFAP-positive astrocytes, located beneath the GCL, are seen in both control and diabetic retinas. DAPI-labeled cell nuclei are shown in red. Scale bars, 20 µm.
Glial-Evoked Vasodilations Are Reduced in the Diabetic Retina
We and others have previously shown that functional hyperemia in the retina and brain is mediated, in part, by glial cells (Metea and Newman, 2006; Mulligan and MacVicar, 2004; Takano et al., 2006; Zonta et al., 2003). In this regard, it is of interest that retinal glial cells undergo pathological changes during the early stages of diabetic retinopathy. Müller cells, the principal macroglial cells of the retina, undergo an upregulation of glial fibrillary acidic protein (GFAP) and downregulation of glutamate transporters (Li and Puro, 2002; Lieth et al., 1998; Mizutani et al., 1998). We tested whether GFAP expression was upregulated in our diabetic animals. We observed an increase in GFAP expression in the Müller cells of some, but not all, diabetic animals (Fig. 2D–G; n = 3 controls and four diabetics), indicating the beginning stages of reactive gliosis.
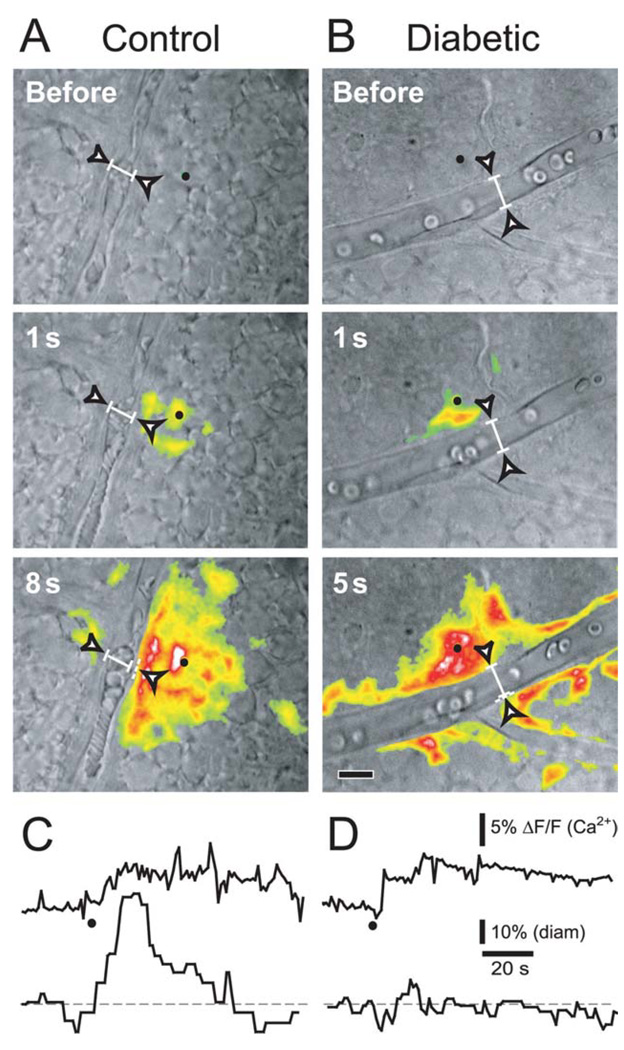
Since the glial cells showed evidence of pathology, we reasoned that abnormal glial regulation of the vasculature could be responsible for the loss of functional hyperemia in the diabetic retina. We tested the role of glia in the loss of functional hyperemia by selectively stimulating glial cells and monitoring the resulting vasomotor responses. Glial cells (both astrocytes and Müller cells) were stimulated by raising intracellular Ca2+ by photolysis of o-nitrophenyl EGTA, a caged Ca2+ compound. Stimulating single glial cells evoked Ca2+ increases that propagated as Ca2+ waves into neighboring astrocytes and Müller cells (Fig. 3A,B). In control retinas, Ca2+ waves that propagated across an arteriole elicited large vasodilations averaging 36.2 ± 7% (Fig. 3A,C; n = 15). In diabetic retinas, these vasodilations were reduced to 15.4 ± 3% (Fig. 3B,D; n = 13, p < 0.02; summarized in Fig. 5J). In addition, glial-evoked vasoconstrictions were seen in 39% of vessels in diabetic retinas compared to only 7% in controls, and the magnitude of constrictions was greater in diabetic animals (Fig. 5I,J). The decrease in glial-evoked vasodilations and increase in vasoconstrictions were not due to reduced glial cell activation. Photolysis of caged Ca2+ produced similar glial Ca2+ increases in control and diabetic retinas (controls: ΔF/F = 15.9 ± 1.7%; diabetics: 13.3 ± 1.9%, p > 0.3; Fig. 5K). These results indicate that the deficit in functional hyperemia seen in diabetic retinas is likely due to compromised glial regulation of vessel diameter.
Fig. 3. Glial-evoked vasodilation is reduced in diabetic retinas.
A,B: IR-DIC images of a normal (A) and diabetic (B) retina showing small arterioles. Pseudocolor images showing glial Ca2+ increases are superimposed. In A and B, the top two images show time points before and 1 s after photolysis of caged Ca2+ in single glial cells (black dots). The last images show time points at which maximum vessel dilation was observed, with maximum ΔF/F Ca2+ projections overlaid. Although the glial Ca2+ increases are similar in control and diabetic retinas, the glial-evoked dilation is smaller in the diabetic retina. Scale bar, 10 µm. C,D: Ca2+ increases (upper traces) and arteriole diameters (lower traces) in a control (C) and a diabetic (D) retina. Photolysis of caged Ca2+ (black dots) produces similar glial Ca2+ increases in control and diabetic retinas. Yet, glial stimulation produces smaller arteriole dilations in the diabetic retina.
iNOS Is Upregulated in the Diabetic Retina
We have previously shown that nitric oxide (NO) is a modulator of functional hyperemia in the retina (Metea and Newman, 2006). Both light stimulation and glial cell stimulation evoke vasodilations when NO levels are low. Vasodilations are reduced and vasoconstrictions are enhanced, however, when NO levels are raised. Notably, iNOS expression is increased and tissue NO concentration is raised in the retinas of diabetic animals (Du et al., 2002; Kowluru et al., 2000). We confirmed that iNOS was upregulated in our diabetic retinas by immunohistochemistry, which indicated an increase in iNOS levels in the ganglion cell, inner plexiform and outer nuclear layers (Fig. 4A–D; n = 3 controls and 4 diabetics). iNOS was expressed in retinal glia as well as neurons. No changes in the expression of neuronal or endothelial NOS were observed (Fig. 4E–L).
Fig. 4. iNOS expression is increased in diabetic retinas.
Immunostaining shows the expression of iNOS, nNOS, and eNOS (green) in control (B,F,J) and diabetic (D,H,L) retinas. DAPI staining (red) shows cell nuclei in control (A,E,I) and diabetic (C,G,K) retinas. iNOS expression in the ONL, IPL and GCL of diabetic retinas (D) is raised compared to controls (B). nNOS (F,H) and eNOS (J,L) expression is unaltered in diabetic retinas. Scale bar, 20 µm.
Inhibition of iNOS Restores Light-Evoked and Glial-Evoked Vasodilations
We reasoned that increased NO levels in the inner retina due to the upregulation of iNOS might be responsible for reduced functional hyperemia in diabetic animals. We tested this by treating diabetic retinas with 1400W and aminoguanidine, two inhibitors of NOS that are relatively selective for iNOS at the concentrations used. In the presence of either 1 µM 1400W or 100 µM aminoguanidine, the amplitude of light-evoked vasodilations in diabetic retinas was restored to control levels (Fig. 5A–E; 1400W: 27.3 ± 3.5%, n = 22: aminoguanidine: 28.3 ± 2.7%, n = 26; neither different from controls, p > 0.3). The incidence and amplitude of light-evoked vasoconstrictions, which were raised in diabetic retinas, were also reduced to control levels by the iNOS inhibitors (Fig. 4D,E). 1400W had no effect on light-evoked vasomotor responses in control retinas (28.4 ± 5%, n = 15, p > 0.4; Supp. Info. Fig. 2), suggesting that NO synthesized by iNOS does not play a significant role in neurovascular coupling in healthy retinas.
If increased NO levels reduce functional hyperemia in the diabetic retina by interfering with glial control of vessel diameter (Metea and Newman, 2006), then inhibiting iNOS should restore glial-evoked vasodilations as well in diabetic animals. This proved to be the case. In the presence of aminoguanidine, glial-evoked vasodilations were restored to control levels in diabetic retinas (27.4 ± 9%, n = 5, p > 0.2 compared with controls) and no glial-evoked vasoconstrictions were observed (Fig. 5F–J). Aminoguanidine did not alter the photolysis-evoked glial Ca2+ response (Fig. 5K).
DISCUSSION
Our results demonstrate that the STZ rat model of diabetes reproduces the deficit in functional hyperemia observed in diabetic patients. Light-evoked vasodilations in the isolated retina are significantly reduced in diabetic animals. This deficit is not due to a loss of retinal neurons or vascular responsiveness, but rather is due to aberrant glia-to-vessel signaling. Notably, the loss of functional hyperemia can be restored by aminoguanidine and 1400W, two inhibitors of iNOS.
The diabetic retinas used in our study showed few overt signs of retinopathy. Although functional hyperemia was reduced and iNOS expression increased, there was no loss of retinal neurons or any evident changes in the morphology of the vasculature. In contrast, several previous studies have reported alterations in neuronal function, changes in the expression patterns of neurons and glial cells, and neuronal and vascular cell death in early diabetic retinopathy (reviewed in Antonetti et al., 2006). Most of these studies were conducted using Sprague Dawley or other albino rat strains, which are susceptible to light-induced retinal damage. Our study, in contrast, used pigmented Long-Evans rats. It is likely that albino strains show a higher level of retinopathy when made diabetic due to the added effect of light damage. Indeed, a recent study demonstrated that albino Sprague Dawley rats showed a large increase in inflammatory cytokines four months after STZ treatment while pigmented rats (Long-Evans and Brown Norway) showed only small increases in just a few of their measures (Kirwin et al., 2009). Moreover, in STZ-treated pigmented mice, neuronal cell death did not differ from controls for up to a year after an initial transient increase in apoptosis (Feit-Leichman et al., 2005). The lack of overt retinal pathology at 7 months in our diabetic animals is, we believe, due to pigmented eyes being less vulnerable to damage.
Our observation that glial-evoked and light-evoked vasodilations were similarly reduced in diabetic retinas suggests that the loss of functional hyperemia was not due to altered neuronal responses to light stimulation. In addition, inhibition of iNOS restored both light- and glial-evoked dilations to control levels, arguing against any significant neuronal dysfunction. It is likely that changes in glial cell signaling to the vasculature underlie the deficits in functional hyperemia that we observed.
iNOS is upregulated in response to injury and pathology in many systems (Cattell and Jansen, 1995). In diabetic retinopathy, iNOS is upregulated in retinal neurons and glial cells and retinal NO levels are raised (Du et al., 2002; Kowluru et al., 2000). We have confirmed that iNOS is upregulated in our model of diabetic retinopathy. Immunolabeling for iNOS, but not nNOS or eNOS, was increased in retinal glial cells and neurons. Although iNOS was upregulated in our diabetic animals, mean arteriole diameter after treatment with the thromboxane analog U-46619 was no larger in diabetic than in control retinas. This is not surprising, as NO has multiple effects on the neurovascular unit. In addition to directly dilating vessels by raising cGMP levels in vascular smooth muscle cells (Ignarro, 2002), NO can also inhibit glial production of vasodilating EETs compounds (Kessler et al., 1999; Roman, 2002).
Our finding that iNOS inhibitors restore both light- and glial-evoked vasodilation indicates that raised NO levels are responsible for the loss of functional hyperemia, most likely by disrupting glial signaling to vessels. We have previously shown that vasodilation in the retina is mediated by glial release of EETs and PGE2 (Metea and Newman, 2006; Mishra et al., 2010) and that raised NO levels reduce vasodilation and enhance vasoconstriction (Metea and Newman, 2006). It is likely that NO reduces vasodilation by inhibiting glial production of EETs (Kessler et al., 1999; Roman, 2002), although this has yet to be tested in the retina.
The STZ rat model of Type I diabetes replicates many of the pathologies associated with human diabetic retinopathy (Yu et al., 2001). However, there are several limitations to this model that should be kept in mind. First, the timeline of disease progression is very different in the STZ rat model, which progresses over months, and Type I diabetes in patients, which can take well over a decade to develop (Alder et al., 1997; Lieth et al., 1998). Second, blood glucose levels are poorly controlled (intentionally) in our model, while they are well controlled in most patients diagnosed with diabetes. Additionally, the observations made in our model of Type I diabetic mellitus may not be directly applicable to Type II diabetes, even though retinopathy can develop in both cases, and therefore should be interpreted with care.
The loss of functional hyperemia is one of the first deficits observed in our diabetic retinas. Reduced vasodilation in response to neuronal activity will create a mismatch between energy supply and demand, depriving neurons of oxygen and nutrients (Gariano and Gardner, 2005). This disparity may be an important contributing factor in the development of diabetic retinopathy (Yu et al., 2001). Restoring functional hyperemia by inhibiting iNOS may slow progression of the pathology. Indeed, aminoguanidine (which also inhibits the formation of advanced glycation end-products; Brownlee et al., 1986), has been shown to prevent transcriptional, morphological and ultrastructural changes in the retina in diabetic animals (Corbett et al., 1992; Du et al., 2004; Hammes et al., 1991). Thus, the use of iNOS inhibitors such as aminoguanidine hold promise in treating diabetic retinopathy and should be explored in future studies.
ACKNOWLEDGMENTS
Grant sponsors: Fondation Leducq, NIH EY004077 and NIH Vision Training Grant.
The authors thank Alfonso Araque, Brian MacVicar and Anja Srienc for helpful discussions and comments on the manuscript, and Michael Burian for technical assistance.
Footnotes
Additional Supporting Information may be found in the online version of this article.
REFERENCES
- Alder VA, Su EN, Yu DY, Cringle SJ, Yu PK. Diabetic retinopathy: Early functional changes. Clin Exp Pharmacol Physiol. 1997;24:785–788. doi: 10.1111/j.1440-1681.1997.tb02133.x. [DOI] [PubMed] [Google Scholar]
- Amruthesh SC, Falck JR, Ellis EF. Brain synthesis and cerebrovascular action of epoxygenase metabolites of arachidonic acid. J Neurochem. 1992;58:503–510. doi: 10.1111/j.1471-4159.1992.tb09749.x. [DOI] [PubMed] [Google Scholar]
- Antonetti DA, Barber AJ, Bronson SK, Freeman WM, Gardner TW, Jefferson LS, Kester M, Kimball SR, Krady JK, LaNoue KF, Norbury CC, Quinn PG, Sandirasegarane L, Simpson IA. Diabetic retinopathy: seeing beyond glucose-induced microvascular disease. Diabetes. 2006;55:2401–2411. doi: 10.2337/db05-1635. [DOI] [PubMed] [Google Scholar]
- Barber AJ, Lieth E, Khin SA, Antonetti DA, Buchanan AG, Gardner TW. Neural apoptosis in the retina during experimental and human diabetes. Early onset and effect of insulin. J Clin Invest. 1998;102:783–791. doi: 10.1172/JCI2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco VM, Stern JE, Filosa JA. Tone-dependent vascular responses to astrocyte-derived signals. Am J Physiol Heart Circ Physiol. 2008;294:H2855–H2863. doi: 10.1152/ajpheart.91451.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brownlee M, Vlassara H, Kooney A, Ulrich P, Cerami A. Aminoguanidine prevents diabetes-induced arterial wall protein cross-linking. Science. 1986;232:1629–1632. doi: 10.1126/science.3487117. [DOI] [PubMed] [Google Scholar]
- Cattell V, Jansen A. Inducible nitric oxide synthase in inflammation. Histochem J. 1995;27:777–784. [PubMed] [Google Scholar]
- Corbett JA, Tilton RG, Chang K, Hasan KS, Ido Y, Wang JL, Sweetland MA, Lancaster JR, Jr, Williamson JR, McDaniel ML. Aminoguanidine, a novel inhibitor of nitric oxide formation, prevents diabetic vascular dysfunction. Diabetes. 1992;41:552–556. doi: 10.2337/diab.41.4.552. [DOI] [PubMed] [Google Scholar]
- Du Y, Sarthy VP, Kern TS. Interaction between NO, COX pathways in retinal cells exposed to elevated glucose and retina of diabetic rats. Am J Physiol Regul Integr Comp Physiol. 2004;287:R735–R741. doi: 10.1152/ajpregu.00080.2003. [DOI] [PubMed] [Google Scholar]
- Du Y, Smith MA, Miller CM, Kern TS. Diabetes-induced nitrative stress in the retina, and correction by aminoguanidine. J Neurochem. 2002;80:771–779. doi: 10.1046/j.0022-3042.2001.00737.x. [DOI] [PubMed] [Google Scholar]
- Ellis EF, Wei EP, Kontos HA. Vasodilation of cat cerebral arterioles by prostaglandins D2, E2, G2, and I2. Am J Physiol. 1979;237:H381–H385. doi: 10.1152/ajpheart.1979.237.3.H381. [DOI] [PubMed] [Google Scholar]
- Feit-Leichman RA, Kinouchi R, Takeda M, Fan Z, Mohr S, Kern TS, Chen DF. Vascular damage in a mouse model of diabetic retinopathy: Relation to neuronal and glial changes. Invest Ophthalmol Vis Sci. 2005;46:4281–4287. doi: 10.1167/iovs.04-1361. [DOI] [PubMed] [Google Scholar]
- Filosa JA, Bonev AD, Nelson MT. Calcium dynamics in cortical astrocytes and arterioles during neurovascular coupling. Circ Res. 2004;95:e73–e81. doi: 10.1161/01.RES.0000148636.60732.2e. [DOI] [PubMed] [Google Scholar]
- Garhofer G, Zawinka C, Resch H, Kothy P, Schmetterer L, Dorner GT. Reduced response of retinal vessel diameters to flicker stimulation in patients with diabetes. Br J Ophthalmol. 2004;88:887–891. doi: 10.1136/bjo.2003.033548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gariano RF, Gardner TW. Retinal angiogenesis in development and disease. Nature. 2005;438:960–966. doi: 10.1038/nature04482. [DOI] [PubMed] [Google Scholar]
- Gordon GRJ, Choi HB, Rungta RL, Ellis-Davies GCR, MacVicar BA. Brain metabolism dictates the polarity of astrocyte control over arterioles. Nature. 2008;456:745–749. doi: 10.1038/nature07525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammes HP, Martin S, Federlin K, Geisen K, Brownlee M. Aminoguanidine treatment inhibits the development of experimental diabetic retinopathy. Proc Natl Acad Sci USA. 1991;88:11555–11558. doi: 10.1073/pnas.88.24.11555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harder DR, Gebremedhin D, Narayanan J, Jefcoat C, Falck JR, Campbell WB, Roman R. Formation and action of a P-450 4A metabolite of arachidonic acid in cat cerebral microvessels. Am J Physiol. 1994;266:H2098–H2107. doi: 10.1152/ajpheart.1994.266.5.H2098. [DOI] [PubMed] [Google Scholar]
- Ignarro LJ. Nitric oxide as a unique signaling molecule in the vascular system: A historical overview. J Physiol Pharmacol. 2002;53:t-14. [PubMed] [Google Scholar]
- Kessler P, Popp R, Busse R, Schini-Kerth VB. Proinflammatory mediators chronically downregulate the formation of the endothelium-derived hyperpolarizing factor in arteries via a nitric oxide/cyclic GMP-dependent mechanism. Circulation. 1999;99:1878–1884. doi: 10.1161/01.cir.99.14.1878. [DOI] [PubMed] [Google Scholar]
- Kirwin SJ, Kanaly ST, Linke NA, Edelman JL. Strain-dependent increases in retinal inflammatory proteins and photoreceptor FGF-2 expression in streptozotocin-induced diabetic rats. Invest Ophthalmol Vis Sci. 2009;50:5396–5404. doi: 10.1167/iovs.09-3474. [DOI] [PubMed] [Google Scholar]
- Koehler RC, Roman RJ, Harder DR. Astrocytes and the regulation of cerebral blood flow. Trends Neurosci. 2009;32:160–169. doi: 10.1016/j.tins.2008.11.005. [DOI] [PubMed] [Google Scholar]
- Kowluru RA, Engerman RL, Kern TS. Abnormalities of retinal metabolism in diabetes or experimental galactosemia VIII. Prevention by aminoguanidine. Curr Eye Res. 2000;21:814–819. doi: 10.1076/ceyr.21.4.814.5545. [DOI] [PubMed] [Google Scholar]
- Li Q, Puro DG. Diabetes-induced dysfunction of the glutamate transporter in retinal Müller cells. Invest Ophthalmol Vis Sci. 2002;43:3109–3116. [PubMed] [Google Scholar]
- Lieth E, Barber AJ, Xu B, Dice C, Ratz MJ, Tanase D, Strother JM. Glial reactivity and impaired glutamate metabolism in short-term experimental diabetic retinopathy. Diabetes. 1998;47:815–820. doi: 10.2337/diabetes.47.5.815. [DOI] [PubMed] [Google Scholar]
- Mandecka A, Dawczynski J, Blum M, Muller N, Kloos C, Wolf G, Vilser W, Hoyer H, Muller UA. Influence of flickering light on the retinal vessels in diabetic patients. Diabetes Care. 2007;30:3048–3052. doi: 10.2337/dc07-0927. [DOI] [PubMed] [Google Scholar]
- Metea MR, Newman EA. Glial cells dilate and constrict blood vessels: A mechanism of neurovascular coupling. J Neurosci. 2006;26:2862–2870. doi: 10.1523/JNEUROSCI.4048-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra A, Srienc AI, Clark BD, Newman EA. Oxygen modulates functional hyperemia in the retina. Soc Neurosci Abstr. 2010 (in press). [Google Scholar]
- Mizutani M, Gerhardinger C, Lorenzi M. Müller cell changes in human diabetic retinopathy. Diabetes. 1998;47:445–449. doi: 10.2337/diabetes.47.3.445. [DOI] [PubMed] [Google Scholar]
- Mizutani M, Kern TS, Lorenzi M. Accelerated death of retinal microvascular cells in human and experimental diabetic retinopathy. J Clin Invest. 1996;97:2883–2890. doi: 10.1172/JCI118746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulligan SJ, MacVicar BA. Calcium transients in astrocyte end-feet cause cerebrovascular constrictions. Nature. 2004;431:195–199. doi: 10.1038/nature02827. [DOI] [PubMed] [Google Scholar]
- Newman EA. Propagation of intercellular calcium waves in retinal astrocytes and Müller cells. J Neurosci. 2001;21:2215–2223. doi: 10.1523/JNEUROSCI.21-07-02215.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter JT, McCarthy KD. Hippocampal astrocytes in situ respond to glutamate released from synaptic terminals. J Neurosci. 1996;16:5073–5081. doi: 10.1523/JNEUROSCI.16-16-05073.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riva CE, Logean E, Falsini B. Visually evoked hemodynamical response and assessment of neurovascular coupling in the optic nerve and retina. Prog Ret Eye Res. 2005;24:183–215. doi: 10.1016/j.preteyeres.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Roman RJ. P-450 metabolites of arachidonic acid in the control of cardiovascular function. Physiol Rev. 2002;82:131–185. doi: 10.1152/physrev.00021.2001. [DOI] [PubMed] [Google Scholar]
- Rungger-Brandle E, Dosso AA. Streptozotocin-induced diabetes—A rat model to study involvement of retinal cell types in the onset of diabetic retinopathy. Adv Exp Med Biol. 2003;533:197–203. doi: 10.1007/978-1-4615-0067-4_25. [DOI] [PubMed] [Google Scholar]
- Takano T, Tian GF, Peng W, Lou N, Libionka W, Han X, Nedergaard M. Astrocyte-mediated control of cerebral blood flow. Nat Neurosci. 2006;9:260–267. doi: 10.1038/nn1623. [DOI] [PubMed] [Google Scholar]
- van Dijk HW, Kok PH, Garvin M, Sonka M, Devries JH, Michels RP, van Velthoven ME, Schlingemann RO, Verbraak FD, Abramoff MD. Selective loss of inner retinal layer thickness in type 1 diabetic patients with minimal diabetic retinopathy. Invest Ophthalmol Vis Sci. 2009;50:3404–3409. doi: 10.1167/iovs.08-3143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu DY, Cringle SJ, Su EN, Yu PK, Jerums G, Cooper ME. Pathogenesis and intervention strategies in diabetic retinopathy. Clin Experiment Ophthalmol. 2001;29:164–166. doi: 10.1046/j.1442-9071.2001.00409.x. [DOI] [PubMed] [Google Scholar]
- Zonta M, Angulo MC, Gobbo S, Rosengarten B, Hossmann KA, Pozzan T, Carmignoto G. Neuron-to-astrocyte signaling is central to the dynamic control of brain microcirculation. Nat Neurosci. 2003;6:43–50. doi: 10.1038/nn980. [DOI] [PubMed] [Google Scholar]