Abstract
Background
The amygdala is a key site where alterations in the regulation of the serotonin transporter (5-HTT) may alter stress response. Deficient 5-HTT function and abnormal amygdala activity have been hypothesized to contribute to the pathophysiology of posttraumatic stress disorder (PTSD), but no study has evaluated the 5-HTT in humans with PTSD. Based upon translational models, we hypothesized that patients diagnosed with PTSD would exhibit reduced amygdala 5-HTT expression as measured with positron emission tomography (PET) and the recently developed 5-HTT-selective radiotracer [11C]AFM.
Methods
Fifteen participants with PTSD and 15 healthy control (HC) subjects without trauma history underwent a resting-state PET scan.
Results
[11C]AFM binding potential (BPND) within the combined bilateral amygdala ROI was significantly reduced in the PTSD group compared to the HC group (p=0.027; 16.3% reduction), which was largely driven by the between-group difference in the left amygdala (p=0.008; 20.5% reduction). Further, amygdala [11C]AFM BPND was inversely correlated with both HAM-A scores (r=−0.55, p=0.035) and MARDS scores (r=−0.56, p=0.029).
Conclusions
Our findings of abnormally reduced amygdala 5-HTT binding in PTSD and its association with higher anxiety and depression symptoms in PTSD patients support a translational neurobiological model of PTSD directly implicating dysregulated 5-HTT signaling within neural systems underlying threat detection and fear learning.
Introduction
Brain serotonin (5-HT) systems have been linked to the neurobiology of posttraumatic stress disorder (PTSD) based upon evidence from both preclinical and clinical studies (1–6). In humans, the 5-HT agonist m-chlorophenylpiperazine (mCPP) was found to transiently evoke panic attacks and trauma-related flashbacks in patients with PTSD (7) that were not observed when mCPP was administered to patients with other psychiatric disorders (8–10). Moreover, the 5-HT transporter protein (5-HTT) is the target of the two U.S. Food and Drug Administration-approved pharmacotherapies for PTSD. The most direct evidence for the importance of 5-HTT function in PTSD can be inferred from recent human genetic studies showing that the short allele of the common repeat polymorphism in the promoter region of the gene coding for the 5-HTT (5-HTTLRP) increases the vulnerability to develop PTSD (1, 5, 11, 12), and may predict poor treatment outcome (13). However, to date no study has directly examined brain 5-HTT in patients with PTSD.
Fear conditioning experiments highlight the role of the amygdala as a key brain structure responsible for processing and storing fear-related memories and for coordinating fear-related behaviors (14–16), leading to the hypothesis that PTSD may be characterized by amygdala over-activity or hyper-responsiveness to threatening stimuli in humans (17–19). Indeed, a convergence of findings from functional neuroimaging investigations in clinical populations supports a neurocircuitry model of PTSD characterized by abnormally elevated amygdala activity coupled with deficient regulation by prefrontal cortical structures (20–27). Studies specifically suggest that amygdala function may be enhanced during the acquisition of conditioned fear in PTSD (26, 28), potentially leading to deficient fear extinction hypothesized to play a role in PTSD (19, 29). Despite an emerging neurocircuitry model of PTSD, the neurochemical regulation of this circuitry remains incompletely understood.
The amygdala is a major forebrain target of 5-HT neurons arising from the dorsal raphe (30) and 5-HT signaling within the amygdala regulates normal fear and threat responsiveness (2, 31, 32), supporting the hypothesis that abnormal 5-HTT function within the amygdala specifically may be an important mechanism in the pathophysiology of PTSD. In support of this hypothesis, common genetic variants which lead to differential expression of 5-HTT are associated with differences in the acquisition of a conditioned fear response and altered startle response in humans (33, 34). In aggregate, these data suggest a model whereby altered 5-HTT function influences amygdala activity to enhance the acquisition of conditioned fear and/or decrease fear extinction, which in turn mediates a vulnerability to PTSD.
Positron emission tomography (PET) imaging is the most direct, sensitive and straightforward means of probing the functional neurochemistry of human subjects and assessing molecular targets in the brain in vivo, provided the proper tracer is available. In the current study we utilized PET and the selective 5-HTT radioligand [11C]AFM (35, 36) to characterize 5-HTT receptor binding in patients with PTSD and matched healthy control (HC) subjects. Given that the low-expressing 5-HTT genotype (the short allele of the 5-HTTLRP) is associated with elevated risk for PTSD and the important role of the amygdala in fear-related neurocircuitry, we hypothesized reduced 5-HTT binding in the amygdala in patients with PTSD.
Methods
Subjects
Fifteen participants with PTSD and 15 age- and sex-matched HC participants without trauma history were recruited through public advertisement. After giving informed consent, participants were screened and diagnosed using Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) criteria and the Structured Clinical Interview for DSM-IV (SCID) (37, 38). PTSD participants suffered both combat and non-combat trauma exposures. Non-combat trauma exposure consisted of physical or sexual assault, domestic violence or natural disaster. PTSD participants were free of co-morbid psychiatric disorders, with the exception of major depressive disorder (MDD) if the primary diagnosis was determined to be PTSD, which was defined by PTSD being the dominant clinical syndrome and the onset of MDD occurred after the onset of PTSD. PTSD symptom severity was measured using the Clinician-Administered PTSD Scale for DSM-IV (CAPS) (39) and trauma history was quantified with the Traumatic Life Events Questionnaire (TLEQ) (40). Depression and anxiety severity was assessed using the Montgomery-Asberg Depression Rating Scale (MADRS) and the Hamilton Rating Scale for Anxiety (HAM-A), respectively (41, 42). All participants were evaluated by physical examination, electrocardiogram, standard laboratory tests, urine analysis and toxicology and were free of significant medical or neurological conditions. None of the participants were receiving psychotherapy or psychotropic medication for at least 4 weeks prior to scanning. The protocol was approved by the Yale University School of Medicine Human Investigation Committee, the Human Subjects Subcommittee of the Veterans Affairs Connecticut Healthcare System, the Magnetic Resonance Research Center and the Yale New Haven Hospital Radiation Safety Committee.
Scanning and Imaging Procedures
MR images were obtained for each subject on a Siemens 3T Trio system to exclude individuals with anatomical abnormalities and for co-registration. Participants subsequently underwent a resting PET scan with 20 mCi of [11C]AFM (35, 36). PET scans were done on a High Resolution Research Tomograph (HRRT) (Siemens Medical Solutions, Knoxville, TN, USA), which acquires 207 slices (1.2 mm slice separation) with a reconstructed image resolution of ~3 mm. Images were reconstructed with corrections for motion, attenuation, scatter, randoms, and deadtime. A summed image (0–10 min post-injection) was created from the motion-corrected PET data and registered to the subject’s MR image, which, in turn, was registered (12-parameter affine transformation) to an MR template (MNI space). The cerebellum ROI was taken from the template for SPM2 (Anatomical Automatic Labeling) and applied to the PET data to produce time- activity curves for the reference region (43). [11C]AFM, pixel-wise BPND images were created by SRTM2 (Simplified Reference Tissue Model). Amygdalar BPND values were extracted from the parametric images using the template. Results from test-retest studies using [11C]AFM BPND from the amygdala ROI demonstrate approximately 3% mean difference, indicating very good reliability (Williams W, et al., unpublished).
Statistical Analysis
Independent sample t-tests were used to compare continuous clinical, demographic variables and [11C]AFM BPND values between PTSD and HC. Data were normally distributed as determined by visual inspection and the Kolmogorov-Smirnov D test. Chi-square was used in the case of dichotomous variables. Tests of association between continuous variables were performed using Pearson’s product-moment correlations. All tests were performed two-tailed, with results considered significant at p<0.05. Means and standard deviations are reported. All statistical analyses were conducted using SPSS version 16.0 (SPSS Inc, Chicago, IL, USA).
Results
Demographics and Clinical Characteristics
Participants in the PTSD and HC groups were matched for age and gender frequency. Participants in the PTSD group had a history of combat (n=5) or non-combat (n=10) index trauma exposure. The mean age of onset of the first criteria A trauma was 15.6 ± 5.1 years (range 8–25) and participants suffered from 5.4 ± 3.0 lifetime criteria A traumas (range 1–25). Participants in the PTSD group experienced moderate to severe PTSD symptom severity as well as significant levels of depression and anxiety symptoms at the time of the PET scan (see Table 1).
Table 1.
Demographic, Clinical and Positron Emission Tomography Procedural Characteristics
| PTSD (n=15) | Healthy Control (n=15) | P Value | |
|---|---|---|---|
| Age (yrs) | 32.9 9.8 | 30.1 10.0 | 0.45 |
| Range | 21 – 51 | 18 – 49 | - |
| Sex (M, F) | 9M, 6F | 10M, 5F | 0.71 |
| Ethnicity | 4C, 5AA, 5H | 10C, 4AA | - |
| BMI | 29.7 5.2 | 26.5 4.6 | 0.09 |
| Smoking status (Y/N) | 2/15 | 1/15 | 0.54 |
| Index Trauma Type(Combat/Non-Combata) | 5/10 | - | - |
| No. Lifetime Criteria A Traumas | 5.4 ± 3.0 | - | |
| CAPS Total Score | 68.4 15.8 | - | - |
| CAPS Re-Experiencing Sub-Score | 18.8 5.6 | - | - |
| CAPS Avoidance Sub- Score | 27.5 8.1 | - | - |
| CAPS Hyperarousal Sub- Score | 22.1 6.2 | - | - |
| HAM-A Total Score | 18.5 6.9 | 2.5 5.1 | <0.001 |
| MADRS Total Score | 26.5 8.6 | 4.2 4.4 | <0.001 |
| Injected Dose (MBQ) | 710 38 | 699 53 | 0.53 |
| Specific Activity(MBQ/nmol) | 203 144 | 219 124 | 0.75 |
| Injected Mass (μg) | 1.57 1.04 | 1.34 0.85 | 0.52 |
Data presented in mean ± standard deviation, unless otherwise indicated. P values determined by independent sample t-tests for continuous variables or by chi-square for dichotomous variables.
AA, African-American; AS, Asian-American; BMI, body mass index; C, Caucasian; F, female; H, Hispanic; HAMA-A, Hamilton Anxiety Scale; M, male; MARDS, Montgomery-Asberg Depression Rating Scale; PTSD, posttraumatic stress disorder; N, nonsmoker; S, smoker.
Non-combat trauma exposure consisted of physical or sexual assault, domestic violence or natural disaster.
Neuroreceptor Imaging and Behavioral Correlations
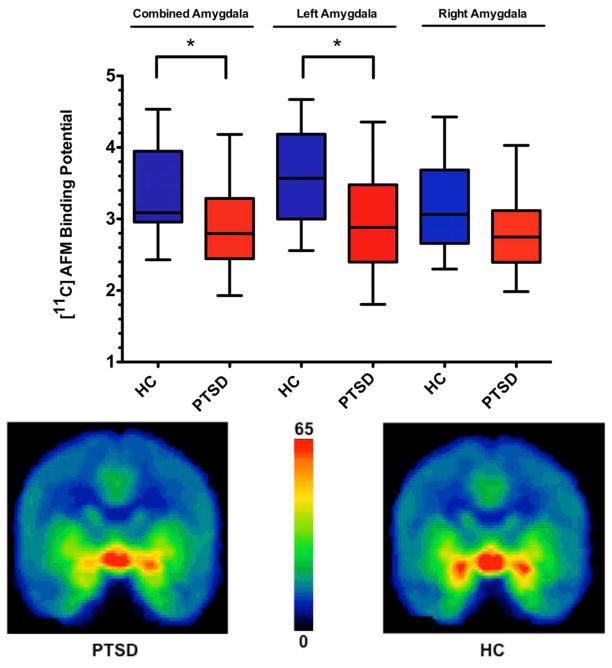
[11C]AFM BPND within the combined bilateral amygdala ROI was significantly reduced in the PTSD group compared to the HC group (HC: 3.38 ± 0.63, PTSD: 2.83 ± 0.64, df=28, t=2.33, p=0.027; 16.3% reduction). This finding was driven by the between- group [11C]AFM BPND difference in the left amygdala (HC: 3.61 ± 0.69, PTSD: 2.87 ± 0.73, df=28, t=2.9, p=0.008; 20.5% reduction). The between-group difference in the right amygdala did not reach statistical significance (HC: 3.17 ± 0.63, PTSD: 2.80 ± 0.59, df=28, t=1.65, p=0.11; 11.7% reduction) (Figure 1).
Figure 1. Reduced amygdala [11C]AFM BPND in PTSD compared to healthy subjects.
Upper panel: plot showing [11C]AFM binding potential (BPND) differences in the combined bilateral amygdala region of interest (ROI) and in both left and right amygdala ROI between patients with posttraumatic stress disorder (PTSD) and healthy control subjects (HC). * indicates p<0.05, two-tailed.
Lower panel: Averaged [11C]AFM positron emission tomography (PET) images (coronal view) illustrate reduced amygdala distribution volume in PTSD (left) relative to HC (right).
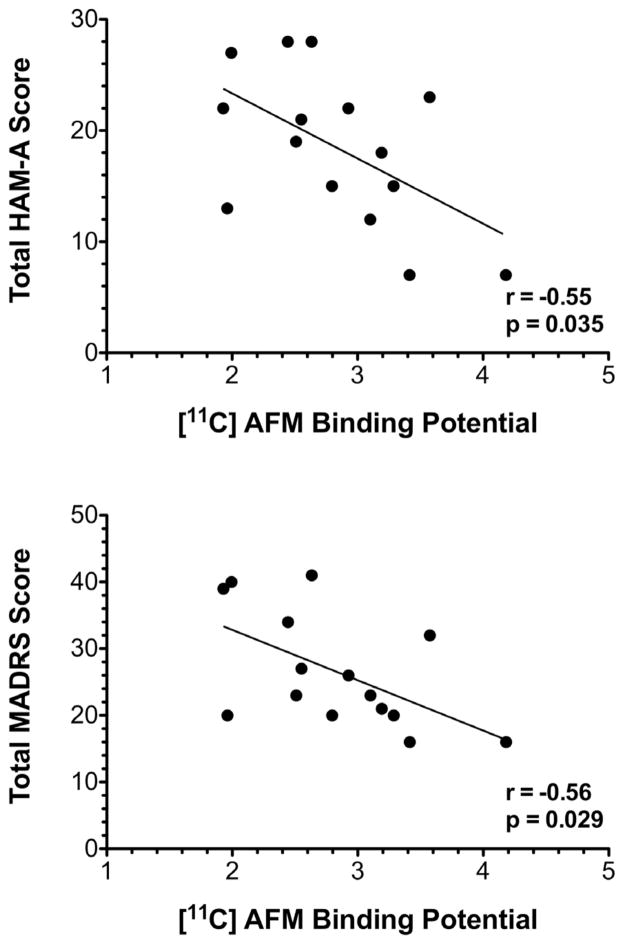
In the PTSD group, amygdala [11C]AFM BPND was inversely correlated with both HAM-A scores (r=−0.55, p=0.035) and MARDS scores (r=−0.56, p=0.029) (Figure 2). There was no correlation between [11C]AFM BPND and total CAPS score (r=−0.21, p=0.45) or sub-score. There were no associations between [11C]AFM BPND and age, gender or BMI in either group and no associations between [11C]AFM BPND and number or age of traumatization in the PTSD group.
Figure 2. Correlation between [11C]AFM BPND and levels of anxiety and depression in individuals with PTSD.
In the PTSD group (n=15) amygdala [11C]AFM BPND was inversely correlated with both Hamilton Rating Scale for Anxiety (HAM-A) scores (r=−0.55, p=0.035) and Montgomery-Asberg Depression Rating Scale (MADRS) scores (r=−0.56, p=0.029). Tests of association between continuous variables were performed using Pearson’s product-moment correlations.
Exploratory analyses of [11C]AFM BPND in brain regions outside of the amygdala did not reveal between-group differences any region (see Table 2).
Table 2.
Regional [11C]-AFM BPND in PTSD and Healthy Control Study Groups Outside of Amygdala a priori ROI
| PTSD (n=49) | Healthy Control(n=27) | df | t value | p value | |
|---|---|---|---|---|---|
| ACC | 0.97 ± 0.20 | 1.02 ± 0.15 | 28 | 0.74 | 0.47 |
| Caudate | 2.11 ± 0.60 | 2.11 ± 0.31 | 28 | 0.007 | 0.99 |
| Hippocampus | 1.21 ± 0.27 | 1.3 ± 0.32 | 28 | 0.69 | 0.49 |
| Occipital Cortex | 0.41 ± 0.09 | 0.46 ± 0.09 | 28 | 1.55 | 0.13 |
| Pallidum | 3.36 ± 0.66 | 3.22 ± 0.76 | 28 | 0.51 | 0.61 |
| Raphe | 6.78 ± 1.06 | 7.34 ± 0.80 | 28 | 1.60 | 0.12 |
| Thalamus | 3.06 ± 0.46 | 2.9 ± 0.72 | 28 | 0.70 | 0.49 |
Independent sample t-tests were used to compare [11C]AFM BPND values between PTSD and HC. Data presented in mean ± standard deviation. ACC: anterior cingulate cortex
Discussion
In this study we demonstrate in vivo reductions in 5-HTT availability in the amygdala with [11C]AFM BPND in patients with PTSD compared to healthy control participants. [11C]AFM BPND reductions are associated with characteristic features of the PTSD phenotype such that lower levels of ligand binding were associated with higher levels of both anxiety and depression. These results support the link between 5-HT regulation and the role of the amygdala in the pathophysiology of PTSD and are consistent with prior studies describing amygdala hyperactivity upon exposure to trauma- or fear-related stimuli in PTSD (20–22, 25, 26, 28). The reduced availability of 5-HTT in patients with PTSD is in line with prior animal studies that predict reductions in 5-HTT would be associated with increased fear (2, 31, 32).
The results of our study are consistent with a model of PTSD whereby reduced functioning of 5-HTT, resulting from inheritance of the lesser-expressing short allele of the 5-HTTLRP (1, 5, 11, 13, 44, 45) or other mechanism, leads to altered amygdala functioning which in turn drives increased anxiety and vulnerability to the effects of stress and trauma. Support for this model comes from preclinical studies showing deficient extinction recall, enhance behavioral vulnerability to stress and altered morphology of basolateral amygdala (BLA) in a 5-HTT knock-out (KO) mouse (2, 3). Conversely, overexpression of the human 5-HTT gene in transgenic mice resulted in a low-anxiety phenotype (46).
It is notable that in the study by Wellman et al (2007), 5-HTT KO mice showed a selective deficit in the recall of an extinguished fear memory 24 hours following a standard fear conditioning and extinction paradigm (2). This selective deficient in extinction mirrors the findings in human PTSD populations of persistently elevated fear responses often in the face of normal fear acquisition (29, 47–49). Further, a recent fear conditioning-extinction functional MRI (fMRI) study comparing PTSD to healthy volunteers found impaired extinction recall in PTSD 24 hours following the conditioning-extinction protocol as indexed by skin conductance response (SCR) whereas there was no difference in the acquisition or early extinction phase in congruence with the selective effects of 5-HTT KO on extinction recall reported Wellman et al (2007) (26). The authors also found greater amygdala activation in the PTSD during fear acquisition, consistent with the hypothesized role of enhanced amygdala activity driving the psychophysiological substrates of the vulnerability to PTSD (26). This finding replicated an earlier PET study of cerebral blood flow in women with childhood sexual abuse demonstrating elevated amygdala blood flow during fear acquisition in a conditioned fear paradigm (28).
Although our model suggests that altered 5-HTT function is a risk factor for PTSD, the current study was not designed to determine the causal relationship between ligand binding to 5-HTT and PTSD. Genetic studies linking reduced 5-HTT gene expression (50) with both increased amygdala activation during processing of emotional salient information with a fear-provoking component (51, 52) and vulnerability to PTSD (1, 5, 11, 13, 44, 45) provide compelling support for this model. However, stress and other environmental factors also may lower 5-HTT gene expression (53). Thus, reductions in 5-HTT may in fact predispose or be a consequence of extreme stress exposure and future studies will be necessary to test these alternative models.
There are several limitations of the current study. This study focused on the amygdala given its significance for PTSD and only secondarily explored [11C]AFM BPND in other regions outside our a priori ROI. Although we did not find evidence for abnormal binding outside of the amygdala, the sample size of the study may have limited our power to detect smaller differences between groups. Our findings of reduced amygdala [11C]AFM BPND in PTSD appeared to be driven by the left amygdala (20.5%), while reductions in the right amygdala (11.7%) did not reach statistical significance. The reason for the laterality of our finding is not clear, although it is possible that the lack of significance in the right amygdala represents a type II error given the modest sample size and the within-group variability in [11C]AFM BPND. Our study did not include a trauma-control group, leaving open the possibility that our findings reflect trauma exposure per se. Future studies utilizing a three-group design will be required to further explore this possibility. Whether our observation of low 5-HTT availability represents a state or trait finding is not addressed by the current cross-sectional study and future longitudinal studies are needed to address this important question. The absence of an association between [11C]AFM BPND and specific PTSD symptoms as measured by CAPS score, and in contrast the association between lower[11C]AFM BPND and both higher depression and anxiety symptoms, suggests that 5-HTT reductions contribute to but do not fully explain the complex phenotype of PTSD. Finally, we did not collect genetic data, which may have contributed to explaining the observed 5-HTT reductions in the PTSD cohort given the findings of human genetic studies.
Altogether, our findings support a translational neurobiological model implicating dysregulated amygdala 5-HTT signaling in the neurobiology of PTSD. Whether reduced 5-HTT binding is a pre-existing condition enhancing the vulnerability to develop PTSD after trauma, or is alternatively a consequence of trauma exposure, remains to be determined.
Acknowledgments
The project described was supported by the National Institutes of Health through the following awards: R21 MH085627, the Department of Veterans Affairs through its support of the Clinical Neurosciences Division of the VA National Center for PTSD, a VA Merit Review Grant and a NARSAD 2007 Independent Investigator Award to Dr. Neumeister. Dr. Murrough receives salary support through a Mount Sinai School of Medicine (MSSM) research fellowship funded with an educational grant from AstraZeneca provided to MSSM. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Mental Health of the National Institutes of Health, the Department of Veterans Affairs or NARSAD.
The authors acknowledge the excellent work of the staff of the Yale PET Center and the nursing support from Sue Kasserman, R.N. for help in recruitment and patient care and Brenda Breault, R.N., B.S.N. for her contributions with patient care during the PET scans.
Footnotes
All other co-authors report no biomedical financial interests or potential conflicts of interest.
Financial Disclosures
Dr. Krystal has been a consultant to the following companies: Aisling Capital, LLC, AstraZeneca Pharmaceuticals, Brintnall & Nicolini, Inc., Easton Associates, Gilead Sciences, Inc., GlaxoSmithKline, Janssen Pharmaceuticals, Lundbeck Research USA, Medivation, Inc., Merz Pharmaceuticals, MK Medical Communications, F. Hoffmann-La Roche Ltd, SK Holdings Co., Ltd, Takeda Industries, Teva Pharmaceutical Industries, Ltd.; and is on the scientific advisory board of the following companies: Abbott Laboratories, Bristol-Myers Squibb, Eisai, Inc., Eli Lilly and Co., Lohocla Research Corporation, Mnemosyne Pharmaceuticals, Naurex, Inc., Pfizer Pharmaceuticals, Shire Pharmaceuticals; Exercisable Warrant Options: Tetragenex Pharmaceuticals (value less than $150). Board of Directors: Coalition for Translational Research in Alcohol and Substance Use Disorders. President Elect: American College of Neuropsychopharmacology (12–2010). Research Support to Department of Veterans Affairs: Janssen Research Foundation (Provided drug and some study support to the Department of Veterans Affairs). Editorial Board: Income Greater than $10,000: Editor - Biological Psychiatry. Employment: Yale University School of Medicine, VA CT Healthcare System. Patents and Inventions: 1) Seibyl JP, Krystal JH, Charney DS. Dopamine and noradrenergic reuptake inhibitors in treatment of schizophrenia. Patent #:5,447,948. September 5, 1995 2) Co-inventor with Dr. Gerard Sanacora on a filed patent application by Yale University related to targeting the glutamatergic system for the treatment of neuropsychiatric disorders (PCTWO06108055A1). 3) Intranasal Administration of Ketamine to Treat Depression (pending).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kilpatrick DG, Koenen KC, Ruggiero KJ, Acierno R, Galea S, Resnick HS, et al. The serotonin transporter genotype and social support and moderation of posttraumatic stress disorder and depression in hurricane-exposed adults. Am J Psychiatry. 2007;164:1693–1699. doi: 10.1176/appi.ajp.2007.06122007. [DOI] [PubMed] [Google Scholar]
- 2.Wellman CL, Izquierdo A, Garrett JE, Martin KP, Carroll J, Millstein R, et al. Impaired stress-coping and fear extinction and abnormal corticolimbic morphology in serotonin transporter knock-out mice. J Neurosci. 2007;27:684–691. doi: 10.1523/JNEUROSCI.4595-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hariri AR, Holmes A. Genetics of emotional regulation: the role of the serotonin transporter in neural function. Trends Cogn Sci. 2006;10:182–191. doi: 10.1016/j.tics.2006.02.011. [DOI] [PubMed] [Google Scholar]
- 4.Holmes A. Genetic variation in cortico-amygdala serotonin function and risk for stress-related disease. Neurosci Biobehav Rev. 2008;32:1293–1314. doi: 10.1016/j.neubiorev.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xie P, Kranzler HR, Poling J, Stein MB, Anton RF, Brady K, et al. Interactive effect of stressful life events and the serotonin transporter 5-HTTLPR genotype on posttraumatic stress disorder diagnosis in 2 independent populations. Arch Gen Psychiatry. 2009;66:1201–1209. doi: 10.1001/archgenpsychiatry.2009.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Martin EI, Ressler KJ, Binder E, Nemeroff CB. The neurobiology of anxiety disorders: brain imaging, genetics, and psychoneuroendocrinology. Psychiatr Clin North Am. 2009;32:549–575. doi: 10.1016/j.psc.2009.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Southwick SM, Krystal JH, Bremner JD, Morgan CA, 3rd, Nicolaou AL, Nagy LM, et al. Noradrenergic and serotonergic function in posttraumatic stress disorder. Arch Gen Psychiatry. 1997;54:749–758. doi: 10.1001/archpsyc.1997.01830200083012. [DOI] [PubMed] [Google Scholar]
- 8.Charney DS, Goodman WK, Price LH, Woods SW, Rasmussen SA, Heninger GR. Serotonin function in obsessive-compulsive disorder. A comparison of the effects of tryptophan and m-chlorophenylpiperazine in patients and healthy subjects. Arch Gen Psychiatry. 1988;45:177–185. doi: 10.1001/archpsyc.1988.01800260095012. [DOI] [PubMed] [Google Scholar]
- 9.Krystal JH, Webb E, Cooney NL, Kranzler HR, Southwick SW, Heninger GR, et al. Serotonergic and noradrenergic dysregulation in alcoholism: m-chlorophenylpiperazine and yohimbine effects in recently detoxified alcoholics and healthy comparison subjects. Am J Psychiatry. 1996;153:83–92. doi: 10.1176/ajp.153.1.83. [DOI] [PubMed] [Google Scholar]
- 10.Price LH, Malison RT, McDougle CJ, McCance-Katz EF, Owen KR, Heninger GR. Neurobiology of tryptophan depletion in depression: effects of m-chlorophenylpiperazine (mCPP) Neuropsychopharmacology. 1997;17:342–350. doi: 10.1016/S0893-133X(97)00084-5. [DOI] [PubMed] [Google Scholar]
- 11.Lee HJ, Lee MS, Kang RH, Kim H, Kim SD, Kee BS, et al. Influence of the serotonin transporter promoter gene polymorphism on susceptibility to posttraumatic stress disorder. Depress Anxiety. 2005;21:135–139. doi: 10.1002/da.20064. [DOI] [PubMed] [Google Scholar]
- 12.Kolassa IT, Ertl V, Eckart C, Glockner F, Kolassa S, Papassotiropoulos A, et al. Association study of trauma load and SLC6A4 promoter polymorphism in posttraumatic stress disorder: evidence from survivors of the Rwandan genocide. J Clin Psychiatry. 2010;71:543–547. doi: 10.4088/JCP.08m04787blu. [DOI] [PubMed] [Google Scholar]
- 13.Bryant RA, Felmingham KL, Falconer EM, Pe Benito L, Dobson-Stone C, Pierce KD, et al. Preliminary evidence of the short allele of the serotonin transporter gene predicting poor response to cognitive behavior therapy in posttraumatic stress disorder. Biol Psychiatry. 2010;67:1217–1219. doi: 10.1016/j.biopsych.2010.03.016. [DOI] [PubMed] [Google Scholar]
- 14.Rogan MT, Staubli UV, LeDoux JE. Fear conditioning induces associative long-term potentiation in the amygdala. Nature. 1997;390:604–607. doi: 10.1038/37601. [DOI] [PubMed] [Google Scholar]
- 15.LeDoux JE. Emotion circuits in the brain. Annu Rev Neurosci. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- 16.Rodrigues SM, Schafe GE, LeDoux JE. Molecular mechanisms underlying emotional learning and memory in the lateral amygdala. Neuron. 2004;44:75–91. doi: 10.1016/j.neuron.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 17.Grillon C, Southwick SM, Charney DS. The psychobiological basis of posttraumatic stress disorder. Mol Psychiatry. 1996;1:278–297. [PubMed] [Google Scholar]
- 18.Phelps EA, LeDoux JE. Contributions of the amygdala to emotion processing: from animal models to human behavior. Neuron. 2005;48:175–187. doi: 10.1016/j.neuron.2005.09.025. [DOI] [PubMed] [Google Scholar]
- 19.Rauch SL, Shin LM, Phelps EA. Neurocircuitry models of posttraumatic stress disorder and extinction: human neuroimaging research--past, present, and future. Biol Psychiatry. 2006;60:376–382. doi: 10.1016/j.biopsych.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 20.Rauch SL, Whalen PJ, Shin LM, McInerney SC, Macklin ML, Lasko NB, et al. Exaggerated amygdala response to masked facial stimuli in posttraumatic stress disorder: a functional MRI study. Biol Psychiatry. 2000;47:769–776. doi: 10.1016/s0006-3223(00)00828-3. [DOI] [PubMed] [Google Scholar]
- 21.Shin LM, Orr SP, Carson MA, Rauch SL, Macklin ML, Lasko NB, et al. Regional cerebral blood flow in the amygdala and medial prefrontal cortex during traumatic imagery in male and female Vietnam veterans with PTSD. Arch Gen Psychiatry. 2004;61:168–176. doi: 10.1001/archpsyc.61.2.168. [DOI] [PubMed] [Google Scholar]
- 22.Shin LM, Wright CI, Cannistraro PA, Wedig MM, McMullin K, Martis B, et al. A functional magnetic resonance imaging study of amygdala and medial prefrontal cortex responses to overtly presented fearful faces in posttraumatic stress disorder. Arch Gen Psychiatry. 2005;62:273–281. doi: 10.1001/archpsyc.62.3.273. [DOI] [PubMed] [Google Scholar]
- 23.Bremner JD, Vermetten E, Schmahl C, Vaccarino V, Vythilingam M, Afzal N, et al. Positron emission tomographic imaging of neural correlates of a fear acquisition and extinction paradigm in women with childhood sexual-abuse-related post-traumatic stress disorder. Psychol Med. 2005;35:791–806. doi: 10.1017/s0033291704003290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Etkin A, Wager TD. Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. Am J Psychiatry. 2007;164:1476–1488. doi: 10.1176/appi.ajp.2007.07030504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liberzon I, Sripada CS. The functional neuroanatomy of PTSD: a critical review. Prog Brain Res. 2008;167:151–169. doi: 10.1016/S0079-6123(07)67011-3. [DOI] [PubMed] [Google Scholar]
- 26.Milad MR, Pitman RK, Ellis CB, Gold AL, Shin LM, Lasko NB, et al. Neurobiological basis of failure to recall extinction memory in posttraumatic stress disorder. Biol Psychiatry. 2009;66:1075–1082. doi: 10.1016/j.biopsych.2009.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shin LM, Liberzon I. The neurocircuitry of fear, stress, and anxiety disorders. Neuropsychopharmacology. 2010;35:169–191. doi: 10.1038/npp.2009.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bremner JD, Vermetten E, Schmahl C, Vaccarino V, Vythilingam M, Afzal N, et al. Positron emission tomographic imaging of neural correlates of a fear acquisition and extinction paradigm in women with childhood sexual-abuse-related post-traumatic stress disorder. Psychol Med. 2005;35:791–806. doi: 10.1017/s0033291704003290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Orr SP, Metzger LJ, Lasko NB, Macklin ML, Peri T, Pitman RK. De novo conditioning in trauma-exposed individuals with and without posttraumatic stress disorder. J Abnorm Psychol. 2000;109:290–298. [PubMed] [Google Scholar]
- 30.Jacobs BL, Azmitia EC. Structure and function of the brain serotonin system. Physiol Rev. 1992;72:165–229. doi: 10.1152/physrev.1992.72.1.165. [DOI] [PubMed] [Google Scholar]
- 31.Muller JM, Morelli E, Ansorge M, Gingrich JA. Serotonin transporter deficient mice are vulnerable to escape deficits following inescapable shocks. Genes Brain Behav. 2011;10:166–175. doi: 10.1111/j.1601-183X.2010.00652.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zanoveli JM, Carvalho MC, Cunha JM, Brandao ML. Extracellular serotonin level in the basolateral nucleus of the amygdala and dorsal periaqueductal gray under unconditioned and conditioned fear states: an in vivo microdialysis study. Brain Res. 2009;1294:106–115. doi: 10.1016/j.brainres.2009.07.074. [DOI] [PubMed] [Google Scholar]
- 33.Lonsdorf TB, Weike AI, Nikamo P, Schalling M, Hamm AO, Ohman A. Genetic gating of human fear learning and extinction: possible implications for gene-environment interaction in anxiety disorder. Psychol Sci. 2009;20:198–206. doi: 10.1111/j.1467-9280.2009.02280.x. [DOI] [PubMed] [Google Scholar]
- 34.Brocke B, Armbruster D, Muller J, Hensch T, Jacob CP, Lesch KP, et al. Serotonin transporter gene variation impacts innate fear processing: Acoustic startle response and emotional startle. Mol Psychiatry. 2006;11:1106–1112. doi: 10.1038/sj.mp.4001908. [DOI] [PubMed] [Google Scholar]
- 35.Huang Y, Hwang DR, Narendran R, Sudo Y, Chatterjee R, Bae SA, et al. Comparative evaluation in nonhuman primates of five PET radiotracers for imaging the serotonin transporters: [11C]McN 5652, [11C]ADAM, [11C]DASB, [11C]DAPA, and [11C]AFM. J Cereb Blood Flow Metab. 2002;22:1377–1398. doi: 10.1097/01.WCB.0000040948.67415.05. [DOI] [PubMed] [Google Scholar]
- 36.Huang Y, Hwang DR, Bae SA, Sudo Y, Guo N, Zhu Z, et al. A new positron emission tomography imaging agent for the serotonin transporter: synthesis, pharmacological characterization, and kinetic analysis of [11C]2-[2-(dimethylaminomethyl)phenylthio]-5-fluoromethylphenylamine ([11C]AFM) Nucl Med Biol. 2004;31:543–556. doi: 10.1016/j.nucmedbio.2003.11.008. [DOI] [PubMed] [Google Scholar]
- 37.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis Disorders (SCID) New York: New York State Psychiatric Institute, Biometrics Research; 1995. [Google Scholar]
- 38.American Psychiatric Association. Task Force on DSM-IV. Diagnostic and statistical manual of mental disorders: DSM-IV-TR. 4. Washington, DC; New York: American Psychiatric Association; 2000. text revision. [Google Scholar]
- 39.Blake DD, Weathers FW, Nagy LM, Kaloupek DG, Gusman FD, Charney DS, et al. The development of a Clinician-Administered PTSD Scale. J Trauma Stress. 1995;8:75–90. doi: 10.1007/BF02105408. [DOI] [PubMed] [Google Scholar]
- 40.Kubany ES, Haynes SN, Leisen MB, Owens JA, Kaplan AS, Watson SB, et al. Development and preliminary validation of a brief broad-spectrum measure of trauma exposure: the Traumatic Life Events Questionnaire. Psychol Assess. 2000;12:210–224. doi: 10.1037//1040-3590.12.2.210. [DOI] [PubMed] [Google Scholar]
- 41.Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382–389. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- 42.HAMILTON M. The assessment of anxiety states by rating. Br J Med Psychol. 1959;32:50–55. doi: 10.1111/j.2044-8341.1959.tb00467.x. [DOI] [PubMed] [Google Scholar]
- 43.Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- 44.Grabe HJ, Spitzer C, Schwahn C, Marcinek A, Frahnow A, Barnow S, et al. Serotonin transporter gene (SLC6A4) promoter polymorphisms and the susceptibility to posttraumatic stress disorder in the general population. Am J Psychiatry. 2009;166:926–933. doi: 10.1176/appi.ajp.2009.08101542. [DOI] [PubMed] [Google Scholar]
- 45.Mellman TA, Alim T, Brown DD, Gorodetsky E, Buzas B, Lawson WB, et al. Serotonin polymorphisms and posttraumatic stress disorder in a trauma exposed African American population. Depress Anxiety. 2009;26:993–997. doi: 10.1002/da.20627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jennings KA, Loder MK, Sheward WJ, Pei Q, Deacon RM, Benson MA, et al. Increased expression of the 5-HT transporter confers a low-anxiety phenotype linked to decreased 5-HT transmission. J Neurosci. 2006;26:8955–8964. doi: 10.1523/JNEUROSCI.5356-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wessa M, Flor H. Failure of extinction of fear responses in posttraumatic stress disorder: evidence from second-order conditioning. Am J Psychiatry. 2007;164:1684–1692. doi: 10.1176/appi.ajp.2007.07030525. [DOI] [PubMed] [Google Scholar]
- 48.Rothbaum BO, Davis M. Applying learning principles to the treatment of post-trauma reactions. Ann N Y Acad Sci. 2003;1008:112–121. doi: 10.1196/annals.1301.012. [DOI] [PubMed] [Google Scholar]
- 49.Milad MR, Orr SP, Lasko NB, Chang Y, Rauch SL, Pitman RK. Presence and acquired origin of reduced recall for fear extinction in PTSD: results of a twin study. J Psychiatr Res. 2008;42:515–520. doi: 10.1016/j.jpsychires.2008.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lesch KP, Bengel D, Heils A, Sabol SZ, Greenberg BD, Petri S, et al. Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science. 1996;274:1527–1531. doi: 10.1126/science.274.5292.1527. [DOI] [PubMed] [Google Scholar]
- 51.Hariri AR, Mattay VS, Tessitore A, Kolachana B, Fera F, Goldman D, et al. Serotonin transporter genetic variation and the response of the human amygdala. Science. 2002;297:400–403. doi: 10.1126/science.1071829. [DOI] [PubMed] [Google Scholar]
- 52.Hariri AR, Drabant EM, Munoz KE, Kolachana BS, Mattay VS, Egan MF, et al. A susceptibility gene for affective disorders and the response of the human amygdala. Arch Gen Psychiatry. 2005;62:146–152. doi: 10.1001/archpsyc.62.2.146. [DOI] [PubMed] [Google Scholar]
- 53.Greenwood BN, Foley TE, Day HE, Burhans D, Brooks L, Campeau S, et al. Wheel running alters serotonin (5-HT) transporter, 5-HT1A, 5-HT1B, and alpha 1b-adrenergic receptor mRNA in the rat raphe nuclei. Biol Psychiatry. 2005;57:559–568. doi: 10.1016/j.biopsych.2004.11.025. [DOI] [PubMed] [Google Scholar]